Key Points
Question
Does providing a simultaneous integrated boost to 63 Gy of radiotherapy for locally advanced esophageal cancer improve treatment efficacy without increasing toxic effects?
Findings
This single-arm phase 1/2 trial of 46 patients treated with chemoradiotherapy and given a simultaneous integrated boost of radiotherapy dose for unresectable locally advanced esophageal cancer showed promising local control rates, higher than rates for 97 similar patients who received a standard 50.4-Gy dose of radiotherapy without a simultaneous integrated boost. Toxic effects were mild, with no grade 4 to 5 adverse events.
Meaning
Use of a simultaneous integrated boost to 63 Gy of radiotherapy for locally advanced esophageal cancer merits further evaluation.
Abstract
Importance
Effective treatment options for locally advanced esophageal cancer are limited, and rates of local recurrence after standard chemoradiotherapy remain high.
Objective
To evaluate toxic effects, local control, and overall survival rates after chemoradiotherapy with a simultaneous integrated boost of radiotherapy dose to the gross tumor and nodal disease for patients with unresectable locally advanced esophageal cancer.
Design, Setting, and Participants
A phase 1/2, single-arm trial was conducted in 46 patients from April 28, 2010, to April 9, 2015 (median follow-up, 52 months [range, 2-86 months]), at a tertiary academic cancer center. Outcomes of the study patients were compared with those of 97 similar patients treated at the same institution from January 10, 2010, to December 5, 2014, as part of the interim analysis. Statistical analysis was performed from December 15, 2018, to February 12, 2019.
Interventions
Chemoradiotherapy with a simultaneous integrated boost of radiotherapy dose (50.4 Gy to subclinical areas at risk and 63.0 Gy to the gross tumor and involved nodes, all given in 28 fractions) with concurrent docetaxel and capecitabine or fluorouracil.
Main Outcomes and Measures
Toxic effects, local (in-field) control, and overall survival rates.
Results
All 46 patients (11 women and 35 men; median age, 65.5 years [range, 37.3-84.4 years]) received per-protocol therapy, as intensity-modulated photon therapy (39 [85%]) or intensity-modulated proton therapy (7 [15%]); 11 patients (24%) ultimately underwent resection. No patients experienced grade 4 or 5 toxic effects; the 10 acute grade 3 toxic events were esophagitis (4), dysphagia (3), and anorexia (3) and the 3 late grade 3 toxic events were all esophageal strictures. The actuarial local recurrence rates were 22% (95% CI, 11%-35%) at 6 months, 30% (95% CI, 18%-44%) at 1 year, and 33% (95% CI, 20%-46%) at 2 years. Overall, 15 patients (33%) experienced local failure, at a median interval of 5 months (range, 1-24 months). The median overall survival time was 21.5 months (range, 2.3-86.4 months). Exploratory comparison with a 97-patient contemporaneous institutional cohort receiving standard-dose (non–simultaneous integrated boost) chemoradiotherapy showed superior local control (hazard ratio, 0.49; 95% CI, 0.26-0.92; P = .03) and overall survival (hazard ratio, 0.66; 95% CI, 0.47-0.94; P = .02) in the group that received chemoradiotherapy with a simultaneous integrated boost.
Conclusions and Relevance
These findings suggest that chemoradiotherapy with a simultaneous integrated boost of radiotherapy dose for locally advanced esophageal cancer is well tolerated, with encouraging local control, and thus warrants further study.
Trial Registration
ClinicalTrials.gov identifier: NCT01102088
This phase 1/2 single-arm trial examined toxic effects, local control, and overall survival rates after chemoradiotherapy with a simultaneous integrated boost of radiotherapy dose to the gross tumor and nodal disease for patients with unresectable locally advanced esophageal cancer.
