Abstract
Five pairs of alkaloid enantiomers (1a/1b–5a/5b) were obtained from Isatis indigotica (I. indigotica) roots. Among them, 1a/1b, 2a/2b and 3a/3b were determined as three pairs of new alkaloid enantiomers. Their structures were elucidated by physicochemical properties and spectroscopic methods. The absolute configurations were deduced by comparison of their experimental circular dichroism (CD) and calculated electronic circular dichroism (ECD) spectra, as well as by single-crystal X-ray crystallography using anomalous scattering of Cu Kα radiation. Alkaloids 1a and 1b possess an unpresented carbon skeleton and their putative biosynthetic pathways are discussed. Moreover, all of the alkaloids were tested for their nitric oxide (NO) inhibitory effects in RAW 264.7 cells, and 4a and 4b showed inhibitory effects with IC50 values of 76.97 μM and 65.88 μM, respectively.
Keywords: Isatis indigotica, alkaloid enantiomers, structure deduction, anti-inflammatory activity
1. Introduction
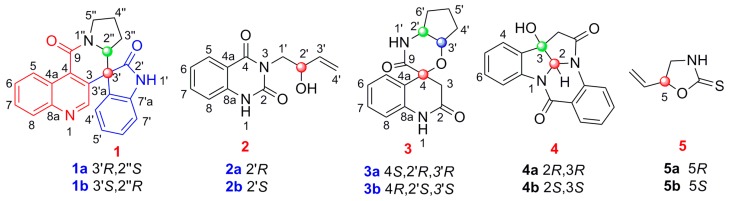
“Ban lan gen”, widely distributed and cultivated in the North of China, is the roots of Isatis indigotica Fort. (Cruciferae) [1,2,3]. As one of the most famous traditional Chinese medicines (TCMs), ban lan gen is usually used for the treatment of various kinds of diseases, such as influenza, fever, epidemic hepatitis and infections [4,5,6]. Previous phytochemical investigations of I. indigotica have led to the isolation of various kinds of natural constituents, including alkaloids, lignans, flavonoids and nucleotides [1,2,3,4,5,6,7,8,9], among which alkaloids have been considered as the most active constituents that possess anti-inflammatory, antiviral, antibacterial, antitumor and antioxidant activities [1,4,5,6,7,8,9]. For our continuous project to explore more bioactive components from I. indigotica [8,9], five pairs of alkaloid enantiomers were isolated from the 80% alcohol exact of I. indigotica roots. Among them, 1a/1b, 2a/2b and 3a/3b were determined as three pairs of new enantiomers, whose structures and absolute configurations were determined by extensive spectroscopic data analysis, including 1D, 2D-NMR and HRESIMS data, optical rotation data, comparison of their experimental circular dichroism (CD) and calculated electronic circular dichroism (ECD) spectra and single-crystal X-ray crystallography using anomalous scattering of Cu Kα radiation. The known forms (4a/4b–5a/5b, Figure 1) were identified by comparison of their spectroscopic and optical rotation data with those reported in the literature as (−)-(2R,3R)-3-hydroxy-2H-pyrrolo[2-b]indolo[5,5a,6-b,a]quinazoline- 9(8H),7′-dione (4a) [1], (+)-(2S,3S)-3-hydroxy-2H-pyrrolo[2-b]indolo[5,5a,6-b,a]quinazoline-9(8H),7′-dione (4b) [1], epigoitrin (5a) [10] and goitrin (5b) [10]. Alkaloids are one of the main types of active constituents in I. indigotica, and they have been reported to possess potential anti-inflammatory effects [1,2,3,4,5,6,7,8,9,10]. This pharmacological action, together with the traditional use for the treatment of epidemic hepatitis, prompted us to test the inhibitory effects on nitric oxide (NO) production of all the isolated alkaloids (1a/1b–5a/5b). Herein, the isolation and structure elucidation, putative biosynthetic pathways and the NO inhibitory activities of these enantiomers are presented.
Figure 1.
Structures of compounds 1a/1b–5a/5b.
