Key Points
Question
What pathophysiologic mechanisms contribute to the clinical effects of sacubitril-valsartan compared with enalapril in patients with heart failure and reduced ejection fraction (HFrEF)?
Findings
In this randomized clinical trial of 464 participants with HFrEF, there was no significant difference in the change in aortic characteristic impedance (a measure of central aortic stiffness) at 12 weeks among patients treated with sacubitril-valsartan vs enalapril (−2.9 vs −0.7 dyne × s/cm5).
Meaning
Treatment of HFrEF with sacubitril-valsartan, compared with enalapril, did not significantly reduce central aortic stiffness.
Abstract
Importance
Compared with enalapril, sacubitril-valsartan reduces cardiovascular mortality and heart failure hospitalization in patients with heart failure and reduced ejection fraction (HFrEF). These benefits may be related to effects on hemodynamics and cardiac remodeling.
Objective
To determine whether treatment of HFrEF with sacubitril-valsartan improves central aortic stiffness and cardiac remodeling compared with enalapril.
Design, Setting, and Participants
Randomized, double-blind clinical trial of 464 participants with heart failure and ejection fraction of 40% or less enrolled across 85 US sites between August 17, 2016, and June 28, 2018. Follow-up was completed on January 26, 2019.
Interventions
Randomization (1:1) to sacubitril-valsartan (n = 231; target dosage, 97/103 mg twice daily) vs enalapril (n = 233; target dosage, 10 mg twice daily) for 12 weeks.
Main Outcomes and Measures
The primary outcome was change from baseline to week 12 in aortic characteristic impedance (Zc), a measure of central aortic stiffness. Prespecified secondary outcomes included change from baseline to week 12 in N-terminal pro–B-type natriuretic peptide, ejection fraction, global longitudinal strain, mitral annular relaxation velocity, mitral E/e′ ratio, left ventricular end-systolic and end-diastolic volume indexes (LVESVI and LVEDVI), left atrial volume index, and ventricular-vascular coupling ratio.
Results
Of 464 validly randomized participants (mean age, 67.3 [SD, 9.1] years; 23.5% women), 427 completed the study. At 12 weeks, Zc decreased from 223.8 to 218.9 dyne × s/cm5 in the sacubitril-valsartan group and increased from 213.2 to 214.4 dyne × s/cm5 in the enalapril group (treatment difference, −2.2 [95% CI, −17.6 to 13.2] dyne × s/cm5; P = .78). Of 9 prespecified secondary end points, no significant between-group difference in change from baseline was seen in 4, including left ventricular ejection fraction (34%-36% with sacubitril-valsartan vs 33 to 35% with enalapril; treatment difference, 0.6% [95% CI, −0.4% to 1.7%]; P = .24). However, greater reductions from baseline were seen with sacubitril-valsartan than with enalapril in all others, including left atrial volume (from 30.4 mL/m2 to 28.2 mL/m2 vs from 29.8 mL/m2 to 30.5 mL/m2; treatment difference, −2.8 mL/m2 [95% CI, −4.0 to −1.6 mL/m2]; P < .001), LVEDVI (from 75.1 mL/m2 to 70.3 mL/m2 vs from 79.1 mL/m2 to 75.6 mL/m2; treatment difference, −2.0 mL/m2 [95% CI, −3.7 to 0.3 mL/m2]; P = .02), LVESVI (from 50.8 mL/m2 to 46.3 mL/m2 vs from 54.1 to 50.6 mL/m2; treatment difference, −1.6 mL/m2 [95% CI, −3.1 to −0.03 mL/m2]; P = .045), and mitral E/e′ ratio (from 13.8 to 12.3 vs from 13.4 to 13.8; treatment difference, −1.8 [95% CI, −2.8 to −0.8]; P = .001). Rates of adverse events including hypotension (1.7% vs 3.9%) were similar in both groups.
Results
Of 464 validly randomized participants (mean age, 67.3 [SD, 9.1] years; 23.5% women), 427 completed the study. At 12 weeks, Zc decreased with sacubitril-valsartan and increased with enalapril; the between-group difference in change from baseline was not statistically significant. Of 9 prespecified secondary end points, no significant between-group difference in change from baseline was seen in 4, including LVEF. Greater reductions from baseline were seen with sacubitril-valsartan in all others, including left atrial volume index, LVEDVI, LVESVI, and mitral E/e′ ratio. Rates of adverse events including hypotension (1.7% vs 3.9%) were similar in both groups.
