Abstract
β-glucans, heterogeneous glucose polymers present in many organisms, have the capability to activate the innate immune system. Efficacy of activation depends on purity of the compound, molecular structure, polymerization degree, and source. One of the purest forms of crystallized β-(1–3)-glucan present in nature is the paramylon extracted from the WZSL non-chloroplastic mutant of Euglena gracilis, which can be processed to produce linear nanofibers capable of interacting with specific receptors present on cell membranes. The effects of these nanofibers, already investigated on plants, animals, and humans, will be analyzed.
Keywords: β-glucans, immune system, PAMPs, PRRs, glucan source, glucan extraction, Euglena gracilis
1. Background
β-glucans are natural polysaccharides present in fungi, algae, bacteria, and plants, which differ in structure, size, branching frequency, and conformation. All these features influence their physiological functions and biological activity. These polysaccharides commonly consist of a main chain of β-(1,3) and/or β-(1,4)-glucopyranosyl units in non-repeating non-random order, with side chains of varying lengths [1,2,3]. β-glucans function as pathogen-associated molecular patterns (PAMPs) and can be non-specifically recognized by pattern recognition receptors (PRRs) present on the surface of the innate immune system cells [4,5]. β-glucans have a potent immunomodulating activity [6,7,8]; their action is mediated through receptors present on immune cells, such as Dectin-1 (a C-type lectin receptor), Toll-like receptors, complement receptor 3, scavenger receptor, and lactosylceramide and elicits specific biological responses [9,10,11].
Dectin-1 is indicated as the preferential receptor for linear β-(1,3)-linked glucans [12,13,14,15]. Upon linkage of the glucan effector with Dectin-1, the innate immune response is activated, which leads to production of both ROS and inflammatory cytokines, through the activation of transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB), enzymes such as phospholipase C, and mitogen-activated protein kinases [10]. This ability to enhance defense mechanisms against infection and simultaneously down-regulate inflammations makes β-glucans a promising alternative to the mainstream use of immunosuppressive drugs for inflammatory diseases.
In order to avoid controversial results on the bioactivity of β-glucans, especially in clinical trials, efforts should be made to use highly purified glucans, free from components that could alter their affinity for innate immunity receptors or counteract their pro-inflammatory potency. Among linear β-(1,3)-linked glucans, paramylon extracted from the non-chloroplastic WZSL mutant of Euglena gracilis can be considered highly pure since it lacks any contamination from cellular components (membranes, proteins, pigments). Such purity is confirmed by optical and scanning electron microscope examination and by NMR spectrum, which indicates the presence of 100% glucose [2] and guarantees the absence of any non-specific immune-modulating effects. Paramylon, either in granular form or as linear β-(1,3)-linked glucan nanofibers, has been used to assess the beneficial potential of the polysaccharide and investigate the structure-function relationship and mechanism of action in animal and plant models.
We will analyze and discuss four experimental cases on which paramylon granules and paramylon nanofibers from the WZSL mutant have been tested. The four cases are
Experimental Case 1: induction of stress resistance in Artemia sp.
Experimental Case 2: activation of innate immune responses in human lymphocytes.
Experimental Case 3: anti-fibrotic effect on CCl4-induced liver damage in mice.
Experimental Case 4: increase of drought resistance and improvement of quality profile in horticultural species.
2. WZSL Mutant and Paramylon
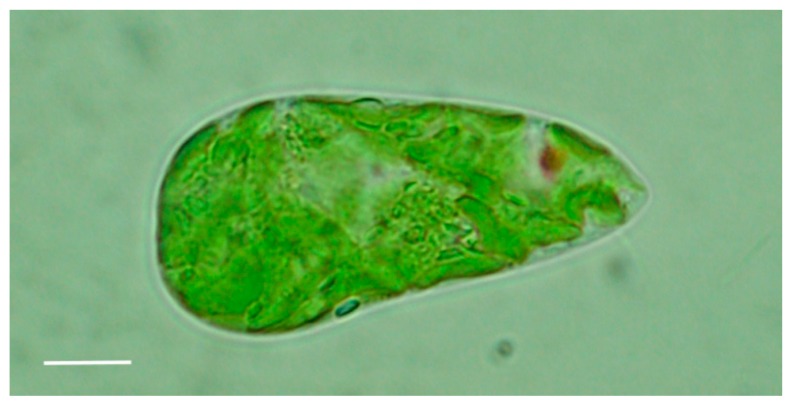
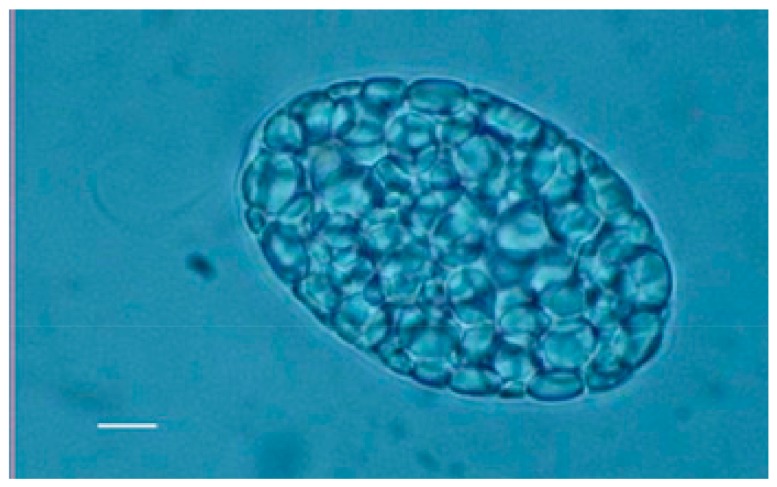
Both wild type (Figure 1) and WZSL mutant cells of Euglena gracilis (Figure 2) synthesize the storage polysaccharide paramylon, which is deposited as granules in the cytoplasm. These granules, ranging from 1 to 2 μm, have a very high degree of crystallinity, due to the concentric deposition of higher order aggregates of elemental 4–10 nm nanofibers consisting of unbranched triple helices of β-(1–3)-glucan chains. The cellular content of paramylon varies greatly with growth conditions (in the light or in the dark) and carbon source. For both cell types, under batch culture conditions and under both growth conditions, the highest concentration of the polysaccharide is reached after 24 h, with higher values in the dark respect to the light, and with glucose as best carbon source. Paramylon content of the mutant can be almost twice the content of the wild type: about 2.5 ng/cell, corresponding to 85% of the dry biomass in the mutant, respect to about 1.4 ng/cell, corresponding to 48% of the dry biomass in the wild type [16].
Figure 1.
Optical microscopy image of Euglena gracilis wild type, showing its photosynthetic machinery. Bar: 10 μm.
Figure 2.
Optical microscopy image of WZSL mutant filled up with paramylon granules. Bar: 10 μm.
WZSL mutant can be cultivated aseptically using a fed-batch technique, a culture technique commonly used in fermentation industry to attain high cell densities and increase the quality of the final product, especially important for algal biomass intended for extraction of high-value pure compounds [17,18]. Fed-batch culture in the dark using Cramer and Myers medium [19], with 10g/L glucose reaches a biomass yield of 8 g L−1 after 20 days of cultivation, with a production of paramylon constantly higher than 80% of the dry biomass.
