Abstract
Increasing evidence indicates that abnormalities in the composition of gut microbiota might play a role in stress-related disorders. In the learned helplessness (LH) paradigm, ~60–70% rats are susceptible to LH in the face of inescapable electric stress. The role of gut microbiota in susceptibility in the LH paradigm is unknown. In this study, male rats were exposed to inescapable electric stress under the LH paradigm. The compositions of gut microbiota and short-chain fatty acids were assessed in fecal samples from control rats, non-LH (resilient) rats, and LH (susceptible) rats. Members of the order Lactobacillales were present at significantly higher levels in the susceptible rats than in control and resilient rats. At the family level, the number of Lactobacillaceae in the susceptible rats was significantly higher than in control and resilient rats. At the genus level, the numbers of Lactobacillus, Clostridium cluster III, and Anaerofustis in susceptible rats were significantly higher than in control and resilient rats. Levels of acetic acid and propionic acid in the feces of susceptible rats were lower than in those of control and resilient rats; however, the levels of lactic acid in the susceptible rats were higher than those of control and resilient rats. There was a positive correlation between lactic acid and Lactobacillus levels among these three groups. These findings suggest that abnormal composition of the gut microbiota, including organisms such as Lactobacillus, contributes to susceptibility versus resilience to LH in rats subjected to inescapable electric foot shock. Therefore, it appears likely that brain–gut axis plays a role in stress susceptibility in the LH paradigm.
Subject terms: Pharmacodynamics, Depression
Introduction
Resilience is adaptation in the face of stress and adversity. It is well known that humans display wide variability in their responses to stress. Increasing amounts of evidence show that resilience might be mediated by adaptive changes in several neural circuits, including numerous molecular and cellular pathways1–11. An understanding of the molecular and cellular mechanisms underlying resilience will facilitate the discovery of new therapeutic drugs for stress-related psychiatric disorders, but the detailed mechanisms underlying resilience and susceptibility remain unclear.
The brain–gut–microbiome axis is a complex, bidirectional signaling system between the brain and the gut microbiota12–16. Accumulating studies suggest that an abnormal composition of the gut microbiota contributes to the pathophysiology of depression17–21 and the antidepressant effects of certain potential compounds22–29. Previously, we reported that the presence of Bifidobacterium in the gut microbiome confers stress resilience in a chronic social defeat stress (CSDS) model30. It has been shown that ~30–40% of rats are resilient to inescapable electric stress in the learned helplessness (LH) paradigm31–34; however, the role of gut microbiota in the production of this resilience has not yet been investigated. Microbes in the gut can produce short-chain fatty acids. The presence and abundance of such acids could possibly be used as an indicator of the types of bacteria present in the gut. However, it is also currently unknown how the altered composition of the gut microbiota affects the concentration of short-chain fatty acids in fecal samples.
The purpose of this study was to investigate the role of gut microbiota on stress resilience using a rat LH paradigm. First, we investigated whether the composition of the gut microbiota was altered in fecal samples from LH (susceptible) and non-LH (resilient) rats compared with control rats. Then we examined whether the levels of short-chain fatty acids—acetic acid, propionic acid, butyric acid, lactic acid, and succinic acid—in the fecal samples from susceptible and resilient rats were altered compared with control rats, since these short-chain fatty acids can produced by the gut microbiota23.
Materials and methods
Animals
Male Sprague-Dawley rats (n = 25, 200–230 g; 7 weeks, Charles River Japan, Co., Tokyo, Japan) were used. The animals were housed under a 12-h light/dark cycle with ad libitum access to food and water. The experimental procedures were approved by the Chiba University Institutional Animal Care and Use Committee (Permission number: 31-341).
Stress paradigm (LH model) and collection of fecal sample
The LH paradigm was performed as previously reported6–8,11,31–34. Animals were initially exposed to uncontrollable stress to create LH rats. When the rats were later placed in a situation in which the shock is controllable; that is, the animal could escape it, an animal exhibiting LH not only fails to acquire an escape response but also often makes no effort to escape the shock at all.