Introduction
Locally advanced esophageal cancer is typically treated with either trimodality therapy or definitive chemoradiotherapy.1,2,3 Local-regional failure rates are at least 50%, especially after definitive treatment.1 Attempts to improve this high failure rate have included escalating the radiotherapy (RT) dose, which was tested in the Intergroup (INT) 0123 trial, which compared standard-dose (50.4 Gy) vs high-dose (64.8 Gy) RT delivered with concurrent chemotherapy.4 The results showed no difference in local-regional control or overall survival (OS), findings that have since been corroborated by large retrospective studies of patients treated after that trial.5
The lack of a dose-response effect in the INT 0123 trial renders its conclusions controversial because randomized trials of other types of cancer indicate that RT dose escalation can directly improve tumor control or survival.6,7,8 Large numbers of premature deaths in the high-dose group of INT 0123 may have partially obscured differences between groups, but most of those deaths occurred at or before the delivery of 50.4 Gy of RT. Whether these findings would be replicated in the contemporary era, however, is questionable. Numerous retrospective studies of more modern RT techniques have shown benefits from RT dose escalation,9,10,11,12,13,14,15,16,17 but, to date, no randomized trials directly testing these techniques have been performed, to our knowledge. Moreover, INT 0123 involved the use of 2-dimensional RT, which is now considered antiquated; the current standard is 3-dimensional conformal RT, which offers a much-improved safety profile vs 2-dimensional RT. The newest RT technologies, namely intensity-modulated RT and proton beam therapy, may be even safer ways of escalating RT doses in esophageal cancer.18,19
Intensity-modulated RT and intensity-modulated proton therapy offer the unique capability of dose escalation by means of a simultaneous integrated boost (SIB), also known as “dose-painting.” Simultaneous integrated boost of RT (SIB-RT) involves the delivery of standard-fraction doses of RT to areas considered to be at low or intermediate risk of disease; delivery of higher doses of RT per fraction to the gross tumor constitutes the “boost.” Unlike sequential boosts, in simultaneous boosting the total number of RT fractions is kept constant. We prospectively evaluated the safety (toxic effects) and efficacy (outcomes) of this approach in a single-arm phase 1/2 trial (NCT01102088); a planned interim analysis of 38 patients with more than 6 months of follow-up was reported previously.20 The updated results presented here are findings from all patients enrolled in this trial.
Methods
Patients
Complete details of this trial, conducted from April 28, 2010, to April 9, 2015, are presented elsewhere.20 Primary inclusion criteria were pathologically confirmed squamous cell carcinoma (SCC) or adenocarcinoma of esophageal origin, diagnosed by means of contrast-enhanced positron emission tomography–computed tomography (PET-CT), and gastroenterologic procedures (ultrasonography, esophagogastroduodenoscopy, or barium esophagram). Rigorous multidisciplinary evaluation was used to identify patients with inoperable disease or at high surgical risk at presentation (although eventual receipt of surgery after chemoradiotherapy was not grounds for exclusion). All disease stages (including stage IV cases with up to 3 distant metastatic sites) were allowed, provided that the multidisciplinary team recommended definitive chemoradiotherapy as the primary management option. Patients with tumors 10 cm or longer were excluded, as were those with disease with more than 2 cm of gastric invasion. Patients with poor performance status (Karnofsky score <60) also were not eligible. This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All patients provided written informed consent.
Study Procedures
Chemotherapy (5 cycles of 200-mg/m2 docetaxel with 300-mg/m2 fluorouracil or capecitabine) was administered concurrently with RT to all patients. Induction chemotherapy was considered prior to concurrent chemoradiotherapy on a case-by-case basis after multidisciplinary evaluation; induction chemotherapy was delivered for all patients with stage IV cancer as well as those with T3-4 N+ nonmetastatic cancer (mean, 2 cycles [range, 1-4 cycles] of docetaxel, oxaliplatin, and fluorouracil [n = 13] or fluorouracil and oxaliplatin [n = 4]). After chemoradiotherapy, patients were reevaluated, both clinically and with diagnostic CT scan, to determine the potential for resection. If esophagectomy was considered appropriate at that time, the techniques and timing of the procedure were chosen at the surgeon’s discretion.
Radiotherapy targets were delineated based on information from the contrast-enhanced simulation CT scan results (3- or 4-dimensional depending on tumor location), PET and CT scan results, and results of gastroenterology procedures. The internal gross tumor volume encompassed both the primary tumor and PET-avid lymph nodes. The clinical target volume was defined as the primary tumor gross tumor volume with a 3-cm craniocaudal margin and 1-cm radial margin, plus an isotropic 0.5-cm expansion from any PET-avid nodes. Addition of a uniform 0.5-cm margin to the clinical target volume resulted in construction of the planning target volume.
