Abstract
Background
We conducted a meta-analysis to evaluate the efficacy of anti-programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) monotherapy or immunotherapy combined with chemotherapy and further estimated the value of PD-L1 expression in predicting the response from anti-PD-1/PD-L1 treatments as monotherapy or in combination with chemotherapy.
Methods
Clinical trial data were searched from electronic databases, which evaluated PD-1/PD-L1 inhibitors in non-small cell lung cancer (NSCLC) and correlated with PD-L1 expression levels.
Results
Fifteen randomized-controlled trials involving 10,074 patients were identified. Comparing anti-PD-1/PD-L1 monotherapy to chemotherapy, the pooled HR for overall survival (OS) was 0.77 (95% CI: 0.69–0.85, P<0.00001). Subgroup analyses revealed that patients had longer OS at ≥1%, ≥5%, ≥10% and ≥50% PD-L1 expression levels. Patients with higher PD-L1 expression may get increased benefit from PD-1/PD-L1 inhibitors. Moreover, patients with PD-L1 ≥50% had an objective response rate (ORR) improvement from anti-PD-1/PD-L1 therapy (RR =1.87, 95% CI: 1.27–2.75, P=0.001), but no ORR benefits were observed in patients with PD-L1 expression <1% (RR =0.82, 95% CI: 0.56–1.22, P=0.33) or 1–49% (RR =0.80, 95% CI: 0.64–0.98, P=0.03). OS was significantly better in patients receiving second-or-third line treatments (P<0.00001) with PD-L1 ≥1%. The efficacy of PD-1 inhibitors was similar to that of PD-L1 inhibitors, with no significant difference (P=0.63, I2=0%). Furthermore, immunotherapy combined with chemotherapy had better OS (HR =0.64, 95% CI: 0.48–0.84, P=0.001) than chemotherapy alone. Subgroup analyses showed that patients benefited from the combined chemo-IO treatment in the first-line setting regardless of PD-L1 expression level.
Conclusions
PD-L1 expression may be a valuable predictor of the efficacy of anti-PD-1/PD-L1 monotherapy in certain NSCLC patients. However, the combination of chemotherapy plus immunotherapy significantly improved survival regardless of the PD-L1 expression level in the first-line treatment of NSCLC.
Keywords: Immunotherapy, meta-analysis, non-small cell lung cancer (NSCLC), programmed cell death ligand 1 (PD-L1)
Introduction
Lung cancer is the most frequent cause of cancer related mortality worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 85% of the lung cancers. Most patients already have advanced-stage or metastatic (stage III or IV) disease at the time of the first diagnosis. The long-term survival of patients with lung cancer was not optimistic, for which the 5-year survival rate was 18%, and only 4% for patients with metastatic disease (stage IV) (1,2). Platinum-based doublet chemotherapy has long been the standard of care for first-line therapy for patients with no oncogenic driver, with ORR ranging from 25–35%, while 5-year OS rates were only about 2%. In addition, chemotherapy is also associated with significant side effects (3). In recent years, the application of targeted therapies brings new hope to lung cancer patients. Patients with an oncogenic driver who received a targeted agent had a median survival of 3.5 years, which was longer than patients with no actionable drivers (4). However, the acquired resistance to tyrosine kinase inhibitors (TKI) inevitably develops. On the other hand, most patients do not have an actionable driver and may not benefit from targeted treatment (5). In this challenging clinical setting, immunotherapy is gradually changing the treatment pattern of NSCLC with improved response rates and impressive efficacy (6).
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is the earliest clinically developed targeted immune checkpoint receptor (7). It is expressed by activated T cells and regulatory T cells (Tregs), and mainly regulates the immune response in the early stages of T cell activation. The major function of CTLA-4 is to down-regulate the activity of helper T cells and enhance the immunosuppressive activity of Tregs (7). Programmed cell death 1 (PD-1), another immune checkpoint receptor, is a member of the B7-CD28 superfamily, and is expressed on activated T cells, B cells and natural killer T cells. It is a negative regulator of T-cell activity that inhibits effector T-cell activity in the effector phase and promotes tumor induced immune suppression when it interacts with its two ligands programmed cell death ligand 1 (PD-L1) and PD-L2 (8-10). It has been found that immunologic checkpoint blockade with monoclonal antibodies that target CTLA-4 and PD-1/PD-L1 (the programmed cell death protein 1 pathway) could enhance antitumour immune responses and prolong survival time (7,11). So far, the clinically available immune checkpoint inhibitors can mostly be broken down into the following major categories: antibodies against PD-1 (nivolumab, pembrolizumab or pidilizumab), antibodies against PD-L1 (atezolizumab, durvalumab or avelumab) and antibodies against CTLA-4 (ipilimumab or tremelimumab) (12). Clinical trials utilizing immunotherapy or immunotherapy combined with chemotherapy have been carried out worldwide to evaluate the survival of lung cancer patients and have shown some promising preliminary results. Recently, several inhibitors such as nivolumab, pembrolizumab, and atezolizumab have been approved by the US Food and Drug Administration (FDA) for treatment of advanced or previously treated, recurrent metastatic NSCLC. In 2018, durvalumab was approved as maintenance therapy in patients with unresectable stage III NSCLC who have not progressed after concurrent chemoradiotherapy (13). In 2019, the FDA also approved pembrolizumab for the first-line treatment of stage III or IV NSCLC patients with tumor PD-L1 expression ≥1%. Besides, chemotherapy plus PD-1/PD-L1 inhibitors have been approved for first-line therapy in NSCLC (such as pembrolizumab or atezolizumab plus chemotherapy).
So far most clinical trials have demonstrated superior efficacy of immunotherapy in advanced NSCLC, and shown a strong predictive association between PD-L1 expression levels and clinical efficacy endpoints such as overall survival (OS), progression free survival (PFS), objective response rate (ORR) (12,14). In a phase III study, PD-L1 positive patients treated with nivolumab vs. docetaxel had longer survival time than PD-L1 negative patients after progression on platinum-based chemotherapy. Furthermore, the benefit was statistically significant across all endpoints at predefined (≥1%, ≥5%, and ≥10%) PD-L1 expression levels (15). In another randomized study, nivolumab treatment demonstrated improved survival compared to docetaxel in patients with advanced, previously treated squamous cell NSCLC, while the level of PD-L1 expression did not predict survival benefit (16). On the other hand, in the CheckMate 026 trial, no PFS benefit was observed with first-line nivolumab monotherapy compared with platinum doublet chemotherapy among patients with PD-L1 ≥5% or the higher 50% level (17). Therefore, in clinical trials targeting the PD-1/PD-L1 axis, there are specific differences in the relationship between PD-L1 expression level and anti-PD-1/PD-L1 antibody efficacy, and the linear relationship between the level of PD-L1 expression and clinical response to anti-PD-1/PD-L1 cannot always be established.
