Abstract
Machine learning (ML) is a software solution with the ability of making predictions without prior explicit programming, aiding in the analysis of large amounts of data. These algorithms can be trained through supervised or unsupervised learning. Cardiology is one of the fields of medicine with the highest interest in its applications. They can facilitate every step of patient care, reducing the margin of error and contributing to precision medicine. In particular, ML has been proposed for cardiac imaging applications such as automated computation of scores, differentiation of prognostic phenotypes, quantification of heart function and segmentation of the heart. These tools have also demonstrated the capability of performing early and accurate detection of anomalies in electrocardiographic exams. ML algorithms can also contribute to cardiovascular risk assessment in different settings and perform predictions of cardiovascular events. Another interesting research avenue in this field is represented by genomic assessment of cardiovascular diseases. Therefore, ML could aid in making earlier diagnosis of disease, develop patient-tailored therapies and identify predictive characteristics in different pathologic conditions, leading to precision cardiology.
Keywords: Cardiac imaging techniques, Cardiology, Electrocardiography, Machine learning, Review
1. Introduction
In the past years, there has been an increasing interest for artificial intelligence (AI) as it offers promising applications in medicine. Machine learning (ML) is a subset of AI, characterized by the ability of software to learn from data and make predictions without explicit prior programming.[1] It is based on algorithms which can be divided based on learning type. In supervised learning, labelled data is available for the training process thus creating a ground truth. In unsupervised learning, the software finds hidden structure in data automatically without prior categorization of the training set. In reinforcement learning, positive and negative reinforcement contribute to the improvement of the predictive model.[2] In other words, it learns from the consequences of its interactions with a dynamic environment. Cluster analysis is an unsupervised ML subtype which enables the creation of homogenous instance groups as it finds hidden relations in data. It could be useful to discover relevant features in diseases with heterogenous phenotypes.[3] A ML algorithm could also be constituted by combinations of different types of learning or classifiers.
Neural networks are themselves a subfield of ML, modelled on the way human's process information.[4] Each neuron (i.e., node) of the network stores a numeric value and all of them form the network architecture. A connection between neurons represents a weight, which represents the strength of connections between neurons. The final layer encodes the desired outcomes. A neural network which contains more than one hidden layer is defined as “deep”, thus enabling higher-level abstractions.[5]
ML has potential applications in multiple fields of medicine, though it has been most frequently applied to imaging. In this setting, it has shown promising performance in cancer patients for lesion detection, characterization and staging.[6]–[10] ML analysis has also been used in non-oncologic pathology, with promising results.[11],[12] Another interesting aim of ML is represented by the automated segmentation of medical images. Though manual segmentation represents the gold standard, AI tools might have higher accuracy and reproducibility.[13]
Although AI has been widely used in imaging, it has other applications as it enables fast and accurate analysis of large amounts of data. This ability can be employed to create predictive models both for classification and regression. Over the years, studies have demonstrated the promise of AI in other fields of medicine.[14]–[17] Hence, it can aid the development of personalized medicine, longevity prediction and treatment failure.[18] ML might also be used to create platforms that help healthcare coordination, based on demographic and socio-economic factors.[19] Moreover, it could be effective in identifying patients who are likely to be nonadherent with medication through the extensive analysis of both pharmacy and clinical data, thus tailoring clinical follow-up.[20]
ML could provide a set of tools that can enhance cardiologist's workflow, as the amount of data available in clinical practice has increased. It can potentially facilitate every step of patient care and make it more efficient.[21] Aim of our narrative review is to give an overview of the current and possible future applications of ML in cardiology, including diagnostic imaging, electrocardiography (ECG), patient cardiovascular risk assessment and prognosis prediction.
2. Applications in cardiology
2.1. Imaging
AI might help the diagnostic work-up of cardiovascular diseases. For example, neural networks classifiers could facilitate the detection of patterns of congestive heart failure on chest radiographs.[22] The study conducted by Seah, et al.,[22] is particularly interesting, as it focused on obtaining a direct visualization of the characteristics used to make the prediction through the use of a generative adversarial network. These allow for the creation of a visual output, in this case employed to highlight relevant abnormal features in chest X-rays.
