Abstract
Background
The purpose of this study was to evaluate baseline demographic characteristics which may be associated with worse health related quality of life (HRQOL) for patients with locally advanced non-small cell lung cancer (NSCLC) receiving definitive chemoradiation (CRT).
Materials
Patients with NSCLC were prospectively enrolled on an Institutional Review Board-approved clinical trial between 2009 and 2012. HRQOL assessments were collected pre-radiation therapy (RT), during RT, and within 3 months post-RT using Euroqol (EQ-5D), MD Anderson Symptom Inventory (MDASI), and Functional Assessment of Cancer Therapy General (FACT-G). HRQOL correlation was assessed with categorical variables by Wilcoxon rank sum tests and with continuous variables by Pearson correlation. P<0.05 was defined as statistically significant.
Results
Forty-three consecutive patients received definitive concurrent CRT and completed assessments at one or more time-points. Patients most commonly had stage IIIB disease (72%), were married or with a partner (70%) and Caucasian (91%). Median patient age was 65 (range: 39–79) years and Charlson comorbidity index (CCI) was 0 (range: 0–5). Female gender, African-American ethnicity, age, chemotherapy type, baseline hemoglobin, and CCI were associated with worse post-treatment HRQOL measures.
Conclusions
We have identified novel characteristics associated with worse quality of life following definitive CRT for lung cancer. Patients at risk for worse post-treatment quality of life may benefit from earlier follow-up and greater supportive measures following treatment.
Keywords: Chemoradiation (CRT), non-small cell lung cancer (NSCLC), quality of life
Introduction
Lung cancer is the leading cause of cancer-related death in the United States, with 224,000 cases and 158,000 deaths in 2016 (1). Approximately one-third of patients present with locally advanced non-small cell lung cancer (LA-NSCLC) (2). These patients receive multi-modality therapy which may include chemotherapy, radiation therapy (RT), and surgery.
Pre-treatment demographic characteristics including race, age, Karnofsky performance status, marital status, education level, income level, and employment have been associated with worse health related quality of life (HRQOL) outcomes in patients with head-and-neck, esophageal, lung, and prostate cancer (3). In lung cancer specifically, age, gender, income, insurance, smoking status, and symptoms of disease have all been shown to contribute to overall quality of life (4).
In lung cancer patients treated with definitive chemoradiation (CRT), quality of life has also been shown to contribute to long-term outcomes. Baseline physical functioning as assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) questionnaire was an independent predictor of overall survival (OS) in patients prospectively treated on the Radiation Therapy Oncology Group (RTOG) 9801 (3). Baseline lung Cancer 13 (LC-13) dyspnea score and Functional Assessment of Cancer Therapy (FACT)-Trial Outcome Index (TOI) have also been shown to independently predict for OS in these patients (5,6).
While demographic characteristics have been shown to correlate with baseline HRQOL measures in patients with LA-NSCLC, few studies have evaluated post-treatment HRQOL in these patients. In this study, we sought to evaluate the impact of demographic characteristics on post-treatment HRQOL in patients with LA-NSCLC treated with definitive CRT, to evaluate changes in HRQOL over time, and study correlation of pre and post-treatment HRQOL measures with OS. We hypothesize that baseline demographic characteristics may be correlated with post-treatment HRQOL measures.
Methods
After informed consent, patients with NSCLC were prospectively enrolled on an Institutional-Review Board (IRB) approved study (IRB 808014) at the Hospital of the University of Pennsylvania between 2009 and 2012. Inclusion criteria included patients with stage I, II, and III NSCLC. Other eligibility criteria included being aged 18 and over and having the ability to read and understand English. Exclusion criteria for the study included stage IV disease or patients treated without radiation.
QOL assessments were collected pre-RT, during RT, and within 3 months post-RT using Euroqol (EQ-5D), MD Anderson Symptom Inventory (MDASI), and functional assessment of cancer therapy-general (FACT-G). Patients were given surveys to complete prior to seeing the physician at each time-point.
Clinical data were abstracted from patients’ electronic medical records. Other pre-specified data abstracted included patient age, sex, race, smoking status, performance status, baseline hemoglobin, tumor histology, Charlson comorbidity index (CCI) (7), body mass index (BMI), and radiation modality. BMI was assessed at the pre-treatment consultation using the standard formula of weight in kilograms divided by height in meters squared. BMI cut-offs were as defined by the Centers for Disease Control including underwent, normal, overweight, and obese were <18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2, respectively.