In this meta-analysis, we systematically evaluated the efficacy of anti-PD-1/PD-L1 monotherapy or the combined immunotherapy with chemotherapy in NSCLC patients. Subgroup analyses were performed to assess the correlation between PD-L1 expression levels and cancer related outcomes. Furthermore, we attempted to find the best cutoff value for PD-L1 positive tumors and other predictive clinical characteristic factors to help guide future clinical practice.
Methods
Search strategy
PubMed, Embase and Google Scholar databases were used to perform our systematic literature search. The date of the last search was May 23, 2019. The search was conducted using the following keywords: “PD-1 or PD-L1” and “nivolumab or pembrolizumab or atezolizumab or durvalumab or avelumab” and “lung cancer”. Finally, we also carried out manual retrieval of references cited in the available articles.
Inclusion and exclusion criteria
Inclusion criteria were the followings:
The study reporting anti-PD-1/PD-L1 therapy or immunotherapy combined with chemotherapy in lung cancer;
The study reporting any of the following information: PFS, OS, ORR;
The study reporting sufficient information for the relationship between PD-L1 expression levels and efficacy measures;
The study was a prospective randomized controlled trial, and the full text was available.
Exclusion criteria for this study were as follows:
Letters, case reports, reviews, retrospective studies and expert opinions;
Duplicate publications;
Study with insufficient data.
If several articles concerned the same study, the study using the most significant or most recent sample of subjects was included.
Data extraction
Two reviewers (Y Xu and B Wan) independently extracted and summarized data from available studies. Disagreements were resolved by discussion among the reviewers. The following information was retrieved: authors, publication year, pathological type, trial phase, number of randomized patients, treatment strategies, clinical outcomes, PD-L1 cut-off values, IHC antibody and IHC assay utilized. We used OS as the primary endpoint, PFS and ORR as the secondary endpoints in the meta-analysis.
Quality assessment and statistical analysis
All the studies included were randomized controlled trials. Therefore, we chose the Cochrane risk of bias tool for assessing the methodological quality (18). Any inconsistencies were resolved by consensus. The pooled hazard ratios (HRs) for PFS and OS, risk ratios (RRs) for ORR were estimated to evaluate the efficacy of anti-PD-1/PD-L1 therapy and the value of PD-L1 expression through a meta-analysis. Statistical analyses were performed using Review Manager (RevMan) Version 5.3 (Nordic Cochrane Center, Copenhagen, Denmark). Heterogeneity was assessed with the Chi2 Q test and I2 statistic. A Chi2 P value less than 0.05 or an I2 value higher than 50% was considered significant heterogeneity (19). The random-effects model analyzed the data when substantial heterogeneity existed; otherwise, the fixed-effects model was used. The potential publication biases were assessed through funnel plots. Sensitivity analysis was also carried out to explore the possible sources of heterogeneity. For all reviews, a P value less than 0.05 was considered statistically significant.
Results
Results selection and study characteristics
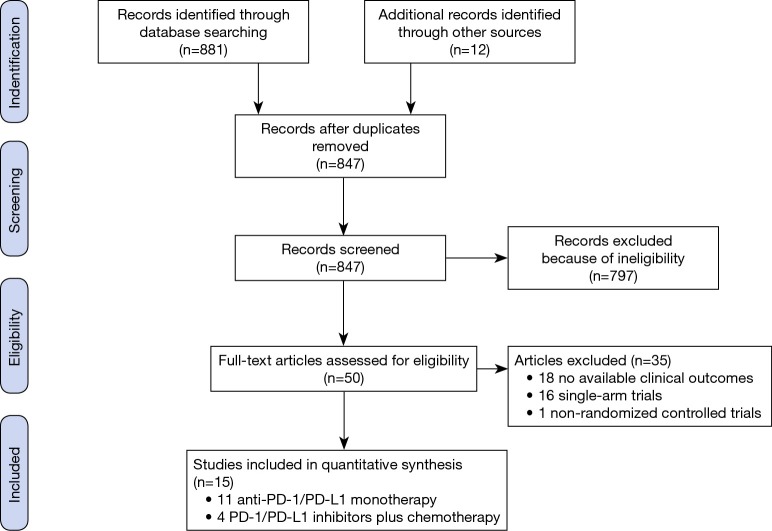
The flowchart of study selection is shown in Figure 1. A total of 893 studies were electronically retrieved from the initial database. All the articles were written in English though there were no language restrictions. After careful manual selection and review of these articles, fifteen studies with full text and available data were identified for inclusion in the final analysis (15-17,20-31): two phase II studies, one phase II/III study and twelve phase III studies. Among these fifteen studies, six studies evaluated pembrolizumab, one study used avelumab, one study used durvalumab, four studies used nivolumab and three studies assessed atezolizumab. In addition, although we did not limit the clinical stage in inclusion or exclusion criteria, all the studies above enrolled patients with advanced (stage III or IV) NSCLC. Detailed baseline characteristics of each trial are summarized in Table 1.
Figure 1.
Flowchart of study selection procedure.
Table 1. Characteristics of the included randomized controlled trials.