2. Results and Discussion
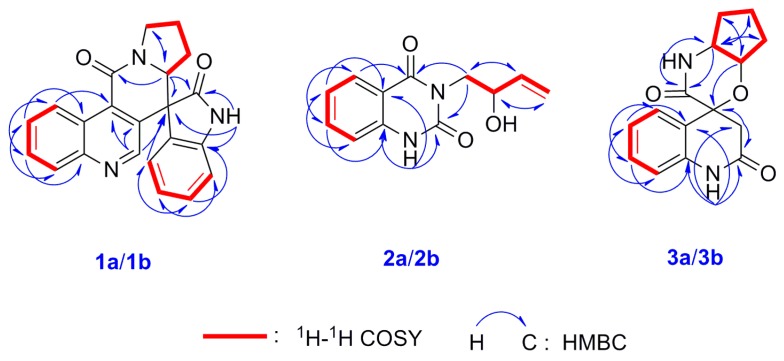
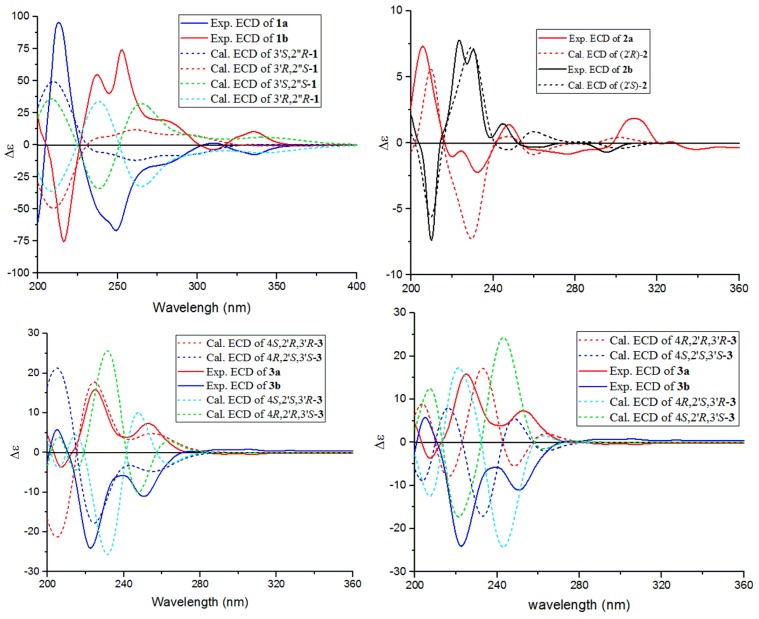
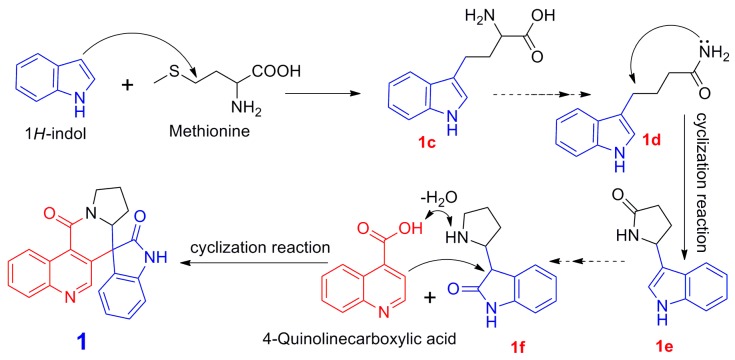
Isatisindigoticanine B (1) was obtained as a primrose yellow amorphous power. Its molecular formula was determined as C22H17N3O2, by the 13C NMR data and the HRESIMS quasimolecular ion peak at m/z 356.1398 [M + H]+ (cacld. 356.1394 [M + H]+). The 1H NMR spectrum (Table 1) of 1 showed signals of two ortho-disubstituted benzene ring at [δH 9.29 (1H, d, J = 8.6 Hz, H-5), 7.77 (1H, dd, J = 8.6, 7.7 Hz, H-6), 7.84 (1H, dd, J = 8.3, 7.7 Hz, H-7) and 8.07 (1H, d, J = 8.3 Hz, H-8)] and [6.76 (1H, d, J = 7.5 Hz, H-4′), 6.95 (1H, dd, J = 7.7, 7.5 Hz, H-5′), 7.30 (1H, dd, J = 7.8, 7.7 Hz, H-6′) and 7.05 (1H, d, J = 7.8 Hz, H-7′)]; a trisubstituted double bond at δH 8.56 (1H, s, H-2) and an exchangeable proton at δH 11.25 (1H, brs, NH-1′). The 13C NMR spectrum (Table 1) displayed 22 carbon signals, and based on the DEPT 135° experiments, 9 × C signals at δC (176.8, 161.6, 148.5, 141.9, 133.4, 132.1, 128.2, 124.6, 55.4), 10 × CH signals at δC (148.3, 130.4, 130.1, 129.8, 128.9, 126.7, 124.4, 123.1, 111.2, 61.8) and 3 × CH2 signals at δC (46.6, 27.1, 22.8) were observed. These spectroscopic features, along with the molecular formula and the degrees of unsaturation (16 index of hydrogen deficiency, IHD), suggested that isatisindigoticanine B was an unusual alkaloid [1,2,3,6,7,8,9]. This inference was confirmed by detailed analysis of the 2D NMR data. The proton and protonated carbon resonances in the NMR spectra of 1 were unambiguously assigned by the HSQC experiments [11,12,13,14]. The 1H-1H COSY correlations of H-4′/H-5′/H-6′/H-7′, along with HMBC correlations (Figure 2) of NH-1′/C-2′, C-3′ and C-7′a, indicate a 1H-indol-2-one unit in 1 [15]; 1H-1H COSY correlations of H-2″/H-3″/H-4″/H-5″, along with the HMBC correlations of H-2″/C-3″ and C-5″, indicated a pyrrolidine unit in 1 [16]; 1H-1H COSY correlations of H-5/H6/H7/H8, along with the HMBC correlations of H-2/C-3 and C-4, H-5/C-4 and C-8a and the remaining molecular formula C10H5NO, indicated a 4-quinolinecarboxylic acid unit in 1 [17]. HMBC correlations of H-2/C-3′, C-3 and C-4 and correlations of H-2″/C-9, C-2′, C-3′ and C-5″, determined the 1H-indol-2-one unit connected with the pyrrolidine unit and the 4-quinolinecarboxylic acid via a six-membered ring of C-3-C-4-C-9-N-1″-C-2″-C-3′. The planar structure of 1 was thus deduced as