Abstract
Background
Lung is a reservoir for megakaryocytes (MKs). The relationship between intra-tumoral MKs and non-small cell lung cancer (NSCLC) is unknown. We investigate relationship between high intra-tumoral MKs with the recurrence of NSCLC.
Methods
The tissue sections of 629 patients with resected NSCLC were stained with hematoxylin, anti-CD61, anti-CD34 and stromal cell-derived factor-1 (SDF-1). CD61+ giant cells localized in CD34+ capillaries were identified as MKs. The impact of MKs and preoperative platelet count on disease-free survival (DFS) was investigated.
Results
Overall, 18.9% of patients were positive for the presence of MKs. In univariate analysis, the median DFS of the MK+ group was shorter than the median DFS of the MK- group (69.1 vs. 80.5 months; P=0.021). Multivariate analysis indicated that MKs in tumor tissue was an unfavorable prognostic factor for DFS (HR 1.351, P=0.065), the impact of which was more significant in non-squamous cell carcinoma (NSCC) (HR 1.710, P=0.008) and in patients with N0 (HR 1.883, P=0.009). Although systemic platelet count of the MK+ group was significantly higher than the MK- group (270.6×109 vs. 243.6×109/L, P=0.007), the stratified subgroup DFS curves (P=0.003) showed that the effect of MKs on prognosis was independent of the blood platelet count.
Conclusions
Our results demonstrate that CD61+ MKs in tumor tissue predict unfavorable prognosis in NSCLC.
Keywords: Disease-free survival (DFS), intra-tumoral thrombopoiesis, megakaryocyte (MK), non-small cell lung cancer (NSCLC), platelet
Introduction
Platelets were discovered by Bizzozero in 1882 and recognized as anucleate blood cells specialized in promoting thrombosis in vivo (1). Platelets have been reported to facilitate tumor growth and metastasis during cancer development (2,3). Tumor cell-activated platelets contribute to opening the endothelial barrier and promote transendothelial migration of tumor cells (4). The effect of tumor-cell-induced platelet aggregation lead to platelets coating tumor cells, isolation of tumor cells from neutralization by tumor-necrosis-factor-α-mediated cytotoxicity and natural killer cells in the circulating blood (5,6), facilitation of the arrest and adherence of tumor cells to endothelial cells for migration to distant sites (7). Elevated platelet count from venous blood has been widely proven to be a predictor for poor prognosis in patients with lung cancer (8). Platelets that were supplied to a malignant neoplasm contributed to cancer metastasis at each phase of the metastasis (9).
Platelets are derived from megakaryocytes (MKs). MKs are polyploid hematopoietic cells, which are unique to mammals and are specialized to produce platelets (10). MKs originate in the marrow and exit from marrow sinusoids through venous vessels and the pulmonary artery to pulmonary microvessels (11,12). The lungs have an intimate relationship with platelets because the lung is the organ of thrombopoiesis at the latest stage of the MK/platelet hematopoietic linage (13,14). Approximately 50% of total platelets that are produced from circulating MKs are retained in the pulmonary capillary bed and sheared by the pulmonary capillary vessel (14,15).
There is evidence demonstrating the existence of pulmonary blood supply to lung cancer lesions (16). Therefore, it’s possible that MKs in lung cancer tissue are transported through the pulmonary artery. Non-small cell lung cancer (NSCLC) tumors tend to overexpress SDF-1, which is a powerful chemotactic factor for mature MKs (17), suggesting that lung cancer cells are more capable of recruiting MKs into tumoral sites. Similar to MKs in a normal lung capillary, MKs supplied to the lung cancer capillary may induce local thrombopoiesis. More MKs trapped in a tumor capillary bed may produce more platelets in the tumor region, and a higher regional platelet level may fuel development and metastasis of tumor. Therefore, we hypothesized that MKs in cancer tissue are linked to metastasis and poor prognosis of patients with lung cancer.
