Key Points
Question
Are chronotypes (evening vs morning preference) and social jet lag (sleep midpoint difference on weekends vs weekdays) associated with adiposity and cardiometabolic risk in young adolescents?
Findings
This cross-sectional study found that evening chronotypes and greater social jet lag were associated with greater adiposity in adolescent girls, but not boys, independent of sleep duration. There were no associations with a cardiometabolic risk score.
Meaning
This study suggests that female adolescents may be more vulnerable to the obesogenic effects of circadian misalignment; obesity prevention efforts should consider regular sleep-wake patterns in addition to sleep extension and sleep quality improvement.
Abstract
Importance
Inadequate sleep duration and quality increase the risk of obesity. Sleep timing, while less studied, is important in adolescents because increasing evening preferences (chronotypes), early school start times, and irregular sleep schedules may cause circadian misalignment.
Objective
To investigate associations of chronotype and social jet lag with adiposity and cardiometabolic risk in young adolescents.
Design, Setting, and Participants
Starting in 1999, Project Viva recruited pregnant women from eastern Massachusetts. Mother-child in-person visits occurred throughout childhood. From January 23, 2012, to October 16, 2016, 804 adolescents aged 12 to 17 years completed 5 days or more of wrist actigraphy, questionnaires, and anthropometric measurements. A cross-sectional analysis using these data was conducted from April 31, 2018, to May 1, 2019.
Exposures
Chronotype, measured via a continuous scale with higher scores indicating greater evening preferences, and social jet lag, measured as the continuous difference in actigraphy sleep midpoint in hours from midnight on weekends vs weekdays, with higher values representing more delayed sleep timing on weekends.
Main Outcomes and Measures
Adiposity, measured via anthropometry and dual-energy x-ray absorptiometry. For a subset of 479 adolescents with blood samples, cardiometabolic risk scores were computed as the mean of 5 sex- and cohort-specific z scores for waist circumference, systolic blood pressure, inversely scaled high-density lipoprotein cholesterol, and log-transformed triglycerides and homeostatic model of insulin resistance.
Results
Among the 804 adolescents in the study, 418 were girls and 386 were boys, with a mean (SD) age of 13.2 (0.9) years. In multivariable models adjusted for age, puberty, season, and sociodemographics, associations of chronotype and social jet lag with adiposity varied by sex. For girls, greater evening preference was associated with a 0.58-cm (95% CI, 0.12-1.03 cm; P = .04 for interaction) higher waist circumference and 0.16 kg/m2 (95% CI, 0.01-0.31 kg/m2; P = .03 for interaction) higher fat mass index as measured by dual-energy x-ray absorptiometry; each hour of social jet lag was associated with a 1.19-cm (95% CI, 0.04-2.35 cm; P = .21 for interaction) higher waist circumference and 0.45 kg/m2 (95% CI, 0.09-0.82 kg/m2; P = .01 for interaction) higher fat mass index as measured by dual-energy x-ray absorptiometry. Associations of social jet lag and evening chronotypes persisted for many measures of adiposity after adjustment for sleep duration and other lifestyle behaviors. By contrast, no associations were observed in boys. There were no associations with the cardiometabolic risk score for either sex, although statistical power was low for this outcome.
Conclusions and Relevance
Evening chronotypes and social jet lag were associated with greater adiposity in adolescent girls but not adolescent boys. Interventions aimed at improving sleep schedules may be useful for obesity prevention, especially in girls.
This cross-sectional analysis of the Project Viva cohort investigates associations of chronotype and social jet lag with adiposity and cardiometabolic risk in young adolescents.
Introduction
There is increasing evidence that short sleep duration and poor-quality sleep increase adiposity and cardiometabolic risk among children.1,2 Exposures associated with sleep timing are less studied and include chronotypes (underlying diurnal preference for evening vs morning that shapes sleep timing and other behaviors) and social jet lag (discrepancy between biological and social rhythms, measured as the difference in sleep midpoint on work or school days and free days). In adults, both evening chronotypes and greater social jet lag are associated with metabolic abnormalities and adiposity.3,4,5
As children enter adolescence, this mismatch between behaviors and circadian rhythms may be particularly important owing to physiologically mediated shifts toward evening preferences6 coupled with early school start times and social factors, such as athletic activities or after-school jobs, that contribute to irregular sleep schedules. However, relatively few studies have examined associations of chronotypes or social jet lag with adiposity in pediatric populations.7,8,9,10 Furthermore, most of these studies have relied on parents to report children’s sleep behaviors and on body mass index (BMI) as the sole indicator of cardiometabolic risk. There is, therefore, a need to measure these sleep characteristics directly from adolescents by combining subjective questionnaires with objective methods, such as actigraphy,9 and to examine more accurate and comprehensive measures of adiposity and cardiometabolic risk.
To address this gap, we conducted a cross-sectional study of 804 adolescents participating in Project Viva, a longitudinal prebirth cohort study of 2128 children and their mothers. We obtained chronotype and sleep timing data via standardized questionnaires and wrist actigraphy to examine associations with adiposity and blood biomarkers of cardiometabolic risk. We hypothesized that evening preferences and greater social jet lag would be associated with adiposity and cardiometabolic risk independent of sleep duration and nonsleep, obesity-related behaviors (ie, diet quality, physical activity, and television viewing). Given differences observed in adults, such as greater evening preferences expressed by men vs women,11 we hypothesized that these associations would differ by sex.