| Parameters | Sacubitril-Valsartan, Mean (SD) | Enalapril, Mean (SD) | Between-Group Difference (95% CI) | ||
|---|---|---|---|---|---|
| Baseline | 12 wk | Baseline | 12 wk | ||
| Primary End Point | |||||
| Aortic Zc, dyne × s/cm5 | 223.8 (112.7) | 218.9 (112.7) | 213.2 (102.6) | 214.3 (95.2) | −2.2 (−17.6 to 13.2) |
| Secondary End Points | |||||
| LVEF, % | 34 (10) | 36 (10) | 33 (10) | 35 (10) | 0.6 (−0.4 to 1.7) |
| LVEDVI, mL/m2 | 75.1 (26.1) | 70.3 (23.5) | 79.1 (25.9) | 75.6 (23.7) | −2.0 (−3.7 to −0.3) |
| LVESVI, mL/m2 | 50.8 (22.6) | 46.3 (20.5) | 54.1 (22.6) | 50.6 (20.0) | −1.6 (−3.1 to −0.03) |
| Left atrial volume index, mL/m2 | 30.4 (9.5) | 28.2 (9.0) | 29.8 (8.7) | 30.5 (9.1) | −2.8 (−4.0 to −1.6) |
| Mitral E/e′ ratio | 13.8 (7.6) | 12.3 (5.6) | 13.4 (6.8) | 13.8 (7.4) | −1.8 (−2.8 to −0.8) |
Conclusions and Relevance
Treatment of HFrEF with sacubitril-valsartan, compared with enalapril, did not significantly reduce central aortic stiffness. The study findings may provide insight into mechanisms underlying the effects of sacubitril-valsartan in HFrEF.
Trial Registration
ClinicalTrials.gov Identifier: NCT02874794
This randomized trial compares the effects of sacubitril-valsartan vs enalapril on central aortic stiffness and other physiologic indexes in patients with heart failure and reduced ejection fraction.
Introduction
Among patients with heart failure and reduced ejection fraction (HFrEF) enrolled in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Trial,1 angiotensin receptor–neprilysin inhibition (ARNI) with sacubitril-valsartan reduced the primary composite outcome of cardiovascular death or heart failure hospitalization relative to angiotensin-converting enzyme (ACE) inhibition with enalapril. The benefits of sacubitril-valsartan were apparent early after randomization, consistent across all examined subgroups, and unrelated to differential changes in blood pressure over the course of the trial.1,2 Treatment guidelines have been updated to encourage substitution of ARNI for ACE inhibitors/angiotensin receptor blockers (ARBs) in patients with symptomatic HFrEF.3
The pathophysiologic mechanisms responsible for benefits of ARNI over ACE inhibition remain unclear. Neprilysin inhibition enhances circulating levels of biologically active natriuretic peptides and other vasoactive peptides that may have favorable vasodilatory, antifibrotic, and antihypertrophic effects. In hypertension, neprilysin inhibition reduces central aortic impedance, a key determinant of ventricular load and cardiac performance.4,5 Rapid reductions in N-terminal pro–B-type natriuretic peptide (NT-proBNP) and biomarkers of collagen turnover during treatment of HFrEF with sacubitril-valsartan are consistent with a direct effect of neprilysin inhibition on ventricular wall stress and cardiovascular structure and function.6 This article summarizes the principal results of a randomized, multicenter trial to examine the effect of sacubitril-valsartan compared with enalapril on central aortic stiffness and cardiac remodeling in patients with HFrEF.
Methods
Study Design and Eligibility
EVALUATE-HF was a multicenter randomized trial conducted at 85 hospital and clinic-based study sites in the United States. The study protocol (Supplement 1) was approved by the institutional review board or ethics committee at each site prior to enrollment of the first participant, and all participants provided written informed consent.
Key criteria for study eligibility included age 50 years or older; history of hypertension; chronic heart failure with left ventricular ejection fraction of 40% or less; New York Heart Association class I, II, or III symptoms; and treatment with stable doses of guideline-directed medical therapy other than ACE inhibitors or ARBs with systolic blood pressure greater than 105 mm Hg at both screening and randomization.
Key exclusion criteria included current or prior treatment with sacubitril-valsartan; persistent or permanent atrial fibrillation at screening or randomization; and inability to secure a technically adequate baseline hemodynamic study. Detailed eligibility criteria are summarized in the eAppendix in Supplement 2.
Participants meeting all eligibility criteria during screening were randomized in a 1:1 fashion to double-blind, double-dummy treatment with sacubitril-valsartan (initial dosage, 24/26 mg twice daily titrated to a target dosage of 97/103 mg twice daily) plus enalapril placebo or enalapril (initial dosage, 2.5 mg twice daily, titrated to a target dosage of 10 mg twice daily) plus sacubitril-valsartan placebo using a computerized permuted-block randomization system (block size of 4) with concealed study group assignments. Patients taking an ACE inhibitor prior to study enrollment underwent 36-hour washout prior to randomization. Because recruitment of participants from racial and ethnic minorities was a goal, race was ascertained at the time of randomization by patient self-report according to predefined study categories. At week 12, all participants were transitioned to open-label sacubitril-valsartan after a 36-hour washout of blinded study drug. Longer duration of randomized follow-up was thought to be unethical in light of the established clinical superiority of sacubitril-valsartan. Details of study drug dosing and titration are further elaborated in the eAppendix in Supplement 2.
Noninvasive Hemodynamic Assessment
Hemodynamic data were acquired using arterial applanation tonometry and echocardiography at baseline, week 4, week 12, and week 24, as previously described.7 All data were digitized during the primary acquisition and transferred to the hemodynamic core laboratory (Cardiovascular Engineering Inc, Norwood, Massachusetts) for analysis by personnel blinded to treatment assignment. Aortic characteristic impedance (Zc), a measure of central aortic stiffness and a key determinant of ventricular wall stress,8 was calculated as the ratio of change in carotid pressure (derived from carotid tonometry waveform) and the change in flow in the proximal aorta (derived from Doppler echocardiography of the left ventricular outflow tract) during early systole. Higher values of Zc represent greater stiffness and ventricular load, and the reference value for a 70-year-old man is 250 dyne × s/cm5.7 Additional details regarding acquisition and analysis of hemodynamic data are provided in the eAppendix in Supplement 2.