HRQOL correlation with categorical variables was assessed for categorical variables by Wilcoxon rank sum tests and for continuous variables by Pearson correlation. CCI was further evaluated by adjusting for co-variables using linear regression analysis. Comparison of means across time was performed by Student’s t-test. The Kaplan-Meier method was used to estimate OS. Univariable analysis was performed using the log-rank test. P<0.05 was considered statistically significant. In this hypothesis-generating study, no adjustments were made for multiple comparisons.
Results
Forty-three consecutive patients with LA-NSCLC treated with definitive CRT were enrolled and completed one or more QOL assessments. Nineteen patients (44%) completed an initial and on-treatment assessment but did not complete a follow-up assessment. The patients who completed follow-up assessments did not differ from those who did not with respect to CCI, BMI, cancer stage, or history of prior RT (P≥0.05).
Patients were a median age of 65 (range: 39–79) years (Table 1). Patients were predominantly male (60%), obese or overweight (77%), married or with a partner (70%), and Caucasian (91%). Patients smoked a median of 40 (range, 0–100) pack-years. Of all patients, 2 were never smokers. Median CCI was 0 (range, 0–5). Seventy-two percent of patients were stage IIIB and 56% were treated with a cisplatin-based doublet chemotherapy regimen. Median radiotherapy dose was 66.6 (range: 45–79.2) Gy using photon (93%) or proton (7%) plans.
Table 1. Patient characteristics.
| Characteristic | N (%) |
|---|---|
| Age (years) | |
| Median, range | 65, 39–79 |
| Sex | |
| Female | 17 (40%) |
| Male | 26 (60%) |
| Smoking status | |
| Median pack-years, range | 40, 0–100 |
| Marital status | |
| Married/with partner | 30 (70%) |
| Divorced/widowed/single/separated | 13 (30%) |
| BMI | |
| Obese | 9 (21%) |
| Overweight | 24 (56%) |
| CCI | |
| Median, range | 0, 0–5 |
| Ethnicity | |
| Caucasian | 39 (91%) |
| African American | 4 (9%) |
| Stage | |
| II/IIIA | 12 (28%) |
| IIIB | 31 (72%) |
| Chemotherapy | |
| Cisplatin-based doublet | 24 (56%) |
| Carboplatin-based doublet | 19 (44%) |
BMI, body mass index; CCI, Charlson comorbidity score.
On univariable analysis, pre-treatment carboplatin-based chemotherapy was associated with worse MDASI dry mouth (2.5 vs. 0.9, P=0.04). Age >60 was associated with worse MDASI sadness scores (3.7 vs. 1.7, P=0.03). Female gender was associated with worse MDASI sadness (3.1 vs. 1.5, P=0.03), distress (3.4 vs. 1.7, P=0.01), and overall severity score (27.2 vs. 15.2, P=0.04). Hemoglobin <12 was associated with worse MDASI pain (4.2 vs. 0.5, P<0.01) and fatigue (3.2 vs. 2.1, P<0.01). Lower tumor stage was associated with worse MDASI sleep interference (2.9 vs. 0.7, P<0.01) and fatigue (3.4 vs. 1.0, P<0.01). Higher tumor stage was associated with worse FACT-G emotional functioning (15.4 vs. 18.6, P<0.01). Non-white patients reported worse MDASI numbness (2.3 vs. 0.5, P<0.01) (Table 2). Higher CCI was associated with worse pre-treatment MDASI memory scores (r=0.4, P=0.03) (Table 3). Higher CCI was also associated with receipt of carboplatin-based chemotherapy, >40 pack-year smoking history, and squamous histology (P≤0.02).
Table 2. Univariable analysis of baseline demographic characteristics and HRQOL outcomes for categorical variables.