Methods
Participants
Data collection started in 1999, when Project Viva recruited pregnant women at in-person visits in the first trimester of pregnancy from Atrius Health in eastern Massachusetts. Mother-child in-person visits occurred throughout childhood and early adolescence, and additional information was collected from medical records and annual questionnaires. Details of the study protocol and recruitment and retention procedures are available elsewhere.12 Of 2128 children enrolled, 1038 participated in the adolescent in-person visit (mean [SD] age, 13.2 [0.9] years; range, 11.9-16.6 years). Of these, 804 adolescents provided valid actigraphy, sleep questionnaire, and anthropometric measurements from January 23, 2012, to October 16, 2016 (eFigure in the Supplement). The 804 families included had higher socioeconomic status than those excluded (608 [76%] vs 662 [50%] with household incomes >$70 000 per year at enrollment; 583 [73%] vs 777 [59%] mothers with a college degree or higher) but were similar with respect to children’s race/ethnicity and sex. Among those included, characteristics were similar whether or not adolescents underwent dual-energy x-ray absorptiometry (DXA) scans or provided blood samples. All procedures were approved by the relevant institutional review boards. Mothers provided written informed consent, and children provided verbal assent.
Exposures
Our main exposures were chronotype and social jet lag. Chronotype was defined by questionnaire results, and social jet lag was defined by actigraphy results.
Actigraphy
The adolescents wore a triaxial GT3X+ actigraph (ActiGraph) on their nondominant wrist for 24 hours per day (except when bathing or swimming) for 7 to 10 consecutive days. Bedtimes, naps, and times when the actigraphs were removed were reported by participants in a paper diary. At the end of the wear period, the participants returned their actigraphs in the mail or in person. The data were downloaded using ActiLife software, version 6.7 (or later) (ActiGraph). Activity counts for the GT3X+ were collected in 1-minute time intervals (ie, epochs), and devices were initialized at a sampling rate of 30 Hz. A recent study validated the GT3X+ actigraph for detecting sleep and wakefulness vs polysomnography in adolescents, showing high sensitivity to detect sleep periods.13 Participants had to provide at least 5 days of recordings to be included into our analysis. Actigraphy records were manually annotated by trained scorers blinded to other data at the Brigham and Women’s Sleep Reading Center.
Chronotype
To assess circadian preferences (chronotype, 1 of 2 main exposures), we adapted the previously validated reduced Morningness-Eveningness Scale for Children, which included 5 questions regarding adolescents’ preferences for when to get in bed and out of bed, what time of day they had the most energy, and how easy it was to get up in the morning (exact wording of each question and the descriptive characteristics for the responses are in eTable 1 in the Supplement).14,15,16 The theoretical range of this abbreviated score was 5 to 23, with higher scores indicating greater morning preferences; for comparability with prior literature, we inverted the score for analysis such that higher scores indicated greater evening preference.
Social Jet Lag
From actigraphy and sleep logs, we defined bed and wake times and calculated sleep midpoint (midpoint time between the start and end of the primary sleep period). The main rest interval was identified using information from the self-completed logs and evidence of a sharp decrease or increase in activity. We considered the primary sleep period invalid if the device was removed for 1 hour or more within the in-bed interval. We then classified epochs as sleep or wakefulness within this time using the Cole-Kripke sleep algorithm.13,17,18 We computed social jet lag, 1 of 2 main exposures, as the difference between mean sleep midpoint on weekend days minus weekdays. Higher values indicate a shift toward later sleep timing (ie, bed and wake times) on weekends compared with weekdays.
Outcomes
Body mass index z scores based on age- and sex-specific reference data19 were available for 804 adolescents. As outlined in Table 1 and the eFigure in the Supplement, other outcomes had smaller sample sizes, including DXA fat mass index (n = 603) and the cardiometabolic risk score (n = 479) and its components (mean of 5 sex- and cohort-specific z scores for waist circumference, systolic blood pressure, high-density lipoprotein cholesterol [scaled inversely], and log-transformed triglycerides and homeostatic model assessment of insulin resistance). Detailed methods of assessment for each of these outcomes have been described previously.20
Table 1. Participant Characteristics, Overall and by Sex.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| Overall (N = 804) | Girls (n = 418) | Boys (n = 386) | |
| Enrollment or early childhood visit | |||
| Mother college graduate | 583 (72.5) | 313 (74.9) | 270 (69.9) |
| Child race/ethnicity | |||
| Black or African American | 126 (15.7) | 62 (14.8) | 64 (16.6) |
| Hispanic or Latino | 35 (4.4) | 18 (4.3) | 17 (4.4) |
| Non-Hispanic white | 517 (64.3) | 275 (65.8) | 242 (62.7) |
| Other | 125 (15.6) | 62 (14.8) | 63 (16.3) |
| Adolescent visit | |||