Echocardiography and Laboratory Assessment
Cardiac structure and function were assessed by 2-dimensional echocardiography during screening, week 4, week 12, and week 24. Parameters prespecified in the statistical analysis plan (Supplement 3) included left ventricular ejection fraction, indexed left ventricular end-systolic and end-diastolic volumes, indexed left atrial volume, lateral early diastolic mitral annular velocity (e′), ratio of mitral peak velocity of early filling (E) to e′, ratio of arterial to end-systolic elastance (Ea/Ees), and global longitudinal strain. Echocardiograms were obtained at study sites according to a standardized protocol and transmitted to the cardiac imaging core laboratory, where they were reviewed in blinded fashion according to American Society of Echocardiography standards (eAppendix in Supplement 2).
Cardiac biomarkers including NT-proBNP, high-sensitivity troponin T, soluble ST2 (a member of the interleukin 1 receptor family expressed by cells in response to myocardial stress), and urinary cyclic guanosine monophosphate/urinary creatinine ratio were analyzed in a central laboratory (eAppendix in Supplement 2) using stored samples collected prior to study drug administration at randomization and at 2, 4, 12, and 24 weeks.
Study End Points
The primary study end point was the between-group difference in change in Zc from baseline to week 12. Prespecified secondary end points included differences between groups in change from baseline to week 12 in NT-proBNP as well as change from baseline to week 12 in left ventricular ejection fraction, global longitudinal strain, left atrial volume index, e′ velocity, mitral E/e′ ratio, left ventricular end-systolic and end-diastolic volume indices, and Ea/Ees ratio. An analysis of the effect of treatment on correlation between change in NT-proBNP and change in Zc at 4 weeks was prespecified but not performed because of a lack of observed change in Zc at that time point. Exploratory end points of interest included difference between groups in change from baseline to week 12 in high-sensitivity troponin T, ST2, and urinary cyclic guanosine monophosphate/urinary creatinine ratio, change from baseline in central and brachial blood pressure and pulse pressure, as well as change from baseline in the overall summary score of the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ), a 0- to 100-point scale in which higher scores represent fewer symptoms and physical limitations due to heart failure.9 Because a change of 5 points or greater on the KCCQ is generally accepted to be clinically meaningful,10 we assessed the proportion of patients achieving this threshold by treatment group in post hoc analyses.
Statistical Analysis
Assuming a clinically important change in Zc of 30 dyne × s/cm5 from baseline to week 12 (based on data from the CHOIR study4), a standard deviation of change in Zc of 80 dyne × s/cm5, a 20% rate of study dropout, a 10% rate of nonevaluable tonometry data, and a 2-sided α = .05, we estimated that a sample size of 432 patients (216 per group) would provide 90% power for the primary end point.
Analyses of the change from baseline in all primary and secondary echocardiographic end points was performed using an analysis-of-covariance model adjusted for baseline values and treatment assignment without imputation for missing values. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as hypothesis-generating. For cardiac biomarkers, proportional change from baseline is expressed as a ratio of geometric means and was analyzed using an analysis-of-covariance model adjusted for baseline values. For the KCCQ, change from baseline in the overall summary score and component scores was analyzed based on a repeated-measures analysis-of-covariance model in which treatment, week, and treatment × week interaction were included as fixed-effect factors and baseline value as a covariate, with a common unstructured covariance for each treatment group. In post hoc analyses, change from baseline in brachial and central blood pressure was analyzed in analysis-of-covariance models adjusted for baseline values. In addition, correlations between changes in echocardiographic parameters, biomarkers, and quality-of-life scores were assessed by linear regression adjusting for the corresponding baseline values of each parameter. All analyses were conducted with Stata version 14.1 (StataCorp), and a 2-sided P<.05 was considered statistically significant.