ML could also be applied to echocardiography. It has been proposed to calculate aortic valve area in aortic stenosis automatically or help the differentiation of different prognostic phenotypes.[23] Narula, et al.[24] used ML to differentiate hypertrophic cardiomyopathy from normal heart hypertrophy in athletes. They studied a cohort of 139 males who underwent 2D-echocardiography and their classifier achieved an overall sensitivity of 87% and specificity of 82%. Deep learning could also help classifying echocardiography views as shown by Madani and colleagues. They trained a convolutional neural network to recognize 15 standard echocardiographic views, using a training and validation set of over 200,000 images and a test set of 20,000. It outperformed board-certified echocardiographers with an overall accuracy of 91.7%.[25] Deep Learning has also been employed to detect and characterize myocardial delayed enhancement on magnetic resonance imaging (MRI). This feature can reveal myocardial disease and help in the differential diagnosis of ischemic and non-ischemic cardiomyopathy. They analyzed a cohort of 200 patients obtaining an accuracy ranging between 78.9%–82.1%.[26] Though these results are not sufficient for introduction in daily clinical practice, they represent promising applications that could be further developed with the availability of multi-institutional and larger datasets.
Another interesting application is automated computation of scores and quantification of heart function. González, et al.[27] developed a convolutional neural network to calculate the Agatston score from a database of 5,973 unenhanced chest CT exams, without prior segmentation of coronary artery calcifications. When compared to standard methods, they were able to obtain the computation of the score faster and more accurately (Pearson correlation coefficient: 0.923). Deep learning has also shown promising performance for automatic calculation of left ventricular function. Tao, et al.[28] trained a convolutional neural network on a dataset of 596 MRI examinations acquired in different institutions and on scanners from various vendors to create a tool which outperformed manual segmentation. Moreover, its accuracy increased with the heterogeneity of included patients.
ML can also be used for automated segmentation of the heart. Segmentation of the epicardium and endocardium from the left ventricle is important for the assessment of the cardiovascular system function. Ngo, et al.[29] used ML for automated segmentation of heart using a dataset of 45 cardiac cine magnetic resonance with ischemic and non-ischemic heart failure, left ventricle hypertrophy and normal cases. Its accuracy was comparable to standard methods.
Table 1 provides a summary of the main findings of the reported studies.
Table 1. Overview of ML algorithms applied to imaging.
| Authors | ML algorithm | Aim | Performance |
| Seah, et al.[22] | Neural network | To visualize chest radiograph features of congestive heart failure | AUC: 0.82 |
| Playford, et al.[23] | Multidimensional clusters | To infer aortic valve area from other echocardiographic data, without the need for any left ventricular outflow tract measurements | AUC: 0.95 |
| AUPRC: 0.73 | |||
| Narula, et al.[24] | Support vector machine | Automated discrimination of hypertrophic cardiomyopathy from physiological hypertrophy of the athletes | Overall sensitivity: 87% |
| Random forest | Overall specificity: 82% | ||
| Artificial neural network | |||
| Madani, et al.[25] | Neural network | View classification of echocardiograms | Accuracy: 97.8% |
| Otha, et al.[26] | Convolutional neural network | To detect and classify myocardial delayed enhancement pattern | Accuracy: 87.2%–88.9% |
| González, et al.[27] | Convolutional neural network | To calculate Agatston score from non-enhanced chest CT without prior segmentation of coronary artery calcification | Pearson correlation coefficient between the reference standard and the computed scores on the test set: 0.932 |
| Tao, et al.[28] | Convolutional neural network | Fully automated quantification of LV from cine MR and to evaluate its performance in a multivendor and multicenter setting | The average perpendicular distance compared with manual analysis was 1.1 ± 0.3 mm |
| Ngo, et al.[29] | Deep neural network | Automated segmentation of LV from cine magnetic resonance imaging | It outperformed manual segmentation |
AUC: area under the curve; AUPRC: area under the precision recall curve; LV: left ventricle; ML: machine learning.
2.2. ECG
Another interesting field of application of ML in cardiology is the automatic detection of anomalies of electrocardiogram, which could be extremely useful as there has been an increase of wearable devices. Isin, et al.[30] used deep learning algorithm for automated detection of arrhythmia on ECG using an online dataset of over 4,000 long-term ECG Holter recordings, including rare conditions. It showed a correct recognition rate of 98.5% and accuracy of 92%. Convolutional neural networks could help identifying patients with asymptomatic left ventricular systolic dysfunction from ECG as well.[31] Galloway, et al.[32] used ML for the screening of hyperkalemia in patients with chronic kidney disease from ECG retrieved from three Mayo Clinic centers in Minnesota, Florida, and Arizona. They analyzed a database of 449,380 patients of different hospitals and obtained a high sensitivity (area under the Receiver Operating Characteristics curve, AUC range: 0.853–0.883). In Table 2, a summary of the reviewed papers is presented.