Thanks to their high crystallinity, paramylon granules can be extracted from the mutant cells very easily by sonication or other mechanical disruption method and purified by using a low concentration detergent to solubilize all the membrane components that could contaminate the final product [15]. Granules are recovered by low-speed centrifugation and rinsed repeatedly to remove any trace of detergent. Paramylon nanofibers are obtained by alkaline degradation of the granules, which leads to the unwinding of the ordered arrays of linear nanofibers, and to their random fragmentation in small unit recognizable by Dectin-1 receptors [12,13]. This processing improves the interaction of the β-(1,3)-linked glucan with PRRs present on cell membranes [4,5,12,13,14,15].
3. Experimental Cases
3.1. Experimental Case 1: Induction of Stress Resistance in Artemia sp.
The first test of paramylon bioactivity was conducted on the brine shrimp Artemia sp. (Figure 3) to investigate whether this glucan could help the animals to cope with and eventually overcome stress conditions during growth [20].
Figure 3.
Optical microscopy image of Artemia sp. adult. Bar: 1 mm.
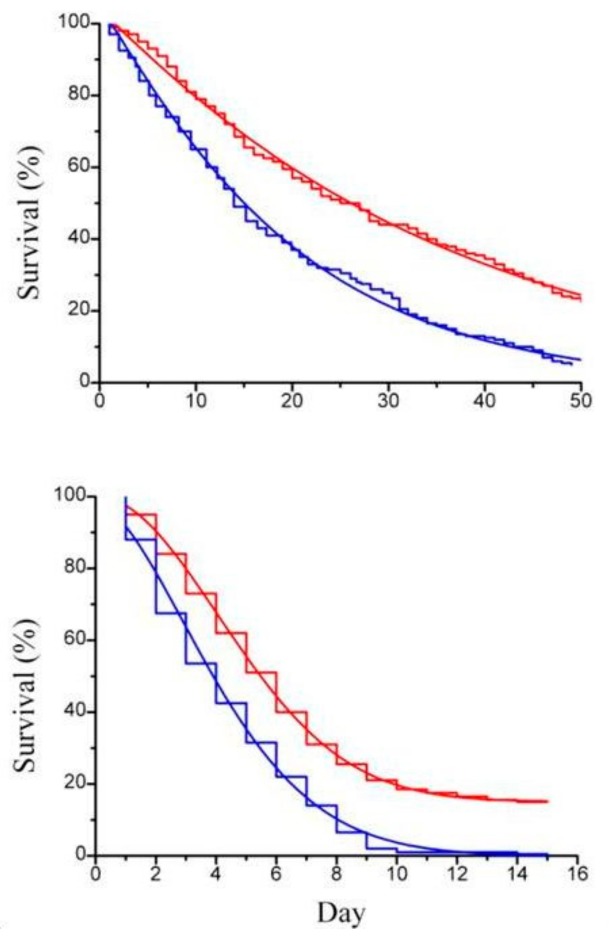
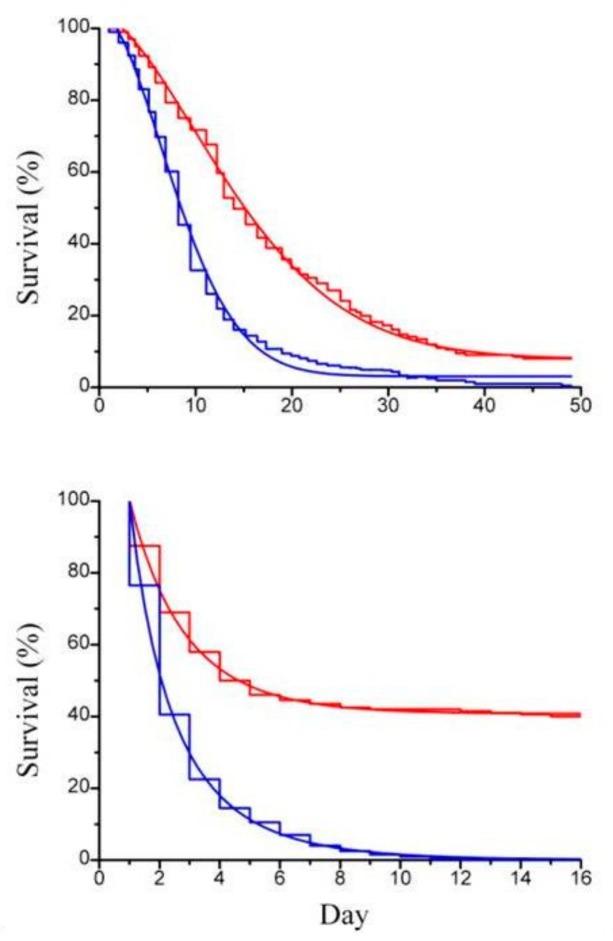
Previous works by Itami et al. [21] and Lopez et al. [22] used β-glucan derived from Bifodobacterium sp. in the form of particulate cell wall fragment preparation as dietary addition to assess its physiological and immunological role in shrimps. In order to compare our result with the result of these authors [20,21], paramylon granules were tested suspended in the artificial sea water to rear the animals. Imposed stress condition were those connected to routine operations, such as daily medium replacement and handling, known to lower animal tolerance to diseases [23]. Paramylon added to the culture medium significantly enhanced the survival performance of both Artemia adults (Figure 4a) and offspring (Figure 4b) to deteriorated environmental conditions (no medium replacement) and increased the ability of the crustaceans to withstand the stress of daily medium replacement (Figure 5a,b).
Figure 4.
(a) Survival curve (step line) of Artemia sp.; (b) survival curve (step line) of Artemia sp. offspring with the corresponding Weibull survival functions (continuous line), with paramylon (red curve) and without paramylon (blue curve), under conditions of no daily medium replacement.
Figure 5.
(a) Survival curve (step line) of Artemia sp.; (b) survival curve (step line) of Artemia sp. offspring with the corresponding Weibull survival functions (continuous line), with paramylon (red curve) and without paramylon (blue curve), under conditions of daily medium replacement.
Paramylon positively influenced also the number of the offspring produced, reducing the number of the encysted animals, both when medium was not replaced and with daily medium replaced, confirming its enhancing action on the reproductive success of the population.
Our results showed that paramylon treatment obtained significant higher survival percentage of the shrimps respect to the treatment with bacterial glucan; this may be due to the penetration of paramylon granules in the digestive tract of the shrimps, which enhances the production of cell activating factors in the hemocytes, increasing the phagocytic activity of the granulocytes, thus providing defense against diseases [20,21,22]. As other commercial products worldwide introduced as feed additive to improve health and performance of farmed shrimp, fish, farmed animals, and pets (e.g., MacroGard), paramylon could therefore be used as non-specific dietary additive in aquaculture, improving the nutritional value of the feed and mitigating negative effects of stressors when they occur by promoting innate immunity of the animals.