Results
Study Participants and Follow-up
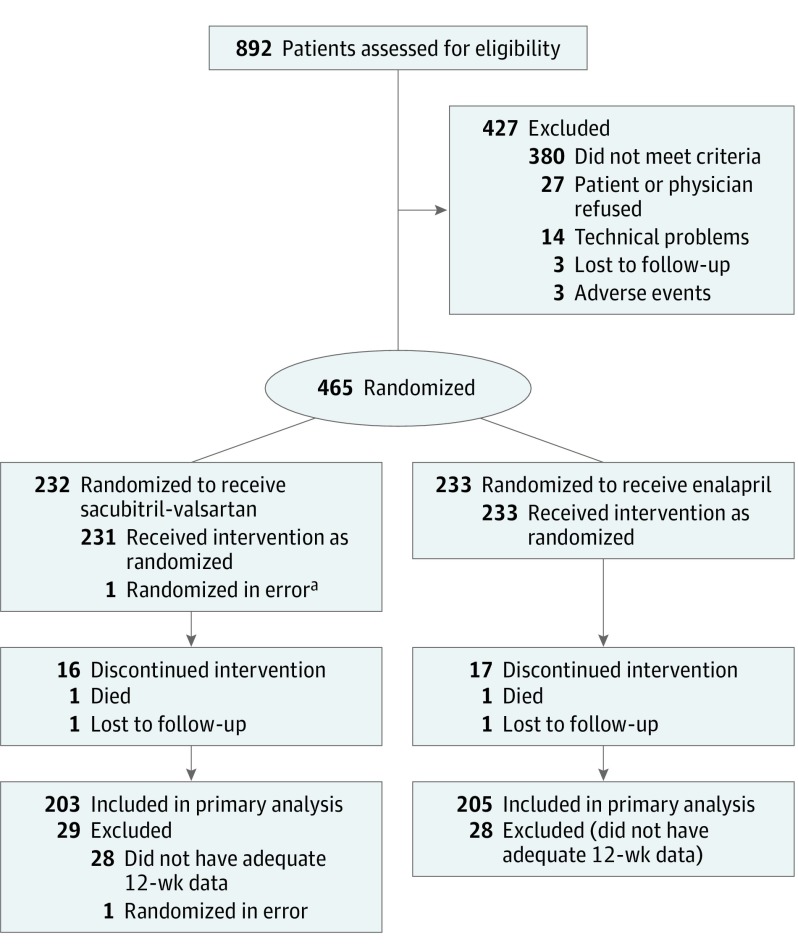
Of 892 participants screened between August 17, 2016, and June 28, 2018, when the recruitment goal was reached, 465 fulfilled criteria for randomization. Excluding 1 participant who was erroneously randomized and did not receive study treatment, 464 participants, including 233 randomly assigned to enalapril and 231 assigned to sacubitril-valsartan, were included in the primary analysis (Figure). Study groups were well matched with regard to key baseline characteristics (Table 1). In the overall study population, the mean age was 67.3 (SD, 9.1) years, 109 (23.5%) were female, 115 (24.8%) were black, 313 (67.4%) reported New York Heart Association class II functional status, and 391 (84.3%) were previously treated with an ACE inhibitor or ARB.
Figure. Participant Flow in the EVALUATE-HF Randomized Clinical Trial.
aA patient whom the site intended to report as a screening failure was inadvertently entered into the computerized randomization system. This patient was excluded from the full analysis set.
Table 1. Baseline Characteristics by Treatment Group.
| Characteristics | Sacubitril-Valsartan (n = 231) | Enalapril (n = 233) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 67.8 (9.8) | 66.7 (8.5) |
| ≥75, No. (%) | 61 (26) | 49 (21) |
| Sex, No. (%) | ||
| Men | 170 (74) | 185 (79) |
| Women | 61 (26) | 48 (21) |
| Race, No. (%) | ||
| White | 166 (72) | 175 (75) |
| Black | 62 (27) | 53 (23) |
| Othera | 3 (1) | 5 (2) |
| Hispanic/Latino, No. (%) | 70 (30) | 82 (35) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 131 (15) | 130 (13) |
| Diastolic | 77 (10) | 78 (10) |
| Heart rate, mean (SD), /min | 68 (11) | 68 (12) |
| Body mass index, mean (SD)b | 30.0 (5.7) | 30.1 (5.8) |
| Current smoking, No. (%) | 50 (22) | 38 (16) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | ||
| Mean (SD) | 70 (22) | 69 (20) |
| <45, No. (%) | 31 (13) | 24 (10) |
| Left ventricular ejection fraction, mean (SD), % | 34 (10) | 33 (10) |
| NT-proBNP, pg/mL | ||
| Median (IQR) | 560 (254-1498) | 595 (244-1438) |
| Geometric mean (95% CI) | 575 (487-680) | 575 (480-687) |
| <450, No. (%) | 94 (41) | 90 (39) |
| Prior heart failure hospitalization, No. (%) | 128 (55) | 115 (49) |
| Ischemic heart disease, No. (%) | 137 (59) | 146 (63) |
| New York Heart Association functional class, No. (%)c | ||
| I | 33 (14) | 28 (12) |
| II | 152 (66) | 161 (69) |
| III | 56 (20) | 44 (19) |
| Medical therapy at randomization, No. (%) | ||
| ACE/ARB | 187 (81) | 204 (88) |
| β-Blocker | 196 (85) | 204 (88) |
| Loop diuretic | 130 (56) | 128 (55) |
| Mineralocorticoid receptor antagonist | 57 (25) | 58 (25) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; IQR, interquartile range; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Includes Asian, Native American, Pacific Islander, specified other, and unknown.
Calculated as weight in kilograms divided by height in meters squared.
A graded scale of functional capacity; class I reflects no limitation with daily activities; class II, mild limitation; and class III, marked limitation, even with less than ordinary activity.
During the double-blind treatment interval, study drug was discontinued in 17 (7.3%) participants assigned to enalapril and 16 (6.9%) assigned to sacubitril-valsartan. Successful titration to the target dosage was achieved in 199 (85.4%) participants assigned to enalapril and 192 (82.8%) assigned to sacubitril-valsartan. One patient in each group died and 1 in each group was lost to follow-up at 12 weeks.