Methods
Patient selection
A total of 629 NSCLC patients underwent radical resection with or without adjuvant chemotherapy in the Department of Thoracic Surgery of The First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China) between September of 2009 and December of 2012. These patients were retrospectively investigated. The median follow-up time was 5.06 years, and 248 events, including relapse or death, occurred during the follow-up period. The protocol of this study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, and written informed consent for the use of cancer tissues was obtained from all patients prior to surgery. The inclusion criteria were as follows: complete resection through a surgical procedure; systematic node dissection or sampling (at least three N2 stations sampled or complete lymph node dissection); microscopically tumor-negative resection margins; pathologically proven stage pT1-3N1-2M0 NSCLC (according to the TNM classification in the UICC 7th ed); and postoperative follow-up duration ≥4 months. The factors evaluated included age, gender and pathologic data (histology, primary tumor stage and node stage). The level of venous platelets was measured using ethylenediaminetetraacetic acid (EDTA)-treated blood by an automated blood cell counter before surgery. The patients were followed every 3 months for the first 2 years and then every 6 months thereafter. The follow-up data consisted of clinical assessments, brain MRI, chest CT, and ultrasound or CT of the abdomen. Disease recurrence at the surgical margin, ipsilateral hilum, and/or mediastinum was defined as local failure. All other sites of failure, including the contralateral hilum and supraclavicular fossa, were considered distant metastasis. Disease-free survival (DFS) was calculated as the time from the date of surgery to the date of disease recurrence.
IHC staining
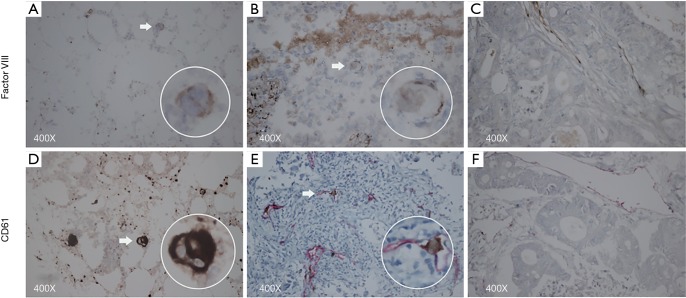
As a preliminary experiment, the staining power of anti-CD61 {Mouse [2f2] Monoclonal Antibody, Leica, Germany} and anti-Factor VIII (Rabbit polyclonal Antibody, MXB, China) for MKs was compared. The result of Factor VIII staining and CD61 staining during the preliminary experiment indicated the presence of MKs in the capillaries of lung tumors; however, Factor VIII staining was weaker than CD61 staining in the tumor and bone marrow (Figure 1). Therefore, this cohort was completely stained by anti-CD61. The sections (5 µm) obtained from formalin-fixed, paraffin-embedded tissue were stained with hematoxylin, anti-CD61, and anti-CD34. Anti-CD61 targets the IIIa subunit of the glycoprotein heterodimer IIb/IIIa which is expressed on the surface membrane of both MKs and platelets. CD61 staining was recommended as the method to detect MKs in the lung tissue (18-20). Anti-CD34 {Mouse [QBEnd/10] Monoclonal Antibody, Leica, Germany} was utilized to visualize endothelial cells stained by red precipitate in the cytoplasm (21). MKs were screened by transmitted light microscope with a ×40 objective and a ×10 eyepiece. Images of 25 random fields at 400× magnification were captured by a camera (Canon EOS 600D, Japan) coupled to an Olympus microscope BX43 (Olympus, Japan). Yellowish-brown CD61+ giant cells (diameter >10 µm) located in CD34+ microvessels were defined as MKs (18). MKs were identified by two independent pathological doctors who were blind to the clinicopathologic symptoms in the patients. Microthrombi in CD34+ microvessels were also identified under 400× magnification. Microthrombi in the area of necrosis were excluded. Cases harboring one microthrombus or more were recognized as positive cases. Sections from the same paraffin-embedded samples were stained with anti-SDF-1 (Rabbit polyclonal Antibody, Abcam, USA). SDF-1 staining was observed under high magnification in malignant cells. The percentage area of positive tumor cells in the observed field was scored as follows: 0 (0–5%), 1 (6–25%), 2 (25–50%), 3 (51–75%) and 4 (>75%). The intensity of tumor cells was scored as 0 (no staining), 1 (yellow), 2 (brown), 3 (dark brown). We categorized the sum of the area score and the intensity score into four grades: 0–1 (-), 2–3 (+), 4–5 (++), 6–7 (+++). For analysis, cases ranking (-) and (+) were defined as having low-absent expression, whereas cases ranking (++) and (+++) were defined as having high expression (22). The images were saved on a computer.
Figure 1.
Factor VIII, magnification 400×: (A) positive control, bone marrow; (B) intravascular factor VIII+ megakaryocyte; (C) negative control, factor VIII+ microvessels, rectal cancer. CD61, magnification 400×: (D) positive control, bone marrow; (E) intravascular CD61+ megakaryocyte; (F) negative control, CD34+ microvessels, rectal cancer. The arrow indicates megakaryocytes.