| Time point | HRQOL measure | Characteristic | Mean | 95% CI | P value |
|---|---|---|---|---|---|
| Pre-treatment | FACT-G emotional | Stage IIIB | 15.4 | 13.2–17.8 | <0.01 |
| 18.6 | 15.6–21.5 | ||||
| MDASI distress | Female gender | 3.4 | 1.8–5.0 | 0.01 | |
| 1.7 | 0.4–3.2 | ||||
| MDASI dry mouth | Carboplatin-based chemo | 2.5 | 0.6–4.4 | 0.04 | |
| 0.9 | −0.1–1.8 | ||||
| MDASI fatigue | Stage II/IIIA | 3.4 | 2.2–4.5 | <0.01 | |
| 1.0 | −1.0–3.0 | ||||
| Hemoglobin <12 | 3.2 | −1.3–7.7 | <0.01 | ||
| 2.1 | 1.1–3.1 | ||||
| MDASI numbness | Non-white ethnicity | 2.3 | −3.4–8.1 | <0.01 | |
| 0.5 | 0.2–0.9 | ||||
| MDASI pain | Hemoglobin <12 | 4.2 | −1.2–9.9 | <0.01 | |
| 0.5 | 0.2–1.1 | ||||
| MDASI sadness | Age >60 | 3.7 | 1.7–4.6 | 0.03 | |
| 1.7 | 0.5–2.3 | ||||
| Female gender | 3.1 | 1.8–4.5 | 0.03 | ||
| 1.5 | 0.5–2.7 | ||||
| MDASI severity | Female gender | 27.2 | 15.2–39.2 | 0.04 | |
| 15.2 | 7.5–25.9 | ||||
| MDASI sleep interference | Stage II/IIIA | 2.9 | 1.8–4.03 | <0.01 | |
| 0.7 | −0.3–3.2 | ||||
| Post-treatment | EQ-5D index value | Carboplatin-based chemo | 0.7 | 0.3–0.9 | <0.01 |
| 0.9 | 0.6–1.0 | ||||
| FACT-G emotional score | Female gender | 16.0 | 12.1–19.9 | 0.02 | |
| 21.3 | 19.4–23.1 | ||||
| FACT-G fatigue | Carboplatin-based chemo | 2.6 | 1.3–3.9 | 0.02 | |
| 5.4 | 3.1–7.6 | ||||
| FACT-G physical functioning | Cisplatin-based chemo | 17.0 | 11.2–22.8 | 0.02 | |
| 23.1 | 20.1–26.1 | ||||
| MDASI distress | Carboplatin-based chemo | 3.0 | 0.5–5.5 | 0.04 | |
| 0.8 | 0.1–1.5 | ||||
| MDASI dry mouth | Age >60 | 2.7 | 0.9–4.5 | 0.03 | |
| 0.8 | −0.5–2.2 | ||||
| MDASI memory | Age >60 | 3.2 | 1.5–4.9 | <0.01 | |
| 0.4 | −0.3–1.1 | ||||
| Carboplatin-based chemo | 3.6 | 1.5–5.8 | <0.01 | ||
| 0.5 | −0.6–1.6 | ||||
| MDASI nausea/vomiting | Non-white ethnicity | 1.0 | −0.8–2.1 | <0.01 | |
| 0.0 | 0.0–0.0 | ||||
| MDASI pain | Non-white ethnicity | 4.3 | −3.6–12.3 | 0.03 | |
| 1.4 | −1.5–4.3 | ||||
| MDASI severity | Carboplatin-based chemo | 39.4 | 20.1–58.1 | 0.02 | |
| 14.9 | 6.2–23.6 | ||||
| MDASI SOB | Hemoglobin <12 | 3.0 | 1.6–4.3 | 0.04 | |
| 0.0 | 0.0–0.0 |
HRQOL, health-related quality of life; FACT-G, functional assessment of cancer therapy general; MDASI, MD Anderson Symptom Inventory; SOB, shortness of breath; chemo, chemotherapy.
Table 3. Univariable analysis of baseline demographic characteristics and HRQOL outcomes for continuous variables.
| Time point | HRQOL measure | Characteristic | R | P value |
|---|---|---|---|---|
| Pre-treatment | MDASI memory | CCI | 0.4 | 0.03 |
| Post-treatment | EQ-5D index value | CCI | −0.5 | 0.01 |
| EQ-5D vas score | CCI | −0.4 | 0.04 | |
| MDASI distress | CCI | 0.6 | <0.01 | |
| MDASI general activity | CCI | 0.5 | 0.03 | |
| MDASI interference | CCI | 0.6 | <0.01 | |
| MDASI mood | CCI | 0.5 | 0.01 | |
| MDASI numbness | CCI | 0.5 | 0.03 | |
| MDASI relationships | CCI | 0.7 | <0.01 | |
| MDASI sadness | CCI | 0.6 | <0.01 | |
| MDASI severity | CCI | 0.4 | 0.04 | |
| MDASI sleep interference | CCI | 0.5 | 0.02 | |
| MDASI SOB | CCI | 0.4 | 0.03 | |
| MDASI walking | CCI | 0.6 | <0.01 | |
| MDASI work interference | CCI | 0.6 | 0.03 |
HRQOL, health-related quality of life; EQ-5D, Euroqol 5-Dimension; VAS, visual analog scale; MDASI, MD Anderson Symptom Inventory; CCI, Charlson comorbidity index; SOB, shortness of breath; chemo, chemotherapy.