| Source | Study | Study type | Pathological type | Line of treatment | Treatment regimen | No. of patients | Outcome |
|---|---|---|---|---|---|---|---|
| Herbst [2016] | KEYNOTE-010 | Phase II/III | PD-L1 ≥1%, advanced NSCLC | ≥2L | Arm A: pembrolizumab group (2 mg/kg); arm B: pembrolizumab group (10 mg/kg); arm C: Docetaxel group | 345; 346; 343 | PFS, OS, ORR |
| Reck [2016] | KEYNOTE-024 | Phase III | PD-L1 ≥50% NSCLC | 1L | Arm A: pembrolizumab group (200 mg every 3 weeks); arm B: chemotherapy group | 154; 151 | PFS, OS, ORR |
| Barlesi [2018] | JAVELIN Lung 200 | Phase III | PD-L1 ≥1% NSCLC | ≥2L | Arm A: avelumab group (10 mg/kg every 2 weeks); arm B: docetaxel group (75 mg/m2 every 3 weeks) | 396; 396 | OS, ORR |
| Carbone [2017] | CheckMate 026 | Phase III | PD-L1 ≥1%, stage IV or recurrent NSCLC | 1L | Arm A: nivolumab group (3 mg/kg every 2 weeks); arm B: chemotherapy group | 271; 270 | PFS, OS, ORR |
| Brahmer [2015] | CheckMate 017 | Phase III | Advanced squamous-cell NSCLC | ≥2L | Arm A: nivolumab group (3 mg/kg every 2 weeks); arm B: docetaxel group (75 mg/m2 every 3 weeks) | 135; 137 | PFS, OS, ORR |
| Borghaei [2015] | CheckMate 057 | Phase III | Stage IIIB/IV or recurrent non-squamous NSCLC | ≥2L | Arm A: nivolumab group (3 mg/kg every 2 weeks); arm B: docetaxel group (75 mg/m2 every 3 weeks) | 292; 290 | PFS, OS, ORR |
| Antonia [2018] | PACIFIC | Phase III | Stage III, unresectable NSCLC | ≥2L | Arm A: durvalumab group (10 mg/kg); arm B: placebo group (every 2 weeks for up to 12 months) | 473; 236 | PFS, OS |
| Fehrenbacher [2018] | OAK | Phase III | NSCLC | ≥2L | Arm A: atezolizumab group; arm B: docetaxel group | 613; 612 | PFS, OS, ORR |
| Fehrenbacher [2016] | POPLAR | Phase II | NSCLC | ≥2L | Arm A: atezolizumab group (1,200 mg fixed dose); arm B: docetaxel group (75 mg/m2) | 144; 143 | PFS, OS, ORR |
| Mok [2019] | KEYNOTE-042 | Phase III | Locally advanced or metastatic NSCLC | 1L | Arm A: pembrolizumab group; arm B: chemotherapy group | 637; 637 | PFS, OS, ORR |
| Wu [2019] | CheckMate 078 | Phase III | Stage IIIB/IV or recurrent NSCLC | ≥2L | Arm A: nivolumab group (3 mg/kg Q2W); arm B: docetaxel group (75 mg/m2 Q3W) | 338; 166 | OS |
| Langer [2016] | KEYNOTE-021 | Phase II | Stage IIIB or IV, non-squamous NSCLC | 1L | Arm A: pembrolizumab plus chemotherapy group (PbCPt); arm B: chemotherapy group (CPt) | 60; 63 | ORR |
| Paz-Ares [2018] | KEYNOTE-407 | Phase III | Stage IV squamous NSCLC | 1L | Arm A: pembrolizumab-combination group (PemCP/PemCN-P); arm B: saline placebo-combination group (PlaCP/PlaCN-P) | 278; 281 | PFS, OS, ORR |
| Gandhi [2018] | KEYNOTE-189 | Phase III | Metastatic non-squamous NSCLC | 1L | Arm A: pembrolizumab combination group (PbPtC); arm B: placebo combination group (PlaPtC) | 410; 206 | PFS, OS, ORR |
| Socinski [2018] | IMpower150 | Phase III | Stage IV non-squamous NSCLC | 1L | Arm A: ACP group; arm B: ABCP group; arm C: BCP group | 402; 400; 400 | PFS |
NSCLC, non-small cell lung cancer; 1L, first-line treatment; ≥2L, second or more line treatment; PbCPt, pembrolizumab + carboplatin + pemetrexed; CPt, carboplatin + pemetrexed; PemCP/PemCN-P, pembrolizumab + carboplatin + paclitaxel or pembrolizumab + carboplatin + nab-paclitaxel; PlaCP/PlaCN-P, placebo + carboplatin + paclitaxel or placebo + carboplatin + nab-paclitaxel; PbPtC, pembrolizumab + pemetrexed + cisplatin/carboplatin; PlaPtC, placebo + pemetrexed + cisplatin/carboplatin; AC, atezolizumab + carboplatin + paclitaxel; ABCP, atezolizumab + carboplatin + paclitaxel + bevacizumab; BCP, carboplatin + paclitaxel + bevacizumab; PFS, progression free survival; OS, overall survival; ORR, objective response rate; PD-L1, programmed death-ligand 1.
PD-L1 testing
In all the trials selected in the current meta-analysis, PD-L1 expression on tumor cells (TC) or tumor-infiltrating immune cells (IC) was detected by clinical trial immunohistochemistry (IHC) assays. Detailed information on the PD-L1 measurement used in each trial is shown in Table 2, which includes sample type, cut-off values, staining locations and antibody used.
Table 2. Technical information of PD-L1 measurement in the included studies.
| Study/authors | Sample type | Cutoff for PD-L1 positive status | PD-L1 measurement | Antibody | ||
|---|---|---|---|---|---|---|
| Company | Source | Clone | ||||
| Herbst et al. [2016] | Fresh or archival tumor-biopsy specimens | 1% and 50% | Tumor cell membrane staining | Merck; Kenilworth, NJ, USA | murine | 22C3 |
| Reck et al. [2016] | Tumor samples were obtained by core-needle or excisional biopsy or from tissue resected at the time the metastatic disease was diagnosed | ≥50% | Tumor cell membrane staining | Dako North America | N/R | 22C3 |
| Barlesi et al. [2018] | N/R | 1%, 50% and 80% | Tumor cell membrane staining | N/R | N/R | 73-10 |
| Carbone et al. [2017] | Fresh or archival tumor-biopsy specimens | 5% and 50% | Tumor cell membrane staining | Dako | N/R | 28-8 |
| Brahmer et al. [2015] | Archival or recent tumor-biopsy specimens | 1%, 5% and 10% | Tumor cell membrane staining | Dako North America | rabbit | 28-8 |
| Borghaei et al. [2015] | Archival or recent tumor biopsies | 1%, 5% and 10% | Tumor cell membrane staining | Epitomics Inc., Burlingame, CA | rabbit | 28-8 |
| Antonia et al. [2018] | Archived tumor tissue samples | 1% and 25% | Tumor cell membrane staining | Ventana | N/R | SP263 |
| Fehrenbacher et al. [2018] | Archival or fresh tumor samples | TC3 or IC3; TC2/3 or IC2/3; TC1/2/3 or IC1/2/3; TC0 and IC0 | Membranous staining to the tumor cells/staining to the tumour-infiltrating immune cells | Ventana Medical Systems, Inc., Tucson, AZ, USA | N/R | SP142 |
| Fehrenbacher et al. [2016] | Formalin-fixed paraffin-embedded sections |
TC3 or IC3; TC2/3 or IC2/3; TC1/2/3 or IC1/2/3; TC0 and IC0 | Membranous staining to the tumor cells/staining to the tumour-infiltrating immune cells | Ventana Medical Systems, Tucson, AZ, USA | N/R | SP142 |
| Mok et al. [2019] | Formalin-fixed tumor samples obtained by core-needle or excisional biopsy of a tumor lesion or from tissue resected at or after the time metastatic disease was diagnosed | 1%, 20% and 50% | Tumor cell membrane staining | Agilent Technologies, Carpinteria, CA, USA | N/R | 22C3 |
| Wu et al. [2019] | N/R | 1% | Tumor cell membrane staining | Dako | N/R | 28-8 |
| Socinski et al. [2018] | Archival or freshly collected tumor tissue [or both] | TC3 or IC3; TC1/2/3 or IC1/2/3; TC1/2 or IC1/2; TC0/1/2 and IC0/1/2; TC0 and IC0 | Membranous staining to the tumor cells/staining to the tumour-infiltrating immune cells | Ventana Medical Systems | N/R | SP142 |
| Langer et al. [2016] | Formalin-fixed tumor samples obtained from core-needle biopsies, excisional biopsies, or resected tissue collected at the time of diagnosis of metastatic disease | 1% and 50% | Tumor cell membrane staining | Dako North America, Carpinteria, CA, USA | N/R | 22C3 |
| Paz-Ares et al. [2018] | Formalin-fixed tumor samples obtained at the time metastatic disease was diagnosed | 1% and 50% | Tumor cell membrane staining | Agilent Technologies | N/R | 22C3 |
| Gandhi et al. [2018] | Formalin-fixed tumor samples obtained by core-needle or excisional biopsy or from tissue resected at the time metastatic disease was diagnosed | 1% and 50% | Tumor cell membrane staining | Agilent | N/R | 22C3 |
TC3 or IC3, PD-L1 expression on ≥50% of TC or ≥10% of IC; TC2/3 or IC2/3, PD-L1 expression on ≥5% of TC or IC; TC1/2/3 or IC1/2/3, PD-L1 expression on ≥1% of TC or IC; TC0 and IC0, PD-L1 expression on <1% of TC or IC. N/R, not reported; TC, tumor cells; IC, tumor-infiltrating immune cells; PD-L1, programmed cell death ligand-1.