depicted in Figure 1. Subsequent HPLC separation of 1 on a chiral column yielded 1a and 1b (Figure S34, Supplementary Information) with opposite optical rotations (+12.6° for 1a and −12.5° for 1b) and mirrored CD spectra curves (Figure 3). To further determine the absolute configurations of 1, the ECD curves were simulated for the four epimers of 1, [(3′R,2″S)-1, (3′S,2″R)-1, (3′R,2″R)-1 and (3′S,2″S)-1] (Figure 3). The experimental CD spectra of 1a and 1b were well matched with the calculated ECD curves of (3′R,2″S)-1 and (3′S,2″R)-1, respectively. Accordingly, the structure of 1a and 1b were elucidated as depicted (Figure 1) and named as (+)-(3′R,2″S)-isatisindigoticanine B (1a) and (−)-(3′S,2″R)-isatisindigoticanine B (1b). This carbon skeleton is the first report from a natural source and the putative biosynthetic pathways were proposed (Figure 4).
Table 1.
1H NMR (600 MHz in DMSO-d6) and 13C NMR data (150 MHz in DMSO-d6) of 1a/1b–3a/3b (ov: overlap signals).
| No. | 1a/1b | 2a/2b | 3a/3b | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | |
| 1 | 11.39, brs | 12.39, brs | ||||
| 2 | 8.56, s | 148.3 | 150.8 | 179.9 | ||
| 3 | 133.4 | 2.43, 2H, ov | 47.9 | |||
| 4 | 132.1 | 162.6 | 71.8 | |||
| 4a | 124.6 | 114.3 | 131.7 | |||
| 5 | 9.29, d (8.6) | 126.7 | 7.93, d (8.0) | 127.9 | 7.27, d (7.3) | 123.7 |
| 6 | 7.77, dd (8.6, 7.7) | 128.9 | 7.22, dd (8.0, 7.7) | 122.9 | 6.87, dd (7.5, 7.3) | 121.9 |
| 7 | 7.84, dd (8.3, 7.7) | 130.4 | 7.76, dd (8.2, 7.7) | 135.4 | 7.17, dd (7.7, 7.5) | 128.5 |
| 8 | 8.07, d (8.3) | 129.8 | 7.16, d (8.2) | 115.5 | 6.76, d (7.7) | 109.4 |
| 8a | 148.5 | 139.9 | 141.3 | |||
| 9 | 161.6 | 174.2 | ||||
| 1′ | 11.25, brs | 3.84, dd (12.6, 5.0) | 45.6 | 10.37, brs | ||
| 4.01, dd (12.6, 5.0) | ||||||
| 2′ | 176.8 | 4.34, dd (12.6, 6.5) | 69.2 | 3.33, m | 46.9 | |
| 3′ | 55.4 | 5.83, ddd (16.9, 10.7, 6.5) | 140.0 | 3.82, dd (7.7, 1.4) | 68.5 | |
| 3′a | 128.2 | |||||
| 4′ | 6.76, d (7.5) | 124.4 | 5.01, dd (10.7, 1.2) | 115.5 | 1.71, m | 31.8 |
| 5.11, dd (16.9, 1.2) | 1.90, m | |||||
| 5′ | 6.95, dd (7.7, 7.5) | 123.1 | 1.73, m; 1.99, m | 28.4 | ||
| 6′ | 7.30, dd (7.8, 7.7) | 130.1 | 2.28, dd (12.5, 6.2) | 43.3 | ||
| 2.43, ov | ||||||
| 7′ | 7.05, d (7.8) | 111.2 | ||||
| 7′a | 141.9 | |||||
| 2′’ | 4.44, dd (9.1, 2.6) | 61.8 | ||||
| 3′’ | 1.11, m; 1.97, m | 27.1 | ||||
| 4′’ | 1.94, 2H, m | 22.8 | ||||
| 5′’ | 3.48, ov; 3.81, m | 46.6 | ||||
| 2′-OH | 5.12, d (5.2) | |||||
Figure 2.