3.2. Experimental Case 2: Activation of Innate Immune Responses in Human Lymphocytes
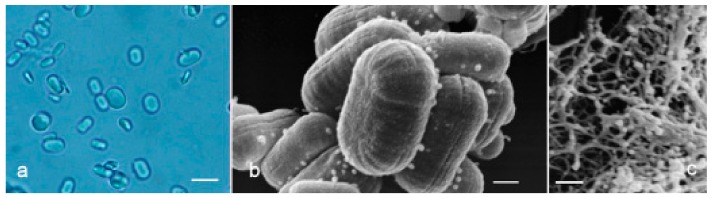
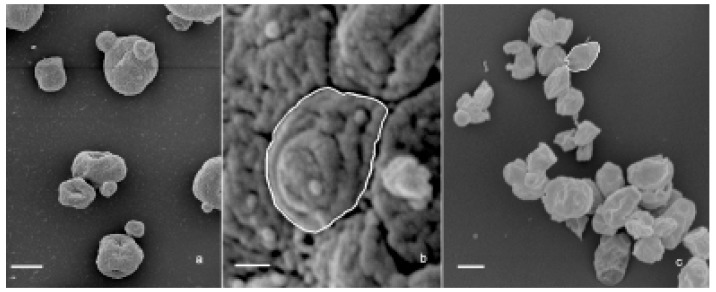
To investigate the influence of source, purity, primary chemical structure, and 3D conformation on β-glucan capacity to activate innate immune system response, we tested three different β-glucan forms i.e., paramylon granules (Figure 6a,b), paramylon nanofibers (Figure 6c), and Saccharomyces cerevisiae, (Figure 7a–c) processed according to Russo et al. [24], in human peripheral blood mononuclear cells (PBMC),
Figure 6.
(a) Optical image of paramylon granules extracted from WZSL mutant. Bar: 2 μm. (b) SEM image of paramylon granules. Bar: 200 nm. (c) SEM image of paramylon sonicated and alkalized showing its linear fibrous structure. Aggregation is due to the SEM preparation procedure (gold coating thickness about 10 nm). Bar: 400 nm.
Figure 7.
(a) SEM image of the aggregates present in S. cerevisiae preparation. Bar: 3 μm. (b) SEM image of an aggregate surface showing its particulate texture. The white contour defines a single particle. Bar: 200 nm. (c) SEM image of the irreducible particles. The white contour defines a single particle. Bar: 1 μm.
Evaluation of the response was carried out by measuring gene expression of inflammation-related cytokines and mediators, transactivation of relevant transcription factors, and phagocytic activity.
The three β-glucan forms greatly differ in purity, size, and 3D conformation. Paramylon granules range in size between 2–8 µm and show no contamination from other cell components (Figure 6a). Paramylon nanofibers show a degree of purity similar to that of the granules and possess a linear structure in sub-nanometric range. The preparation of S. cerevisiae shows aggregates of about 50 µm consisting of a patchwork of irreducible particles of heterogeneous yeast components of about 2 µm (highlighted in Figure 7b,c). According to Hunter et al. [25], these particles consist of conglomerates of several different molecules such as hexoses, proteins, lipids, and nucleic acids, which can make difficult to interpret experimental results on the effects of S. cerevisiae glucan.
Paramylon nanofibers increased transactivation of NF-κB (rapid acting primary transcription factor, first responder to harmful stimuli, [26]), as shown by the evident immunofluorescence nuclear labelling of treated PBMC (Figure 8), whereas both Saccharomyces preparation and paramylon granules had almost no effect [24].
Figure 8.
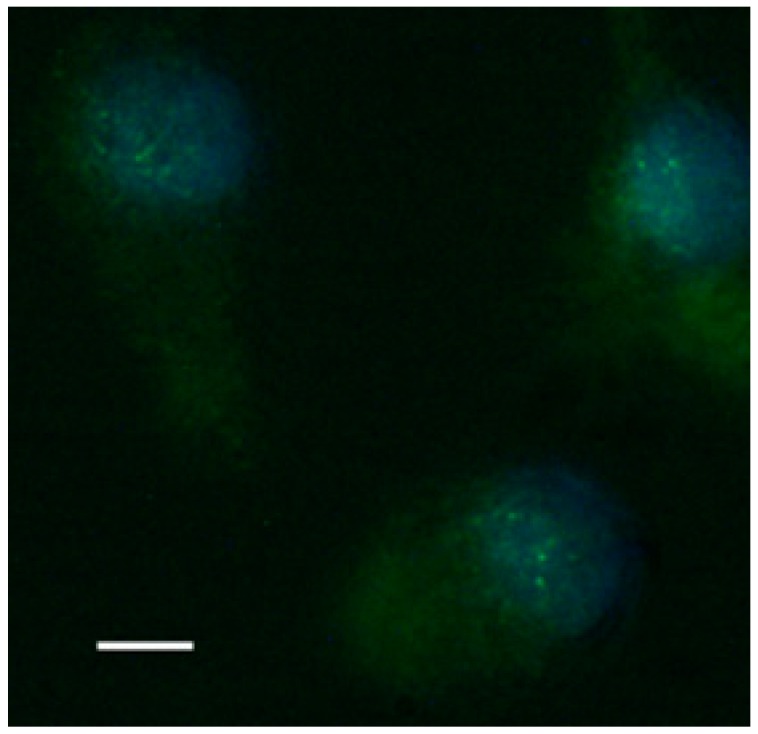
Fluorescence image of translocation of nuclear factor NF-κB in PBM cells stimulated by paramylon nanofibers. Bar 10 μm.
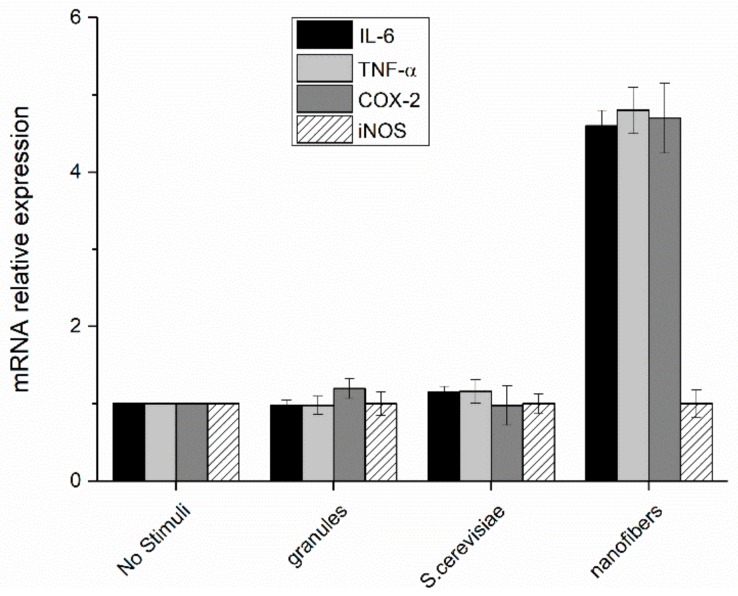
The increase in the transcription factor translocation led to a consequent increase of the expression of pro-inflammatory mediators (TNF-α, IL-6, COX-2, and iNOS) in cells treated with paramylon nanofibers, whereas the level of expression in the cells treated with paramylon granules and S. cerevisiae preparation did not significantly differ from that of non-treated control cells. These mediators are high inflammatory and are indispensable when priming immune responses and licensing dendritic cells (Figure 9).