We used the Gemini Avoidance System (San Diego Instruments, San Diego, CA) for LH paradigm. This apparatus has two compartments by a retractable door. On day 1 and day 2, rats were subjected to 30 inescapable electric foot shocks (0.65 mA, 30 s duration, at random intervals averaging 18–42 s). On day 3, a post-shock test using a two-way conditioned avoidance test was performed to determine whether the rats would exhibit the predicted escape deficits (Fig. 1a). This session consisted of 30 trials, in which electric foot shocks (0.65 mA, 6 s duration, at random intervals with a mean of 30 s) were preceded by a 3-s conditioned stimulus tone that remained on until the shock was terminated. The numbers of escape failures and the latency to escape in each of the 30 trials were counted. Animals with more than 25 escape failures in the 30 trials were regarded as having met the criterion for LH rats (susceptible). Animals with fewer than 24 failures were defined as non-LH rats (resilient)31–34. Fresh fecal samples were collected in a blind manner before post-shock stress on day 4, and stored at −80 °C until use (Fig. 1A).
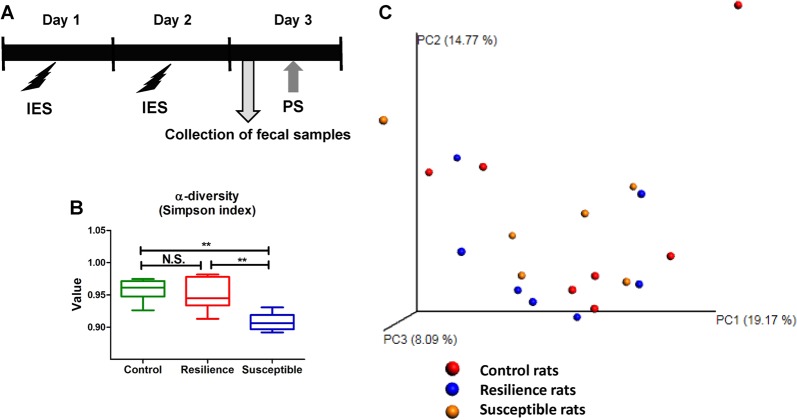
Fig. 1. Experimental schedule of LH paradigm and profiles of gut microbiota.
a Schedule of LH paradigm and collection of fecal samples. Rats received inescapable electric shock (IES) treatments on 2 days (days 1 and 2). On day 3, fecal samples from rats were collected. Subsequently, rats passed a post-shock test (PS), and were designated as resilient rats and susceptible rats. b Simpson index (an α-diversity indicator: one-way ANOVA: F2,17 = 11.531, P = 0.001) among the three groups. α-Diversity data are shown as mean ± S.E.M. (n = 6 or 7). **P < 0.01. N.S.: not significant. c Principal coordinates analysis (PCA)
16S rDNA analysis
DNA extraction from fecal samples and the 16S rDNA analysis were performed at the TechnoSuruga Laboratory, Co., Ltd. (Shizuoka, Japan), as reported previously35. Briefly, the samples were suspended in a buffer containing 4 M guanidium thiocyanate, 100 mM Tris-HCl (pH 9.0) and 40 mM EDTA and broken up in the presence of zirconia beads using the FastPrep-24 5G homogenizer (MP Biomedicals, Irvine, CA). Then, DNA was extracted using GENE PREP STAR PI-480 (KURABO, Japan). The final concentration (10 ng/μL) of the DNA sample was used. Briefly, the V3-V4 hypervariable regions of the 16S rRNA were amplified from microbial genomic DNA using PCR with the bacterial universal primers (341F/R806)35 and the dual-index method36. For bioinformatics analysis, the overlapping paired-end reads were merged using the fastq-join program with default settings37. The reads were processed for quality and chimera filtering as follows. Only reads with quality value scores of 20 for >99% of the sequence were extracted, and chimeric sequences were removed using the program usearch6.138. Non-chimeric reads were submitted for 16S rDNA-based taxonomic analysis using the Ribosomal Database Project (RDP) Multiclassifier tool39. Reads obtained in the Multi-FASTA format were assigned to genus or phylum levels with an 80% confidence threshold. Principal component analysis (PCA) was performed using Metagenome@KIN software (World Fusion Co., Ltd., Tokyo, Japan) based on data obtained from the bacterial family using the RDP taxonomic analysis software.