Efficacy outcomes of PD-1/PD-L1 inhibitor therapy versus chemotherapy in the intention-to-treat population
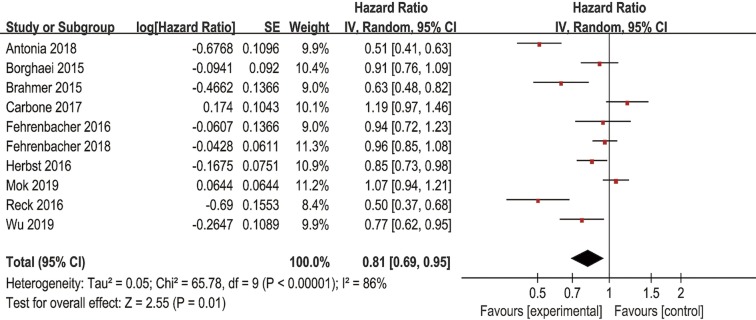
Ten of the fifteen studies including 6,737 patients and another ten of the fifteen studies including 7,025 patients reported PFS and OS data of anti-PD-1/PD-L1 antibodies, respectively. Pooled results showed that PD-1/PD-L1 inhibitors significantly improved the PFS (HR =0.81, 95% CI: 0.69–0.95, P=0.01) (Figure S1) and OS (HR =0.77, 95% CI: 0.69–0.85, P<0.00001) (Figure 2) in all patients when compared with chemotherapy. Random effect models were chosen to analyze the PFS and OS because significant heterogeneity was observed. Ten of the fifteen studies including 6,667 patients reported ORR data. The pooled RR for ORR was 1.53 (95% CI: 1.24–1.89, P<0.0001) in a random effect model (Figure S2), which showed that anti-PD-1/PD-L1 antibodies had higher ORR than chemotherapy.
Figure S1.
Forest plots of HR of PFS for PD-1/PD-L1 inhibitors in the intention-to-treat population. HR, hazard ratio; PFS, progression free survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Figure 2.
Forest plots of HR of OS for PD-1/PD-L1 inhibitors in the intention-to-treat population. HR, hazard ratio; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Figure S2.
Forest plots of RR of ORR for PD-1/PD-L1 inhibitors in the intention-to-treat population. RR, risk ratio; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Subgroup analyses by PD-L1 expression in anti-PD-1/PD-L1 monotherapy
All the eleven RCTs included evaluated the correlation between the PD-L1 expression level and clinical outcomes of PD-1/PD-L1 inhibitors, of which three studies focused on first-line therapy, and eight focused on second or more lines of therapy. A total of 3,981 patients in the anti-PD-1/PD-L1 therapy group and 3,227 in the chemotherapy group were included in the subgroup analyses.
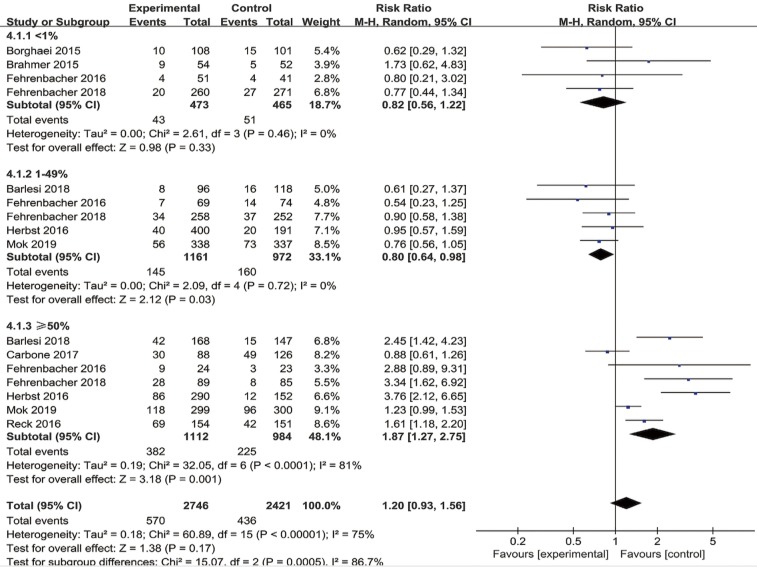
Subgroup analyses showed that immunotherapy significantly improved OS when compared with chemotherapy in the PD-L1 ≥1% population (HR =0.74, 95% CI: 0.65–0.84, P<0.00001), the PD-L1 ≥5% population (HR =0.62, 95% CI: 0.44–0.87, P=0.006), the PD-L1 ≥10% population (HR =0.42, 95% CI: 0.30–0.59, P<0.00001), and the PD-L1 ≥50% population (HR =0.63, 95% CI: 0.55–0.72, P<0.00001). However, there was no statistically significant benefit between PD-1/PD-L1 inhibitors and chemotherapy in evaluating OS when cutoffs of PD-L1 <1% (HR =0.85, 95% CI: 0.72–1.01, P=0.07), PD-L1 <5% (HR =0.86, 95% CI: 0.59–1.25, P=0.43) and PD-L1 <10% (HR =0.85, 95% CI: 0.60–1.21, P=0.37) were utilized (Table 3). Moreover, immunotherapy predicted significantly longer PFS when compared with chemotherapy in the PD-L1 ≥1% population (HR =0.83, 95% CI: 0.70–0.99, P=0.03), the PD-L1 ≥5% population (HR =0.73, 95% CI: 0.54–0.98, P=0.04), the PD-L1 ≥10% population (HR =0.54, 95% CI: 0.40–0.73, P<0.0001), and the PD-L1 ≥50% population (HR =0.68, 95% CI: 0.54–0.85, P=0.0009). Nevertheless, for the PD-L1 <1% (HR =0.97, 95% CI: 0.78–1.21, P=0.81), PD-L1 <5% (HR =1.01, 95% CI: 0.58–1.75, P=0.98) and PD-L1 <10% population (HR =0.94, 95% CI: 0.53–1.66, P=0.84), there was no statistically significant differences on PFS between PD-1/PD-L1 inhibitor therapy and chemotherapy (Figure S3). Furthermore, subgroup analyses of ORR with tumor expression of PD-L1 <1%, 1–49% and ≥50% were also carried out. In patients with a PD-L1 tumor proportion score of <1% and 1–49%, the pooled RRs for ORR were 0.82 (95% CI: 0.56–1.22, P=0.33) and 0.80 (95% CI: 0.64–0.98, P=0.03), respectively. While the pooled RR for ORR was 1.87 (95% CI: 1.27–2.75, P=0.001) in the PD-L1 ≥50% population (Figure 3).