Key 1H-1H COSY and HMBC correlations of compounds 1a/1b–3a/3b.
Figure 3.
Experimental and calculated electronic circular dichroism (ECD) spectra of compounds 1a/1b–3a/3b.
Figure 4.
Putative biosynthetic pathway of compound 1.
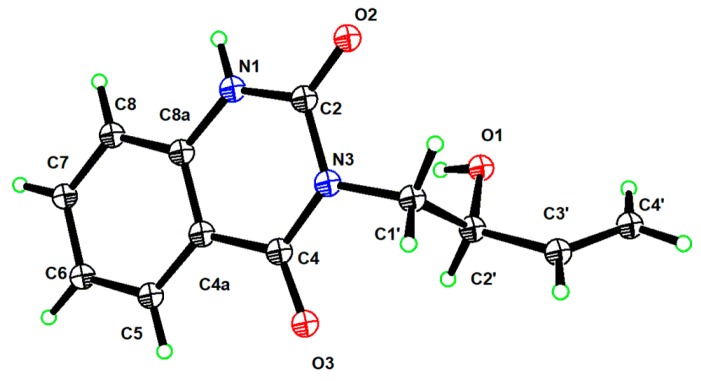
Isatisindigoticanine C (2) was obtained as a white amorphous power. Its molecular formula was assigned as C12H12N2O3 by the 1D NMR data and the HRESIMS quasimolecular ion peak at m/z 231.0771 [M − H]− (cacld. 231.0775 [M − H]−). The 1H NMR spectrum (Table 1) of 2 showed signals of an ortho-disubstituted benzene ring at δH 7.93 (1H, d, J = 8.0 Hz, H-5), 7.22 (1H, dd, J = 8.0, 7.7 Hz, H-6), 7.76 (1H, dd, J = 8.2, 7.7 Hz, H-7) and 7.16 (1H, d, J = 8.2 Hz, H-8) [8,9,15]; a monosubstituted double bond at δH 5.83 (1H, ddd, J = 16.9, 10.7, 6.5 Hz, H-3′), 5.01 (1H, dd, J = 10.7, 1.2 Hz, H-4′a) and 5.11 (1H, dd, J = 16.9, 1.2 Hz, H-4′b) and an exchangeable proton at δH 11.39 (brs, NH-1) [15]. Analysis of the 13C NMR, DEPT 135° and HSQC data (Table 1) of 2, a 2′-hydroxybut-3′-en-1′-yl (45.6, CH2; 69.2, CH; 140.0, CH; 115.5, CH2) [4,10] and a quinazoline-2,4(1H,3H)-dione moiety (150.8, C; 162.6, C; 114.3, C; 127.9, CH; 122.9, CH; 135.4, CH; 115.5, CH; 139.9, C) were observed [1,2,3]. HMBC correlations of H-1′/C-2 and C-4 indicated the 2′-hydroxybut-3′-en-1′-yl unit connected with the quinazoline-2,4(1H,3H)-dione unit via a N-3-C-1 bond [2,3]. This inference was supported by detailed analysis of the 2D NMR data including HSQC, HMBC (Figure 2) and 1H-1H COSY (Figure 2) experiments. The planar structure of 2 was thus deduced as depicted in Figure 1. Subsequent HPLC separation of 2 on a chiral column yielded 2a and 2b in a ratio of approximately 1:1, with opposite optical rotations (−31.1° for 2a and +33.1° for 2b) and cotton effects in their experimental ECD spectra (Figures S29 and S35, Supplementary Information). The comparison of the experimental CD spectra and the calculated ECD spectra of 2a and 2b (Figure 3) confirmed the two enantiomers as (−)-(2′R)-isatisindigoticanine C (2a, Figure 1) and (+)-(2′S)-isatisindigoticanine C (2b, Figure 1), respectively [2,3]. Finally, a single-crystal X-ray experiment with Cu Kα radiation analysis confirmed the structure of (+)-(2′S)-isatisindigoticanine C (2b, Figure 5).