Study Outcomes
From baseline to 12 weeks, the primary end point of Zc decreased from 223.8 to 218.9 dyne × s/cm5 in the sacubitril-valsartan group and increased from 213.2 to 214.4 dyne × s/cm5 in the enalapril group. There was no statistically significant difference between groups in the change from baseline (between-group difference, −2.2 dyne × sec/cm5; 95% CI, −17.6 to 13.2 dyne × sec/cm5; P = .78), despite observed reduction in brachial systolic blood pressure by 6.4 mm Hg in the sacubitril-valsartan group and by 1.6 mm Hg in the enalapril group (between-group difference, −4.8 mm Hg; 95% CI, −7.6 to −2.1 mm Hg; P = .001) and in central systolic blood pressure by 4.9 mm Hg and 2.3 mm Hg, respectively (between-group difference, −2.6 mm Hg; 95% CI, −5.8 to 0.5 mm Hg; P = .10) in post hoc analyses (Table 2).
Table 2. Change in Hemodynamic Parameters From Baseline to 12 Weeks by Treatment Group.
| Parameters | Sacubitril-Valsartan | Enalapril | Between-Group Difference in Change From Baseline (95% CI) | P Value Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Baseline, Mean (SD) | 12 wk, Mean (SD) | Change, Baseline to 12 wk (95% CI) | No. | Baseline, Mean (SD) | 12 wk, Mean (SD) | Change, Baseline to 12 wk (95% CI) | |||
| Primary End Point | ||||||||||
| Zc, dyne × s/cm5 | 203 | 223.8 (112.7) | 218.9 (112.7) | − 2.9 (−13.8 to 8.0) | 205 | 213.2 (102.6) | 214.4 (95.2) | −0.7 (−11.6 to 10.1) | −2.2 (−17.6 to 13.2) | .78 |
| Exploratory End Points | ||||||||||
| Central aortic pressure, mm Hg | ||||||||||
| Systolic | 219 | 130.6 (24.2) | 125.0 (24.1) | −4.9 (−7.2 to −2.7) | 231 | 127.3 (21.3) | 125.6 (20.2) | −2.3 (−4.5 to −0.1) | −2.6 (−5.8 to 0.5) | .10 |
| Pulse pressure | 219 | 58.2 (21.5) | 55.9 (22.1) | −2.0 (−3.9 to −0.2) | 231 | 56.2 (20.0) | 55.3 (17.1) | −1.3 (−3.1 to 0.5) | −0.7 (−3.3 to 1.8) | .57 |
| Brachial blood pressure, mm Hga | ||||||||||
| Systolic | 209 | 132.0 (21.4) | 125.1 (20.1) | −6.4 (−8.4 to −4.5) | 212 | 128.8 (18.3) | 127.7 (19.1) | −1.6 (−3.5 to 0.3) | −4.9 (−7.6 to −2.1) | .001 |
| Diastolic | 209 | 72.3 (11.4) | 68.9 (11.1) | −3.2 (−4.3 to −2.0) | 212 | 70.8 (10.7) | 70.0 (11.3) | −1.1 (−2.2 to 0.1) | −2.1 (−3.8 to −0.4) | .013 |
| Pulse pressure, mm Hga | 209 | 59.7 (19.2) | 56.2 (18.3) | −3.3 (−4.7 to −1.9) | 212 | 58.0 (16.9) | 57.7 (15.9) | − 0.5 (−1.9 to 0.9) | −2.8 (−4.8 to −0.7) | .007 |
Abbreviation: Zc, aortic characteristic impedance.
Parameters not prespecified in statistical analysis plan.
Greater reductions from baseline were seen among participants assigned to sacubitril-valsartan compared with those assigned to enalapril in left ventricular end-diastolic volume index (from 75.1 to 70.3 mL/m2 with sacubitril-valsartan vs from 79.1 to 75.6 mL/m2 with enalapril; between-group difference, −2.0 mL/m2; 95% CI, −3.7 to −0.3 mL/m2; P = .02), left ventricular end-systolic volume index (from 50.8 to 46.3 mL/m2 with sacubitril-valsartan vs from 54.1 to 50.6 mL/m2 with enalapril; between-group difference, −1.6 mL/m2; 95% CI, −3.1 to −0.03 mL/m2; P = .045), left atrial volume index (from 30.4 to 28.2 mL/m2 with sacubitril-valsartan vs from 29.8 to 30.5 mL/m2 with enalapril; between-group difference, −2.8 mL/m2; 95% CI, −4.0 to −1.6 mL/m2; P < .001), and mitral E/e′ ratio (from 13.8 to 12.3 with sacubitril-valsartan vs from 13.4 to 13.8 with enalapril; between-group difference, −1.8; 95% CI, −2.8 to −0.8; P = .001) (Table 3). Although ejection fraction increased modestly by 1.9% in the sacubitril-valsartan group and by 1.3% in the enalapril group, we observed no significant between-group differences in change from baseline to 12 weeks in left ventricular ejection fraction (between-group difference, 0.6%; 95% CI, −0.4% to 1.7%; P = .24) or in other measured parameters, including global longitudinal strain, mitral e′ velocity, or Ea/Ees ratio (Table 3).