Table 2. Overview of ML algorithms used to assess ECG analysis.
| Authors | ML algorithm | Aim | Performance |
| Isin, et al.[30] | Deep neural network | To detect automatically arrhythmia on ECG | Correct recognition rate: 98.5% |
| Accuracy: 92% | |||
| Attia, et al.[31] | Convolutional neural network | To identify asymptomatic left ventricular systolic dysfunction | AUC: 0.93 |
| Sensitivity: 86.3% | |||
| Specificity: 85.7% | |||
| Accuracy: 85.7% | |||
| Galloway, et al.[32] | Convolutional neural network | Screening of hyperkalemia in patients with chronic kidney disease | AUC: 0.853–0.883 |
AUC: area under the curve; ECG: electrocardiography; ML: machine learning.
2.3. Risk assessment and predictions
An important challenge for ML is to aid cardiologists in making accurate predictions and assess cardiovascular risk in different settings thus leading to personalized medicine. Przewlocka-Kosmala, et al.[33] used a ML classifier to identify prognostic phenotypes among patients with heart failure and preserved ejection fraction. Deep learning could also be used to create tools capable of predicting specific cardiovascular events. Kwon, et al.[34] developed a deep learning algorithm to detect in-hospital cardiac arrest and death without attempted resuscitation. They used a dataset of 52,131 patients admitted to two different hospitals over a period of 91 months. It outperformed standard methods (AUC: 0.850; area under the Precision Recall Curve: 0.044), showing higher sensitivity and lower false alarm rates. A ML-based model has shown promising results in predicting in-hospital length of stay among cardiac patients with high accuracy (80%) and sensitivity (80%).[35] Mortazavi, et al.[36] investigated whether ML could help predict 30-day all-cause of hospital readmission of patients with heart failure. Though it performed better than classical statistical analysis, the difference was not sufficient for its introduction in daily clinical practice probably because several other elements should be taken into account during the development of the algorithm. Another possible application of ML is risk assessment of ventricular arrhythmia in hypertrophic cardiomyopathy, though its performance is still not sufficient for clinical practice as well.[37]
The real challenge is to characterize cardiovascular risk in asymptomatic population. This requires the extensive evaluation of numerous variables in order to identify patterns which are possibly not recognizable by standard statistical analysis. Different studies have shown high potential of ML in this field. In particular, Alaa, et al.[38] created an automated ML tool based on a dataset of more than 400,000 people and over 450 variables. It improves cardiovascular risk prediction compared to Framingham score. Plus, it discovered new cardiovascular risk predictors and interactions between different features of an individual.
Table 3 shows an overview of the above-mentioned studies.
Table 3. ML classifiers used to evaluate risk assessment and make predictions.
| Authors | ML algorithms | Aim | Performance |
| Przewlocka-Kosmala, et al.[33] | Cluster analysis | To identify prognostic phenotypes among patients with heart failure and preserved ejection fraction | Lower left ventricle systolic reserve may have a prognostic role in heart failure and preserved ejection fraction |
| Kwon, et al.[34] | Deep neural network | To detect in-hospital cardiac arrest and death without attempted resuscitation | AUC: 0.85 |
| AUPRC: 0.044 | |||
| Daghistani, et al.[35] | Random forest | To predict in-hospital length of stay among cardiac patients | Random forest outperformed among the other models with |
| Artificial neural network | Sensitivity: 80% | ||
| Support vector machine | Accuracy: 80% | ||
| Bayesian network | AUC: 0.94 | ||
| Mortazavi, et al.[36] | Logistic regression | To predict 30-day all-cause of hospitalreadmission of patients with heart failure | Random forest outperformedclassical statistical methods |
| Poisson regression | |||
| Random forest | |||
| Boosting | |||
| Bhattacharya, et al.[37] | Logistic regression | To assess the risk of ventricular arrhythmia in hypertrophic cardiomyopathy | Sensitivity: 0.73 |
| Naıve bayes | Specificity: 0.76 | ||
| Decision tree | AUC: 0.83 | ||
| Random forest | |||
| Alaa, et al.[38] | Supprto vector machines | To evaluate cardiovascular risk in asymptomatic people | AUC: 0.724 |
| Random forest | |||
| Neural network | |||
| AdaBoost | |||
| Gradient boosting |
AUC: area under the curve; AUPRC: area under the precision recall curve; ML: machine learning.