Table 3. Subgroup analyses of OS by PD-L1 expression for PD-1/PD-L1 inhibitors.
| Subgroup | No. of studies | HR (95% CI) | Heterogeneity (I2) | Subgroup differences (P value) |
|---|---|---|---|---|
| Cutoff value: 1% | 0.17 | |||
| PD-L1 expression <1% | 6 | 0.85 (0.72–1.01) | 25% | |
| PD-L1 expression ≥1% | 10 | 0.74 (0.65–0.84) | 63% | |
| Cutoff value: 5% | 0.21 | |||
| PD-L1 expression <5% | 2 | 0.86 (0.59–1.25) | 60% | |
| PD-L1 expression ≥5% | 5 | 0.62 (0.44–0.87) | 78% | |
| Cutoff value: 10% | 0.005 | |||
| PD-L1 expression <10% | 2 | 0.85 (0.60–1.21) | 57% | |
| PD-L1 expression ≥10% | 2 | 0.42 (0.30–0.59) | 0% | |
| Cutoff value: 50% | ||||
| PD-L1 expression ≥50% | 6 | 0.63 (0.55–0.72) | 45% |
HR, hazard ratio; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Figure S3.
Forest plot for the subgroup analyses of HR of PFS by PD-L1 expression for PD-1/PD-L1 inhibitors. (A) PD-L1 expression <1% vs. PD-L1 expression ≥1%; (B) PD-L1 expression <5% vs. PD-L1 expression ≥5%; (C) PD-L1 expression <10% vs. PD-L1 expression ≥10%; (D) PD-L1 expression ≥50%. HR, hazard ratio; PFS, progression free survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Figure 3.
Forest plot for the subgroup analyses of RR of ORR by PD-L1 expression for PD-1/PD-L1 inhibitors. RR, risk ratio; ORR, objective response rate; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Similar efficacy results on OS, PFS, ORR were observed after excluding the trials of Reck et al. (23), Mok et al. (22) and Carbone et al. (17) (which used PD-1/PD-L1 inhibitors in the first-line treatment) (data not shown).
Subgroup analyses by clinicopathologic features in the PD-L1-positive population
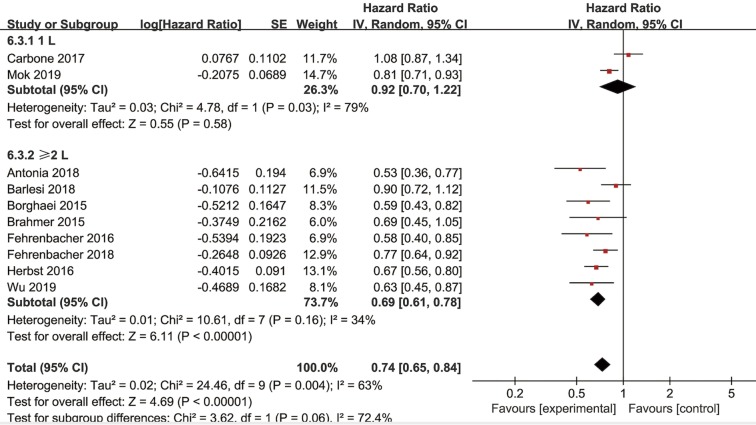
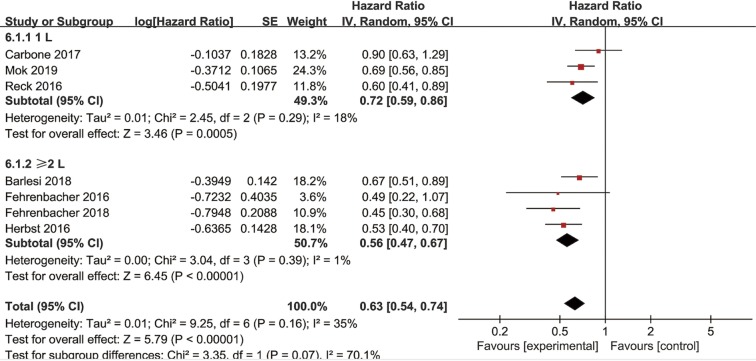
Subgroup analyses for OS according to histology and line of therapy were conducted in NSCLC patients. In the PD-L1 ≥1% subpopulation receiving second-line treatment, four trials reported OS data of squamous-cell carcinoma and another four trials reported OS data of non-squamous NSCLC. The pooled HRs in squamous and non-squamous cell carcinoma were 0.74 (95% CI: 0.62–0.89, P=0.002) and 0.73 (95% CI: 0.58–0.92, P=0.008), respectively (Figure 4). In the PD-L1 ≥1% subpopulation, two studies investigated OS in first-line (1L) PD-1/PD-L1 inhibitor therapy while eight trials investigated OS in second or more line (≥2L) treatment. The combined HRs of studies received 1L and ≥2L treatment were 0.92 (95% CI: 0.70–1.22, P=0.58) and 0.69 (95% CI: 0.61–0.78, P<0.00001), respectively (Figure 5). Moreover, in the PD-L1 ≥50% subpopulation, three studies investigated OS in first-line therapy while four trials in second or more line treatment. The combined HRs of studies received 1 L and ≥2 L treatment were 0.72 (95% CI: 0.59–0.86, P=0.0005) and 0.56 (95% CI: 0.47–0.67, P<0.00001), respectively (Figure S4).
Figure 4.
Forest plot for the subgroup analyses of HR of OS by tumor histology for the PD-L1 ≥1% population in second-or-third line treatment. HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death ligand-1.
Figure 5.
Forest plot for the subgroup analyses of HR of OS by the line of treatment for the PD-L1 ≥1% population. HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death ligand-1.
Figure S4.
Forest plot for the subgroup analyses of HR of OS by the line of treatment for the PD-L1 ≥50% population. HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death ligand-1.