Table 3. Change in Echocardiographic Parameters and Quality of Life From Baseline to 12 Weeks by Treatment Group.
| Parameters | Sacubitril-Valsartan | Enalapril | Between-Group Difference in Change From Baseline (95% CI) | P Value Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Baseline, Mean (SD) | 12 wk, Mean (SD) | Change, Baseline to 12 wk (95% CI) | No. | Baseline, Mean (SD) | 12 wk, Mean (SD) | Change, Baseline to 12 wk (95% CI) | |||
| Secondary End Points | ||||||||||
| Left ventricular ejection fraction, %a | 194 | 34 (10) | 36 (10) | 1.9 (1.2 to 2.6) | 200 | 33 (10) | 35 (10) | 1.3 (0.6 to 2.0) | 0.6 (−0.4 to 1.7) | .24 |
| Global longitudinal strain, %b | 195 | −10.4 (3.2) | −10.6 (3.1) | −0.3 (−0.7 to −0.02) | 204 | −9.6 (3.2) | −10.0 (3.4) | −0.2 (−0.5 to 0.1) | −0.13 (−0.58 to 0.33) | .58 |
| LVEDVI, mL/m2c | 194 | 75.1 (26.1) | 70.3 (23.5) | −5.2 (−6.4 to −3.9) | 200 | 79.1 (25.9) | 75.6 (23.7) | −3.2 (−4.4 to −2.0) | −2.0 (−3.7 to −0.3) | .02 |
| LVESVI, mL/m2d | 194 | 50.8 (22.6) | 46.3 (20.5) | −4.9 (−6.0 to −3.8) | 200 | 54.1 (22.6) | 50.6 (20.0) | −3.3 (−4.4 to −2.2) | −1.6 (−3.1 to −0.03) | .045 |
| Left atrial volume index, mL/m2e | 205 | 30.4 (9.5) | 28.2 (9.0) | −2.2 (−3.0 to −1.3) | 208 | 29.8 (8.7) | 30.5 (9.1) | 0.6 (−0.2 to 1.5) | −2.8 (−4.0 to −1.6) | <.001 |
| Mitral e′ velocity, cm/sf | 179 | 5.9 (2.0) | 5.9 (2.0) | −0.0 (−0.2 to 0.2) | 184 | 6.0 (1.9) | 6.0 (1.8) | −0.0 (−0.2 to 0.2) | −0.03 (−0.3 to 0.3) | .86 |
| Mitral E/e′ ratiog | 170 | 13.8 (7.6) | 12.3 (5.6) | −1.4 (−2.1 to −0.7) | 169 | 13.4 (6.8) | 13.8 (7.4) | 0.3 (−0.4 to 1.0) | −1.8 (−2.8 to −0.8) | .001 |
| Ea/Ees ratioh | 147 | 0.84 (0.20) | 0.87 (0.20) | 0.02 (−0.01 to 0.05) | 155 | 0.89 (0.23) | 0.91 (0.27) | 0.03 (−0.001 to 0.06) | −0.005 (−0.05 to 0.04) | .82 |
| Exploratory End Point | ||||||||||
| KCCQ overall summary scorei | 216 | 64.7 (23.1) | 73.8 (21.3) | 8.7 (6.7 to 10.7) | 222 | 67.7 (20.8) | 71.5 (21.0) | 4.2 (2.2 to 6.2) | 4.5 (1.7 to 7.3) | .002 |
Abbreviations: e′, lateral mitral annular relaxation velocity; Ea, arterial elastance; Ees, end-systolic elastance; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index.
Reference range: men, 52%-72%; women, 54%-74%.11 Higher values indicate better ejection fraction.
Values <−20% indicate likely normal.11 More negative values indicate better ventricular function.
Reference range: men, 34-74 mL/m2; women, 29-61 mL/m2.11 Higher values indicate larger volumes.
Reference range: men, 11-31 mL/m2; women, 8-24 mL/m2.11 Higher values indicate higher volumes.
Reference range: 16-34 mL/m2. Higher values indicate larger volumes.
Normal = ≥10 cm/s.12 Higher values indicate better diastolic function.
Normal = ≤13.12 Mitral E/e′ ratio correlates with left ventricular filling pressure; higher values indicate higher filling pressures.
Reference range: 0.6-1.2.13 Lower values indicate improved ventricular-vascular coupling. Methods for calculation of Ea/Ees ratio are described in the eAppendix in Supplement 2.
Score range: 0-100. Higher values reflect better quality of life.
Levels of NT-proBNP, soluble ST2, and high-sensitivity troponin T were reduced to a greater extent at 12 weeks in the sacubitril-valsartan group than in the enalapril group, while the urinary cyclic guanosine monophosphate/urinary creatinine ratio was increased in the sacubitril-valsartan group (Table 4). In post hoc analyses, changes in NT-proBNP were significantly correlated with changes in left ventricular volume (eFigure in Supplement 2).