2.4. Genomics
One of the main aims of genomics is to characterize gene function, identifying connections between genotype and phenotype. This is crucial for the creation of predictive models and the development of precision medicine, but the complexity of DNA still constitutes a limit. Deep learning could be used to make accurate and fast large-scale genome-wide association studies.[39],[40]
Oguz, et al.[41] developed a neural network to predict advanced coronary artery calcium through a large-scale genome-wide association study of single-nucleotide polymorphism. They analyzed both clinical and genotype data. Furthermore, they tested their model on multiple network typologies, with high accuracy (AUC > 0.8).
Genetics is thought to play a major role in atherosclerosis, as an increased number of long non-coding RNA has been associated to its pathogenesis. Many of the tools employed to make these analysis are based on ML.[42]
Burghardt, et al.[43] used neural network to analyze SNPs associated to inheritable heart diseases. Ventricular myosin and cardiac myosin binding protein C emerged to be the most frequent implicated proteins. Therefore, this tool can be employed to identify genotypes associated with precocious or more severe phenotypes of heart diseases.
2.5. Current applications
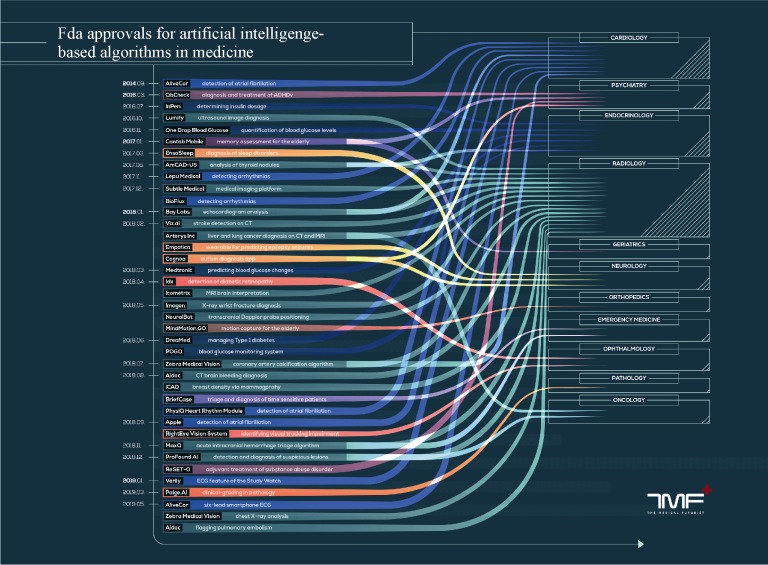
Several AI algorithms have already been approved for medical usage by the American Food and Drug Administration. This does not necessarily implicate that a software is available for daily clinical practice as both de novo and premarket approvals are possible. The first is used for novel devices which still require demonstration of safety and effectiveness. The second is the process of review and validation for practical clinical use. There are seven algorithms approved for cardiology up to June 2019, second only to the field of radiology in medicine. In particular, two of these have been developed for early and remote detection of atrial fibrillation, two for automated diagnosis of arrhythmias, one for echocardiogram analysis and one for coronary artery calcification detection and quantification (Figure 1).[44]
Figure 1. FDA-approved AI software for medical usage as of June 2019.
Courtesy of the medical futurist (Creative commons 4.0 license). AI: artificial intelligence; FDA: Food and Drug Administration (American).