Measurement of fecal short-chain fatty acids
Measurement of short-chain fatty acids—acetic acid, propionic acid, butyric acid, lactic acid, and succinic acid—in fecal samples was performed at the TechnoSuruga Laboratory, Co., Ltd. (Shizuoka, Japan). For the determination of these short-chain fatty acids, feces were suspended in distilled water, heated at 85 °C for 15 min to inactivate viruses, and then centrifuged according to previously reported methods40,41. The concentrations of these short-chain fatty acids in feces were measured using a high-performance liquid chromatography organic acid analysis system with a Prominence CDD-10A conductivity detector (Shimadzu, Kyoto, Japan), two tandemly arranged Shim-pack SCR-102(H) columns [300 mm × 8 mm inner diameter (ID)], and a Shim-pack SCR-102(H) guard column (50 mm × 6 mm ID)40,41. The HPLC calibration curves for the measurement of the short-chain fatty acids were created using prepared standard solutions.
Statistical analysis
The data are presented as the mean ± standard error of the mean (S.E.M.). Analysis was performed using the PASW Statistics 20 software (now SPSS statistics; SPSS, Tokyo, Japan). Comparisons between groups were performed using one-way analysis of variance, followed by post hoc Fisher’s Least Significant Difference test. A P value < 0.05 was considered statistically significant.
Results
Composition of gut microbiota in control, resilient, and susceptible rats
We used 16S rDNA gene sequencing to determine differences in the gut microbiota composition among the three groups of rats. α-diversity refers to the diversity of bacteria or species within a community or habitat. The susceptible rats showed a significant decrease in the α-diversity value compared with control rats or resilient rats (Fig. 1b). In the three-dimensional PCoA data, the measurements from susceptible rats were well separated from those of control rats and resilient rats. Four measurements from the susceptible group were close to those of the sham group, whereas the other three measurements were close to those of the resilient group (Fig. 1c).
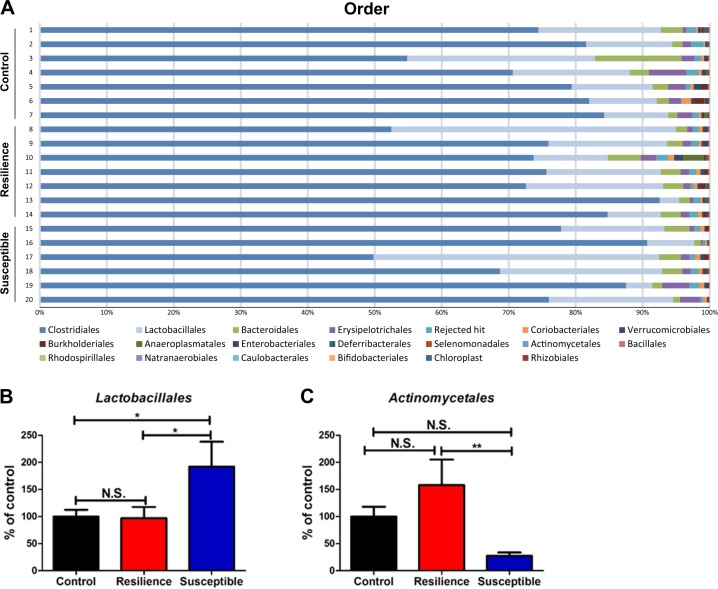
Firmicutes were the most dominant phylum, comprising >85% of the total sequences. There were no significant differences in the levels of this phylum among the three groups. The order levels of gut bacterium in control rats, resilient rats, and susceptible rats were identified (Fig. 2a). Clostridiales and Lactobacillales were the most dominant orders, with >80% of total sequences. The number of Lactobacillales was significantly increased in the susceptible rats compared with that in control and resilient rats (Fig. 2b). In contrast, the number of Lactobacillales in the resilient rats was similar to that in the control rats (Fig. 2b). The number of Actinomycetales in the susceptible rats was significantly lower than that in the resilient rats (Fig. 2c). Although the number of Actinomycetales in the resilient rats was higher than that in the control rats, the difference did not reach statistical significance.