| Household income >$70 000/y | 608 (75.6) | 317 (75.8) | 291 (75.4) |
| Season of early teen visit | |||
| Winter | 164 (20.4) | 82 (19.6) | 82 (21.2) |
| Spring | 217 (27.0) | 109 (26.1) | 108 (28.0) |
| Summer | 268 (33.3) | 141 (33.7) | 127 (32.9) |
| Fall | 155 (19.3) | 86 (20.6) | 69 (17.9) |
| Age, mean (SD), y | 13.2 (0.9) | 13.2 (0.9) | 13.1 (0.9) |
| Child-reported Tanner stage, mean (SD), pointsa | 3.6 (1.1) | 3.8 (1.0) | 3.4 (1.0) |
| Television viewing, mean (SD), h/d | 2.0 (1.4) | 1.9 (1.3) | 2.2 (1.4) |
| Fast food, mean (SD), servings/wk | 0.7 (1.1) | 0.7 (1.1) | 0.7 (1.1) |
| Sugary drinks, mean (SD), servings/d | 0.8 (0.9) | 0.6 (0.8) | 0.9 (1.0) |
| Parent-reported | |||
| Child sleep duration, mean (SD), h/d | 8.8 (0.9) | 8.8 (0.9) | 8.8 (0.9) |
| Child physical activity, mean (SD), h/d | 1.3 (1.0) | 1.1 (0.9) | 1.4 (1.0) |
| Sleep measures, median (IQR) | |||
| Sleep duration | |||
| min/d | 441 (413-468) | 450 (423-475) | 432 (404-457) |
| h/d | 7.3 (6.9-7.8) | 7.5 (7.1-7.9) | 7.2 (6.7-7.6) |
| Social jet lag, hb | 0.9 (0.3-1.5) | 0.8 (0.3-1.5) | 0.9 (0.4-1.6) |
| Reduced Morningness-Eveningness Scale for Childrenc | 15 (14-16) | 15 (14-16) | 15 (14-16) |
| Mean sleep midpoint, time | |||
| On weekdays | 2:56 (2:23-3:50) | 2:57 (2:24-3:32) | 2:56 (2:22-3:37) |
| On weekends | 3:58 (3:19-4:53) | 4:05 (3:24-4:55) | 3:35 (3:16-4:51) |
| Adiposity and cardiometabolic outcomes, mean (SD) | |||
| BMId (n = 804) | 20.9 (4.5) | 21.0 (4.8) | 20.7 (4.2) |
| BMI z score | 0.37 (1.07) | 0.34 (1.08) | 0.41 (1.06) |
| Waist circumference, cm (n = 804) | 73.0 (11.5) | 72.7 (11.3) | 73.2 (11.6) |
| Sum of skinfolds (subscapular and triceps), mm (n = 803) | 28.2 (13.7) | 29.8 (13.4) | 26.5 (13.9) |
| DXA, kg/m2 (n = 603) | |||
| Fat mass index | 6.3 (3.0) | 6.6 (3.0) | 5.9 (3.0) |
| Trunk fat index | 2.4 (1.5) | 2.6 (1.5) | 2.2 (1.4) |
| Cardiometabolic risk score (n = 479)e | −0.01 (0.60) | 0.00 (0.60) | −0.02 (0.59) |
| HDL cholesterol, mg/dL (n = 541) | 55.3 (13.1) | 54.6 (12.6) | 56.0 (13.6) |
| Triglycerides, mg/dL (n = 541) | 70.6 (34.4) | 73.0 (33.4) | 68.3 (35.2) |
| HOMA-IR (n = 483) | 3.2 (2.1) | 3.5 (2.3) | 2.8 (1.8) |
| Systolic blood pressure, mm Hg (n = 799) | 107.3 (8.9) | 105.8 (8.9) | 108.9 (8.7) |
Abbreviations: BMI, body mass index; DXA, dual-energy x-ray absorptiometry; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; IQR, interquartile range.
SI conversion factors: To convert HDL cholesterol to millimoles per liter, multiply by 0.0259; and triglycerides to millimoles per liter, multiply by 0.0113.
Child-reported Tanner stage (5-point pubic hair scale).
Social jet lag is the difference in the mean sleep midpoint on weekend days minus the mean sleep midpoint on weekdays.
Morningness-Eveningness Scale is derived from 5 questions regarding adolescents’ preferences for when to get into bed and out of bed, what time of day they had the most energy, and how easy it was to get up in the morning. In this table, higher scores indicate greater morning preference; in multivariable models, we inverted the scores so that higher scores indicate stronger evening preferences.
Calculated as weight in kilograms divided by height in meters squared.
Cardiometabolic risk score is calculated as the mean of 5 sex- and cohort-specific z scores for HDL cholesterol (inverted), log HOMA-IR, log triglycerides, systolic blood pressure, and waist circumference. Higher scores indicate greater risk.
Covariates
At the enrollment visit, mothers reported their educational attainment. At the adolescent visit, mothers reported their household income, as well as the number of hours their children spent on an average weekday and weekend day in the past month in moderate to vigorous physical activities. Adolescents reported how many hours on an average weekday and weekend day in the past month that they spent watching television, from which we computed mean hours per day of television viewing. In separate questions, adolescents reported the number of days per week in the past month they ate something from a fast food restaurant (eg, Burger King, McDonald’s, Dunkin’ Donuts, Taco Bell, or a pizza place) and/or consumed sugar-sweetened beverages (including soda, flavored milk, and/or sports, fruit, and energy drinks), from which we computed mean servings per week of fast food and servings per day of sugary drinks. We defined season of actigraphy by a 4-level categorical variable according to the meteorological definition of seasons. Adolescents reported their pubertal status using a self-administered, validated scale (5-point rating on pubic hair growth, with higher values indicating greater pubertal development).21
Statistical Analysis
Statistical analysis was conducted from April 31, 2018, to May 1, 2019. We first examined correlations among the sleep variables from actigraphy (duration, bed and wake times, and sleep midpoints) with evening preference and social jet lag. Next, we assessed the normality of the biomarker measurements and log transformed where appropriate. Social jet lag was treated continuously, with results reported per hour. Chronotype cutpoints for the reduced Morningness-Eveningness Scale for Children depend on the population examined22,23; thus, we treated evening preferences continuously in analysis and also defined strong evening and morning preferences based on the extreme quintiles.