Post-treatment, carboplatin-based chemotherapy was associated with worse EQ-5D index value (0.7 vs. 0.9, P<0.01) and MDASI distress (3.0 vs. 0.8, P=0.04), memory (3.6 vs. 0.5, P<0.01), and overall severity scores (39.4 vs. 14.9, P=0.02). Carboplatin-based chemotherapy was also associated with worse FACT-G fatigue (2.6 vs. 5.4, P=0.02). Cisplatin-based chemotherapy was associated with worse FACT-G physical functioning score (17.0 vs. 23.1, P=0.02).
Non-white ethnicity was associated with worse MDASI nausea (1.0 vs. 0.0, P<0.01) and pain (4.3 vs. 1.4, P=0.03). Age >60 was associated with worse MDASI dry mouth (2.7 vs. 0.8, P=0.03) and memory scores (3.2 vs. 0.4, P<0.01). Hemoglobin <12 mg/dL was associated with worse MDASI shortness of breath (SOB) (3.0 vs. 0.0, P=0.04). Female gender was associated with worse FACT-G emotional score (16.0 vs. 21.3, P=0.02) (Table 2).
CCI was associated with worse post-treatment EQ-5D index score (r=−0.5, P=0.01) and visual analog scores (r=−0.4, P=0.04). CCI was associated with worse MDASI distress (r=0.6, P<0.01), sleep interference (r=0.5, P=0.02), general activity (r=0.5, P=0.03), mood (r=0.5, P=0.01), numbness (r=0.5, P=0.03), relationships (r=0.7, P<0.01), sadness (r=0.6, P<0.01), SOB (r=0.4, P=0.03), work interference (r=0.6, P=0.03), overall interference (r=0.6, P<0.01), walking (r=0.6, P<0.01), and overall severity scores (r=0.4, P=0.04) (Table 3).
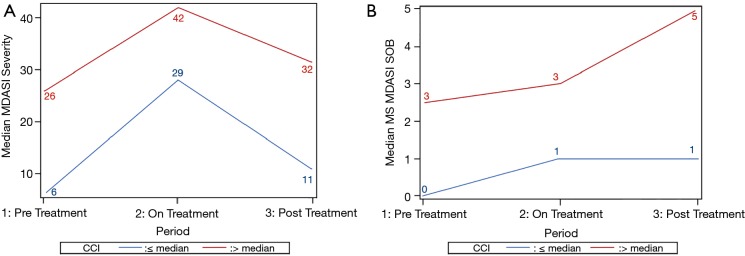
After adjustment for co-variables, CCI remained significantly associated with post-treatment MDASI SOB (P=0.01) and overall severity score (P=0.04) (Table 4, Figure 1).
Table 4. Association of CCI and post-treatment HRQOL after adjustment for co-variables.
| HRQOL measure | P value |
|---|---|
| EQ-5D index value | 0.05 |
| MDASI distress | 0.09 |
| MDASI severity score | 0.01 |
| MDASI SOB | 0.04 |
HRQOL, health-related quality of life; EQ-5D, Euroqol 5-Dimension; VAS, visual analog scale; MDASI, MD Anderson Symptom Inventory; SOB, shortness of breath; CCI, Charlson comorbidity index.
Figure 1.
MDASI severity (A) and MDASI SOB (B) by CCI above or below the median over time. MDASI, MD Anderson Symptom Inventory; SOB, shortness of breath; CCI, Charlson comorbidity index.
There was no significant change in HRQOL parameters post-treatment as compared to baseline (Table 5).