Fig. 2. Changes in the composition of gut microbiota at the order level.
a The order levels of gut microbiota among the three groups. b Lactobacillales (one-way ANOVA: F2,17 = 4.021, P = 0.044) among the three groups. c Actinomycetales (one-way ANOVA: F2,17 = 4.000, P = 0.038) among the three groups. The data are shown as mean ± S.E.M. (n = 6 or 7). *P < 0.05, **P < 0.01. N.S.: not significant
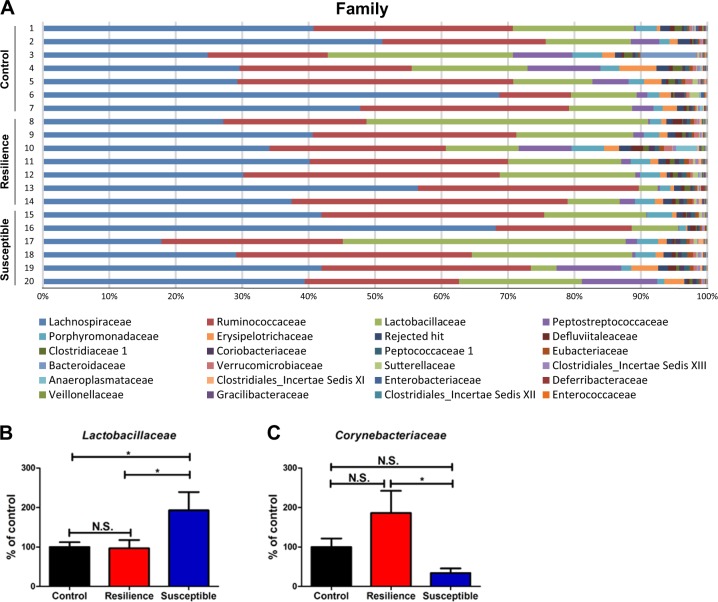
The families of the gut bacteria in control, resilient, and susceptible rats are shown (Fig. 3a). Lactobacillaceae were significantly more highly represented in the susceptible rats than in the control and resilient rats, although the number of Lactobacillaceae in the resilient rats was similar to that in the control rats (Fig. 3b). In contrast, the number of Corynebacteriaceae was significantly lower in the susceptible rats than that in the resilient rats, although these two did not significantly differ from the control rats (Fig. 3c).
Fig. 3. Changes in the composition of gut microbiota at the family level.
a The family levels of gut microbiota among the three groups. b Lactobacillaceae (one-way ANOVA: F2,17 = 4.039, P = 0.043) among the three groups. c Corynebacteriaceae (one-way ANOVA: F2,17 = 4.159, P = 0.034) among the three groups. The data are shown as mean ± S.E.M. (n = 6 or 7). *P < 0.05, **P < 0.01. N.S.: not significant
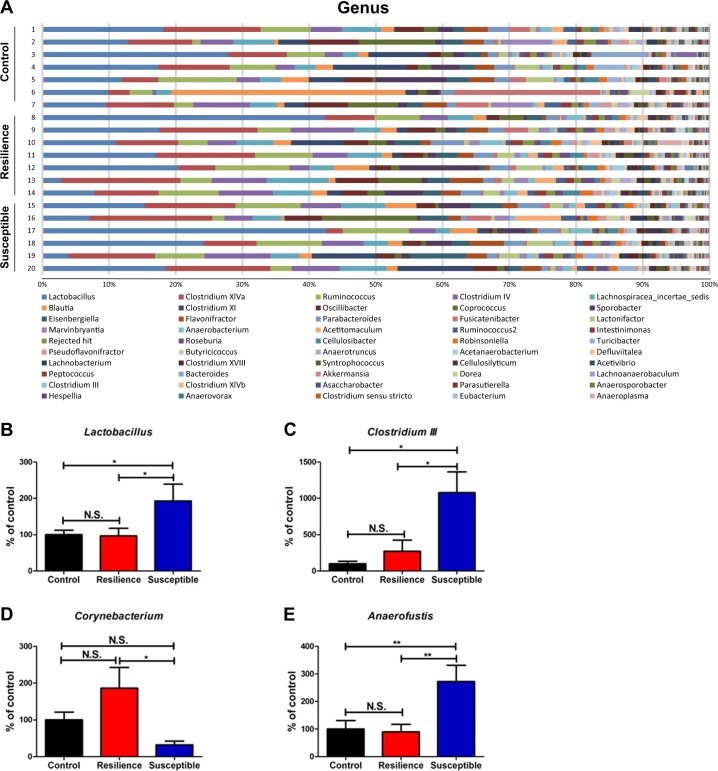
The genera of gut bacteria in the control, resilient, and susceptible rats are shown (Fig. 4a). Lactobacillus, Clostridium cluster III, and Anaerofustis numbers were significantly higher in the susceptible rats than in the control and resilient rats (Fig. 4b, c, e). Corynebacterium numbers were significantly lower in the susceptible rats than in the resilient rats, although these two groups were not significantly altered compared with the control rats (Fig. 4d).