Figure 5.
ORTEP darning of (+)-(2′S)-isatisindigoticanine C (2b).
Isatisindigoticanine D (3), a white amorphous powder, has the molecular formula of C15H16N2O3, which was supported by the positive HRESIMS ion at m/z 273.1245 [M + H]+ (cacld. 273.1239 [M + H]+) and 1D NMR data. The 1H NMR spectrum (Table 1) of 3 showed signals of an ortho-disubstituted benzene ring at δH 7.27 (1H, d, J = 7.3 Hz, H-5), 6.87 (1H, dd, J = 7.5, 7.3 Hz, H-6), 7.17 (1H, dd, J = 7.7, 7.5 Hz, H-7) and 6.76 (1H, d, J = 7.7 Hz, H-8) and two exchangeable protons at δH 12.39 (brs, NH-1) and 10.37 (brs, NH-1′) [1,15]. The 13C NMR spectrum (Table 1) displayed 15 carbon signals based on the DEPT 135° experiments, and 5 × C signals at δC (179.9, 174.2, 141.3, 131.7, 71.8), 6 × CH signals at δC (128.5, 123.7, 121.9, 109.4, 68.5, 46.9) and 4 × CH2 signals at δC (47.9, 43.3, 31.8, 28.4) were observed. The 2D NMR spectra of 3 showed the 1H-1H COSY correlations of H-5/H-6/H-7/H-8 and HMBC correlations from NH-1/C-2, C-3, C-4a and C-8a, and those from H-3/C-2 and C-4 indicated a 4-hydroxy-2-oxo-1,2,3,4-tetrahydroquinoline-4-carboxylic acid unit in 3 [18], while 1H-1H COSY correlations of H-2′/H-3′/H-4′/H-5′/H-6′ indicated a cyclopentanamine unit in 3 [19]. HMBC correlations from NH-1′/C-9 and C-6 and from H-2′/C-4 determined the 4-hydroxy-2-oxo-1,2,3,4-tetrahydroquinoline-4-carboxylic acid unit connected with the cyclopentanamine unit via a six-membered ring of N-1′-C-2″-C-3-O-C-4-C-9. The planar structure of 3 was thus determined as depicted in Figure 1. Subsequent HPLC separation of 3 on a chiral column yielded 3a and 3b (Figure S36, Supplementary Information) with opposite optical rotations (−65.7° for 3a and +63.2° for 3b) and mirrored CD spectra curves (Figure 3). Subsequently, the absolute configurations of 3a and 3b were determined by comparison of their experimental and calculated ECD spectra at the b3lyp/6-31g(d) level. As shown in Figure 3, the theoretically calculated ECD of (4S,2′R,3′R)-3 and (4R,2′S,3′S)-3 matched well with the experimental CD of 3a and 3b, respectively. Thus, the structures of the two enantiomers were given and named as (−)-(4S,2′R,3′R)-isatisindigoticanine D (3a, Figure 1) and (+)-(4R,2′S,3′S)-isatisindigoticanine D (3b, Figure 1), respectively [2,3].
For our continuous project to explore more anti-inflammatory components from I. indigotica [8,9], compounds 1a/1b–5a/5b were tested for their inhibitory effects on the NO production in LPS activated RAW 264.7 cells, a primary indicator in assessing inflammatory activities [20]. The results suggested that only 4a and 4b exhibited inhibitory activities, with IC50 values of 76.97 μM and 65.88 μM.
Isatisindigoticanine B (1) is the first example of a 1H-indol-2-one unit connected with a 4-quinolinecarboxylic acid unit and a pyrrolidine unit via a six-membered ring of C-3-C-4-C-9-N-1″-C-2″-C-3′. On the basis of its unique structural features, the putative biosynthetic pathways for isatisindigoticanine B (1) are proposed in Figure 4. The biosynthetic precursor of 1 is proposed from 1H-indol [4]. First, 1H-indol was connected with methionine moiety by sequential or simultaneous enzymatic catalysis to give 1c [4,5], and then 1c was modified via an enzyme-catalyzed reaction to give 1d [20]. Then, 1e was obtained by cyclization reaction of 1d [8] and then changed via a reduction reaction to give 1f, and finally 1f was modified by steps of dehydration and cyclization reactions [5,8] to give 1. Compound 1 was separated by chiral analysis to give 1a and 1b [2,3].