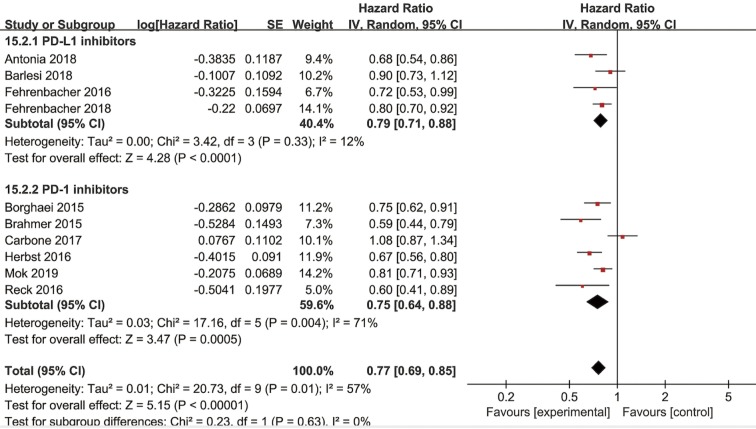
Subgroup analyses by the inhibitors used in anti-PD-1/PD-L1 monotherapy
Among the studies included, four studies received PD-L1 inhibitors therapy and six studies assessed PD-1 inhibitors therapy. The combined OS in studies with PD-L1 inhibitors and PD-1 inhibitors were 0.79 (95% CI: 0.71–0.88, P<0.0001) and 0.75 (95% CI: 0.64–0.88, P=0.0005), respectively (Figure 6). Random-effect models were applied because of the significant heterogeneity. The subgroup difference was not significant between PD-L1 inhibitors and PD-1 inhibitors (P=0.63, I2=0%).
Figure 6.
Forest plot for the subgroup analyses of HR of OS by the inhibitors used in anti-PD-1/PD-L1 monotherapy. HR, hazard ratio; OS, overall survival; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand-1.
Efficacy outcomes of combined immunotherapy versus chemotherapy in the intention-to-treat population
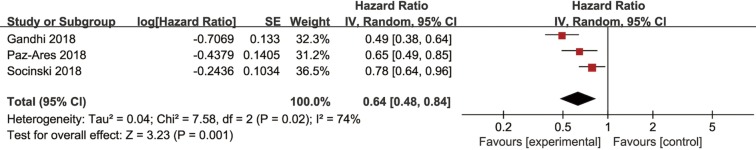
Four studies including a total of 2,500 patients reported efficacy outcomes of combined immunotherapy in the first-line treatment of NSCLC. Among them, three studies evaluated pembrolizumab plus chemotherapy versus chemotherapy alone, and one study evaluated the combination of atezolizumab and chemotherapy versus chemotherapy alone.
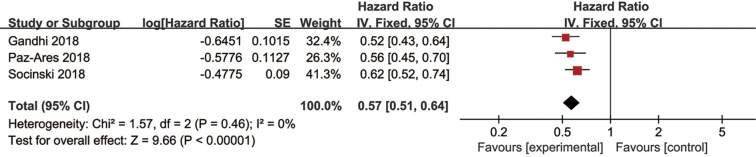
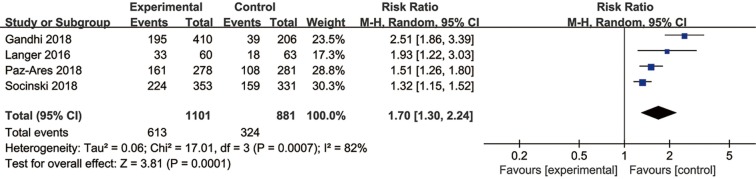
Three trials had usable data for OS. A random-effects model was applied as a significant heterogeneity was observed. Immunotherapy combined with chemotherapy had better OS (HR =0.64, 95% CI: 0.48–0.84, P=0.001) than chemotherapy alone (Figure 7). The same three trials reported PFS data, and the pooled PFS was 0.57 (95% CI: 0.51–0.64, P<0.00001) without heterogeneity (Figure S5). Four studies reported ORR data of NSCLC and the pooled RR for ORR was 1.70 (95% CI: 1.30–2.24, P=0.0001) in a random effect model (Figure S6).
Figure 7.
Forest plots of HR of OS for the combined immunotherapy in the intention-to-treat population. HR, hazard ratio; OS, overall survival.
Figure S5.
Forest plots of HR of PFS for the combined immunotherapy in the intention-to-treat population. HR, hazard ratio; PFS, progression free survival.
Figure S6.
Forest plots of RR of ORR for the combined immunotherapy in the intention-to-treat population. RR, risk ratio; ORR, objective response rate.
Subgroup analyses by PD-L1 expression in combined immunotherapy
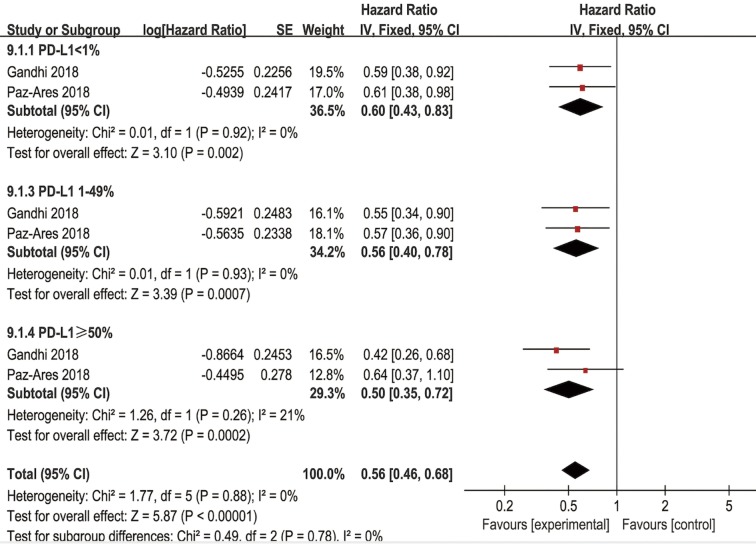
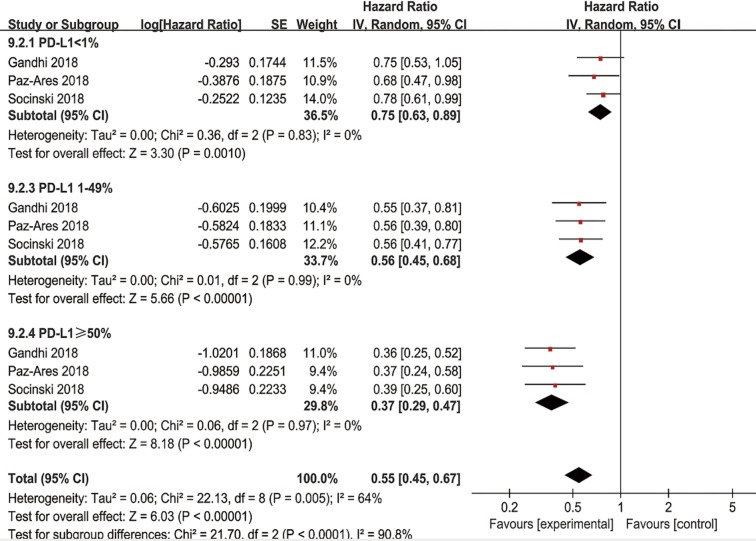
In the tumor PD-L1 <1% subpopulation, pooled results revealed that the combination of immunotherapy with chemotherapy significantly improved OS (HR =0.60, 95% CI: 0.43–0.83, P=0.002) and PFS (HR =0.75, 95% CI: 0.63–0.89, P=0.0010) in NSCLC patients. In patients with a PD-L1 tumor proportion score of 1–49%, significantly higher OS (HR =0.56, 95% CI: 0.40–0.78, P=0.0007) and PFS (HR =0.56, 95% CI: 0.46–0.68, P<0.00001) were observed in patients receiving combined chemo-IO treatment compared to chemotherapy alone. In the tumor PD-L1 ≥50% subpopulation, the pooled HRs for OS (Figure 8) and PFS (Figure S7) were 0.50 (95% CI: 0.35–0.72, P=0.0002) and 0.37 (95% CI: 0.29–0.47, P<0.00001), respectively.