Figure 9.
Relative expression of interleukin-6, tumor necrosis factor alpha, cyclooxygenase 2, and inducible nitric oxide synthase in PBM cells after 24 h stimulation with paramylon granules, S. cerevisiae preparation, and paramylon nanofibers. Results were expressed as fold-increase respect to control and plotted as the mean ± SD.
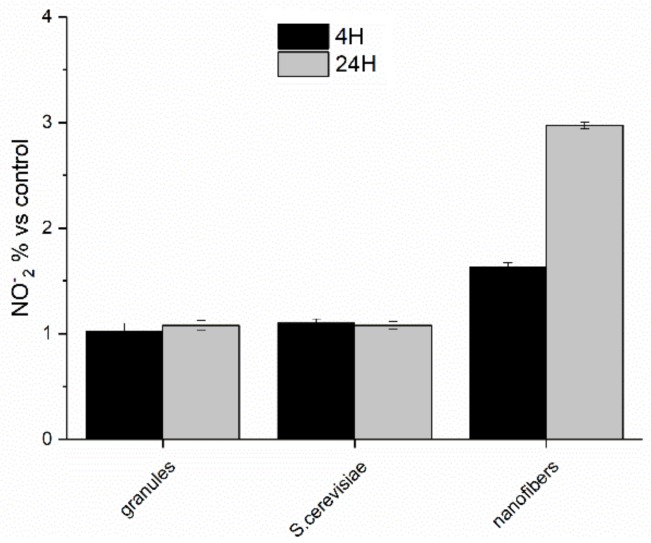
Paramylon nanofibers also induced the production of high level of NO (via transcription of iNOS gene), which increased from 4 to 24 h and exerted an inhibitory effect on cytokine expression via inhibition of NF-κB transactivation (Figure 10) [27], thus preventing a dangerous cytokine storm.
Figure 10.
Nitric oxide production by PBMC cells stimulated with paramylon granules, S. cerevisiae preparation, and paramylon nanofibers 4 and 24 h, expressed as percent nitrite concentration versus control and plotted as the mean ± SD.
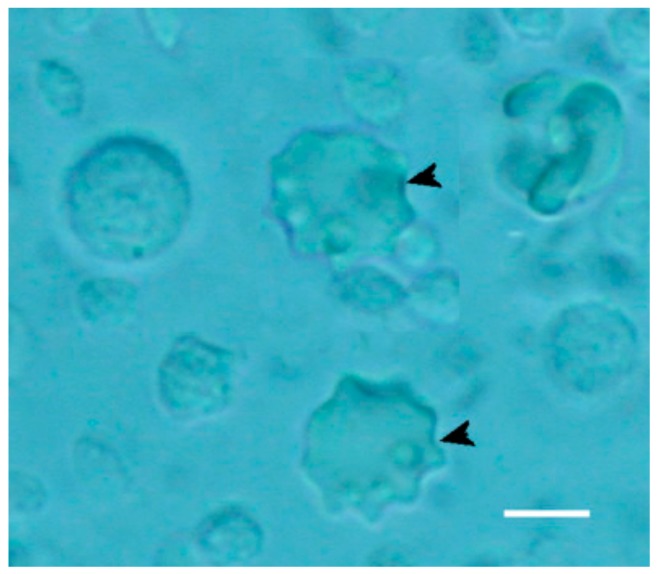
This signaling cascade guarantees a safe activation of the innate immune system, as demonstrated by the presence of newly differentiated dendritic cells (Figure 11, arrowheads). Paramylon granules and particles of Saccharomyces were not absorbed to the cell surface of PBMC due to their sizes, which did not fit with Dectine-1 receptors. Though both these preparations were internalized, this phagocytic activity did not lead to any activation of the immune system, i.e., no dendritic cells differentiated [8,24].
Figure 11.
Newly differentiated dendritic cells (arrowheads) indicating activation of the innate immune system in PBM cells treated with paramylon nanofibers. Bar 10 μm.
The immune activation response obtained by paramylon nanofibers treatment fits well with evidences reported by many scientific groups [12,13,28] that the minimum β-glucan unit recognized by Dectin-1 is a 9- or 10-mer of glucose.
3.3. Experimental Case 3: Anti-Fibrotic Effect on CCl4-Induced Liver Damage in Mice
Hepatic fibrosis is one of the most severe pathology worldwide clinically associated with the eventual development of hepatocarcinogenesis and expected to become a major global burden [29]. To date, no definitive therapeutic options are available, and functional foods containing bioactive ingredients are expected to play a very important role in treating and preventing this pathology. Therefore, we explored the feasibility of paramylon nanofibers to act as a potential functional food by investigating their anti-fibrotic effect in mice with liver fibrosis induced by 4 weeks treatment of CCl4 administered via intraperitoneal injection [30]. Liver fibrosis induced by CCl4 is a reliable in vivo experimental model to investigate the progression of liver disease and monitor the effects of different therapeutic strategies [29].
One group of animals was administered 150 μL of paramylon nanofibers suspension 2 weeks before the beginning of the challenging, via voluntary suction (90 mg/kg body weight/die). The paramylon administration continued over the entire experimental period (2 + 4 weeks). Since the progression of fibrosis is mainly due to inflammation and collagen accumulation, we evaluated liver damage markers and collagen content (Picrosirius red staining and hydroxyproline determination).