All patients received SIB-RT delivered with intensity-modulated RT or intensity-modulated proton therapy. The planning target volume was to receive 50.4 Gy in 28 daily fractions. The area to receive the SIB was defined as the internal gross tumor volume with an isotropic 3-mm expansion; this volume was to receive 58.8 Gy (to evaluate toxic effects in the first 3 patients) or 63.0 Gy (to evaluate the rest of the patients, if no grade 4-5 toxic events occurred with 58.8 Gy), all delivered in 28 daily fractions. All patients underwent daily image guidance prior to receiving each RT fraction, and did not consume anything orally for 3 hours prior to RT to avoid changes in anatomy caused by gastric distension.
Patients were evaluated weekly during chemoradiotherapy and at 1 month afterward; subsequent follow-up was every 3 months for the next 2 years, and every 6 months thereafter. Follow-up visits consisted of interval history and physical examination for purposes of assessment of toxic effects (per the Common Terminology Criteria for Adverse Events, version 4.0321); laboratory tests and imaging studies were performed if clinically indicated.
End Points and Statistical Analysis
The primary end point of this phase 1/2 study was to characterize the toxic effect profile of concurrent chemotherapy and SIB-RT; enrolling at least 42 patients would also provide 90% power to detect an improvement in 1-year tumor control from 70% to 85% (1-sided α < .10). Secondary end points included local recurrence rate and OS. Local recurrence (in-field relapse) was based on results of follow-up CT imaging, endoscopic ultrasonography-guided biopsy, or residual disease in the surgical specimen (if applicable). Overall survival was defined from the time of enrollment to death from any cause, or censored at last contact.
Statistical analysis was performed from December 15, 2018, to February 12, 2019. Statistics were analyzed with Stata/MP, version 14 (StataCorp) and R Studio, version 1.1 (R Foundation for Statistical Computing). All statistical tests considered P < .05 to reflect statistical significance. The reverse Kaplan-Meier method was used to calculate follow-up.22 Cumulative local control incidence estimates were computed using the Aalen-Johansen method; hazard ratios (HRs) were estimated using Fine-Gray proportional hazards competing risks regression analysis. Overall survival was analyzed by the Kaplan-Meier method, with estimates compared with log-rank tests. Also, because this nonrandomized trial did not enroll patients who received standard-dose (non-SIB) chemoradiotherapy, we compared outcomes of our study patients with those of 97 similar patients treated at the same institution from January 10, 2010, to December 5, 2014, as part of the interim analysis.20
Results
Patient Characteristics
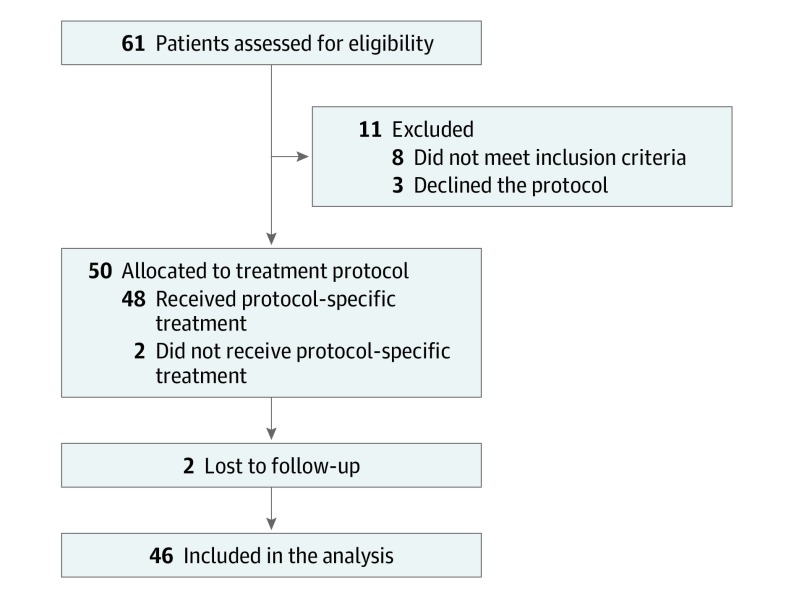
From April 28, 2010, through April 9, 2015, 61 patients were screened for the trial and 46 were ultimately enrolled (Figure 1 and Table 1). Ten patients (22%) had N2 disease and 8 (17%) had M1 disease. Thirty-nine patients (85%) received intensity-modulated RT and 7 patients (15%) received intensity-modulated proton therapy. Eight patients (17%) had disease that was classified as metastatic; all had nonregional nodal disease but no visceral organ metastasis. All patients completed per-protocol treatment, and the median follow-up for all patients was 52 months (range, 2-86 months).