Statistical analysis
Categorical data were analyzed by Pearson’s chi-squared test and continuous data by an unpaired t-test. Univariate analyses of DFS were undertaken using the Kaplan-Meier product-limit method with the log-rank test. Variables that were found to be significant in the univariate analysis were included in a multivariate Cox proportional hazard model to determine the independence. The 95% confidence interval (CI) for the DFS was calculated using Greenwood’s method. All analyses were performed using the statistical package SPSS, version 19.0 (SPSS, IBM SPSS Statistics, Chicago, IL, USA). A two-sided P value of less than 0.05 was considered statistically significant.
Results
Patient baseline characteristics
Retrospective investigation was performed on a total of 629 patients with NSCLC at pathological stages I–III. The 629 patients included 142 patients with SCC, 441 patients with non-squamous cell carcinoma (NSCC) (adenocarcinoma/large cell carcinoma/adenosquamous carcinoma), 45 patients with all other tumor types, and 1 patient with unclear pathology. Of the total patients, 80.8% were younger than 70 years old and 58.0% were male. The distribution of tumor size was as follows: <3 cm, 281 (44.7%); 3–5 cm, 193 (30.7%); 5–7 cm, 98 (15.6%); ≥7 cm, 49 (7.8%); and unknown size, 8 (1.3%). The distribution of pN stage was as follows: pN0, 378 (60.1%); pN1, 84 (13.4%); pN2, 139 (22.1%); and unknown pN, 28 (4.5%). There were 119 out of 629 (18.9%) patients with CD61+ MKs located in red-stained CD34+ microvessels. The baseline data of the study patients are provided in Table 1.
Table 1. General characteristics of study population.
| Characteristic | N=629 (%) |
|---|---|
| Age, years | |
| <70 | 508 (80.8) |
| ≥70 | 121 (19.2) |
| Gender | |
| Male | 365 (58.0) |
| Female | 264 (42.0) |
| Histological type | |
| Squamous cell carcinoma | 142 (22.6) |
| Non-squamous cell carcinoma | 441 (70.1) |
| Others | 45 (7.2) |
| Unknown | 1 (0.2) |
| Size, cm | |
| <3 | 281 (44.7) |
| 3–5 | 193 (30.7) |
| 5–7 | 98 (15.6) |
| ≥7 | 49 (7.8) |
| Unknown | 8 (1.3) |
| pN stage | |
| pN0 | 378 (60.1) |
| pN1 | 84 (13.4) |
| pN2 | 139 (22.1) |
| Unknown | 28 (4.5) |
General characteristics of MK- group and MK+ group
Endothelial cells were identified as positive for CD34 if there was a moderate to strong red staining. CD61+ MKs were found in CD34+ alveolar capillaries in hematoxylin-stained tissue sections, shown as a giant cell nucleus (>10 µm) with limited cytoplasm. General characteristics of the MK- group and MK+ group are shown in Table 2. Most variables, including age (P=0.455), size (P=0.062), and pN stage (P=0.097) were not significantly different between the MK- group and MK+ group. More MK+ cases were related to larger tumor size, but no significant difference was detected (size <3 cm, 15.3%; 3–5 cm, 19.2%; 5–7 cm, 24.5%; ≥7 cm 28.6%). The 142 patients with SCC consisted of 79.6% males and 20.4% females (MK+ in the males: 26.5% vs. MK+ in the females: 10.3%; P=0.065). The 441 patients with NSCC consisted of 49.9% males and 50.1% females (MK+ in the males: 18.2% vs. MK+ in the females: 13.6%; P=0.186). Male patients showed a significantly higher proportion of MK+ cases than female patients (male: 22.2% vs. female 14.4%; P=0.014). Patients with NSCC showed significantly less MK+ cases compared to the patients with SCC and patients with other tumors (NSCC, 15.9%; SCC, 23.2%; others 33.3%; P=0.005).
Table 2. General characteristics of MK- group and MK+ group.
| Characteristic | MK- group, n=510 (%) | MK+ group, n=119 (%) | P |
|---|---|---|---|
| Age, years | 0.455 | ||
| <70 | 409 (80.5) | 99 (19.5) | |
| ≥70 | 101 (83.5) | 20 (16.5) | |
| Gender | 0.014 | ||
| Male | 284 (77.8) | 81 (22.2) | |
| Female | 226 (85.6) | 38 (14.4) | |
| Histological type | 0.005 | ||
| SCC | 109 (76.8) | 33 (23.2) | |
| NSCC | 371 (84.1) | 70 (15.9) | |
| Others | 30 (66.7) | 15 (33.3) | |
| Size, cm | 0.062 | ||
| <3 | 238 (84.7) | 43 (15.3) | |
| 3–5 | 156 (80.8) | 37 (19.2) | |
| 5–7 | 74 (75.5) | 24 (24.5) | |
| ≥7 | 35 (71.4) | 14 (28.6) | |
| pN stage | 0.097 | ||
| pN0 | 303 (80.2) | 75 (19.8) | |
| pN1 | 63 (75.0) | 21 (25.0) | |
| pN2 | 120 (86.3) | 19 (13.7) | |
| Chemotherapy | 0.097 | ||
| Yes | 270 (78.7) | 73 (21.3) | |
| No | 240 (83.9) | 46 (16.1) |
SCC, squamous cell carcinoma; NSCC, non-SCC; MK, megakaryocyte.