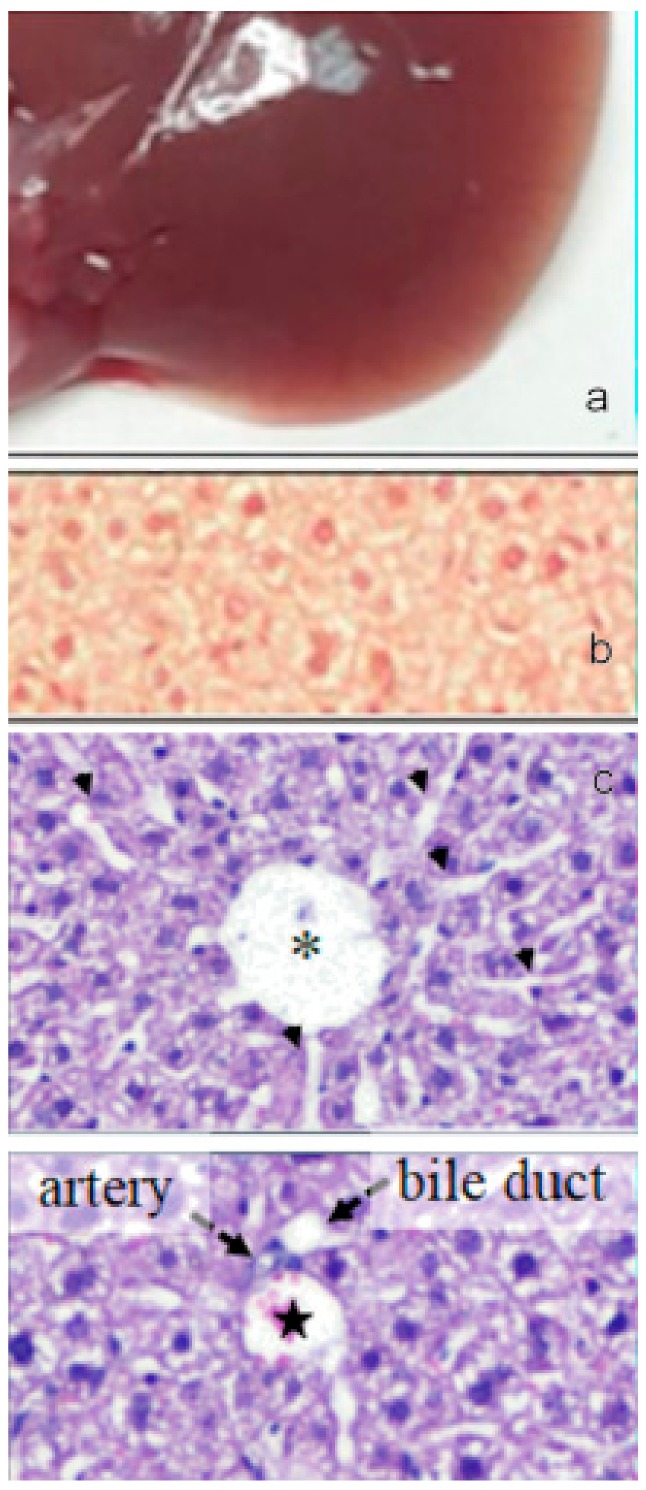
The oral administration of paramylon nanofibers ameliorated the overall clinical picture of the animals; in particular, paramylon dampened the CCl4-induced loss of weight and prevented the increase of aspartate aminotransferase (AST) typical of hepatocyte damage. It also reduced the hyper-echoic areas caused by the toxic treatment, restoring the normal tissue consistency and appearance. CCl4-treated liver had a trabecular ‘fishnet’ texture typical of mild to severe edema, while normal color and consistency was restored in paramylon treated livers, indicating a relieved hepatic injury (Figure 12a, Figure 13a and Figure 14a).
Figure 12.
(a) Image of the gross morphology of the liver showing normal anatomical features in the control group. (b) Picrosirius red staining of liver sections: no evidence of collagen deposition in the parenchyma. (c) Hematoxylin-eosin staining of liver sections: normal parenchyma architecture with sinusoids (upper image) along with the portal triad (lower image), composed by a branch of the hepatic artery, a branch of the portal vein, and the bile duct. Asterisk indicates the central vein and star indicates the portal vein. Bar 50 μm.
Figure 13.
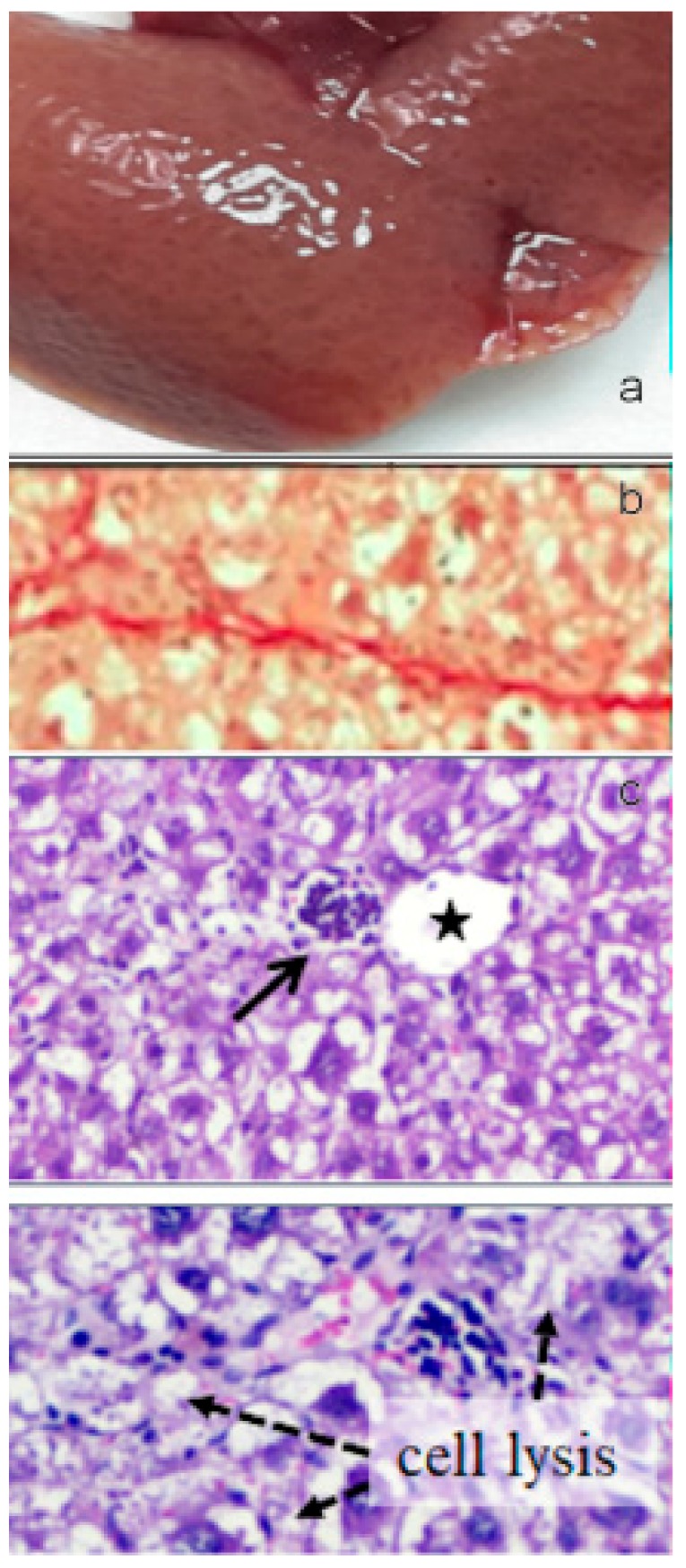
(a) Image of the gross morphology of the liver showing altered macroscopic characteristics in CCl4 challenged group with swollen and spongy appearance of the liver and lobules clearly visible on the surface. (b) Picrosirius red staining of liver sections: marked parenchymal deposition of collagen fibers with portal-to-portal bridging fibrosis. (c) Several glomerular clusters of inflammatory cells were present in the peri-portal region (upper image, arrow) and nearby focal areas of hepatocellular lysis (bottom image). Asterisk indicates the central vein and star indicates the portal vein. Bar 50 μm.
Figure 14.