Figure 1. CONSORT Diagram of the Trial.
Table 1. Patient Characteristics.
| Characteristics | Value (n = 46)a |
|---|---|
| Age, y | |
| ≤50 | 4 (9) |
| >50 to ≤70 | 22 (48) |
| >70 | 20 (44) |
| Median, y (range) | 65.5 (37.3-84.4) |
| Sex | |
| Male | 35 (76) |
| Female | 11 (24) |
| Karnofsky Performance Status score | |
| ≥80 | 40 (87) |
| ≥60 to <80 | 6 (13) |
| AJCC disease stage | |
| I | 4 (9) |
| II | 6 (13) |
| III | 28 (61) |
| IV | 8 (17) |
| T category | |
| T1 | 0 |
| T2 | 5 (11) |
| T3 | 39 (85) |
| T4 | 1 (2) |
| Tx | 1 (2) |
| N category | |
| N0 | 14 (30) |
| N1 | 18 (39) |
| N2 | 10 (22) |
| N3 | 3 (7) |
| Nx | 1 (2) |
| M category | |
| M0 | 38 (83) |
| M1 | 8 (17) |
| Tumor histologic characteristics | |
| Adenocarcinoma | 22 (48) |
| Squamous cell | 24 (52) |
| Tumor grade | |
| Moderate | 24 (52) |
| Moderate-poor to poor | 22 (48) |
| Tumor length, median (range), cm | 5.0 (1.9-11.2) |
| Chemotherapy | |
| Induction | 17 (37) |
| Concurrent | 46 (100) |
| Radiotherapy modality | |
| IMRT | 39 (85) |
| IMPT | 7 (15) |
| Follow-up time, median (range), mo | 20.0 (3.3-53.0) |
| Surgery after chemoradiotherapy | |
| Yes | 11 (24) |
| No | 35 (76) |
Abbreviations: AJCC, American Joint Committee on Cancer; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated (photon) radiation therapy.
Data are presented as number (percentage) of patients unless otherwise indicated.
Eleven patients (24%) ultimately underwent esophagectomy, 5 (45%) of which were adenocarcinoma and 6 (55%) of which were SCC, including 6 that had evidence of malignancy within the gross tumor volume on imaging. Median follow-up time for these patients was 23 months (range, 2.3-86.4 months). All 11 patients experienced distant (out-of-field) recurrence. The 6 local failures all occurred within the first year. No patient experienced intraoperative complications, but 10 patients experienced at least 1 of the following postoperative complications: acute respiratory distress syndrome, small-bowel obstruction, wound infection, gastric outlet obstruction, aspiration pneumonia, and atrial fibrillation. None of these 11 patients died within the first 90 days after surgery.
Adverse Events
Details of toxic effects are shown in Table 2. Thirteen patients (28%) had adverse events at baseline, before starting SIB-RT (dysphagia, 9; anorexia 4). No patient experienced grade 4 to 5 acute or late toxic effects. Ten patients experienced grade 3 acute toxic events (esophagitis, 4; dysphagia, 3; anorexia, 3) and 3 experienced late grade 3 events (esophageal stricture).
Table 2. Toxic Events Among 46 Patients Given a Simultaneous Integrated Boost for Locally Advanced Esophageal Cancer.
| Adverse Eventa | Grade 1 | Grade 2 | Grade 3 | Grade 4 or 5 |
|---|---|---|---|---|
| Acute toxic effects | ||||
| Esophagitis | 17 (37) | 12 (26) | 4 (9) | 0 |
| Dysphagia | 20 (43) | 8 (17) | 3 (7) | 0 |
| Weight loss | 16 (35) | 19 (41) | 0 | 0 |
| Fatigue | 21 (46) | 16 (35) | 0 | 0 |
| Nausea | 10 (22) | 19 (41) | 0 | 0 |
| Anorexia | 10 (22) | 16 (35) | 3 (7) | 0 |
| Late toxic effects | ||||
| Esophageal | ||||
| Stricture | 10 (22) | 4 (9) | 3 (7) | 0 |
| Hemorrhage | 1 (2) | 1 (2) | 0 | 0 |
| Pain | 3 (7) | 3 (7) | 0 | 0 |
Scored with the Common Terminology Criteria for Adverse Events, version 4.0.