Prognostic significance of MK+ in NSCLC patients
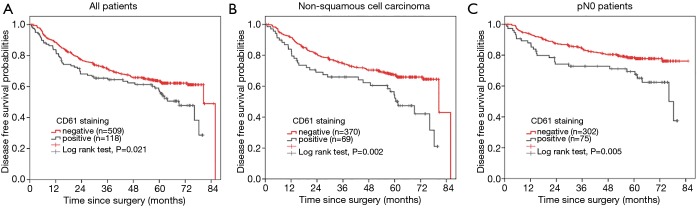
We performed survival analyses of DFS for the study patients. In univariate analysis, the DFS significantly declined in patients with positive MK staining (MK+ group 80.5 months vs. MK- group 69.1 months; P=0.021) (Figure 2A). After we excluded the impact of age, gender, histological type, tumor size, pN stage and adjuvant chemotherapy in the Cox proportional hazard model, it was revealed that the existence of MKs in tumor tissue was still an unfavorable prognostic factor for DFS (HR, 1.351, 95% CI: 0.982–1.859, P=0.065). Further stratified analysis demonstrated that internal MKs in the tumor tissue was strongly associated with poorer prognosis in the NSCC group, which mainly consisted of adenocarcinoma (MK+ group 61.5 months vs. MK- group 80.5 months, P=0.002; multivariable cox model, HR, 1.710, 95% CI: 1.148–2.545, P=0.008) (Figure 2B), However, the difference between the MK-positive and MK-negative group was not significant in the SCC group (long rank P=0.538; HR=0.860, 95% CI: 0.436–1.700, P=0.665). In patients with pN0, MK+ significantly correlated with poor prognosis (MK+ group 76.2 months vs. MK- group N.A. months, P=0.005; HR, 1.883, 95% CI: 1.173–3.024, P=0.009) (Figure 2C); however, no similar correlation was observed in the patients with pN1 or pN2. No significance was found in other stratified groups.
Figure 2.
The DFS curve. (A) The DFS curve of 627 NSCLC patients demonstrated that CD61+ MKs lead to poorer prognosis (P=0.021); (B) the DFS curve of 439 non-squamous cell carcinoma patients demonstrated that CD61+ MKs lead to poorer prognosis (P=0.002); (C) the DFS curve of 377 N0 patients demonstrated that CD61+ MKs lead to poorer prognosis (P=0.005). DFS, disease-free survival; NSCLC, non-small cell lung cancer; MK, megakaryocyte.
Relation of MK and venous platelet level
Systemic blood platelet count in the MK+ group was significantly higher than the MK- group (P=0.007). The cases of patients with a platelet level equal or greater than 300 (×109 L−1) in the MK+ group were significantly higher than those in the MK- group (MK+ group 26.3% vs. MK- group 16.5%; P=0.027) (Table 3).
Table 3. Preoperative platelet count of MK- group and MK+ group.
| Platelet | MK- group, n=391 (%) | MK+ group, n=89 (%) | P |
|---|---|---|---|
| Platelet count (×109/L) | 243.6±80.8 | 270.6±101.5 | 0.007 |
| Platelet level (×109/L) | |||
| <300 | 318 (83.5) | 63 (16.5) | 0.027 |
| ≥300 | 73 (73.7) | 26 (26.3) |
MK, megakaryocyte.
We still found MK to be an unfavorable prognostic factor for lung cancer even after including the platelet count into the Cox proportional hazard model, which was independent from the influence of the systemic platelet count (HR, 1.432, 95% CI: 0.994–2.065, P=0.054). Furthermore, the DFS curves for four groups stratified according to the MK and platelet level were clearly divided (Figure 3), by which we speculated that MKs inside the tumor and platelets outside the tumor exerted their impact on lung cancer through different mechanisms.
Figure 3.