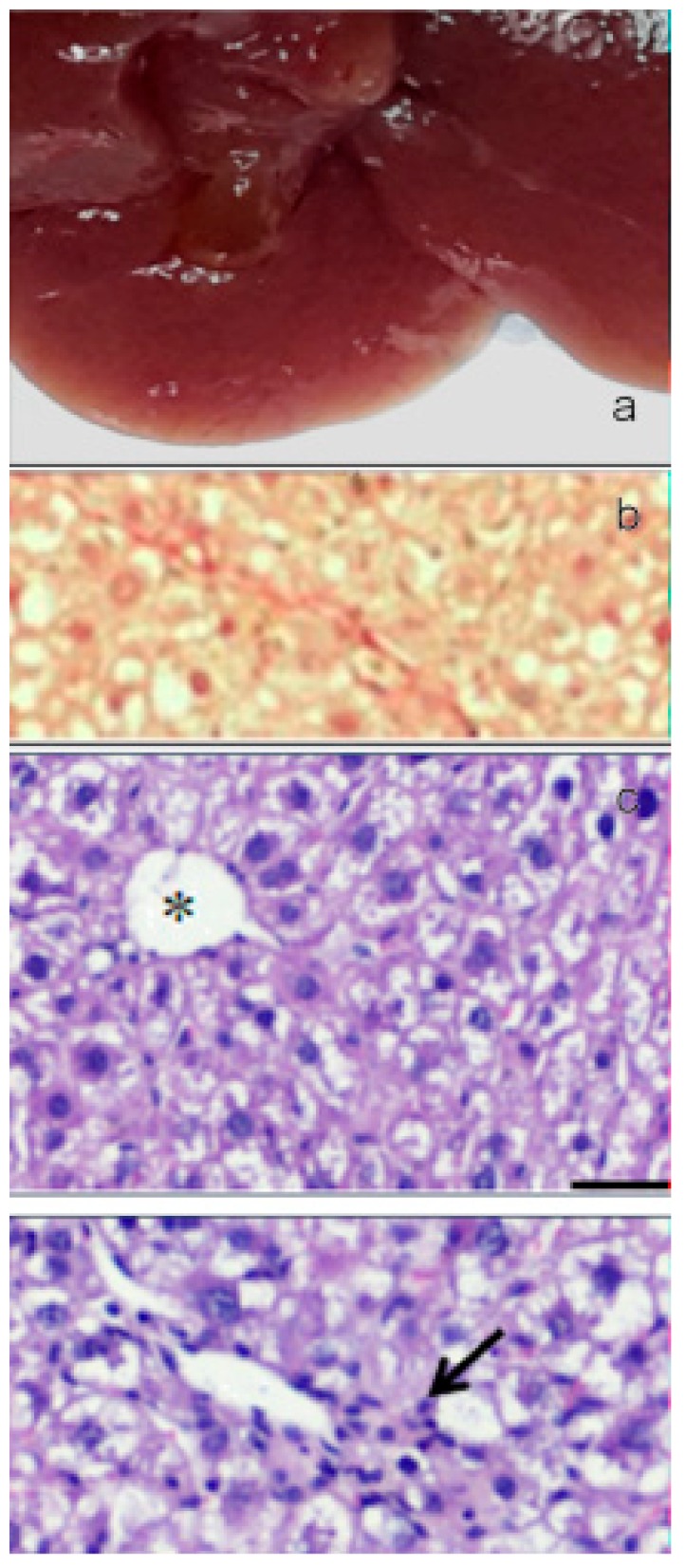
(a) Image of the gross morphology of the liver showing an improvement of the pathological changes induced by CCl4 challenge in the CCl4+paramylon nanofibers group in which the normal tissue consistency was almost restored. (b) Picrosirius red staining of liver sections: reduction of the amount of collagen deposition respect to the challenged group by paramylon nanofibers treatment. (c) Reversal of severe inflammation and vein congestion along with a milder vacuolization of hepatocytes (upper image). The infiltration of inflammatory cells, although still present, is greatly reduced (bottom image, arrow). Asterisk indicates the central vein and star indicates the portal vein. Bar 50 μm.
Collagen deposition in Picrosirius red staining sections of liver of paramylon treated animals was lower than in CCl4-treated animals (Figure 12b, Figure 13b and Figure 14b). This positive effect was confirmed by the content of hydroxyproline, which was comparable with that of the control. Moreover, hematoxylin-eosin staining of CCl4-damaged liver sections revealed prominent evident signs of hepatocyte suffering with diffuse distribution of inflammatory cells particularly around the portal vein. Paramylon nanofibers treatment greatly reduced the overall alteration of tissue parenchyma, and the lobular architecture was alike that non-damaged liver, with mild hepatocyte ballooning respect to CCl4-damaged livers and almost no necrosis regions nor infiltration of inflammatory cells (Figure 12c,d, Figure 13c,d and Figure 14c,d).
The tissue damage induced by CCl4 eventually leads to inflammation, which evolves as a consequence of tissue damage and is the main cause of liver injury [31]. Reduction of inflammation and collagen production by liver stellate cells is due to the inhibiting action of hepatic γδT cells upon Dectin-1 ligation by paramylon nanofibers, which promotes hepatocyte regeneration [32].
Our results cannot discriminate between a curative or preventive role of paramylon; still, they show an important protective effect of this glucan in sterile inflammation, which could be exploited for experimental therapeutics in hepatic fibrosis and inflammation. Moreover, they confirm the higher affinity of nanofibers for Dectin-1 receptor respect to granules, already tested on rat acute hepatic injury for their protective effect [33].
3.4. Experimental Case 4: Increase of Drought Resistance and Improvement of Quality Profile in Horticultural Species.
Drought (low water availability) is a major environmental factor limiting photosynthesis and contributing to the progressive salinization of world arable land, consequently reducing crop production all over the world [34], and tomato, one of the most economically and nutritionally important crops worldwide, is severely affected by water deficits, which compromise both fruit yield and quality. To overcome drought effects, research mainly focuses on the selection of tolerant genotypes that have less demand for water.
Paramylon nanofibers applied to the root system of tomato plants have already shown to modulate hormone levels, photosynthesis, and water use efficiency [35]. To verify the possibility of using paramylon as a novel root treatment to help tomato plants to cope with low water availability, plants of Solanum lycopersicum cultivar Micro-Tom, chosen because of its short life cycle (≤3 months) and small size, were challenged with drought stress with or without root treatment with paramylon nanofibers [36]. Plants were grown under controlled conditions in an aereoponics system, a soil-less air-water system particularly suited to study plant roots since roots grow suspended in a closed chamber in the dark, and the nutrient solution is directly sprayed onto them by atomizers.
Ecophysiological, physical chemical and quality parameters were monitored throughout the life cycle of tomatoes and compared with those of well-watered, untreated plants. Flowering and fruit ripening of stressed paramylon treated plants were about 2 weeks precocious respect to untreated well-watered plants, whereas fruits of stressed untreated plants do not ripe beyond category II (Figure 15).
Figure 15.
The representative features of the tomato plants (aerial parts) cultivated under water-stressed conditions coupled with root treatment with paramylon nanofibers (A) and under water-stressed conditions without paramylon nanofibers (B) 60 days after germination.