Table 5. Pre and post-treatment HRQOL values.
| HRQOL variable | Pre-RT (mean) | Post-RT (mean) | P |
|---|---|---|---|
| EQ-5D VAS score | 66 | 72 | 0.34 |
| EQ-5D index value | 0.86 | 0.83 | 0.34 |
| FACT-G emotional score | 16 | 18 | 0.18 |
| FACT-G functional score | 15 | 17 | 0.25 |
| FACT-G physical score | 22 | 21 | 0.80 |
| FACT-G social score | 23 | 25 | 0.09 |
| FACT-G sum | 75 | 81 | 0.80 |
| MDASI interference score | 13 | 15 | 0.56 |
| MDASI severity score | 20 | 23 | 0.54 |
HRQOL, health-related quality of life; EQ-5D, Euroqol 5-Dimension; VAS, visual analog scale; FACT-G, Functional Assessment of Cancer Therapy General; MDASI, MD Anderson Symptom Inventory; RT, radiation therapy.
Median follow up was 50.0 (range: 11.0–67.3) months. Median OS was 22.0 (range: 2.4–67.3) months (Figure 2). No HRQOL parameters were found to be significantly associated with OS.
Figure 2.
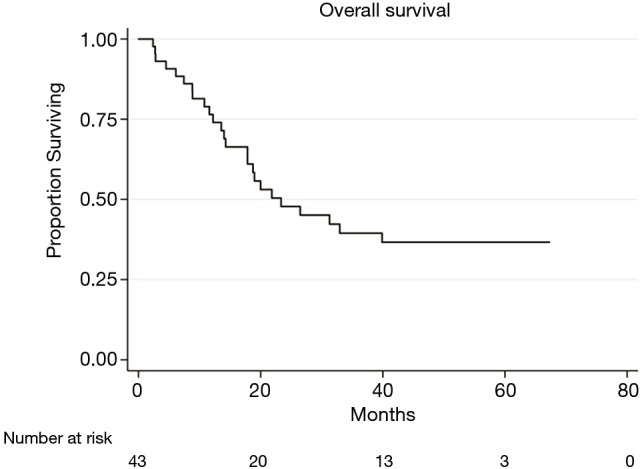
Overall survival in patients with LA-NSCLC treated with definitive concurrent chemoradiation. LA-NSCLC, locally advanced non-small cell lung cancer.
Discussion
In this study we examine the effects of demographic characteristics on pre and post-treatment HRQOL in patients with LA-NSCLC treated with definitive intent CRT. Post-treatment, CCI was found to be associated with numerous HRQOL parameters on univariable analysis and with EQ-5D index value and MDASI SOB and distress after adjustment for co-variables. Other clinical characteristics which may impact HRQOL include non-white ethnicity, age, baseline hemoglobin, chemotherapy subtype, and gender.
Many of the characteristics associated with HRQOL outcomes on this study have been shown to be significant in other settings. In a study of inoperable NSCLC patients including those treated with concomitant CRT, women reported significantly worse emotional functioning at baseline with non-significantly lower values on treatment and three months after baseline assessments (8). Male gender has also been correlated with better emotional and physical well-being in patients with metastatic cancers (9). Our study similarly suggests that female gender may be a risk factor for worse emotional functioning following definitive CRT for LA-NSCLC.
Advanced age has been correlated with improvement in some HRQOL domains and decreased outcomes in other. Studies of terminally ill cancer patients have demonstrated decreased pain and improved emotional functioning in older patients as compared to younger patients (9,10). However, age greater than 65 has also been associated with worse HRQOL in lung cancer patients with solitary nodules and metastatic disease (5,11,12). Patients with advanced NSCLC receiving chemotherapy only were found to have a decrease in physical function score by 0.44 as assessed by the EORTC QLQ-C30 for each increase additional year of age (13). In our study, age greater than 60 was associated with worse symptoms including dry mouth and memory dysfunction without significant differences in physical, emotional, functional, or social scores.
Race has been associated with worse outcome in patients with cancer including lung cancer. In their analysis, Movsas et al. found significantly worse pre-treatment FACT scores in Hispanic patients as compared to Caucasians, and not significantly worse scores in African Americans (3). Studies have previously demonstrated that the severity of cancer-related chronic pain may be higher in African American patients (14,15). Racial disparities have also been demonstrated in social functioning in patients with advanced cancer diagnoses and lung cancer specifically (16,17). We similarly find that African American race is associated with increased symptom burden including numbness, pain, and nausea/vomiting.