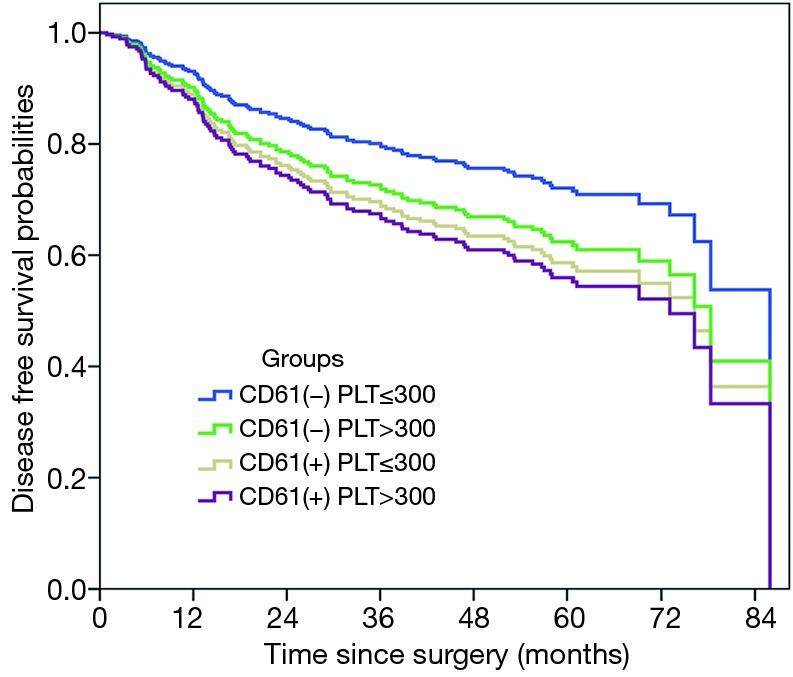
The DFS curves of four subgroups separated (P<0.001): MK(-)Platelet(-) group > MK(-)Platelet(+) group > MK(+)Platelet(-) group > MK(+)Platelet(+). DFS, disease-free survival; MK, megakaryocyte.
SDF-1 staining and microthrombus
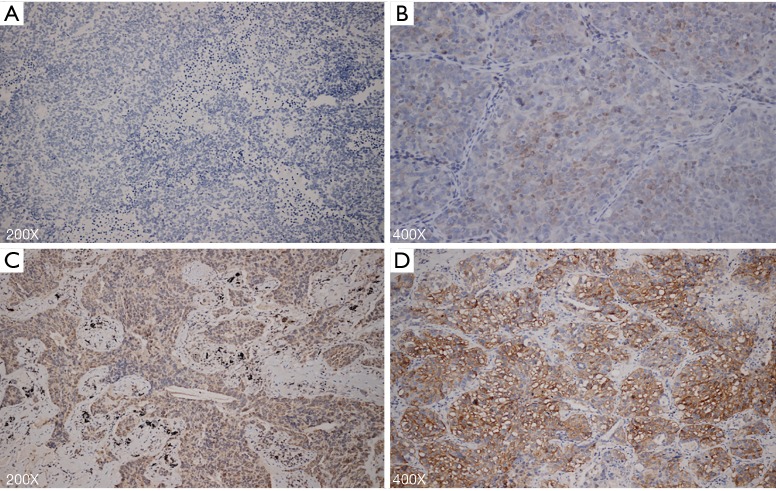
As a potential chemotactic cytokine for MK, SDF-1 showed a high expression of 24.1% in MK- tumors, which was lower than 33.6% expression in MK+ tumors (P=0.033) (Table 4) (Figure 4). Multivariate analysis indicated that DFS was not influenced by SDF-1 in this cohort (HR, 1.110, 95% CI: 0.821–1.501 P=0.498). After SDF-1 was included into the multivariate Cox proportional hazard model, we found that the presence of MK was still a predictor for poor prognosis of NSCLC (HR, 1.375, 95% CI: 0.997–1.898 P=0.052). Microthrombus formation was observed to reflect the local platelet level inside the tumor. Microthrombus formation was significantly greater in the MK+ group than that in the MK- group (MK+ 24.6% vs. MK- 12.7%; P=0.001) (Table 4) (Figure 5).
Table 4. SDF-1 and microthrombus between MK- group and MK+ group.
| Characteristic | MK- | MK+ | P |
|---|---|---|---|
| SDF-1 | 0.033 | ||
| Low-absent | 387 (75.9) | 79 (66.4) | |
| High-level | 123 (24.1) | 40 (33.6) | |
| Microthrombus | 0.001 | ||
| Negative | 445 (87.3) | 90 (75.4) | |
| Positive | 65 (12.7) | 29 (24.6) |
SDF-1, stromal cell-derived factor-1; MK, megakaryocyte.
Figure 4.