Regarding all long-term events (regardless of grade), no patients experienced late cardiopulmonary toxic effects at the time of last follow-up. Seventeen patients (37%) developed esophageal stricture (at a median of 12 months [range, 1.2-28.6 months]) requiring a median of 2 dilation procedures (range, 1-8). The rate of stricture development was statistically similar to that in the 97-patient cohort treated with 50.4 Gy of RT (30 [31%]; P = .47). Two patients developed self-limited (grade 1 or 2) esophageal hemorrhage.
Local Recurrence
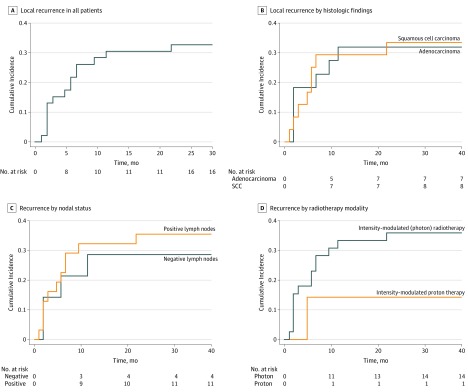
Cumulative local recurrence rates were 22% (95% CI, 11%-35%) at 6 months, 30% (95% CI, 18%-44%) at 1 year, and 33% (95% CI, 20%-46%) at 2 years (Figure 1). At the time of last follow-up, 15 patients (33%) experienced local failure at a median of 5 months (range, 1-24 months) (eFigure 1 in the Supplement). On univariate analysis, local recurrence was not associated with tumor histologic characteristics (HR, 1.1; 95% CI, 0.4-2.8; P = .74; Figure 2B), nodal status (HR, 1.3; 95% CI, 0.4-3.9; P = .48; Figure 2C), or RT modality (HR, 0.4; 95% CI, 0.0-2.7; P = .31; Figure 2D).
Figure 2. Local Recurrence Rate of the Study Population.
A, Local recurrence in all patients. B, Local recurrence by histologic findings. Survival at 12 months was 32% in patients with adenocarcinoma (n = 22) and 29% in patients with squamous cell carcinoma. C, Local recurrence by nodal status. Survival at 12 months was 29% in patients with negative lymph nodes (n = 14) and 32% in patients with positive lymph nodes (n = 31). D, Local recurrence by radiotherapy type. Survival at 12 months was 33% in patients who received intensity-modulated (photon) radiotherapy (n = 39) and 14% in patients who received intensity-modulated proton therapy. Death was used as a competing risk. SCC indicates squamous cell carcinoma.
An exploratory analysis comparing our study patients with 97 similar patients who received modern but standard-dose (50.4 Gy) chemoradiotherapy (eTable in the Supplement) showed reduced local recurrence in the patients who received SIB-RT (HR, 0.49; 95% CI, 0.26-0.92; P = .03; eFigure 2A in the Supplement). This benefit seemed to be more pronounced in patients with adenocarcinoma (HR, 0.44; 95% CI, 0.18-1.10; P = .08; eFigure 2B in the Supplement) than those with SCC (HR, 0.62; 95% CI, 0.24-1.55; P = .30; eFigure 2C in the Supplement). The difference in local recurrence persisted when the patients who eventually underwent resection were excluded from the analysis (HR, 0.39; 95% CI, 0.17-0.86; P = .02; eFigure 2D in the Supplement).
Overall Survival
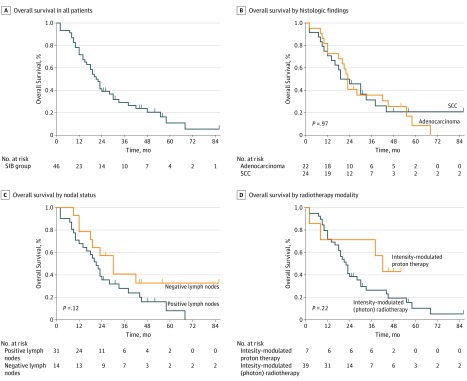
The median OS of the study population was 21.5 months (range, 2.3-86.4 months); OS rates were 78.3% (95% CI, 68.0%-93.2%) at 1 year, 41.3% (95% CI, 27.7%-55.5%) at 2 years, and 29.1% (95% CI, 15.4%-42.7%) at 3 years (Figure 3A). No differences in OS were noted based on tumor histologic characteristics (HR, 0.98; 95% CI, 0.52-1.88; P = .97; Figure 3B), nodal status (HR, 1.79; 95% CI, 0.91-3.51; P = .12; Figure 3C), or RT modality (HR, 0.54; 95% CI, 0.24-1.24; P = .22; Figure 3D).