It should also be noted that commercially available software employing machine learning are also currently employed. For example, an application is represented by cardiac motion analysis on MRI, though significant differences in most parameters are still present. Hence, the introduction of a more robust, standard method of validation is still necessary.[45]
3. Discussion
Techniques employing AI could become essential for improving cardiologist workflow and performance in all aspects of daily practice. Moreover, predictive ML algorithms could help in making earlier disease diagnosis, in turn improving prognosis. For comparison, promising results have recently emerged for the early diagnosis of Alzheimer disease, which is extremely prevalent among the elderly.[46]
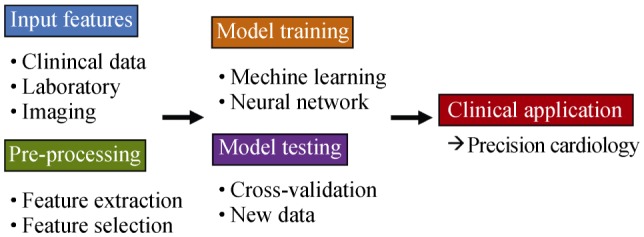
As shown in our review, the development pipeline of ML tools usually follows a typical path. Clinical and instrumental data are employed to train a classifier in a specific task. If the dataset is large, usually a dimensionality reduction technique is necessary for the identification of useful information and elimination of potentially harmful redundancies. During the training phase, in which different algorithms may be compared, different training and testing schemes may be chosen. A popular approach consists in k-fold cross-validation, in which data is divided in k subsets followed by training on k-1 patients and testing on the remaining.[47] This subsampling allows to improve algorithm generalization through multiple tests from which an average accuracy can be derived, in comparison to the information obtainable from a single train-test data split. Ideally, the final step should consist of testing the model on an external dataset, possibly obtained from a different Institutions, to correctly assess its usefulness in clinical practice (Figure 2).
Figure 2. Schematic depiction of a typical machine learning algorithm development and testing pipeline.
3.1. Limitations
AI still presents limitations that should be acknowledged. Firstly, the training phase often requires large amount of data, good informatics skills and an adequate definition of the reference standard.[48] Secondly, models might change over time thus requiring frequent updating. Another relevant problem in their training is overfitting. It occurs when the algorithm is excessively tailored to the training sample, rather than finding actually relevant features and relations. Hence, it makes almost perfect predictions on it, but at the price of generalization, therefore its performance decreases on other populations. This issue can be solved by the inclusion of more data or subtle modifications to the training set.[49] During the training, large amounts of low-quality data are often used limiting the potential of classifiers, as they may find patterns which are not useful in real-life clinical practice.[50] Another significant barrier to the adoption of ML algorithms is represented by their black box nature, i.e., the difficulty, especially for clinicians, in understanding and therefore trusting the interpretation of data leading to the result. This last limitation may represent the largest hurdle to overcome for the future development of AI in medicine.
Despite these limitations, if the current trend of ML tool development and validation continues, they could become essential for every step of patient management. This could also lead to personalized medicine enabling the elaboration of patient-tailored therapies and identification of currently unknown predictive phenotypes of various health conditions.[51]
3.2. Conclusion
In the near future, the increasing use of ML tools in cardiologist daily practice will probably continue. After adequate validation, these could potentially facilitate daily workflow, patient satisfaction, early detection and correct interpretation of findings, leading to improved patient outcomes.
Acknowledgments
All authors had no conflicts of interest to disclose.
References
- 1.Cuocolo R, Ugga L. Imaging applications of artificial intelligence. Health Manage J. 2018;18:484–487. [Google Scholar]
- 2.Choy G, Khalilzadeh O, Michalski M, et al. Current applications and future impact of machine learning in radiology. Radiology. 2018;288:318–328. doi: 10.1148/radiol.2018171820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1317–1335. doi: 10.1016/j.jacc.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaharchuk G, Gong E, Wintermark M, et al. Deep learning in neuroradiology. AJNR Am J Neuroradiol. 2018;39:1776–1784. doi: 10.3174/ajnr.A5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Chang P, Grinband J, Weinberg BD, et al. Deep-learning convolutional neural networks accurately classify genetic mutations in gliomas. AJNR Am J Neuroradiol. 2018;39:1201–1207. doi: 10.3174/ajnr.A5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanzione A, Cuocolo R, Cocozza S, et al. Detection of extraprostatic extension of cancer on biparametric MRI combining texture analysis and machine learning: preliminary results. Acad Radiol. doi: 10.1016/j.acra.2018.12.025. Published Online First: 14 January 2019. [DOI] [PubMed] [Google Scholar]