Fig. 4. Changes in the composition of gut microbiota at the genus level.
a The genus levels of gut microbiota among the three groups. b Lactobacillus (one-way ANOVA: F2,17 = 4.030, P = 0.043) among the three groups. c Clostridium III (one-way ANOVA: F2,17 = 5.625, P = 0.021) among the three groups. d Corynebacterium (one-way ANOVA: F2,17 = 4.283, P = 0.031) among the three groups. c Anaerofustis (one-way ANOVA: F2,17 = 6.608, P = 0.008) among the three groups. The data are shown as mean ± S.E.M. (n = 6 or 7). *P < 0.05, **P < 0.01. N.S.: not significant
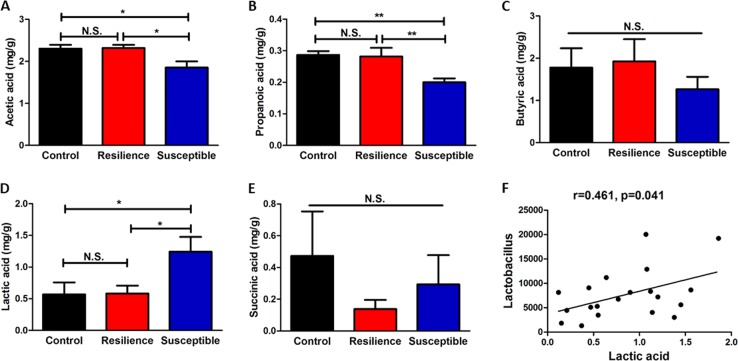
Measurement of short-chain fatty acids in fecal samples
Levels of acetic acid and propionic acid in the susceptible rats were significantly lower than those in control rats and resilient rats, and there were no significant differences between control rats and resilient rats (Fig. 5a, b). There were no changes in butyric acid and succinic acid among the three groups (Fig. 5c, e). In contrast, levels of lactic acid in the susceptible rats were significantly higher than those of control rats and resilient rats, although there were no changes between control rats and resilient rats (Fig. 5d). There was a positive correlation (r = 0.461, P = 0.041) between lactic acid and Lactobacillus levels among three groups (Fig. 5f). There were no correlations between other short-chain fatty acids and the microbiome composition among the three experimental groups.
Fig. 5. Levels of short-chain fatty acids in fecal samples and correlation with microbiota.
a Acetic acid (one-way ANOVA: F2,17 = 5.898, P = 0.016) among the three groups. b Propionic acid (one-way ANOVA: F2,17 = 8.175, P = 0.004) among the three groups. c Butyric acid (one-way ANOVA: F2,17 = 0.559, P = 0.582) among the three groups. d Lactic acid (one-way ANOVA: F2,17 = 4.219, P = 0.041) among the three groups. e Succinic acid (one-way ANOVA: F2,17 = 0.763, P = 0.488) among the three groups. The data are shown as mean ± S.E.M. (n = 6 or 7). *P < 0.05, **P < 0.01. N.S.: not significant. f There is a positive correlation (r = 0.461, P = 0.041) between lactic acid and Lactobacillus in fecal samples
Discussion
The major findings of this study are as follows. At the order level, susceptibility to LH in rats exposed to inescapable shock might be associated with an increase of Lactobacillales and a decrease of Actinomycetales in the host gut. At the family level, stress susceptibility might be associated with an increase in Lactobacillaceae and decrease of Corynebacteriaceae in the host gut. At the gene level, stress susceptibility might be associated with the increase of Lactobacillus, Clostridium cluster III and Anaerofustis, and the decrease of Corynebacterium in the host gut. Levels of acetic acid and propionic acid in the feces from susceptible rats were lower than those in the feces of control and resilient rats, whereas levels of lactic acid in the susceptible rats were higher than those of control and resilient rats. There was a positive correlation between lactic acid and Lactobacillus levels among these three groups. These findings suggest that alterations in the composition of these microbiota contribute to susceptibility versus resilience in rats in the LH situation.