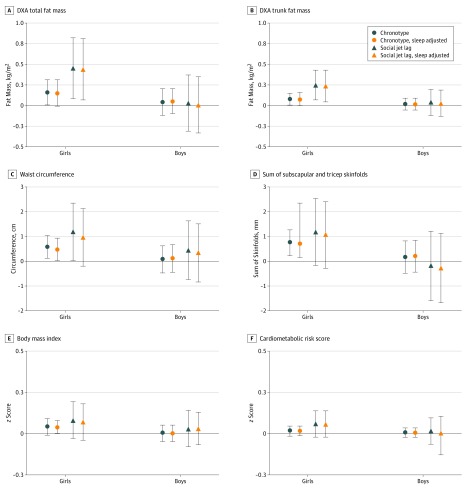
Using multivariable linear regression models, we evaluated the associations of our main exposures (evening preference and social jet lag) with our main outcomes (adiposity measures and the cardiometabolic risk score). All models adjusted for adolescents’ age at the in-person visit, race/ethnicity, pubertal status, maternal educational level, household income, and season of measurement. Prior research suggests that circadian biology varies by sex, with the consequences of inadequate sleep often more pronounced in women24,25; thus, we tested for effect modification in these models using product terms of sex with each of the sleep timing exposures and then stratified analyses by sex. In addition, we considered adjustment for possible mediators in subsequent models: first, actigraphy-measured sleep duration (Figure) and second, adolescent physical activity, indicators of diet quality (eg, sugary drinks and fast food), and television viewing (Table 2).
Figure. Association of Evening Chronotype Score and Social Jet Lag With Adiposity by Sex.
A, Dual energy x-ray absorptiometry (DXA) fat mass index (P = .03 for interaction for chronotype and P = .01 for interaction for social jet lag). B, DXA trunk fat mass index (P = .02 for interaction for chronotype and P = .01 for interaction for social jet lag). C, Waist circumference (P = .04 for interaction for chronotype and P = .21 for interaction for social jet lag). D, Sum of skinfolds and triceps (P = .01 for interaction for chronotype and P = .02 for interaction for social jet lag). E, Body mass index z score (P = .02 for interaction for chronotype and P = .24 for interaction for social jet lag). F, Cardiometabolic risk z score (mean of 5 sex- and cohort-specific z scores for high-density lipoprotein cholesterol [inverted], log homeostatic model assessment of insulin resistance, log triglycerides, systolic blood pressure, and waist circumference; P = .23 for interaction for chronotype and P = .39 for interaction for social jet lag). Interaction P values are based on multivariable models before adding sleep as a covariate. Associations with adiposity measures are reported per additional point of evening preference on the inverted Morningness–Eveningness Scale for Children (higher scores indicate greater evening preferences, shown with circular markers and referred to as chronotype) and per 1 hour of social jet lag (difference in the mean sleep midpoint on weekend days minus the mean sleep midpoint on weekdays, shown with triangular markers). Blue markers indicate adjustment for adolescent age, pubertal status, race/ethnicity, season of measurement, maternal educational level, and household income; orange, “sleep-adjusted” markers indicate additional adjustment for actigraphy-measured sleep duration.
Table 2. Associations of Greater Evening Preferences and Social Jet Lag With Adiposity, Adjusted for Television Viewing, Diet Quality, and Physical Activity.
| Characteristic | Multivariable-Adjusted β (95% CI)a | |
|---|---|---|
| Girls | Boys | |
| Evening preferencesb | ||
| BMI z score | 0.04 (−0.01 to 0.08) | 0.00 (−0.05 to 0.05) |
| DXA, kg/m2 | ||
| Total fat mass index | 0.14 (−0.02 to 0.30) | 0.08 (−0.08 to 0.23) |
| Trunk fat mass index | 0.07 (−0.01 to 0.15) | 0.03 (−0.04 to 0.10) |
| Waist circumference, cm | 0.42 (−0.05 to 0.90) | 0.14 (−0.40 to 0.68) |
| Sum of skinfolds, cm | 0.64 (0.07 to 1.20) | 0.32 (−0.31 to 0.95) |
| Cardiometabolic risk score, z score | 0.01 (−0.02 to 0.05) | 0.00 (−0.04 to 0.03) |
| HDL cholesterol, mg/dL | −0.21 (−0.92 to 0.51) | −0.31 (−1.10 to 0.48) |
| Log HOMA-IR | 0.01 (−0.02 to 0.04) | 0.00 (−0.03 to 0.03) |
| Log triglycerides, mg/dL | 0.02 (0.00 to 0.05) | 0.01 (−0.02 to 0.03) |
| Systolic blood pressure, mm Hg | −0.08 (−0.52 to 0.36) | −0.36 (−0.75 to 0.03) |
| Social jet lagc | ||
| BMI z score | 0.08 (−0.03 to 0.19) | 0.06 (−0.05 to 0.17) |
| DXA, kg/m2 | ||
| Total fat mass index | 0.46 (0.08 to 0.84) | 0.10 (−0.27 to 0.46) |
| Trunk fat mass index | 0.24 (0.05 to 0.44) | 0.07 (−0.10 to 0.23) |
| Waist circumference, cm | 1.08 (−0.09 to 2.26) | 0.71 (−0.52 to 1.94) |
| Sum of skinfolds, cm | 1.29 (−0.08 to 2.67) | 0.24 (−1.20 to 1.68) |
| Cardiometabolic risk score, z score | 0.07 (−0.02 to 0.15) | 0.00 (−0.08 to 0.08) |
| HDL cholesterol, mg/dL | 0.57 (−1.28 to 2.43) | −0.23 (−2.04 to 1.58) |
| Log HOMA-IR | 0.02 (−0.05 to 0.10) | −0.04 (−0.12 to 0.04) |
| Log triglycerides, mg/dL | 0.01 (−0.05 to 0.07) | −0.01 (−0.07 to 0.05) |
| Systolic blood pressure, mm Hg | 0.77 (−0.31 to 1.84) | 0.27 (−0.62 to 1.17) |
Abbreviations: BMI, body mass index; DXA, dual-energy x-ray absorptiometry; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance.