CCI has most often been prognostic for treatment response and survival outcomes, although has been related to HRQOL in some settings. Using comorbidity defined as number of comorbid conditions, simplified comorbidity score, and CCI (less than three versus three or more), less comorbidity has been associated with better disease outcomes (18-20). In NSCLC patients treated surgically, higher CCI was associated with lower HRQOL as assessed by 15D (21). CCI has been associated with worse physical functioning and long-term global health outcomes in prostate cancer patients treated with RT as well as overall quality of life, general health, physical functioning, bodily pain, and vitality in breast cancer patients, the majority of whom received RT (22,23). In this study, patients with higher CCI had higher MDASI memory dysfunction scores at baseline. Patients with higher CCI were more likely to receive carboplatin-based chemotherapy, which was associated with worse pre-treatment MDASI dry mouth and post-treatment EQ-5D index value, MDASI distress, fatigue, memory, and overall severity scores. However, after adjustment for covariables including chemotherapy subtype, higher CCI remained significantly associated with worse HRQOL outcomes.
Prior analyses have demonstrated significant changes in HRQOL over time which were not observed in the present study. In a pooled analysis of the RAKET and Satellite trials, Hallqvist et al. found significant decreases in physical, role, emotional, cognitive, social functions and global QOL 4–6 weeks and 3 months after chemoradiotherapy as compared to baseline (5). Symptoms including fatigue, nausea, appetite loss, constipation, diarrhea, dyspnea, dysphagia, and cough were also significantly worse at 4–6 weeks and 3 months post-CRT as compared to baseline. Patients in both the RAKET and Satellite trials received induction chemotherapy prior to definitive CRT, which may have contributed to increased treatment-related toxicity and greater declines in HRQOL over time as compared to patients in the current study.
Analyses of randomized studies of patients treated with definitive CRT have demonstrated significant associations of baseline HRQOL measures and OS. In an analysis of RTOG 9801, Movsas et al. found that patients with a global QOL score less than 66.7 had an approximately 70% higher risk of death than patients with scores ≥66.7 (24). Authors found that lower baseline physical functioning score and dyspnea scores increased the risk of death. In the combined RAKET and Satellite analysis, patient-reported baseline physical function was significantly associated with OS (5). In addition, on RTOG 0617 each 10 point increase in FACT-TOI at baseline corresponded to a 10% decreased risk of death (6).
Limitations of this study include a small patient sample size and highly selected patient population. There are confounding factors that we may not have adjusted for in this analysis including education level, income, and physical activity. This study may also be limited by inflation of Type I error due to multiple testing and some significant associations may be due to chance from multiple testing. While findings from this study are promising, further investigation is needed to validate any single association.
Conclusions
In this exploratory analysis, we demonstrate that CCI is associated with multiple HRQOL outcomes in patients with LA-NSCLC treated with definitive CRT. We also demonstrate associations of HRQOL with gender, baseline hemoglobin, race, chemotherapy subtype, and patient age. These metrics may be used to predict post-treatment quality of life and select patients who may require additional supportive care during or after treatment, closer follow up after treatment or treatment de-intensification. Future studies evaluating associations between radiotherapy dosimetric parameters and patient reported outcomes may allow therapy and follow-up to be tailored on an individual patient basis. In addition to further study in this patient population, validation of these findings in patients treated with surgery followed by CRT is warranted.
Acknowledgments
None.
Ethical Statement: After informed consent, patients with NSCLC were prospectively enrolled on an Institutional-Review Board (IRB) approved study (IRB 808014) at the Hospital of the University of Pennsylvania. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cancer Facts & Figures 2016. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf. Accessed on July 31, 2019.