3. Experimental Section
The general experimental procedures and extraction and isolation sections are listed in the Supplementary Information section. The plant material (I. indigotica roots) was used in the same way we described previously [8,9].
3.1. Physical and Spectroscopic Data of Isatisindigoticanines B–D
Isatisindigoticanine B (1), a primrose yellow amorphous power; IR (KBr) νmax: 3407, 2923, 1720, 1666, 1613, 1501, 1460, 1350, 1267, 1023, 954, 759 cm−1; m/z 356.1398 [M + H]+ (cacld. 356.1394 [M + H]+); 1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) (Table 1); [α] +12.6° (c 0.15, MeOH) for (+)-(3′R,2″S)-isatisindigoticanine B (1a) and [α] −12.5° (c 0.07, MeOH) for (−)-(3′S,2″R)-isatisindigoticanine B (1b).
Isatisindigoticanine C (2), a white amorphous power; IR (KBr) νmax: 3420, 2939, 1636, 1598, 1514, 1461, 1261, 1139, 1025, 859, 813 cm−1; m/z 233.0771 [M − H]− (cacld. 233.0775 [M − H]−); 1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) (Table 1); [α] −31.1° (c 0.21, MeOH) for (−)-(2′R)-isatisindigoticanine C (2a) and [α] +33.1° (c 0.13, MeOH) for (+)-(2′S)-isatisindigoticanine C (2b).
Isatisindigoticanine D (3), a white amorphous power; IR (KBr) νmax: 3363, 2924, 1703, 1667, 1514, 1443, 1408, 1260, 1026, 957, 726 cm−1; m/z 273.1245 [M + H]+ (cacld. 273.1239 [M + H]+); 1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) (Table 1); [α] −65.7° (c 0.18, MeOH) for (−)-(4S,2′R,3′R)-isatisindigoticanine D (3a) and [α] +63.2° (c 0.10, MeOH) for (+)-(4R,2′S,3′S)-isatisindigoticanine D (3b).
3.2. ECD Calculation of Compounds 1a/1b–3a/3b
The conformers of compounds 1a/1b–3a/3b were obtained using the MM2 force field with ChemBio3D software. Gaussian 09 software was utilized for the semiempirical PM3 quantum mechanical geometry optimizations and the time-dependent density functional theory (TDDFT). ECD was calculated at the b3lyp/6-31g(d) level [20,21,22]. The ECD spectra conformers of 1a/1b–3a/3b were obtained using SpecDis 1.62 and were compared with the experimental data; the calculation details are listed in the supporting information (Figures S26–S33).
3.3. X-ray Crystallography of (+)-(2′S)-isatisindigoticanine C (2b)
A crystal of 2b was obtained in MeOH. The crystallographic data of 2b were obtained with Cu Kα (λ = 1.54178 Å) radiation at 130 K on a Bruker Apex II CCD diffractometer. The structures were solved by a direct method and refined with the full-matrix least-squares technique using SHELX-2014 software. Nonhydrogen atoms were refined with anisotropic displacement parameters, and hydrogen atoms were placed in calculated positions and refined with a riding model. The flack parameter was 0.15(7) (Figure S37, Supplementary Information). The crystallographic data of 2b were deposited in the Cambridge Crystallographic Data Centre (CCDC) with deposition number 1941685.
3.4. Inhibitory Assay of NO Production
Compounds 1a/1b–5a/5b were tested for their NO inhibitory effects in the LPS activated RAW 264.7 cells using the previously reported method [8,9,23]. The IC50 values showed that only 4a and 4b showed inhibitory effects with IC50 values of 76.97 μM and 65.88 μM (aminoguanidine hydrochloride was used as the positive control, IC50 22.67 μM).
4. Conclusions
In this study, six new alkaloids: (+)-(3′R,2″S)-isatisindigoticanine B (1a), (−)-(3′S,2″R)-isatisindigoticanine B (1b), (−)-(2′R)-isatisindigoticanine C (2a), (+)-(2′S)-isatisindigoticanine C (2b), (−)-(4S,2′R,3′R)-isatisindigoticanine D (3a) and (+)-(4R,2′S,3′S)-isatisindigoticanine D (3b), together with four known ones: (−)-(2R,3R)-3-hydroxy-2H-pyrrolo[2,3-b]indolo[5,5a,6-b,a]quinazoline-9(8H),7′-dione (4a), (+)-(2S,3S)-3-hydroxy-2H-pyrrolo[2-b]indolo[5,5a,6-b,a]quinazoline-9(8H),7′-dione (4b), epigoitrin (5a) and goitrin (5b), were isolated from the roots of I. indigotica. The alkaloids 1a and 1b possess an unpresented carbon skeleton of a 1H-indol-2-one unit connected with a 4-quinolinecarboxylic acid unit and a pyrrolidine unit via a six-membered ring of C-3-C-4-C-9-N-1″-C-2″-C-3′. Alkaloids 4a and 4b showed NO inhibitory effects in the LPS activated RAW 264.7 cells, with IC50 values of 76.97 μM and 65.88 μM.