Models adjust for adolescent age, race/ethnicity, pubertal status, season of measurement, parental socioeconomic status, actigraphy-measured sleep duration, parent report of adolescent physical activity, and adolescent report of television viewing and indicators of diet quality (intake of sugary drinks and fast food).
Reduced Morningness-Eveningness Scales for Children is derived from 5 questions regarding adolescents’ preferences for when to get into bed and out of bed, what time of day they had the most energy, and how easy it was to get up in the morning; in multivariable models, the scores were inverted such that higher scores indicate stronger evening preferences.
Social jet lag is the mean sleep midpoint on weekend days minus the mean sleep midpoint on weekdays.
In sensitivity analyses, we considered actigraphy-recorded (instead of parent-reported) physical activity as a covariate. We also categorized age- and sex-specific BMI percentiles19 (normal weight, <85th percentile; overweight, 85th to <95th percentile; and obese, ≥95th percentile) to examine differences in sleep characteristics and associations with overweight and obesity in multinomial logistic regression models.
Results
The mean (SD) age of participants was 13.2 (0.9) years, 418 were girls, and 386 were boys (Table 1). Mean (SD) sleep duration measured by actigraphy was 7.3 (0.7) hours per night. Half of adolescents had both longer sleep duration and later sleep timing on weekends vs weekdays. However, other weekly sleep patterns were also common, with one-fifth of adolescents having later sleep timing but shorter sleep duration on weekends vs weekdays (eTable 2 in the Supplement). Consistent with this finding, the median social jet lag (difference in sleep midpoint on weekends minus weekdays) was 0.9 hours (interquartile range, 0.3-1.5 hours), with 112 adolescents (13.9%) exhibiting a difference of more than 2 hours. Greater evening preferences and social jet lag had modest inverse correlations with sleep duration (evening preferences, Pearson ρ = −0.14; social jet lag, Pearson ρ = −0.12). Adolescents with evening preferences also had later sleep onset and greater social jet lag (eTable 3 in the Supplement).
Many of the associations of evening preference and social jet lag varied by sex, with stronger associations with adiposity observed for girls vs boys (Figure). Greater evening preference as a continuous measure was associated with higher indices on every measure of adiposity among girls; for example, girls had 0.16 kg/m2 (95% CI, 0.01-0.31 kg/m2; P = .03 for interaction) higher DXA fat mass index and 0.58 cm (95% CI, 0.12-1.03 cm; P = .04 for interaction) higher waist circumference for each point on the inverse-coded Morningness-Eveningness Scale for Children. Similarly, with each additional hour of social jet lag, girls had 0.45 kg/m2 (95% CI, 0.09-0.82 kg/m2; P = .01 for interaction) higher DXA fat mass index and 1.19 cm (95% CI, 0.04-2.35 cm; P = .21 for interaction) higher waist circumference. Meanwhile, neither evening preferences nor social jet lag was significantly associated with any measure of adiposity in boys; however, associations were generally in the same direction as observed in girls.
The associations of evening preferences with adiposity were attenuated slightly with adjustment for potential mediating factors: for example, the continuous association of evening preferences with total DXA fat mass index in girls was attenuated from 0.16 kg/m2 (95% CI, 0.01-0.31 kg/m2) to 0.15 kg/m2 (95% CI, 0.00-0.31 kg/m2) with adjustment for sleep duration (Figure) and was further attenuated to 0.14 kg/m2 (95% CI, −0.02 to 0.30 kg/m2) with adjustment for physical activity, television viewing, and diet quality (Table 2). However, social jet lag remained associated with DXA fat mass indices: per 1 hour of social jet lag, girls had a 0.46-kg/m2 (95% CI, 0.08-0.84 kg/m2) total DXA fat mass index and a 0.24-kg/m2 (95% CI, 0.05-0.44 kg/m2) trunk DXA fat mass index after adjustment for these potential mediators (Table 2).
When examining the cardiometabolic risk score and its components in the subset of 479 children who provided fasting blood samples, we found no associations of evening preferences or social jet lag with any of the outcomes examined (Figure and Table 3).