Table 4. Change in Cardiac Biomarkers From Baseline to 12 Weeks by Treatment Group.
| Parameters | Sacubitril-Valsartan | Enalapril | Ratio of Sacubitril-Valsartan vs Enalapril, Geometric Mean (95% CI) | P Value Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Baseline, Geometric Mean (95% CI) | Week 12, Geometric Mean (95% CI) | Ratio of Geometric Means, 12 wk/Baseline (95% CI) | No. | Baseline, Geometric Mean (95% CI) | Week 12, Geometric Mean (95% CI) | Ratio of Geometric Means, 12 wk/Baseline (95% CI) | |||
| Secondary End Points | ||||||||||
| NT-proBNP, pg/mL | 211 | 574 (482-682) | 363 (307-430) | 0.63 (0.58-0.69) | 215 | 569 (474-683) | 541 (449-651) | 0.95 (0.87-1.0) | 0.67 (0.59-0.76) | <.001 |
| Exploratory End Points | ||||||||||
| Soluble ST2, ng/mLa | 206 | 27.6 (26.0-29.2) | 26.1 (24.7-27.6) | 0.95 (0.92-0.98) | 211 | 25.8 (24.4-27.2) | 26.3 (24.8-27.9) | 1.01 (0.98-1.05) | 0.94 (0.89-0.98) | .006 |
| hs-TnT, ng/L | 204 | 0.017 (0.016-0.019) | 0.015 (0.014-0.017) | 0.87 (0.84-0.91) | 208 | 0.018 (0.017-0.019) | 0.019 (0.018-0.021) | 1.05 (1.0-1.09) | 0.83 (0.78-0.88) | <.001 |
| Urinary cGMP/creatinine, μmol/mol | 205 | 67.0 (62.0-72.4) | 89.4 (83.1-96.2) | 1.34 (1.27-1.41) | 205 | 60.6 (56.0-65.5) | 58.2 (53.9-63.0) | 0.96 (0.91-1.02) | 1.44 (1.33-1.55) | <.001 |
Abbreviations: cGMP, cyclic guanosine monophosphate; hs-TnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
ST2 is a member of the interleukin 1 receptor family and is expressed by cells in response to myocardial stress.
The KCCQ overall summary score improved by 8.9 points in the sacubitril-valsartan group and by 4.3 points in the enalapril group (between-group difference, 4.5 points; 95% CI, 1.7-7.3 points; P = .002). The proportion of patients experiencing improvement of 5 points or more in the KCCQ overall summary score was similarly higher in the sacubitril-valsartan group (58% vs 43%; P = .001). In a post hoc analysis, changes in quality of life were observed to be correlated with improvements in NT-proBNP (eFigure in Supplement 2).
Adverse Events
Rates of hypotension (3.9% with sacubitril-valsartan vs 1.7% with enalapril), hyperkalemia (16.0% vs 12.9%, respectively), and worsening renal function (5.2% vs 6.0%, respectively) were similar in both treatment groups. One adjudicated episode of angioedema was noted in the enalapril group (eTable in Supplement 2).
Discussion
In this randomized study of participants with HFrEF, ARNI therapy with sacubitril-valsartan did not reduce the primary study end point of Zc at 12 weeks relative to ACE inhibition with enalapril. Significant reductions were seen with sacubitril-valsartan in selected secondary echocardiographic end points, including left ventricular end-diastolic and end-systolic volumes, left atrial volume, and mitral E/e′ ratio, suggesting improvement in cardiac remodeling and estimated filling pressures, but no difference was noted in measures of contractile function (left ventricular ejection fraction, global longitudinal strain) or ventricular-vascular coupling (Ea/Ees ratio). The favorable cardiac structural changes paralleled reductions in NT-proBNP and improvements in overall quality of life assessed by the KCCQ overall summary score. These data suggest that clinical benefits of sacubitril-valsartan compared with enalapril in patients with HFrEF are likely unrelated to changes in central aortic stiffness or pulsatile load, despite favorable effects of neprilysin inhibition on myocardial remodeling and wall stress.
Central aortic stiffness is known to be increased in heart failure14 and is a key contributor to pulsatile load and wall stress in the left ventricle.8 Although composite ACE-neprilysin inhibition with omapatrilat has been shown to reduce Zc in patients with hypertension,4 a similar effect was not seen with ARNI in this HFrEF population. One possible explanation is that impedance was lower than anticipated at baseline,15 perhaps due to greater basal activation of the natriuretic peptide system in HFrEF (compared with hypertension), which may have lessened the effect of additional elaboration of vasoactive peptides with neprilysin inhibition. Alternatively, high levels of pretreatment with ACE inhibitors/ARBs (>80%) may have facilitated previous aortic remodeling and reduced the opportunity for additional improvement, even with effective medical therapy. It is also possible that 12 weeks was insufficient time to observe an aortic remodeling effect, and a reduction in Zc might have been apparent over longer-duration follow-up. The lack of reduction in Zc with neprilysin inhibition in this study does not obviate the possibility that other drugs might reduce aortic stiffness and Zc, with favorable effects on the failing heart. Nonetheless, these data suggest that substantial clinical benefits of neprilysin inhibition in HFrEF are likely not mediated through effects on aortic stiffness or pulsatile load.