Figure 3. Overall Survival of the Study Population.
SCC indicates squamous cell carcinoma; SIB, simultaneous integrated boost.
Exploratory analyses comparing OS in the study population with that of 97 similar patients who underwent standard-dose RT (without an SIB) echoed the results of local recurrence (eFigure 3 in the Supplement), in that receipt of SIB-RT was associated with higher OS rates in all patients (HR, 0.66; 95% CI, 0.47-0.94; P = .02) and in patients who did not undergo resection (HR, 0.58; 95% CI, 0.37-0.91; P = .04). The difference in OS was more pronounced for patients with adenocarcinoma (HR, 0.70; 95% CI, 0.39-1.26; P = .02) than those with SCC (HR, 1.56; 95% CI, 1.03-2.36; P = .23).
Discussion
These results of a phase 1/2 trial of dose-escalated SIB-RT with concurrent chemotherapy for locally advanced esophageal cancer demonstrate that this approach was well tolerated and provides local control relative to standard-dose RT in contemporaneously treated patients. These results merit evaluation in randomized trials in the future.
The concept of safe dose escalation for esophageal cancer has been understudied, in part because of the negative results of the INT 0123 trial.4 However, extrapolation of the results from that trial to present-day treatment should be done carefully, because esophageal cancer is no longer treated with 2-dimensional RT, as in the INT 0123 trial, and most patients with esophageal cancer (at least in the United States) do not have SCC. Moreover, radiobiological principles suggest that a dose of 45 Gy is the minimum dose for sterilizing microscopic disease; thus, the current standard of care of 50.4 Gy for esophageal cancer could be considered inadequate from a radiobiological perspective.23 Definitive treatment of unresected solid tumors—that is, most locally advanced esophageal cancers—would require doses of at least 54 to 60 Gy, if not higher.24,25,26,27,28 Hence, the favorable results of this trial underscore the need to revisit this issue for esophageal cancer with randomized trials of modern-day treatment techniques.
Compared with the previous interim report,20 our study found that that SIB-RT was associated with durable in-field control (more pronounced with longer follow-up; eFigure 2 in the Supplement). Although competing risks to achieving meaningful local control are duly acknowledged, most failures occur within 2 years after therapy, if not sooner.3 As survival times for locally advanced esophageal cancer continue to improve with contemporary RT techniques, improved systemic therapies, and broader salvage options, durable local control will become increasingly important to address.
Although our results are unique owing to the long-term follow-up for this trial, other prospective studies have attempted to deliver dose-escalated RT for locally advanced esophageal cancer. A phase 1 trial of 25 Chinese patients with a median follow-up time of 9 months concluded that the maximum tolerated dose was 70 Gy delivered in 25 fractions.29 That investigation used a standardized uptake value cutoff on pretreatment PET and CT to guide target delineation for SIB, unlike our trial. In that trial, the rate of local control was 77.4% and OS was 69.2% at 1 year. Another study using 66 Gy in 30 fractions to treat gross disease led to a 2-year local control rate of 78.6% and OS of 72.7% at a median follow-up time of 24 months.30 Although those rates are higher than those found in the current report, patients in both of those studies had only N0-1 disease, whereas 10 patients (22%) enrolled in the current trial had N2 disease and 8 (17)% had M1 disease.
Findings from our exploratory analysis of the 97-patient comparison group should be interpreted cautiously because that comparison was not included in the design of the original phase 1/2 trial and data for the comparison group were collected retrospectively; however, the outcomes were similar to those of other established results.1,2 Nevertheless, that exploratory analysis showed that adenocarcinomas seemed to benefit more from SIB-RT than did SCC. Although the relatively small numbers of patients in both the current trial and the comparison group preclude robust conclusions, the fact that most patients in INT 0123 had SCC tumors4 could imply that SCC could be more resistant to RT; this finding may explain why patients with SCC derived a greater proportional benefit from trimodality therapy vs surgery alone in the CROSS (Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study) investigation.1 However, both of the aforementioned studies29,30 evaluated predominantly SCC and still found SIB-RT to produce encouraging outcomes.