Table 3. Association of Evening Preferences and Social Jet Lag With Cardiometabolic Biomarkersa.
| Characteristic | β (95% CI) | |||
|---|---|---|---|---|
| Girls | Boys | |||
| Multivariable-Adjustedb | Sleep-Adjustedc | Multivariable-Adjustedb | Sleep-Adjustedc | |
| Evening preferencesd | ||||
| HDL cholesterol, mg/dL | −0.15 (−0.81 to 0.50) | −0.12 (−0.80 to 0.57) | −0.33 (−1.10 to 0.44) | −0.33 (−1.09 to 0.43) |
| Log HOMA-IR | 0.01 (−0.01 to 0.04) | 0.01 (−0.02 to 0.04) | 0.01 (−0.03 to 0.04) | 0.01 (−0.03 to 0.04) |
| Log triglycerides, mg/dL | 0.02 (0.00 to 0.04) | 0.02 (0.00 to 0.04) | 0.01 (−0.01 to 0.04) | 0.01 (−0.01 to 0.04) |
| Systolic blood pressure, mm Hg | 0.00 (−0.40 to 0.41) | −0.08 (−0.50 to 0.33) | −0.34 (−0.72 to 0.05) | −0.32 (−0.71 to 0.06) |
| Social jet lage | ||||
| HDL cholesterol, mg/dL | 0.67 (−1.05 to 2.39) | 0.74 (−1.00 to 2.48) | −0.40 (−2.03 to 1.23) | −0.31 (−1.92 to 1.30) |
| Log HOMA-IR | 0.03 (−0.04 to 0.11) | 0.03 (−0.04 to 0.10) | −0.01 (−0.09 to 0.06) | −0.02 (−0.09 to 0.05) |
| Log triglycerides, mg/dL | −0.01 (−0.06 to 0.05) | −0.01 (−0.06 to 0.05) | 0.00 (−0.05 to 0.05) | 0.00 (−0.05 to 0.05) |
| Systolic blood pressure, mm Hg | 0.74 (−0.28 to 1.76) | 0.59 (−0.44 to 1.61) | −0.03 (−0.86 to 0.79) | −0.07 (−0.89 to 0.76) |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance.
Cardiometabolic biomarkers are the components of the cardiometabolic risk score, which consists of the mean of 5 sex- and cohort-specific z scores for HDL cholesterol (inverted), log HOMA-IR, log triglycerides, systolic blood pressure, and waist circumference (shown in the Figure and Table 2).
Multivariable-adjusted models include adolescent age, race/ethnicity, pubertal status, season of measurement, maternal educational level, and household income.
Sleep-adjusted models additionally include actigraphy-measured sleep duration.
Reduced Morningness-Eveningness Scales for Children is derived from 5 questions regarding adolescents’ preferences for when to get into bed and out of bed, what time of day they had the most energy, and how easy it was to get up in the morning; in multivariable models, the scores were inverted such that higher scores indicate stronger evening preferences.
Social jet lag is the difference in mean sleep midpoint on weekend days minus the mean sleep midpoint on weekdays.
In sensitivity analyses, adjustment for actigraphy-recorded (vs parent-reported) physical activity yielded similar results; that is, per 1 hour of social jet lag, girls had 0.47 kg/m2 (95% CI, 0.09-0.84 kg/m2) vs 0.46 kg/m2 (95% CI, 0.08-0.84 kg/m2) greater DXA fat mass index. When examining BMI categories, adolescents with obesity or overweight (vs normal weight) had greater social jet lag and shorter sleep duration and were more likely to have evening preferences (eTable 4 in the Supplement). Greater social jet lag was associated with increased odds of obesity (odds ratio, 1.62; 95% CI, 1.02-2.56 per 1 hour of social jet lag) independent of sociodemographic and lifestyle covariates, including sleep duration. Associations with evening preferences were nonsignificant but in the expected direction (odds ratio, 1.13; 95% CI, 0.95-1.34) (eTable 4 in the Supplement).
Discussion
Among 804 adolescents, we found that evening chronotypes and greater social jet lag were associated with adiposity among girls independent of sociodemographic factors. Chronotype associations were slightly attenuated after adjustment for sleep duration, whereas many of the associations with social jet lag persisted regardless of adjustment for sleep duration and other potential mediating factors including diet quality, physical activity, and television viewing. We observed weaker, nonsignificant associations between chronotypes and social jet lag and adiposity among boys. Among a subset of 479 children with blood biomarkers, we found no associations of chronotype or social jet lag with a cardiometabolic risk score or its components. This is one of the largest adolescent samples to date. Our results add to the evidence that, in addition to sleep duration and quality, sleep timing preferences and behaviors should be considered risk factors for adiposity. Furthermore, the adverse effects of circadian misalignment exhibit sex differences such that impairment is greater in girls.