In this study, we found an increase in the abundance of members of the order Lactobacillales and the family Lactobacillaceae in susceptible rats, in comparison with control and resilient rats. It is possible that increased abundance of members of the Lactobacillales order and Lactobacillaceae family might play a role in susceptibility versus resilience of rats to LH after inescapable electric stress.
At the genus level, susceptibility in rats exposed to inescapable shock might be associated with an increase in Lactobacillus, Clostridium cluster III, and Anaerofustis and a decrease of Corynebacterium in the host gut. Clostridium, a genus of Gram-positive bacteria, includes several significant human pathogens, such as the causative agent of botulism. High levels of members of the genus Clostridium have been reported in patients with major depressive disorder (MDD) compared with controls42,43, suggesting that increased levels of Clostridium play a role in depression. We reported that susceptible mice after CSDS have higher levels of Clostridium, and that the novel antidepressant candidate (R)-ketamine attenuated the increased levels of Clostridium in susceptible mice25. This study shows that the antidepressant effects of (R)-ketamine might be partly mediated by the restoration of altered composition of the gut microbiota in the CSDS susceptible mice. Although the role of members of Clostridium cluster III in depression is currently unclear, it appears that Clostridium cluster III may contribute to susceptibility in rats subjected to inescapable electric stress.
Anaerofustis is a strictly anaerobic, Gram-positive, rod-shaped, non-spore-forming bacterial genus of the family Eubacteriaceae44. In this study, we found decreased levels of Anaerofustis in the susceptible rats compared with the control and resilient rats. At present, there have been no reports showing alterations in Anaerofustis in patients with MDD, or in rodents with depression-like phenotypes. Therefore, it is unclear how decreased levels of Anaerofustis play a role in susceptibility to LH. Further study into the role of Anaerofustis in depression is needed.
In this study, we found lower levels of Corynebacterium in the susceptible rats compared with control and resilient rats. It has been reported that sub-chronic and chronic exposure to glyphosate-based herbicides decreases the composition of microbiota, such as Corynebacterium, resulting in behavioral abnormalities including depression and anxiety45. Low levels of Corynebacterium were also reported in a chronic variable stress-induced rat model of depression46. It appears likely that lowered levels of Corynebacterium might play a role in a depression-like phenotype in rodents, although further study into the role of Corynebacterium in depression is needed.
Short-chain fatty acids—acetic acid, propionic acid, butyric acid, lactic acid, and succinic acid—are generated as the end products of the degradation and fermentation of indigestible carbohydrates by the gut microbiota47. Measurement of these short-chain fatty acids could therefore serve as an indirect method for the analysis of microbiota composition. These organic acids have specific anti-microbial activities48. It has been reported that levels of acetic acid and propionic acid in feces from women with depression are lower than those in control subjects, and that there are negative correlations between acetic acid or propionic acid and depression scores49. In this study, we found decreased levels of acetic acid and propionic acid in rats susceptible to LH, compared with control and resilient rats, consistent with the results from a recent clinical study49. We also found decreased levels of butyric acid in the susceptible rats, although the difference did not reach statistical significance. Three short-chain fatty acids containing C2–C4, acetic acid, propionic acid, and butyric acid, account for over 95% of the pool of short-chain fatty acids23. It is likely that decreased levels of acetic acid and propionic acid in feces may be associated with susceptibility to LH, and with depression in patients with MDD.
Elevated levels of lactic acid in the blood, cerebrospinal fluid and brain have been reported in patients with MDD50–52. Higher levels of lactic acid in rodents with depression-like behaviors have also been reported53. In contrast, peripheral administration of lactic acid produced antidepressant-like effects in different models of depression54. In this research we found higher levels of lactic acid in feces from susceptible rats compared with those from control and resilient rats. We found a positive correlation between Lactobacillus, microbes which produce lactic acid, and the amounts of lactic acid in fecal samples. It appears likely that the increased levels of lactic acid produced by Lactobacillus might contribute to susceptibility to inescapable electric stress, although further study is needed.