Supplementary Materials
The following are available online. Copies of 1H NMR and 13C NMR spectra of 1–5, IR, HREIMS, DEPT 135°, HSQC, HMBC, 1H-1H COSY spectra of compounds 1–3; ECD calculation details of 1a/1b–3a/3b; Chiral separation chromatography of 1–5; Crystallographic data of 2b.
Author Contributions
R.W., Y.L. and K.C. conducted the experiments; R.X. and K.D. carried out the anti-inflammatory activity experiments; Y.S. collected the ECD data; F.G. analyzed the MS data; D.Z. performed the isolation, analyzed the structures and wrote the paper; R.W. oversaw the research project and drafted the paper.
Funding
This work was supported by the National Natural Science Foundation of China (81573571, 81673570), the Excellent Academic Leaders Program of Shanghai (16XD1403500), the programs of the High Level University Innovation Team, the Shanghai E-Research Institute of Bioactive Constituents in Traditional Chinese Medicine and the Shanghai Scientific and Technological Innovation Program (18401931100).
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Sample Availability: Samples of the compounds 1a/1b–5a/5b are available from the authors.
References
- 1.Chen M.H., Gan L.S., Lin S., Wang X.L., Li L., Li Y.H., Zhu C.G., Wang Y.A., Jiang B.Y., Jiang J.D. Alkaloids from the root of Isatis indigotica. J. Nat. Prod. 2012;75:1167–1176. doi: 10.1021/np3002833. [DOI] [PubMed] [Google Scholar]
- 2.Xi Y.F., Zhou L., Bai M., Wang J., Lin B., Wang X.B., Huang X.X., Song S.J. N-acylanthranilic acid derivatives with anti-Aβ1–42 aggregation activity from the leaves of Isatis indigotica fortune. Fitoterapia. 2018;128:169–174. doi: 10.1016/j.fitote.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Liu S.F., Zhang Y.Y., Zhou L., Lin B., Huang X.X., Wang X.B., Song S.J. Alkaloids with neuroprotective effects from the leaves of Isatis indigotica collected in the Anhui Province, China. Phytochemistry. 2018;149:132–139. doi: 10.1016/j.phytochem.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Chen M.H., Lin S., Li L., Zhu C.G., Wang X.L., Wang Y.A., Jiang B.Y., Wang S.J., Li Y.H., Jiang J.D. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org. Lett. 2015;45:1523–7052. doi: 10.1021/ol302660t. [DOI] [PubMed] [Google Scholar]
- 5.Yang L.G., Wang G., Wang M., Jiang H.M., Chen L.X., Zhao F., Qiu F. Indole alkaloids from the roots of Isatis indigotica and their inhibitory effects on nitric oxide production. Fitoterapia. 2014;95:175–181. doi: 10.1016/j.fitote.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Meng L.J., Guo Q.L., Liu Y.F., Shi J.G. 8,4′-Oxyneolignane glucosides from an aqueous extract of “ban lan gen” (Isatis indigotica root) and their absolute configurations. Acta Pharm. Sin. B. 2017;7:638–646. doi: 10.1016/j.apsb.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D.D., Li J.Y., Shi Y.H., Chen K.X., Li Y.M., Wang R. Glycosides from roots of Isatis indigotica. Chin. Tradit. Herbal Drugs. 2019;50:3575–3580. [Google Scholar]
- 8.Zhang D.D., Li J.Y., Ruan D.Q., Chen Z.Q., Zhu W.L., Shi Y.H., Chen K.X., Li Y.M., Wang R. Lignans from Isatis indigotica roots and their inhibitory effects on nitric oxide production. Fitoterapia. 2019:1–7. doi: 10.1016/j.fitote.2019.104189. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D.D., Du K., Zhao Y.T., Shi S.S., Wu Y.C., Jia Q., Chen K.X., Li Y.M., Wang R. Indole alkaloid glycosides from Isatis tinctoria roots. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1624960. [DOI] [PubMed] [Google Scholar]