Immunohistochemical staining for SDF-1. (A) Malignant cells exhibit absent SDF-1 staining in MK- group, magnification 200×; (B) malignant cells exhibit (+) SDF-1 staining in MK- group, magnification 400×; (C) malignant cells exhibit (++) SDF-1 staining in MK+ group, magnification 200×; (D) malignant cells exhibit (+++) SDF-1 staining in MK+ group, magnification 400×. SDF-1, stromal cell-derived factor-1; MK, megakaryocyte.
Figure 5.
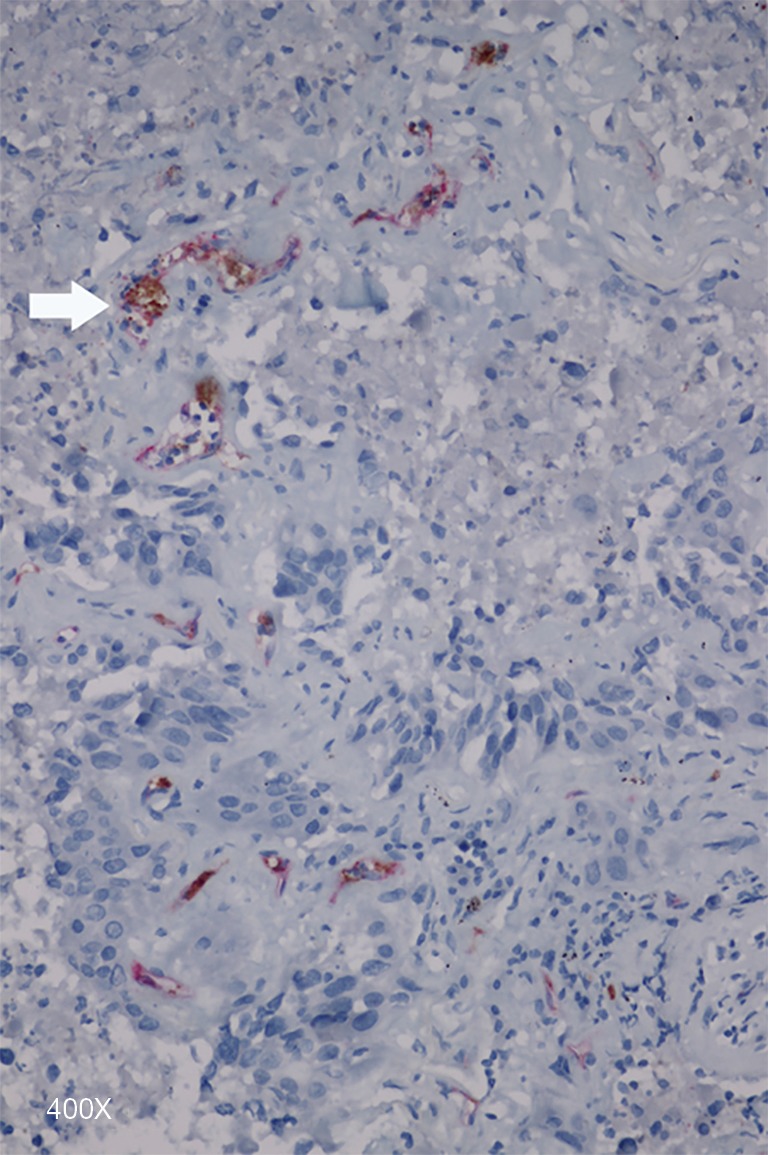
Microthrombi (arrow) located in CD34+ capillary, magnification 400×.
Discussion
To the best of our knowledge, this is the first study to show the existence of CD61+ MKs in the cancer microenvironment and to examine its prognostic impact. Previously, it was assumed that higher the systemic platelet count was, poorer the prognosis for cancer patients (8,23). However, the old concept of the lungs being a reservoir for MKs resulted in overlooking of the existence of regional platelets and their impact on tumors (24).
The concept that platelets are released from MKs in the lung was not universally accepted until Dorothea Zucker-Franklin directly observed, using an electron microscope, that intact platelets were disaggregated from MKs (15). A recent study reported that more than 10 million platelets per hour were released from the lungs of a mouse, accounting for approximately 50% of total platelet production (14). Most lung cancers are nourished by the pulmonary artery (16), which carries and pours MKs into the capillary bed of cancer. Once MKs are sheared by intra-tumoral capillary bed, hundreds of platelets per MK are released that boost growth and early metastasis of tumors.