Although social jet lag is often modeled as an absolute value, we chose to preserve the directionality of the misalignment between social and biological times by using the raw continuous value. Prior studies of evening chronotypes and social jet lag with adiposity in children are few and have shown variable results.7,8,9,26 For example, social jet lag was positively associated with DXA fat mass index among 341 preadolescent children in New Zealand26 but inversely associated with BMI among 83 Dutch adolescents aged 16 years.27 Similar to our study, a cross-sectional analysis of 511 UK children aged 11 to 13 years found that evening vs morning chronotypes were positively associated with BMI z score7; meanwhile, a study of 69 US adolescents found no association of evening chronotypes with BMI z scores despite detecting positive associations with social jet lag.9
The mechanisms through which these sleep timing exposures influence adiposity are not fully understood. Adults and adolescents with evening vs morning preferences tend to have shorter sleep duration, disturbed and irregular sleep,28,29 and less healthy lifestyles, including less physical activity, poor dietary habits, and greater electronic media use.7,9,29,30 Consistent with this finding, adjustment for sleep duration as well as physical activity, diet quality, and television viewing attenuated most associations in our study. However, the association of social jet lag with higher DXA fat mass in girls persisted even after adjustment for these factors.
Associations of chronotype and social jet lag with adiposity varied by sex, with stronger associations observed among girls than boys. The factors driving sex differences in the association of sleep exposures with adiposity are not understood but may include both sociocultural and biological influences. Molecular oscillations of the central circadian clock (ie, the suprachiasmatic nucleus of the hypothalamus) and peripheral clocks (in adipose, liver, muscle, and other tissues) influence the expression of a large number of genes associated with metabolic function.31,32 Steroid hormones modulate both circadian rhythms as well as genes involved in metabolism.24,25,33 Consistent with this finding, sex differences have been reported in associations between sleep duration and/or circadian misalignment and the incidence of adverse outcomes, such as cardiovascular disease and depressive disorders in adults.11,24,25,30 In addition, social and environmental factors may modulate susceptibility to the association of circadian and sleep-related stressors with obesity. Obesity-related behaviors, such as food intake and meal timing, exercise, stress, and mood, may differ by sex and augment or mitigate the influence of circadian misalignment. For example, adolescent girls with short sleep duration were reported to be more likely to eat high-fat diets compared with their male peers, which was postulated to reflect greater emotional eating.34 Future studies are needed to provide insight into the hormonal, social, and environmental factors contributing to sex differences in sensitivity to the effects of circadian misalignment. Such research can inform behavioral interventions that mitigate the associations of evening chronotypes and social jet lag with adolescent health and prevent adult diseases.
To our knowledge, this is the first study in a pediatric population to examine the association of chronotype or social jet lag with components of the metabolic syndrome. In adults, accumulating evidence indicates that evening chronotypes and social jet lag are associated with metabolic disorders and excess adiposity.30,35,36 We report associations of evening chronotype and social jet lag with adiposity in girls but no associations with cardiometabolic risk in either sex. We may have been underpowered to detect these associations given that blood biomarkers were available only in 479 of the adolescents (59.6%). Our findings warrant confirmation in larger longitudinal studies.
Strengths and Limitations
To our knowledge, this is the largest study to examine the association of chronotype and social jet lag with accurate measures of adiposity (eg, DXA fat mass index) and the first to examine associations of chronotype and social jet lag with biomarkers of cardiometabolic risk. Although this cross-sectional study cannot determine the temporality or causality of associations (adiposity and biomarker outcomes could reflect a prior or simultaneous interval to the sleep exposures), these results are consistent with a sexually dimorphic association of sleep timing with adiposity. Other strengths include measuring social jet lag via actigraphy rather than subjective report and controlling for potential confounding by sequentially adjusting for actigraphy-measured sleep duration and for nonsleep, obesity-related behaviors including diet quality, physical activity, and television viewing; few prior studies have had such rich covariate data.
Conclusions
This study suggests that sleep preferences and timing, in addition to sleep duration and quality, are associated with adiposity in adolescents. This finding is particularly important for adolescents, who have increasing evening preferences and may have difficulty falling asleep early on weekdays when they still need to rise early in the morning for school.6 Regular sleep-wake patterns and earlier bed-wake times may extend sleep duration, reduce social jet lag, and benefit adolescents’ cardiometabolic health. Families can support adolescents by encouraging consistency in sleep schedules and improving sleep hygiene (eg, limiting electronic media and caffeine use in the evening and establishing earlier and more consistent bed and wake times). Chrono-interventions, such as bright-light therapy and scheduled meals, may also be appropriate for adolescents with severe social jet lag. From a policy perspective, delaying school start times is a strategy to increase weeknight sleep duration that may also improve the regularity of sleep-wake patterns,37 particularly for students with evening preferences. In addition, the sexually dimorphic associations observed in this and other studies suggest the need to further understand moderating factors, such as sex hormones, that drive these differences in young adolescents.