Thanks to paramylon action, we observed that the optimal water regimen of about 8.64 L plant−1 day−1 can be lowered down to 0.36 L plant−1 day−1. Ecophysiological parameters, (i.e., leaf water potential, stomatal conductance, and photosynthetic yield), were dramatically influenced by water stress, all of them undergoing a continuous decrease to saturation. Root treatment with paramylon nanofibers allowed all the parameters to recover to the values of well-watered plants. These results indicate an effective action of paramylon nanofibers on stomatal behavior, whose control improves water use efficiency, hence prevents dehydration. This action is associated with a transient modification of the content of main plant hormones, i.e., abscissic acid, jasmonic acid and salicylic acid [35].
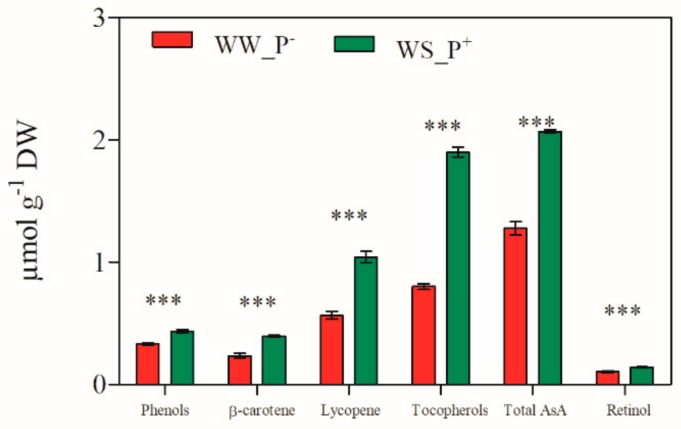
The great increase of physical-chemical and quality parameters such as the antioxidant compounds (Vitamin A/C/E, lycopene, β-carotene, and phenols) [35,36,37,38,39] (Figure 16), together with the increase of carbohydrates (glucose, fructose, and sucrose), in fruits of paramylon treated plants improves their nutritional value and sensory quality [40,41].
Figure 16.
Antioxidant compounds in red ripe fruits of S. lycopersicum cv. Micro-Tom grown under well-watered conditions (WW_P−) and water-stressed conditions coupled with root treatment with paramylon nanofibers (WS_P+). Data are shown as mean ± SD. The significant differences are for: *** P < 0.001.
Moreover, the higher dry matter content (i.e., lower moisture) allows a better post-harvest storage capability extending the commercial period and increasing the commercial product value. These results confirm the involvement of paramylon nanofibers as elicitors of plant response to abiotic stress and their bio-stimulant activity in increasing plant adaptation capacity to environmental stimuli.
4. Conclusions and Outlook
Both paramylon granules and nanofibers obtained by processing the granules have been tested in our research, and nanofibers have shown the capability to effectively interact with PRRs eliciting responses in animal and plant models. Our results indicate that even though the dimensions of paramylon granules and S. cerevisiae particles are in the range of pathogenic bacteria (2–3 µm); still, they are not capable to interact with the Dectin-1 receptors present on the surface of immune system cells. Phagocytosis of these two preparations does not lead to any activations of the immune system since no dendritic cells differentiate [8,24]. Besides playing this important role in immune-system activation, paramylon nanofibers show also a protective effect in sterile liver inflammation, promoting hepatocyte regeneration upon Dectin-1 ligation. Moreover, they have the capability to enhance the plant defense mechanisms against abiotic stress, such as drought, by modulating hormone levels and CO2 diffusion from air, which eventually leads to an improvement of water use efficiency. Activation is induced only by paramylon nanofibers because they consist of a moiety of 9- or 10-mer of glucose, the minimum β-glucan recognized by Dectin-1 receptor [12,13,28].
The WZSL Euglena mutant can be considered an effective and desirable producer of paramylon due to its ability to convert and conserve glucose (the best carbon source for WZSL mutant, [16]) in the paramylon granules at a faster rate than the wild type, and with about half of the glucose added to the culture converted in paramylon. It is easy to grow under controlled conditions, and the cultivation in fed-batch system with glucose addition allows the production of a very high amount of paramylon per gram of dry biomass, i.e., 80%. All these features together with the experimental evidences of paramylon effects provide a reliable framework to further investigate the structure-function relation of β-(1,3)-linked glucans, offering the possibility to test a high purity and well-established preparation with no unexpected or negative side effects on different models, and opening new routes of protection and treatment against diseases and stress agents.
Author Contributions
Conceptualization, methodology, investigation, and writing, L.B. and P.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Myklestad S.M., Granum E. Biology of (1,3)-β-Glucans and Related Glucans in Protozoans and Chromistans. In: Bacic A., Fincher G.B., Stone B.A., editors. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides. Academic Press; Cambridge, MA, USA: 2009. pp. 353–385. [Google Scholar]
- 2.Barsanti L., Passarelli V., Evangelista V., Frassanito A.M., Gualtieri P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat. Prod. Rep. 2011;28:457–466. doi: 10.1039/c0np00018c. [DOI] [PubMed] [Google Scholar]
- 3.Synytsya A., Novak M. Structural analysis of glucans. Ann. Transl. Med. 2014;2:17–31. doi: 10.3978/j.issn.2305-5839.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Thompson I.J., Oyston P.C.F., Williamson D.E. Potential of the β-glucans to enhance innate resistance to biological agents. Expert Rev. Anti. Infect. Ther. 2010;8:339–352. doi: 10.1586/eri.10.10. [DOI] [PubMed] [Google Scholar]
- 6.Vetvicka V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011;2:115–119. doi: 10.5306/wjco.v2.i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesenak M., Banovcin P., Rennerova Z., Majtan J. Β-glucans in the treatment and prevention of allergic diseases. Allergol. Immunopathol. 2014;42:149–156. doi: 10.1016/j.aller.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Soares E., Groothuismink Z.M.A., Boonstra A., Borges O. Glucan Particles Are a Powerful Adjuvant for the HBsAg, Favoring Antiviral Immunity. Mol. Pharm. 2019;16:1971–1981. doi: 10.1021/acs.molpharmaceut.8b01322. [DOI] [PubMed] [Google Scholar]
- 9.Goodridge H.S., Wolf A.J., Underhill D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kankkunen P., Teirilä L., Rintahaka J., Alenius H., Wolff H., Matikainen S. (1,3)-β-glucans activate both Dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010;184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 11.Baert K., Sonck E., Goddeeris B.M., Devriendt B., Cox E. Cell type-specific differences in β-glucan recognition and signaling in porcine innate immune cells. Dev. Comp. Immunol. 2015;48:192–203. doi: 10.1016/j.dci.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Tsoni S.V., Brown G.D. β-glucans and Dectin-1. Ann. N. Y. Acad. Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 13.Adams E.L., Rice P.J., Graves B., Ensley H.E., Yu H., Brown G.D., Williams D.L. Differential high-affinity interaction of Dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharm. Exp. Ther. 2008;325:115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 14.Noss I., Doekes G., Thorne P.S., Heederik D.J., Wouters I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013;19:10–19. doi: 10.1177/1753425912447129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legentil L., Paris F., Ballet C., Trouvelot S., Daire X., Vetvicka V., Ferrières V. Molecular interactions of β-(1→3)-glucans with their receptors. Molecules. 2015;20:9745–9766. doi: 10.3390/molecules20069745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsanti L., Vismara R., Passarelli V., Gualtieri P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001;13:59–65. doi: 10.1023/A:1008105416065. [DOI] [Google Scholar]