Figure 8.
Forest plot for the subgroup analyses of HR of OS by PD-L1 expression for the combined immunotherapy. HR, hazard ratio; OS, overall survival; PD-L1, programmed cell death ligand-1.
Figure S7.
Forest plot for the subgroup analyses of HR of PFS by PD-L1 expression for the combined immunotherapy. HR, hazard ratio; PFS, progression free survival; PD-L1, programmed cell death ligand-1.
Sensitivity analysis and publication bias
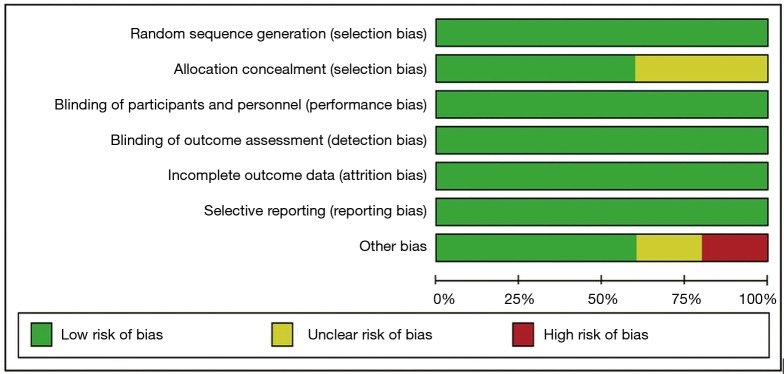
All included studies were RCTs and were considered to present high-quality data (Figure 9). The funnel plot of the studies assessing OS is presented in Figure 10. Visual inspection of the graphical funnel plots revealed no substantial publication bias.
Figure 9.
Risk of bias graph.
Figure 10.
Funnel plot for publication bias.
Sensitivity analyses were performed to evaluate the stability of our results by removing one study at a time. The results indicated that no individual research significantly influenced the combined effects on OS in the intention-to-treat population, which suggested the stability of all trials in the meta-analysis.
Discussion
Different from chemotherapy, immunotherapy can inhibit and kill tumor cells by mobilizing the body’s immune system and enhancing or normalizing the body’s anti-tumor immune response. Immunotherapy represented by PD-1/PD-L1 checkpoint inhibitors is one of the most promising research directions in the field of tumor therapy because it can provide lasting efficacy and make long-term survival possible. Currently, clinical trials on various anti-PD-1/PD-L1 antibodies such as pembrolizumab, nivolumab, atezolizumab, and durvalumab have been conducted to evaluate the clinical efficacy as the first-line, second-line, and adjuvant therapy (32,33). Trials of monotherapies or combination therapies are also carried out worldwide. The continuously updated data show the promising anti-tumor activity of PD-1/PD-L1 inhibitors in NSCLC (14). However, we have learned that immunotherapy is not sufficient for all patients, and only a small proportion of NSCLC patients (20%) benefit from immunotherapy (34). As a result, choosing an accurate, predictive biomarker for treatment selection remains an urgent problem to be solved.
Zhao et al. reported that anti-PD-1/PD-L1 monotherapy significantly increased OS in NSCLC patients even when tumor PD-L1 was <1% (35), whereas another meta-analysis revealed that only the patient population with PD-L1 >1% benefited from anti-PD-1/PD-L1 therapy (14). Therefore, whether PD-L1 expression can be used as a potential biomarker to predict immunotherapy efficacy and the optimal cut-off value remain still controversial. Moreover, some other associated factors such as treatment type, histological subtype of the tumor and line of therapy in patients who got survival benefit with a specific PD-L1 expression have not been analyzed in the previous studies due to the insufficient trials reporting relevant results. With the accumulation of updated clinical data, we systematically evaluated the relationship between PD-L1 expression levels and the cancer related outcomes in both monotherapies and combination therapies with PD-1/PD-L1 inhibitors. Furthermore, we tried to integrate multiple clinical characteristics to select suitable patients for anti-PD-1/PD-L1 treatment effectively.
In our study, pooled results confirmed that anti-PD-1/PD-L1 monotherapy significantly improved OS, PFS, and ORR when compared with chemotherapy in the intention-to-treat population. Our analysis also stressed the value of positive PD-L1 expression in predicting improved clinical outcome from anti-PD-1/PD-L1 treatment. PD-1/PD-L1 inhibitors demonstrated higher OS and PFS at ≥1%, ≥5%, ≥10% and ≥50% PD-L1 expression levels. Patients with higher tumor PD-L1 expression may experience increased clinical benefit from an anti-PD-1/PD-L1 antibody. However, no statistical survival benefit was observed for the PD-L1 <1% population who have received anti-PD-1/PD-L1 monotherapy compared to chemotherapy alone.
Moreover, subgroup analyses showed a proportion of patients with PD-L1 ≥50% had a significant improvement of ORR from immunotherapy. Nonetheless, for patients with PD-L1 expression <1%, the pooled results of ORR showed no difference. Even for patients with PD-L1 expression 1–49%, chemotherapy showed better ORR when compared with anti-PD-1/PD-L1 monotherapy. It is also possible that improvement in OS is distinct from response rate when it comes to the currently available checkpoint inhibitors. These results may imply that patients with PD-L1 positive tumors could achieve an improved response rate and survival benefit, and PD-L1 expression of 50% may be the most suitable cutoff value to be used in future clinical practice.
Furthermore, we have attempted to personalize these treatments in the PD-L1-positive population. For the PD-L1 ≥1% population, PD-1/PD-L1 inhibitor therapy showed similar benefits compared to chemotherapy for both squamous-cell carcinoma and non-squamous NSCLC in the second line setting. Patients who received second or higher lines of therapies had better OS benefits in the PD-L1 ≥1% population, while both first line immunotherapy and second-or-third line anti-PD-1/PD-L1 monotherapy were associated with better OS in the PD-L1 ≥50% population. As the number of studies on first-line therapy in this meta-analysis was relatively small, the results of the subgroup analyses have to be taken cautiously, and more prospective trials are needed to verify this conclusion. Besides, results from subgroup analyses, according to the type of immune checkpoint inhibitor utilized, implied that there was no difference between PD-1 inhibitors and PD-L1 inhibitors.