We utilized CD61 to mark giant cells in tumoral microvessels and excluded monocytes with a diameter of less than 10 µm. The IHC method produced reliable staining. The size of most MKs in lung cancer looked smaller than that of MKs in the bone marrow possibly due to the loss of one portion of cytoplasm. Howell & Donahue proposed that the giant cells with retained cytoplasm represent a state of active platelet production if frequently detected in pulmonary circulation (13). Therefore, we speculated that similar to normal lung tissue, tumor tissue containing MKs with limited cytoplasm was actively involved in thrombopoiesis.
Positive staining for MKs represented a higher density of MKs within the tumor, enabling easier detection of MKs on the sections. However, negative staining did not mean that no MKs existed in the tumor; their absence from the microscopic field was due to a lower density of MKs. Positive staining reflected a high density and negative staining reflected a low density of MKs in a tumor. MK+ patients accounted for 18.9% of the total 629 patients. There were more MK+ cases in patients with SCC than with NSCC which mainly consisted of adenocarcinoma (SCC 23.3% vs. NSCC 15.9%); this may be due to richer pulmonary supply to SCC. Both univariate and multivariate analysis demonstrated MK to be a poor prognostic factor for lung cancer. There are two possibilities for these findings: (I) intra-tumoral MKs improved the regional concentration of platelets; (II) regional higher density of MKs was derived from a larger amount of MKs in the pulmonary blood, which was associated with a higher total platelet count in the blood (18,25), and as a result of elevated platelet count in blood, the regional concentration of platelets increased. Positive relation between regional MKs and the total platelet count was confirmed in Table 3. Diameter of every platelet that ranged from 2 to 4 µm rendered them passing the capillary bed without any obstacles. Capillary platelets can coat tumor cells, protect them from neutralization by tumor-necrosis-factor-α-mediated cytotoxicity and natural killer cells, and release various cytokines to promote growth of tumor. Elevated platelet count pre-operatively has been recognized as a negative impact factor on patients with resectable NSCLC (26). Subsequent Cox regression analysis indicated that the prognostic effect of MKs on NSCC was obvious. Maybe adenocarcinoma itself showed greater tendency to metastasize than SCC. The prognostic effect is more significant in early stage (pN0), where relatively smaller proportion of patients have actual metastases.
Was poor prognosis of the MK+ group caused by high systemic platelet level or by high regional platelet level disaggregated from MKs within the tumor? We considered that the existence of regional MKs might have an additional impact on tumor progression. Figure 3 revealed that DFS curves of four subgroups were significantly separated: MK(-)Platelet(-) group > MK(-)Platelet(+) group > MK(+)Platelet(-) group > MK(+)Platelet(+) (P=0.003). Two conclusions can be drawn from Figure 3: (I) total thrombocytosis and intra-tumoral MKs were an unfavorable factor of prognosis for NSCLC; (II) the effect of intra-tumoral MKs on tumor was independent from those of blood platelets. Further investigation showed that the cases of microthrombus formation were significantly improved in the MK+ group than those in the MK- group (P=0.001); however, microthrombus formation was weakly elevated in the group with platelet level <300×109/L than that in the group with platelet level ≥300×109/L (P=0.355). More microthrombus cases reflected higher regional platelet level. There are no previous studies reporting such oncological function of mature MKs in the lung. The only possibility was that intra-tumoral MKs produced platelets into the tumor region, thus leading to more cases of microthrombus formation.
Did NSCLC have the ability to attract MKs? Our study revealed that the MK+ group tended to overexpress stronger SDF-1 than the MK- group (Table 4) (Figure 4). SDF-1 was a powerful chemotactic factor for mature MKs, suggesting that NSCLC is capable of recruiting MKs into the sites where tumor cells need platelets. The mechanisms underlying the unfavorable prognostic impact of MKs in NSCLC are as follows: MKs produced platelets, which increased the concentration of platelets in tumor tissues, or MKs directly supplied platelets to the site where the platelets can fuel the metastasis of cancer cells. Whether MKs have a direct effect on a tumor or not needs to be further investigated. Our study indicated that malignant tumors containing MKs have the capacity to produce platelets. Intra-tumoral thrombopoiesis is hypothesized as a newly discovered mechanism for continuous growth and distant metastasis of malignant tumors.
In conclusion, presence of intra-tumoral CD61+ MKs is an independent predictive factor for poor prognosis in NSCLC. Especially for NSCC, CD61+ MKs imply a high possibility of metastasis in early-stage tumors. It can be used as a marker for predicting the risk of recurrence.
Acknowledgments
We would like to thank Mrs. Lindsey Hamblin from our department for her assistance in revising the manuscript.
Funding: This work was supported by the Science and Technology Planning Project of Guangdong Province (2007B031515017) and the Guangdong Doctoral Launching Program (2014A030310460).