In secondary analyses, treatment with sacubitril-valsartan was associated with improvements in atrial and ventricular remodeling, lower NT-proBNP, and lower Doppler-derived filling pressures. Although these changes were modest, they occurred earlier than typically observed after initiation of pharmacologic treatment, were observed in a population without advanced cardiac remodeling at baseline, and tracked with changes in cardiac biomarkers and measures of quality of life. Reduction in atrial and ventricular volumes in the absence of an observed effect on load, ejection fraction, or longitudinal strain suggests a possible acute effect on filling pressures, perhaps related to increased venous capacitance or natriuresis. This hypothesis is supported by early and sustained reductions in cardiac biomarkers of wall stress and injury that mirror changes previously observed in PARADIGM-HF6,16 and suggests that reduction in congestion or favorable remodeling effects, rather than changes in after-load or contractile function, may account for the early reductions in heart failure events with sacubitril-valsartan seen in recent trials.1,17
The potential clinical relevance of these early hemodynamic changes is illuminated by exploratory analyses suggesting robust and concordant improvements in quality of life. The observed between-group difference of 4.5 points in change from baseline to 12 weeks in the KCCQ overall summary score is greater than that reported at 8 months in PARADIGM-HF,18 in which quality-of-life questionnaires were administered after a run-in period during which all patients received sacubitril-valsartan and reflects a large proportion of participants with a clinically meaningful (≥5-point) improvement over time. Collectively, these data from a lower-risk heart failure sample provide additional support for current guideline directives to substitute ARNI for ACE inhibitors/ARBs even in the face of apparent clinical stability.
Limitations
This study has several limitations. First, randomized treatment exposure was limited to 12 weeks on ethical grounds because of the established benefits of ARNI over ACE inhibitors/ARBs in patients with HFrEF. It is possible that additional benefits on cardiovascular structure and function may have been apparent over a longer treatment duration, as was recently suggested in a small study of Korean patients with HFrEF and functional mitral regurgitation.19 Second, the study population reflects a mildly symptomatic HFrEF population without persistent atrial fibrillation, and results may not be generalizable to unselected patients with heart failure in clinical practice. Third, because this study was not powered to examine clinical outcomes, the contribution of observed changes in cardiac structure and biomarkers to clinical benefits observed in PARADIGM-HF cannot be directly assessed.
Conclusions
Among patients with heart failure and reduced ejection fraction, treatment with sacubitril-valsartan, compared with enalapril, did not significantly reduce central aortic stiffness. The study findings may provide insight into mechanisms underlying the effects of sacubitril-valsartan in HFrEF.
Trial Protocol
eAppendix. Supplemental Methods
eFigure. Correlation Between Change in NTproBNP and Ventricular Volumes/KCCQ
eTable. Adverse Events, by Treatment
Statistical Analysis Plan
Data Sharing Statement
References
- 1.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 2.Böhm M, Young R, Jhund PS, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. 2017;38(15):1132-1143. doi: 10.1093/eurheartj/ehw570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Izzo JL Jr, Lacourcière Y, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the Conduit Hemodynamics of Omapatrilat International Research Study. Circulation. 2002;105(25):2955-2961. doi: 10.1161/01.CIR.0000020500.77568.3C [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Cockcroft JR, Kario K, et al. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. 2017;69(3):411-420. doi: 10.1161/HYPERTENSIONAHA.116.08556 [DOI] [PubMed] [Google Scholar]
- 6.Zile MR, O’Meara E, Claggett B, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73(7):795-806. doi: 10.1016/j.jacc.2018.11.042 [DOI] [PubMed] [Google Scholar]
- 7.Mitchell GF, Wang N, Palmisano JN, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122(14):1379-1386. doi: 10.1161/CIRCULATIONAHA.109.914507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirinos JA, Segers P, Gillebert TC, et al. ; Asklepios Investigators . Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60(1):64-70. doi: 10.1161/HYPERTENSIONAHA.112.190710 [DOI] [PubMed] [Google Scholar]
- 9.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 10.Spertus J, Peterson E, Conard MW, et al. ; Cardiovascular Outcomes Research Consortium . Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707-715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin. 2011;29(3):447-459. doi: 10.1016/j.ccl.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Tardif JC, Arnold JM, et al. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38(6):1433-1439. doi: 10.1161/hy1201.098298 [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Arnold JM, Dunlap ME, et al. Pulsatile hemodynamic effects of candesartan in patients with chronic heart failure: the CHARM program. Eur J Heart Fail. 2006;8(2):191-197. doi: 10.1016/j.ejheart.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 16.Packer M, McMurray JJ, Desai AS, et al. ; PARADIGM-HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61. doi: 10.1161/CIRCULATIONAHA.114.013748 [DOI] [PubMed] [Google Scholar]
- 17.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators . Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 18.Lewis EF, Claggett BL, McMurray JJV, et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10(8):e003430. doi: 10.1161/CIRCHEARTFAILURE.116.003430 [DOI] [PubMed] [Google Scholar]
- 19.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139(11):1354-1365. doi: 10.1161/CIRCULATIONAHA.118.037077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Supplemental Methods
eFigure. Correlation Between Change in NTproBNP and Ventricular Volumes/KCCQ
eTable. Adverse Events, by Treatment
Statistical Analysis Plan
Data Sharing Statement