- 2.Bite Stat: Lung Cancer Stage at Diagnosis in the United States, 1995-2001. J Natl Cancer Inst 2005;97:1805. 10.1093/jnci/dji454 [DOI] [PubMed] [Google Scholar]
- 3.Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence quality of life in cancer patients: A Radiation Therapy Oncology Group (RTOG) analysis. Int J Radiat Oncol Biol Phys 2006;65:830-5. 10.1016/j.ijrobp.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Ozturk A, Sarihan S, Ercan I, et al. Evaluating quality of life and pulmonary function of long-term survivors of non-small cell lung cancer treated with radical or postoperative radiotherapy. Am J Clin Oncol 2009;32:65-72. 10.1097/COC.0b013e31817e6ec2 [DOI] [PubMed] [Google Scholar]
- 5.Hallqvist A, Bergman B, Nyman J. Health related quality of life in locally advanced NSCLC treated with high dose radiotherapy and concurrent chemotherapy or cetuximab--pooled results from two prospective clinical trials. Radiother Oncol 2012;104:39-44. 10.1016/j.radonc.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 6.Movsas B, Hu C, Sloan J, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol 2016;2:359-67. 10.1001/jamaoncol.2015.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 8.Lövgren M, Tishelman C, Sprangers M, et al. Symptoms and problems with functioning among women and men with inoperable lung cancer-A longitudinal study. Lung Cancer 2008;60:113-24. 10.1016/j.lungcan.2007.09.015 [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann C, Burman D, Swami N, et al. Determinants of quality of life in patients with advanced cancer. Support Care Cancer 2011;19:621-9. 10.1007/s00520-010-0866-1 [DOI] [PubMed] [Google Scholar]
- 10.Jordhøy MS, Fayers P, Loge JH, et al. Quality of life in advanced cancer patients: the impact of sociodemographic and medical characteristics. Br J Cancer 2001;85:1478-85. 10.1054/bjoc.2001.2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karczmarek-Borowska B, Pelc M, Rabiej E, et al. The quality of life of non-small cell lung cancer patients treated with chemotherapy. Pneumonol Alergol Pol 2014;82:349-57. 10.5603/PiAP.2014.0044 [DOI] [PubMed] [Google Scholar]
- 12.Lemonnier I, Baumann C, Jolly D, et al. Solitary pulmonary nodules: Consequences for patient quality of life. Qual Life Res 2011;20:101-9. 10.1007/s11136-010-9719-0 [DOI] [PubMed] [Google Scholar]
- 13.Dai YL, Yang CT, Chen KH, et al. Changes to and Determinants of Quality of Life in Patients With Advanced Non-Small-Cell Lung Cancer Undergoing Initial Chemotherapy. J Nurs Res 2017;25:203-15. [DOI] [PubMed] [Google Scholar]
- 14.Martinez KA, Snyder CF, Malin JL, et al. Is race/ethnicity related to the presence or severity of pain in colorectal and lung cancer? J Pain Symptom Manage 2014;48:1050-9. 10.1016/j.jpainsymman.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain. Cancer 2011;117:1994-2003. 10.1002/cncr.25761 [DOI] [PubMed] [Google Scholar]
- 16.Rao D, Debb S, Blitz D, et al. Racial/Ethnic Differences in the Health-Related Quality of Life of Cancer Patients. J Pain Symptom Manage 2008;36:488-96. 10.1016/j.jpainsymman.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poghosyan H, Stock S, Kennedy Sheldon L, et al. Racial Disparities in Health-Related Quality of Life After Lung Cancer Surgery: Findings From the Cancer Care Outcomes Research and Surveillance Consortium. J Thorac Oncol 2015;10:1404-12. 10.1097/JTO.0000000000000629 [DOI] [PubMed] [Google Scholar]
- 18.Li J, Chen P, Dai CH, et al. Prognostic factors in elderly patients with advanced non-small cell lung cancer treated with chemotherapy. Oncology 2009;76:355-62. 10.1159/000210024 [DOI] [PubMed] [Google Scholar]
- 19.Lilenbaum RC, Herndon JE, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (study 9730). J Clin Oncol 2005;23:190-6. 10.1200/JCO.2005.07.172 [DOI] [PubMed] [Google Scholar]
- 20.Jacot W, Colinet B, Bertrand D, et al. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol 2008;19:1458-64. 10.1093/annonc/mdn064 [DOI] [PubMed] [Google Scholar]
- 21.Rauma V, Salo J, Sintonen H, et al. Patient features predicting long-term survival and health-related quality of life after radical surgery for non-small cell lung cancer. Thorac Cancer 2016;7:333-9. 10.1111/1759-7714.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlgren T, Levitt S, Kowalski J, et al. Use of the Charlson combined comorbidity index to predict postradiotherapy quality of life for prostate cancer patients. Int J Radiat Oncol Biol Phys 2011;81:997-1004. 10.1016/j.ijrobp.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 23.Fu MR, Axelrod D, Guth AA, et al. Comorbidities and Quality of Life among Breast Cancer Survivors: A Prospective Study. J Pers Med 2015;5:229-42. 10.3390/jpm5030229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. J Clin Oncol 2009;27:5816-22. 10.1200/JCO.2009.23.7420 [DOI] [PMC free article] [PubMed] [Google Scholar]