Limitations
Despite the favorable findings of this trial, its limitations must be acknowledged. In addition to the small number of patients and the exploratory nature of the comparison group, the inclusion of patients with advanced disease (ie, N2M0 and M1) may have led to underestimation of competing risks for local failure and toxic effects. Underestimating the influence of these factors could also result from our inclusion of patients who eventually received resection, as both local failure (relative to definitive chemoradiotherapy) and areas at risk of late toxic effects (eg, stricture) are reduced by esophagectomy.31,32 Last, we posit that the results of this trial should not be considered negative because the preestimated improvement in 1-year local control from 70% to 85% did not occur; rather, if the reference 1-year local control had been set around 55%, as in the INT 0123 trial,4 these results represent a clear improvement. Nevertheless, corroborative prospective data are welcomed to test this modern treatment paradigm in phase 3 trials.
Conclusions
These results of a phase 1/2 trial of dose-escalated SIB-RT with concurrent chemotherapy for locally advanced esophageal cancer demonstrate that this approach was well tolerated and provides encouraging local control relative to historical data and standard-dose RT in contemporaneously treated patients. These results merit randomized evaluation in the future.
eTable. Patient and Treatment Characteristics
eFigure 1. Time to Local Recurrence for Each Patient on the Trial
eFigure 2. Cumulative Incidence of Local Recurrence of the Trial Patients Vs a 97-Patient, Contemporaneously Treated, Standard-Dose (50.4 Gy Without a Simultaneous Integrated Boost) Group, Stratified for Adenocarcinoma Histology, Squamous Cell Histology, and for Receipt of Chemoradiotherapy Without Surgery
eFigure 3. Overall Survival of the Trial Patients Vs a 97-Patient, Contemporaneously Treated, Standard-Dose (50.4 Gy Without a Simultaneous Integrated Boost) Group, Stratified for Adenocarcinoma Histology, Squamous Cell Histology, and for Receipt of Chemoradiotherapy Without Surgery
References
- 1.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. ; CROSS study group . Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090-1098. doi: 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 2.Tepper J, Krasna MJ, Niedzwiecki D, et al. . Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086-1092. doi: 10.1200/JCO.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh J, Settle SH, Amini A, et al. . Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118(10):2632-2640. doi: 10.1002/cncr.26586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minsky BD, Pajak TF, Ginsberg RJ, et al. . INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167-1174. doi: 10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 5.Brower JV, Chen S, Bassetti MF, et al. . Radiation dose escalation in esophageal cancer revisited: a contemporary analysis of the National Cancer Data Base, 2004 to 2012. Int J Radiat Oncol Biol Phys. 2016;96(5):985-993. doi: 10.1016/j.ijrobp.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 6.Perez CA, Pajak TF, Rubin P, et al. . Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy: report by the Radiation Therapy Oncology Group. Cancer. 1987;59(11):1874-1881. doi: [DOI] [PubMed] [Google Scholar]
- 7.Kuban DA, Tucker SL, Dong L, et al. . Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67-74. doi: 10.1016/j.ijrobp.2007.06.054 [DOI] [PubMed] [Google Scholar]
- 8.Lacas B, Bourhis J, Overgaard J, et al. ; MARCH Collaborative Group . Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 2017;18(9):1221-1237. doi: 10.1016/S1470-2045(17)30458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Allen PK, Potter A, et al. . Re-evaluating the optimal radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol. 2014;9(9):1398-1405. doi: 10.1097/JTO.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Liao Z, Jin J, et al. . Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(3):656-664. doi: 10.1016/j.ijrobp.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Suh Y-G, Lee YC, et al. . Dose-response relationship between radiation dose and loco-regional control in patients with stage ii-iii esophageal cancer treated with definitive chemoradiotherapy. Cancer Res Treat. 2017;49(3):669-677. doi: 10.4143/crt.2016.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C-Y, Li C-C, Chien C-R. Does higher radiation dose lead to better outcome for non-operated localized esophageal squamous cell carcinoma patients who received concurrent chemoradiotherapy? a population based propensity-score matched analysis. Radiother Oncol. 2016;120(1):136-139. doi: 10.1016/j.radonc.2016.04.042 [DOI] [PubMed] [Google Scholar]