The crosstalk between the brain and the gut is predominately influenced by the gut bacteria55. Imbalance of gut microbiota has been found to cause abnormalities in the brain–gut axis in several neurological and psychiatric diseases13,55. Multiple lines of evidence suggest that an abnormal composition of the gut microbiota contributes to the resilience or susceptibility to LH in rodents after repeated stress30,56–59. It is well recognized that gut microbiota plays a role in animal behaviors14,60–62, although the precise mechanisms underlying the microbiome-mediated behaviors are currently unknown. For example, it has been reported that the vagus nerve plays a major role in modulating the constitutive communication pathway between the brain and the bacteria in the gut63,64. It is likely that altered composition of microbiota might play a key role in the stress-induced disorders although further study is needed.
This research has some limitations. In this study, we did not identify the specific microbiome which can affect susceptibility or resilience in the LH experiments. Therefore, from the present data, we do not know how the specific microbiome can affect behaviors under the LH paradigm. In the future, it will be necessary to identify the specific microbiome, using approaches such as shotgun metagenomics sequencing. It will also be of interest to investigate the way in which specific microbiomes affect behaviors related to LH.
In conclusion, the present study suggests that an altered composition of the gut microbiota, including organisms, such as Lactobacillus, Clostridium cluster III, Anaerofustis, and Corynebacterium, contributes to resilience and susceptibility to learned helplessness in rats subjected to inescapable electric foot shock.
Acknowledgements
This study was supported by SENSHIN Medical Research Foundation, Japan (to K.Z.), Smoking Research Foundation, Japan (to K.H.), and AMED, Japan (to K.H., JP19dm0107119). L.C. was supported by Japan China Sasakawa Medical Fellowship (Tokyo, Japan). S.W. was supported by TAKASE Scholarship Foundation (Tokyo, Japan).
Conflict of interest
K.H. has received research support from Dainippon-Sumitomo, Otsuka, and Taisho. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Ann. Rev. Clin. Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 2.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taliaz D, et al. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2012;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int. J. Neuropsychopharmacol. 2015;18:pyu121. doi: 10.1093/ijnp/pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015;27:312–316. doi: 10.1017/neu.2015.36. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, et al. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266:765–769. doi: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R, Cohen S, Russo SJ, Dinan TG. Resilience and immunity. Brain Behav. Immun. 2018;74:28–42. doi: 10.1016/j.bbi.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu Y, et al. Regional differences in dendritic spine density confer resilience to chronic social defeat stress. Acta Neuropsychiatr. 2018;30:117–122. doi: 10.1017/neu.2017.16. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JC, et al. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur. Arch. Psychiatry Clin. Neurosci. 2018;268:865–870. doi: 10.1007/s00406-017-0848-0. [DOI] [PubMed] [Google Scholar]
- 12.Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom. Med. 2017;79:920–926. doi: 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 13.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front. Neuroendocrinol. 2018;51:80–101. doi: 10.1016/j.yfrne.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Torres G, Rodriguez-Arrastia M, Roman P, Sanchez-Labraca N, Cardona D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019;30(2 and 3 Special Issue):187–202. doi: 10.1097/FBP.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 16.Cussotto Sofia, Strain Conall R., Fouhy Fiona, Strain Ronan G., Peterson Veronica L., Clarke Gerard, Stanton Catherine, Dinan Timothy G., Cryan John F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology. 2018;236(5):1671–1685. doi: 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 19.Wong ML, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TT, et al. Current understanding of gut microbiota in mood disorders: an update of human studies. Front. Genet. 2019;10:98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry. 2019;9:57. doi: 10.1038/s41398-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burokas A, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 23.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho P, Ross DA. More than a gut feeling: the implications of the gut microbiota in psychiatry. Biol. Psychiatry. 2017;81:e35–e37. doi: 10.1016/j.biopsych.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu Y, et al. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci. Rep. 2017;7:15725. doi: 10.1038/s41598-017-16060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry. 2017;7:1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JC, et al. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl. Psychiatry. 2017;7:e1138. doi: 10.1038/tp.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang N, et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol. Biochem. Behav. 2019;176:93–100. doi: 10.1016/j.pbb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Lukić I, et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry. 2019;9:133. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, et al. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017;7:45942. doi: 10.1038/srep45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muneoka K, Shirayama Y, Horio M, Iyo M, Hashimoto K. Differential levels of brain amino acids in rat models presenting learned helplessness or non-learned helplessness. Psychopharmacol. (Berl.) 2013;226:63–71. doi: 10.1007/s00213-013-3080-2. [DOI] [PubMed] [Google Scholar]
- 32.Shirayama Y, et al. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur. Neuropsychopharmacol. 2015;25:2449–2458. doi: 10.1016/j.euroneuro.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267:177–182. doi: 10.1007/s00406-016-0718-1. [DOI] [PubMed] [Google Scholar]
- 34.Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: Comparison with (R)-ketamine. Int. J. Neuropsychopharmacol. 2018;21:84–88. doi: 10.1093/ijnp/pyx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next‐generation sequencing. PLoS ONE. 2014;9:e105592. doi: 10.1371/journal.pone.0105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hisada T, Endoh K, Kuriki K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 2015;197:919–934. doi: 10.1007/s00203-015-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erik A. ea-utils “Command-line tools for processing biological sequencing data”. http://code.google.com/p/ea-utils (2011).