- 8.Ciompi F, Chung K, van Riel SJ, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep. 2017;7:46479–46479. doi: 10.1038/srep46479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357–366. doi: 10.1016/j.crad.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Romeo V, Maurea S, Cuocolo R, et al. Characterization of adrenal lesions on unenhanced MRI using texture analysis: a machine-learning approach. J Magn Reson Imaging. 2018;48:198–204. doi: 10.1002/jmri.25954. [DOI] [PubMed] [Google Scholar]
- 11.Romeo V, Ricciardi C, Cuocolo R, et al. Machine learning analysis of MRI-derived texture features to predict placenta accreta spectrum in patients with placenta previa. Magn Reson Imaging. doi: 10.1016/j.mri.2019.05.017. Published Online First: 15 May 2019. [DOI] [PubMed] [Google Scholar]
- 12.Islam MM, Nasrin T, Walther BA, et al. Prediction of sepsis patients using machine learning approach: a meta-analysis. Comput Methods Programs Biomed. 2019;170:1–9. doi: 10.1016/j.cmpb.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Deniz CM, Xiang S, Hallyburton RS, et al. Segmentation of the proximal femur from MR images using deep convolutional neural networks. Sci Rep. 2018;8:16485–16485. doi: 10.1038/s41598-018-34817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollon TC, Parikh A, Pandian B, et al. A machine learning approach to predict early outcomes after pituitary adenoma surgery. Neurosurg Focus. 2018;45:E8–E8. doi: 10.3171/2018.8.FOCUS18268. [DOI] [PubMed] [Google Scholar]
- 15.Gubbi S, Hamet P, Tremblay J, et al. Artificial intelligence and machine learning in endocrinology and metabolism: the dawn of a new era. Front Endocrinol (Lausanne) 2019;10:185–185. doi: 10.3389/fendo.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das N, Topalovic M, Janssens W. Artificial intelligence in diagnosis of obstructive lung disease. Curr Opin Pulm Med. 2018;24:117–123. doi: 10.1097/MCP.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 17.Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103:167–175. doi: 10.1136/bjophthalmol-2018-313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fröhlich H, Balling R, Beerenwinkel N, et al. From hype to reality: data science enabling personalized medicine. BMC Med. 2018;16:150–150. doi: 10.1186/s12916-018-1122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover M, 4th, Daye D, Khalilzadeh O, et al. Socioeconomic and demographic predictors of missed opportunities to provide advanced imaging services. J Am Coll Radiol. 2017;14:1403–1411. doi: 10.1016/j.jacr.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 20.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–2679. doi: 10.1016/j.jacc.2018.03.521. [DOI] [PubMed] [Google Scholar]
- 22.Seah JCY, Tang JSN, Kitchen A, et al. Chest radiographs in congestive heart failure: visualizing neural network learning. Radiology. 2019;290:514–522. doi: 10.1148/radiol.2018180887. [DOI] [PubMed] [Google Scholar]
- 23.Playford D, Bordin E, Talbot L, et al. Analysis of aortic stenosis using artificial intelligence. Heart Lung Circ. 2018;27:S216–S216. [Google Scholar]
- 24.Narula S, Shameer K, Salem Omar AM, et al. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287–2295. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 25.Madani A, Arnaout R, Mofrad M, et al. Fast and accurate view classification of echocardiograms using deep learning. NPJ Digit Med. doi: 10.1038/s41746-017-0013-1. Published Online First: 21 March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta Y, Yunaga H, Kitao S, et al. Detection and classification of myocardial delayed enhancement patterns on mr images with deep neural networks: a feasibility study. Radiology. 2019;1:e180061–e180061. doi: 10.1148/ryai.2019180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González G, Washko GR, Estépar RSJ, et al. Automated Agatston score computation in non-ECG gated CT scans using deep learning. Proceedings of the Medical Imaging 2018: Image Processing; Texas, USA. March 2018; pp. 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao Q, Yan W, Wang Y, et al. Deep learning–based method for fully automatic quantification of left ventricle function from cine MR Images: a multivendor, multicenter study. Radiology. 2019;290:81–88. doi: 10.1148/radiol.2018180513. [DOI] [PubMed] [Google Scholar]
- 29.Ngo TA, Lu Z, Carneiro G. Combining deep learning and level set for the automated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance. Med Image Anal. 2017;35:159–171. doi: 10.1016/j.media.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Isin A, Ozdalili S. Cardiac arrhythmia detection using deep learning. Procedia Comput Sci. 2017;120:268–275. [Google Scholar]