Ethical Statement: The protocol of this study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. Kls2015-25). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schwertz H, Koster S, Kahr WH, et al. Anucleate platelets generate progeny. Blood 2010;115:3801-9. 10.1182/blood-2009-08-239558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho MS, Bottsford-Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood 2012;120:4869-72. 10.1182/blood-2012-06-438598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA 1968;61:46-52. 10.1073/pnas.61.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013;24:130-7. 10.1016/j.ccr.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295-300. [PubMed] [Google Scholar]
- 6.Placke T, Orgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 2012;72:440-8. 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- 7.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 8.Cox G, Walker RA, Andi A, et al. Prognostic significance of platelet and microvessel counts in operable non-small cell lung cancer. Lung Cancer 2000;29:169-77. 10.1016/S0169-5002(00)00124-0 [DOI] [PubMed] [Google Scholar]
- 9.Mezouar S, Frere C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res 2016;139:65-76. 10.1016/j.thromres.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol 2013;75:569-91. 10.1146/annurev-physiol-030212-183752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavassoli M, Aoki M. Migration of entire megakaryocytes through the marrow--blood barrier. Br J Haematol 1981;48:25-9. 10.1111/j.1365-2141.1981.00025.x [DOI] [PubMed] [Google Scholar]
- 12.Lichtman MA, Chamberlain JK, Simon W, et al. Parasinusoidal location of megakaryocytes in marrow: a determinant of platelet release. Am J Hematol 1978;4:303-12. 10.1002/ajh.2830040402 [DOI] [PubMed] [Google Scholar]
- 13.Howell WH, Donahue DD. The production of blood platelets in the lungs. J Exp Med 1937;65:177-203. 10.1084/jem.65.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefrançais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017;544:105-9. 10.1038/nature21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol 2000;157:69-74. 10.1016/S0002-9440(10)64518-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savai R, Wolf JC, Greschus S, et al. Analysis of Tumor Vessel Supply in Lewis Lung Carcinoma in Mice by Fluorescent Microsphere Distribution and Imaging with Micro- and Flat-Panel Computed Tomography. Am J Pathol 2005;167:937-46. 10.1016/S0002-9440(10)61184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamada T, Mohle R, Hesselgesser J, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med 1998;188:539-48. 10.1084/jem.188.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandal RV, Mark EJ, Kradin RL. Megakaryocytes and platelet homeostasis in diffuse alveolar damage. Exp Mol Pathol 2007;83:327-31. 10.1016/j.yexmp.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 19.Lunetta P, Penttila A. Immunohistochemical identification of syncytiotrophoblastic cells and megakaryocytes in pulmonary vessels in a fatal case of amniotic fluid embolism. Int J Legal Med 1996;108:210-4. 10.1007/BF01369794 [DOI] [PubMed] [Google Scholar]
- 20.Ozbudak IH, Shilo K, Hale S, et al. Alveolar airspace and pulmonary artery involvement by extramedullary hematopoiesis: a unique manifestation of myelofibrosis. Arch Pathol Lab Med 2008;132:99-103. [DOI] [PubMed] [Google Scholar]
- 21.Bing Z, Jian-ru Y, Yao-quan J, et al. Evaluation of angiogenesis in non-small cell lung carcinoma by CD34 immunohistochemistry. Cell Biochem Biophys 2014;70:327-31. 10.1007/s12013-014-9916-5 [DOI] [PubMed] [Google Scholar]
- 22.Fromowitz FB, Viola MV, Chao S, et al. ras p21 expression in the progression of breast cancer. Hum Pathol 1987;18:1268-75. 10.1016/S0046-8177(87)80412-4 [DOI] [PubMed] [Google Scholar]
- 23.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012;366:610-8. 10.1056/NEJMoa1110352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine RF, Eldor A, Shoff PK, et al. Circulating megakaryocytes: delivery of large numbers of intact, mature megakaryocytes to the lungs. Eur J Haematol 1993;51:233-46. 10.1111/j.1600-0609.1993.tb00637.x [DOI] [PubMed] [Google Scholar]
- 25.Kristensen SD, Bath PM, Gladwin AM, et al. The relationship between increased platelet count and megakaryocyte size in bronchial carcinoma. Br J Haematol 1992;81:247-51. 10.1111/j.1365-2141.1992.tb08215.x [DOI] [PubMed] [Google Scholar]
- 26.Tomita M, Shimizu T, Hara M, Ayabe T, et al. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2008;7:613-5. 10.1510/icvts.2007.174391 [DOI] [PubMed] [Google Scholar]