- 38.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;72:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higashimura Y, et al. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G367–G375. doi: 10.1152/ajpgi.00324.2015. [DOI] [PubMed] [Google Scholar]
- 41.Aoe S, Nakamura F, Fujiwara S. Effect of wheat bran on fecal butyrate-producing bacteria and wheat bran combined with barley on Bacteroides abundance in Japanese healthy adults. Nutrients. 2018;10:1980. doi: 10.3390/nu10121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin P, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017;207:300–304. doi: 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Rong H, et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019;113:90–99. doi: 10.1016/j.jpsychires.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Finegold SM, et al. Anaerofustis stercorihominis gen. nov., sp. nov., from human feces. Anaerobe. 2004;10:41–45. doi: 10.1016/j.anaerobe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Aitbali Y, et al. Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018;67:44–49. doi: 10.1016/j.ntt.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Yu M, et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017;138:231–239. doi: 10.1016/j.jpba.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 48.Dibner JJ, Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002;11:453–463. doi: 10.1093/japr/11.4.453. [DOI] [Google Scholar]
- 49.Skonieczna-Żydecka K, et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 2018;10:E1939. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levine J, Panchalingam K, McClure RJ, Gershon S, Pettegrew JW. Stability of CSF metabolites measured by proton NMR. J. Neural Transm. (Vienna). 2000;107:843–848. doi: 10.1007/s007020070064. [DOI] [PubMed] [Google Scholar]
- 51.Regenold WT, et al. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol. Psychiatry. 2009;65:489–494. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ernst J, et al. Increased pregenual anterior cingulate glucose and lactate concentrations in major depressive disorder. Mol. Psychiatry. 2017;22:113–119. doi: 10.1038/mp.2016.73. [DOI] [PubMed] [Google Scholar]
- 53.Shi B, et al. A ¹H-NMR plasma metabonomic study of acute and chronic stress models of depression in rats. Behav. Brain. Res. 2013;241:86–91. doi: 10.1016/j.bbr.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 54.Carrard A, et al. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry. 2018;23:392–399. doi: 10.1038/mp.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann. Epidemiol. 2016;26:366–372. doi: 10.1016/j.annepidem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC. Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav. Immun. 2017;66:45–55. doi: 10.1016/j.bbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019;104:132–142. doi: 10.1016/j.psyneuen.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Kentner AC, Cryan JF, Brummelte S. Resilience priming: Translational models for understanding resiliency and adaptation to early life adversity. Dev. Psychobiol. 2019;61:350–375. doi: 10.1002/dev.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gururajan Anand, van de Wouw Marcel, Boehme Marcus, Becker Thorsten, O'Connor Rory, Bastiaanssen Thomaz F.S., Moloney Gerard M., Lyte Joshua M., Ventura Silva Ana Paula, Merckx Barbara, Dinan Timothy G., Cryan John F. Resilience to chronic stress is associated with specific neurobiological, neuroendocrine and immune responses. Brain, Behavior, and Immunity. 2019;80:583–594. doi: 10.1016/j.bbi.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Ezewa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. Animal behavior and microbiome. Science. 2012;338:198–199. doi: 10.1126/science.1227412. [DOI] [PubMed] [Google Scholar]
- 61.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 62.Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry. 2019;9:40. doi: 10.1038/s41398-019-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dinan TG, Cryan JF. Gut microbiomes and depression: still waiting for godot. Brain Behav. Immun. 2019;79:1–2. doi: 10.1016/j.bbi.2019.02.007. [DOI] [PubMed] [Google Scholar]