- 31.Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 32.Galloway CD, Valys AV, Shreibati JB, et al. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol. 2019;4:428–436. doi: 10.1001/jamacardio.2019.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Przewlocka-Kosmala M, Marwick TH, Dabrowski A, et al. Contribution of cardiovascular reserve to prognostic categories of heart failure with preserved ejection fraction: a classification based on machine learning. J Am Soc Echocardiogr. 2019;32:604–615. doi: 10.1016/j.echo.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Kwon J, Lee Y, Lee Y, et al. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. doi: 10.1161/JAHA.118.008678. Published Online First: 26 June 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daghistani TA, Elshawi R, Sakr S, et al. Predictors of in-hospital length of stay among cardiac patients: a machine learning approach. Int J Cardiol. 2019;288:140–147. doi: 10.1016/j.ijcard.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 36.Mortazavi BJ, Downing NS, Bucholz EM, et al. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9:629–640. doi: 10.1161/CIRCOUTCOMES.116.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya M, Lu DY, Kudchadkar SM, et al. Identifying ventricular arrhythmias and their predictors by applying machine learning methods to electronic health records in patients with hypertrophic cardiomyopathy (HCM-VAr-Risk Model) Am J Cardiol. 2019;123:1681–1689. doi: 10.1016/j.amjcard.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Alaa AM, Bolton T, Di Angelantonio E, et al. Cardiovascular disease risk prediction using automated machine learning: a prospective study of 423,604 UK Biobank participants. PLoS One. 2019;14:e0213653–e0213653. doi: 10.1371/journal.pone.0213653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eraslan G, Avsec Ž, Gagneur J, et al. Deep learning: new computational modelling techniques for genomics. Nat Rev Genet. 2019;20:389–403. doi: 10.1038/s41576-019-0122-6. [DOI] [PubMed] [Google Scholar]
- 40.Ho DSW, Schierding W, Wake M, et al. Machine learning SNP based prediction for precision medicine. Front Genet. doi: 10.3389/fgene.2019.00267. Published Online First: 27 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oguz C, Sen SK, Davis AR, et al. Genotype-driven identification of a molecular network predictive of advanced coronary calcium in ClinSeq® and Framingham Heart Study cohorts. BMC Syst Biol. 2017;11:99–99. doi: 10.1186/s12918-017-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner AW, Wong D, Khan MD, et al. Multi-omics approaches to study long non-coding RNA function in atherosclerosis. Front Cardiovasc Med. 2019;6:9–9. doi: 10.3389/fcvm.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burghardt TP, Ajtai K. Neural/Bayes network predictor for inheritable cardiac disease pathogenicity and phenotype. J Mol Cell Cardiol. 2018;119:19–27. doi: 10.1016/j.yjmcc.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Meskó B. FDA approvals for smart algorithms in medicine in one giant infographic, 2019. The Medical Futurist. [(accessed June 28, 2019)]. https://medicalfuturist.com/fda-approvals-for-algorithms-in-medicine.
- 45.Almutairi HM, Khanji MY, Boubertakh R, et al. A comparison of cardiac motion analysis software packages: application to left ventricular deformation analysis in healthy subjects. J Cardiovasc Magn Reson. 2016;18:47–47. [Google Scholar]
- 46.Ding Y, Sohn JH, Kawczynski MG, et al. A deep learning model to predict a diagnosis of Alzheimer disease by using 18F-FDG PET of the brain. Radiology. 2019;290:456–464. doi: 10.1148/radiol.2018180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Little MA, Varoquaux G, Saeb S, et al. Using and understanding cross-validation strategies. Perspectives on Saeb et al. Gigascience. 2017;6:1–6. doi: 10.1093/gigascience/gix020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manrai AK, Patel CJ, Ioannidis JPA. In the era of precision medicine and big data, Who is normal? JAMA. 2018;319:1981–1982. doi: 10.1001/jama.2018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seetharam K, Shrestha S, Sengupta PP. Artificial intelligence in cardiovascular medicine. Curr Treat Options Cardiovasc Med. 2019;21:25–25. doi: 10.1007/s11936-019-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Y, Paul AK, Kim N, et al. Computer-aided diagnosis for classifying benign versus malignant thyroid nodules based on ultrasound images: A comparison with radiologist-based assessments. Med Phys. 2016;43:554–567. doi: 10.1118/1.4939060. [DOI] [PubMed] [Google Scholar]
- 51.Miotto R, Li L, Kidd BA, et al. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6:26094–26094. doi: 10.1038/srep26094. [DOI] [PMC free article] [PubMed] [Google Scholar]