- 17.Coelho R., Vidotti A., Marques Reis É., Franco T.T. High cell density cultures of microalgae under fed-batch and continuous growth. Chem. Eng. Trans. 2014;38:313–318. [Google Scholar]
- 18.Xie Y., Jin Y., Zeng X., Chen J., Lu Y., Jing K. Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour. Technol. 2015;180:281–287. doi: 10.1016/j.biortech.2014.12.073. [DOI] [PubMed] [Google Scholar]
- 19.Cramer M., Myers J. Growth and photosynthetic characteristics of Euglena gracilis. Arch. Mikrobiol. 1952;17:384–402. doi: 10.1007/BF00410835. [DOI] [Google Scholar]
- 20.Vismara R., Vestri S., Frassanito A.M., Barsanti L., Gualtieri P. Stress resistance induced by paramylon treatment in Artemia sp. J. Appl. Phycol. 2004;16:61–67. doi: 10.1023/B:JAPH.0000019113.77013.0d. [DOI] [Google Scholar]
- 21.Itami T., Asano M., Tokushige K., Jubono K., Nakagawa A., Takeno N., Nishimura H., Maeda M., Kondo M., Takahashi Y. Enhancement of disease resistance of karuma shrimp, Penaeus japonicus, after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture. 1998;164:277–288. doi: 10.1016/S0044-8486(98)00193-8. [DOI] [Google Scholar]
- 22.Lopez N., Cuzon G., Gaxiola G., Taboada G., Valenzuela M., Pascual C., Sanchez A., Rosas C. Physiological, nutritional and immunological role of dietary β 1–3 glucan and ascorbic acid 2-monophosphate in Litopenaeus vannamei juveniles. Aquaculture. 2003;224:223–243. doi: 10.1016/S0044-8486(03)00214-X. [DOI] [Google Scholar]
- 23.Newman S.G. [(accessed on 1 April 2019)]; Available online: http://www.aqua-in-tech.com.
- 24.Russo R., Barsanti L., Evangelista V., Frassanito A.M., Longo V., Pucci L., Penno G., Gualtieri P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2016;20:205–214. doi: 10.1002/fsn3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter K.W., Gault R.A., Berner M.D. Preparation of microparticulate β-glucan from Saccharomyces cerevisiae for use in immune potentiation. Lett. Appl. Microbiol. 2002;35:267–271. doi: 10.1046/j.1472-765X.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- 26.Nishikori M. Classical and alternative NF-κB activation pathways and their roles in lymphoid malignancies. J. Clin. Exp. Hematop. 2005;45:15–24. doi: 10.3960/jslrt.45.15. [DOI] [Google Scholar]
- 27.Katsuyama K., Shichiri M., Marumo F., Hirata Y. NO inhibits cytokine-induced iNOS expression and NF-κB activation by interfering with phosphorylation and degradation of IκB-α. Atertiocler. Thromb. Vasc. Biol. 1998;18:1796–1802. doi: 10.1161/01.ATV.18.11.1796. [DOI] [PubMed] [Google Scholar]
- 28.Palma A.S., Feizi T., Zhang Y., Stoll M.S., Lawson A.M., Díaz-Rodríguez E., Chai W. Ligands for the β-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (Neoglycolipids) generated from glucan polysaccharides. J. Biol. Chem. 2006;281:5771–5779. doi: 10.1074/jbc.M511461200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D.Y., Friedman S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusmic C., Barsanti L., Di Lascio N., Faita F., Evangelista V., Gualtieri P. Anti-fibrotic effect of paramylon nanofibers from the WZSL mutant of Euglena gracilis on liver damage induced by CCl4 in mice. J. Funct. Foods. 2018;46:538–545. doi: 10.1016/j.jff.2018.05.021. [DOI] [Google Scholar]
- 31.Hammerich L., Bangen J.M., Govaere O., Zimmermann H.W., Gassler N. Chemokine receptor CCR6-dependent accumulation of gamma-delta T-cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59:630–642. doi: 10.1002/hep.26697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifert L., Deutsch M., Alothman S., Alqunaibit D., Werba G., Pansari M., Miller G. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep. 2015;13:1909–1921. doi: 10.1016/j.celrep.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama A., Suzuki K., Mitra S., Arashida R., Yoshida E., Nakano R., Takeuchi T. Hepatoprotective effects of paramylon, a β-1, 3-d-glucan isolated from Euglena gracilis Z, on acute liver injury induced by carbon tetrachloride in rats. J. Vet. Med. Sci. 2009;71:885–890. doi: 10.1292/jvms.71.885. [DOI] [PubMed] [Google Scholar]
- 34.Leng G., Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019;654:811–821. doi: 10.1016/j.scitotenv.2018.10.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scartazza A., Picciarelli P., Mariotti L., Curadi M., Barsanti L., Gualtieri P. The role of Euglena gracilis paramylon in modulating xylem hormone levels, photosynthesis and water-use efficiency in Solanum lycopersicum L. Physiol. Plant. 2017;161:486–501. doi: 10.1111/ppl.12611. [DOI] [PubMed] [Google Scholar]
- 36.Barsanti L., Coltelli P., Gualtieri P. Paramylon treatment improves quality profile and drought resistance in Solanum lycopersicum L. cv. Micro-Tom. Agronomy. 2019;9:394. doi: 10.3390/agronomy9070394. [DOI] [Google Scholar]
- 37.Dumas Y., Dadomo M., Licca G.D., Grolier P. Effects of environmental factors and agricultural techniques of antioxidant content of tomatoes. J. Sci. Food Agric. 2003;83:369–382. doi: 10.1002/jsfa.1370. [DOI] [Google Scholar]
- 38.Slimestad R., Verheul M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum L.) cultivars. J. Sci. Food Agric. 2009;89:1255–1270. doi: 10.1002/jsfa.3605. [DOI] [Google Scholar]
- 39.Raiola A., Tenore G.C., Barone A., Frusciante L., Rigano M.M. Vitamin E content and composition in tomato fruits: Beneficial roles and bio-fortification. Int. J. Mol. Sci. 2015;8:29250–29264. doi: 10.3390/ijms161226163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malundo T.M.M., Shewfelt R.L., Scott J.W. Flavor quality of fresh tomato (Lycopersicon-Esculentum Mill) as affected by sugar and acid levels. Postharvest Biol. Technol. 1995;6:103–110. doi: 10.1016/0925-5214(94)00052-T. [DOI] [Google Scholar]
- 41.Sivakumar P., Sharmila P., Jain V., Pardha-Saradhi P. Sugars have potential to curtail oxygenase acitivity of Rubisco. Biochem. Biophys. Res. Commun. 2002;298:247–250. doi: 10.1016/S0006-291X(02)02437-3. [DOI] [PubMed] [Google Scholar]