- 10.Guo Q.L., Xu C.B., Chen M.H., Lin S., Li Y.H., Zhu C.G., Jiang J.D., Yang Y.C., Shi J.G. Sulfur-enriched alkaloids from the root of Isatis indigotica. Acta Pharm. Sin. B. 2018;8:933–943. doi: 10.1016/j.apsb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X.M., Zhang D.D., He H., Li Y.Z., Yang X.J., Deng C., Tang Z.S., Cui J.C., Yue Z.G. Steroidal glycosides from Reineckia carnea. Fitoterapia. 2015;105:240–245. doi: 10.1016/j.fitote.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D.D., Wang W., Li Y.Z., Li Z., Jiang Y., Tang Z.S., Song X.M., Yue Z.G. Two new pregnane glycosides from Reineckia carnea. Phytochem. Lett. 2016;15:142–146. doi: 10.1016/j.phytol.2015.12.005. [DOI] [Google Scholar]
- 13.Li Y.Z., Wang X., He H., Zhang D.D., Jiang Y., Yang X.J., Wang F., Tang Z.S., Song X.M., Yue Z.G. Steroidal saponins from the roots and rhizomes of Tupistra chinensis. Molecules. 2015;20:13659–13669. doi: 10.3390/molecules200813659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y.W., Yang X.J., Zhang D.D., Li Y.Z., Zhang L., Song B., Yue Z.G., Song X.M., Tang H.F. Steroidal constituents from roots and rhizomes of Smilacina japonica. Molecules. 2018;23:798. doi: 10.3390/molecules23040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y.F., Chen M.H., Guo Q.L., Lin S., Xu C.B., Jiang Y.P., Li Y.H., Jiang J.D., Shi J.G. Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 2015;17:689–704. doi: 10.1080/10286020.2015.1055729. [DOI] [PubMed] [Google Scholar]
- 16.Hou W.J., Sun H., Ma Y.F., Liu C.Y., Zhang Z.Y. Identification and optimization of novel cathepsin C inhibitors derived from EGFR inhibitors. J. Med. Chem. 2019;62:5901–5919. doi: 10.1021/acs.jmedchem.9b00631. [DOI] [PubMed] [Google Scholar]
- 17.Cho J.H., Chi H., Hoi S. Antimicrobial activity of quinoline derivatives isolated from Ruta chalepensis toward human intestinal bacteria. J. Microbiol. Biotechnol. 2005;13:646–651. [Google Scholar]
- 18.Feng C., Shi L., Chen D.Z., Zhang H.C., Zhao R.Q. Chemical constituents of effective part in Celosia cristata for treatment of hemostatic. Chin. Tradit. Herbal Drugs. 2017;48:653–656. [Google Scholar]
- 19.Couly F., Harari M., Dubouilh-Benard C., Bailly L., Petit E., Diharce J., Bonnet P., Meijer L., Fruit C., Besson T. Development of kinase inhibitors via metal-catalyzed C–H arylation of 8-alkyl-thiazolo[5,4-f]-quinazolin-9-ones designed by fragment-growing studies. Molecules. 2018;23:2181. doi: 10.3390/molecules23092181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin X.J., Jin L., Yu Y.Q., Liu H., Khan A., Yan H., Hao X.J., An L.K., Liu H.Y. Eucalypglobulusals A–J, formyl-phloroglucinol–terpene meroterpenoids from Eucalyptus globules fruits. J. Nat. Prod. 2018;81:2638–2646. doi: 10.1021/acs.jnatprod.8b00430. [DOI] [PubMed] [Google Scholar]
- 21.Wang W.X., Lei X.X., Ai H.L., Bai X., Li J., He J., Li Z.H., Zheng Y.S., Feng T., Liu J.K. Cytochalasans from the Endophytic fungus xylaria cf. curta with resistance reversal activity against fluconazole-resistant candida albicans. Org. Lett. 2019;21:1108–1111. doi: 10.1021/acs.orglett.9b00015. [DOI] [PubMed] [Google Scholar]
- 22.Chen X.Y., Zhang T., Wang X., Hamann M.T., Kang J., Yu D.Q., Chen R.Y. A chemical investigation of the leaves of Morus alba L. Molecules. 2018;23:1018. doi: 10.3390/molecules23051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D.D., Shi Y.H., Shi S.S., Wu X.M., Zhang L.Q., Chen K.X., Li Y.M., Wang R. Isatisindigoticanine A, a novel indole alkaloid with an unpresented carbon skeleton from the roots of Isatis tinctoria. Nat. Prod. Res. 2019:1–7. doi: 10.1080/14786419.2019.1644632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.