- 13.Chuong MD, Hallemeier CL, Jabbour SK, et al. . Improving outcomes for esophageal cancer using proton beam therapy. Int J Radiat Oncol Biol Phys. 2016;95(1):488-497. doi: 10.1016/j.ijrobp.2015.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma V, Lin SH, Simone CB II, Mehta MP. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol. 2016;7(4):644-664. doi: 10.21037/jgo.2016.05.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma V, Moreno AC, Lin SH. Advances in radiotherapy management of esophageal cancer. J Clin Med. 2016;5(10):E91. doi: 10.3390/jcm5100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SH, Merrell KW, Shen J, et al. . Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol. 2017;123(3):376-381. doi: 10.1016/j.radonc.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 17.Haque W, Verma V, Butler EB, Teh BS. Utilization of neoadjuvant intensity-modulated radiation therapy and proton beam therapy for esophageal cancer in the United States. J Gastrointest Oncol. 2018;9(2):282-294. doi: 10.21037/jgo.2017.11.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh J, Gomez D, Palmer MB, et al. . Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys. 2011;81(5):1336-1342. doi: 10.1016/j.ijrobp.2010.07.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh J, Palmer MB, Ajani JA, et al. . Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82(1):468-474. doi: 10.1016/j.ijrobp.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh JW, Seyedin SN, Allen PK, et al. . Local control and toxicity of a simultaneous integrated boost for dose escalation in locally advanced esophageal cancer: interim results from a prospective phase I/II trial. J Thorac Oncol. 2017;12(2):375-382. doi: 10.1016/j.jtho.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services Common terminology criteria for adverse events (CTCAE), version 4.03. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Updated June 14, 2010. Accessed August 13, 2019.
- 22.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343-346. doi: 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 23.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 8th ed Philadelphia, PA: Wolters Kluwer; 2019. [Google Scholar]
- 24.Ettinger DS, Aisner DL, Wood DE, et al. . NCCN guidelines insights: non–small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821. doi: 10.6004/jnccn.2018.0062 [DOI] [PubMed] [Google Scholar]
- 25.Nabors LB, Portnow J, Ammirati M, et al. . NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(11):1331-1345. doi: 10.6004/jnccn.2017.0166 [DOI] [PubMed] [Google Scholar]
- 26.Carroll PH, Mohler JL. NCCN guidelines updates: prostate cancer and prostate cancer early detection. J Natl Compr Canc Netw. 2018;16(5S):620-623. doi: 10.6004/jnccn.2018.0036 [DOI] [PubMed] [Google Scholar]
- 27.Colevas AD, Yom SS, Pfister DG, et al. . NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw. 2018;16(5):479-490. doi: 10.6004/jnccn.2018.0026 [DOI] [PubMed] [Google Scholar]
- 28.Kalemkerian GP, Loo BW, Akerley W, et al. . NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(10):1171-1182. doi: 10.6004/jnccn.2018.0079 [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Cai XW, Liu Q, et al. . Safety of dose escalation by simultaneous integrated boosting radiation dose within the primary tumor guided by (18)FDG-PET/CT for esophageal cancer. Radiother Oncol. 2015;114(2):195-200. doi: 10.1016/j.radonc.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Guo H, Zhai T, et al. . Radiation dose escalation by simultaneous modulated accelerated radiotherapy combined with chemotherapy for esophageal cancer: a phase II study. Oncotarget. 2016;7(16):22711-22719. doi: 10.18632/oncotarget.8050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl M, Stuschke M, Lehmann N, et al. . Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310-2317. doi: 10.1200/JCO.2005.00.034 [DOI] [PubMed] [Google Scholar]
- 32.Bedenne L, Michel P, Bouché O, et al. . Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160-1168. doi: 10.1200/JCO.2005.04.7118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Patient and Treatment Characteristics
eFigure 1. Time to Local Recurrence for Each Patient on the Trial
eFigure 2. Cumulative Incidence of Local Recurrence of the Trial Patients Vs a 97-Patient, Contemporaneously Treated, Standard-Dose (50.4 Gy Without a Simultaneous Integrated Boost) Group, Stratified for Adenocarcinoma Histology, Squamous Cell Histology, and for Receipt of Chemoradiotherapy Without Surgery
eFigure 3. Overall Survival of the Trial Patients Vs a 97-Patient, Contemporaneously Treated, Standard-Dose (50.4 Gy Without a Simultaneous Integrated Boost) Group, Stratified for Adenocarcinoma Histology, Squamous Cell Histology, and for Receipt of Chemoradiotherapy Without Surgery