eTable 1. Individual Questionnaire Items on the Adapted Morningness-Eveningness Scale: Descriptive Statistics by Sex
eTable 2. Weekly Sleep Patterns, Chronotype, and Social Jetlag
eTable 3. Sleep Characteristics by Chronotype and Sex
eTable 4. Associations of Sleep Characteristics with Overweight and Obesity by Sex
eFigure. Participants in Project Viva Analyses of Social Jetlag, Chronotype, and Cardiometabolic Health
References
- 1.Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Med. 2016;18:36-49. doi: 10.1016/j.sleep.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quist JS, Sjödin A, Chaput J-P, Hjorth MF. Sleep and cardiometabolic risk in children and adolescents. Sleep Med Rev. 2016;29:76-100. doi: 10.1016/j.smrv.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 3.Koopman ADM, Rauh SP, van ’t Riet E, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the New Hoorn Study. J Biol Rhythms. 2017;32(4):359-368. doi: 10.1177/0748730417713572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100(12):4612-4620. doi: 10.1210/jc.2015-2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939-943. doi: 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 6.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871-881. doi: 10.1093/sleep/21.8.871 [DOI] [PubMed] [Google Scholar]
- 7.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes (Lond). 2015;39(1):39-44. doi: 10.1038/ijo.2014.157 [DOI] [PubMed] [Google Scholar]
- 8.Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol Int. 2013;30(5):682-690. doi: 10.3109/07420528.2013.782311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malone SK, Zemel B, Compher C, et al. Social jet lag, chronotype and body mass index in 14-17-year-old adolescents. Chronobiol Int. 2016;33(9):1255-1266. doi: 10.1080/07420528.2016.1196697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AL, Lumeng JC, LeBourgeois MK. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):41-47. doi: 10.1097/MED.0000000000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randler C. Gender differences in morningness–eveningness assessed by self-report questionnaires: a meta-analysis. Pers Individ Dif. 2007;43(7):1667-1675. doi: 10.1016/j.paid.2007.05.004 [DOI] [Google Scholar]
- 12.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37-48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quante M, Kaplan ER, Cailler M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13-20. doi: 10.2147/NSS.S151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adan A, Almirall H. Horne & Östberg Morningness–Eveningness Questionnaire: a reduced scale. Pers Individ Dif. 1991;12(3):241-253. doi: 10.1016/0191-8869(91)90110-W [DOI] [Google Scholar]
- 15.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97-110. [PubMed] [Google Scholar]
- 16.Randler C. German version of the reduced Morningness–Eveningness Questionnaire (rMEQ). Biol Rhythm Res. 2013;44(5):730-736. doi: 10.1080/09291016.2012.739930 [DOI] [Google Scholar]
- 17.Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35(1):159-166. doi: 10.5665/sleep.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res. 2010;19(4):612-619. doi: 10.1111/j.1365-2869.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 20.Cespedes Feliciano EM, Quante M, Rifas-Shiman SL, Redline S, Oken E, Taveras EM. Objective sleep characteristics and cardiometabolic health in young adolescents. Pediatrics. 2018;142(1):e20174085. doi: 10.1542/peds.2017-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271-280. doi: 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- 22.Caci H, Deschaux O, Adan A, Natale V. Comparing three morningness scales: age and gender effects, structure and cut-off criteria. Sleep Med. 2009;10(2):240-245. doi: 10.1016/j.sleep.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 23.Levandovski R, Sasso E, Hidalgo MP. Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatry Psychother. 2013;35(1):3-11. doi: 10.1590/S2237-60892013000100002 [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Silver R. Neuroendocrine underpinnings of sex differences in circadian timing systems. J Steroid Biochem Mol Biol. 2016;160:118-126. doi: 10.1016/j.jsbmb.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111-139. doi: 10.1016/j.yfrne.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoner L, Castro N, Signal L, et al. Sleep and adiposity in preadolescent children: the importance of social jetlag. Child Obes. 2018;14(3):158-164. doi: 10.1089/chi.2017.0272 [DOI] [PubMed] [Google Scholar]
- 27.de Zwart BJ, Beulens JWJ, Elders P, Rutters F. Pilot data on the association between social jetlag and obesity-related characteristics in Dutch adolescents over one year. Sleep Med. 2018;47:32-35. doi: 10.1016/j.sleep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 28.Bruni O, Sette S, Fontanesi L, Baiocco R, Laghi F, Baumgartner E. Technology use and sleep quality in preadolescence and adolescence. J Clin Sleep Med. 2015;11(12):1433-1441. doi: 10.5664/jcsm.5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11(3):191-199. doi: 10.1046/j.1365-2869.2002.00302.x [DOI] [PubMed] [Google Scholar]
- 30.Fabbian F, Zucchi B, De Giorgi A, et al. Chronotype, gender and general health. Chronobiol Int. 2016;33(7):863-882. doi: 10.1080/07420528.2016.1176927 [DOI] [PubMed] [Google Scholar]
- 31.Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008-1015. doi: 10.1126/science.aah4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang AH, Barclay JL, Oster H. Interactions between endocrine and circadian systems. J Mol Endocrinol. 2013;52(1):R1-R16. doi: 10.1530/JME-13-0118 [DOI] [PubMed] [Google Scholar]
- 33.Duffy JF, Cain SW, Chang A-M, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(suppl 3):15602-15608. doi: 10.1073/pnas.1010666108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33(9):1201-1209. doi: 10.1093/sleep/33.9.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutters F, Lemmens SG, Adam TC, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377-383. doi: 10.1177/0748730414550199 [DOI] [PubMed] [Google Scholar]
- 36.Yu JH, Yun CH, Ahn JH, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100(4):1494-1502. doi: 10.1210/jc.2014-3754 [DOI] [PubMed] [Google Scholar]
- 37.Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115(6):1555-1561. doi: 10.1542/peds.2004-1649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Individual Questionnaire Items on the Adapted Morningness-Eveningness Scale: Descriptive Statistics by Sex
eTable 2. Weekly Sleep Patterns, Chronotype, and Social Jetlag
eTable 3. Sleep Characteristics by Chronotype and Sex
eTable 4. Associations of Sleep Characteristics with Overweight and Obesity by Sex
eFigure. Participants in Project Viva Analyses of Social Jetlag, Chronotype, and Cardiometabolic Health