Moreover, several studies have shown that chemotherapeutic drugs can enhance tumor antigen presentation (36), sensitize tumor cells to the cytotoxicity of CTLs (37), promote anti-tumor immunity (38), and trigger immunogenic variation of cell apoptosis (39). Some trials have also been conducted to integrate immunotherapy with current conventional cytotoxic chemotherapy to explore whether chemotherapy has synergistic effects on immunotherapy (40). Our meta-analysis showed that treatment-naive patients could get a significant benefit from the chemo-immunotherapy combination when compared with chemotherapy alone, both in the intention-to-treat population and in subgroups with tumor PD-L1 <1%, 1–49% and ≥50%. This implies that a broader range of NSCLC patients could benefit from the combination of immunotherapy with chemotherapy than from monotherapy alone.
Currently, immunohistochemistry is the only method to evaluate PD-L1 expression in tumor tissues. The United States FDA has approved 22C3 pharmDx as companion diagnostics for pembrolizumab, while 28-8 pharmDx and Ventana SP142 were used as companion diagnostics for PD-L1 detection of nivolumab and atezolizumab, respectively, which is consistent with our findings. Recent studies showed that SP263 had detection consistency with 22C3 and 28-8, and the overall consistency rate of the three methods was >90% when comparing the different cell membrane staining thresholds (1%, 10%, 25%, and 50%) of PD-L1 tumors. In contrast, SP142 requires a higher threshold for the detection of PD-L1 expression in tumor cells. Moreover, the variability of immune cell staining with the four assays is greater than that of tumor cell staining (41,42). Herein, PD-L1 detection, which is clinically verified and suitable for more related drugs, is worthy of exploration for the preliminary evaluation of PD-L1 expression level. At the same time, it is necessary to standardize the testing platform and testing standards (PD-L1 cutoff value and which cells should be included in the score, such as tumor cells or immune cells). And then, because of the heterogeneity in tumors, PD-L1 expression in different tumor sites (the primary lesions versus metastatic lesions) or detected with different sample types (surgical resection versus biopsy) may be frequently different. As more than one third of NSCLC patients are diagnosed with cytological materials, it is important to use the same material for PD-L1 analyses (43,44). Thus, choosing the appropriate tumor site and sample specimen for detecting PD-L1 expression needs more in-depth study and discussion (45).
There are also some limitations in this meta-analysis. First, among the fifteen RCTs, KEYNOTE-010, KEYNOTE-042, CheckMate 026 and JAVELIN Lung 200 trials comprise NSCLC patients with PD-L1 expression level at 1% or more, and the phase III KEYNOTE 024 trial includes patients with PD-L1 expression ≥50%, which are in contrast to the other studies that contain both PD-L1 positive and negative patients. Herbst et al. (46) have found that high expression of PD-L1 in tumor tissue correlates with response to PD-1/PD-L1 inhibitors. Thus, the high number of patients with PD-L1 positive carcinoma (approximately 50% of the patients) may lead to an overestimation of the efficacy of anti-PD-1/PD-L1 agents in the intention-to-treat population. Second, results from CheckMate 026 in the entire population, and even in patients with PD-L1 ≥50%, were inconsistent with those of the first-line anti-PD-1/PD-L1 therapy arms in other trials. The results of KEYNOTE 024 and KEYNOTE 042 indicated that immunotherapy (pembrolizumab) had better PFS and OS than chemotherapy, while Checkate 026 trial suggested that nivolumab did not significantly improve PFS when compared with chemotherapy and that the OS was similar between the two groups. These contrasting results may increase the heterogeneity of the meta-analysis.
Conclusions
IHC PD-L1 serves as a valuable predictor of anti-PD-1/PD-L1 antibody efficacy, and ORR benefit was observed only in patients with PD-L1 ≥50% but OS benefit was seen in broader population. Furthermore, immunotherapy combined with chemotherapy significantly improved survival regardless of tumor PD-L1 expression level in treatment-naive patients. These findings may further help select individualized and precise treatment regimens for NSCLC patients.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81401903, 81572937 and 81572273); China Postdoctoral Science Foundation 64th batch (Postdoctoral number: 45786); Jiangsu Provincial Postdoctoral Science Foundation in 2018; the Natural Science Foundation of Jiangsu province (grant number BK20180139 and BK20161386); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), the Jiangsu Provincial Key Research and Development Program (No. BE2016721).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:849-61. 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. 10.6004/jnccn.2016.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. 10.1016/S0140-6736(16)00004-0 [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann S, Peters S. Present standards and future perspectives in the treatment of metastatic non-small cell lung cancer. Cancer Metastasis Rev 2015;34:173-82. 10.1007/s10555-015-9560-6 [DOI] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alatrash G, Jakher H, Stafford PD, et al. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf 2013;12:631-45. 10.1517/14740338.2013.795944 [DOI] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheema PK, Rothenstein J, Melosky B, et al. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr Oncol 2019;26:37-42. 10.3747/co.26.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol 2016;101:75-85. 10.1016/j.critrevonc.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehrenbacher L, von Pawel J, Park K, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1156-70. 10.1016/j.jtho.2018.04.039 [DOI] [PubMed] [Google Scholar]
- 21.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 22.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 25.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 27.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 28.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468-79. 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 29.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 30.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 31.Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 32.Mayor M, Yang N, Sterman D, et al. Immunotherapy for non-small cell lung cancer: current concepts and clinical trials. Eur J Cardiothorac Surg 2016;49:1324-33. 10.1093/ejcts/ezv371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh K, Eid R, Haddad FG, et al. New developments in the management of head and neck cancer - impact of pembrolizumab. Ther Clin Risk Manag 2018;14:295-303. 10.2147/TCRM.S125059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seya T, Takeda Y, Takashima K, et al. Adjuvant immunotherapy for cancer: both dendritic cell-priming and check-point inhibitor blockade are required for immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci 2018;94:153-60. 10.2183/pjab.94.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Xie R, Lin S, et al. Anti-PD-1/PD-L1 Antibody Therapy for Pretreated Advanced or Metastatic Nonsmall Cell Lung Carcinomas and the Correlation between PD-L1 Expression and Treatment Effectiveness: An Update Meta-Analysis of Randomized Clinical Trials. Biomed Res Int 2018;2018:3820956. 10.1155/2018/3820956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015;3:436-43. 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010;120:1111-24. 10.1172/JCI40269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kareva I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int J Mol Sci 2017;18(10). doi: . 10.3390/ijms18102134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pol J, Vacchelli E, Aranda F, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology 2015;4:e1008866. 10.1080/2162402X.2015.1008866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assal A, Kaner J, Pendurti G, et al. Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy 2015;7:1169-86. 10.2217/imt.15.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:3585-91. 10.1158/1078-0432.CCR-16-2375 [DOI] [PubMed] [Google Scholar]
- 42.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravaccini S, Tumedei MM, Ulivi P, et al. ALK translocation detection in non-small cell lung cancer cytological samples obtained by TBNA or EBUS-TBNA. Cytopathology 2016;27:103-7. 10.1111/cyt.12237 [DOI] [PubMed] [Google Scholar]
- 44.Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol 2017;25:453-9. 10.1097/PAI.0000000000000540 [DOI] [PubMed] [Google Scholar]
- 45.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]