Abstract
Introduction
The value of neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (Kim-1), and liver-type fatty acid binding protein (L-FABP) was assessed in early diagnosis of gentamicin-induced acute kidney injury (AKI) in dogs.
Material and Methods
Subcutaneous gentamicin injection in 16 healthy adult beagles made the AKI model. Blood was sampled every 6 h to detect NGAL, Kim-1, L-FABP, and serum creatinine (SCr) concentrations. Kidney tissue of two dogs was taken before the injection, as soon as SCr was elevated (78 μmol/L), and when it had risen to 1.5 times the baseline, and haematoxylin-eosin staining and transmission electron microscopy (TEM) were used to observe changes.
Results
NGAL, Kim-1, and SCr levels were significantly increased (P < 0.05) at 18, 30, and 78 h post injection, but L-FABP concentration was not associated with renal injury. At the earliest SCr elevation stage, findings were mild oedema, degeneration, and vacuolisation in renal tubular epithelial cells in pathology, and mild cytoplasmic and mitochondrial oedema in TEM. At this time point, NGAL and Kim-1 concentrations were significantly increased (P < 0.05), indicating that these two molecules biomark early kidney injury in dogs. Using receiver operating characteristic curve analysis, their warning levels were > 25.31 ng/mL and > 48.52 pg/mL.
Conclusion
Plasma NGAL and Kim-1 above warning levels are early indicators of gentamicin-induced AKI in dogs.
Keywords: dog, gentamicin, kidney pathology, NGAL, Kim-1, L-FABP
Introduction
Acute kidney injury (AKI) is characterised by a sudden decrease in glomerular filtration rate (8). In dogs, the mortality rate of AKI (about 50%) is closely related to the severity of kidney injury. The prognosis of AKI in dogs is influenced by the time of diagnosis and treatment (1). One of the main reasons for mortality due to AKI is the diagnosis coming too late, which is a missed opportunity for early intervention. This failure to make early diagnosis is attributable to the lack of a valuable early diagnostic marker for AKI in dogs. Conventional laboratory markers for AKI include blood urea nitrogen and serum creatinine (SCr) levels. However, any abnormality of these indices is only seen when the renal tubular epithelial cells have 70%–80% damage (12) and when the kidneys have already suffered serious degradation or have even failed. Moreover, SCr concentration is also related to the total muscle mass of the body, as closely as it is to severe kidney damage, and diseased animals with a low total muscle mass due to malnutrition might not show a significant increase in this biomarker (3). Therefore, the development and screening of early diagnostic markers is of great significance for early diagnosis and prognosis of AKI.
In this study, we established an AKI model by injecting experimental dogs with gentamicin and then performed comparative analysis for screening early diagnostic markers of canine AKI. This study was a preliminary exploration of early diagnostic and warning indicators of this renal impairment in dogs, meant to provide a scientific basis for further studies on early diagnosis of canine acute kidney injury.
Material and Methods
Animals. For this study, sixteen healthy 12- to 16-month-old beagles (eight males and eight females weighing 5.1 ± 0.2 kg) were chosen, and routine immunisation and deworming were performed. The animals were housed under the same conditions for four weeks, and the feeding and management regimens were consistent throughout the experiment. The dogs were subjected to physical examination before the experiment to confirm their good health.
Establishment of an experimental animal model. Before injecting gentamicin, the plasma concentration of SCr in a test dog was measured as the baseline value. This experiment employed the Kidney Disease: Improving Global Outcomes (KDIGO) standard (9), which defines an increase in SCr level to ≥ 1.5 times the baseline level within seven days as an indicator for AKI. The experimental dogs were subcutaneously injected with 20 mg/kg of gentamicin every 8 h to induce nephrotoxicity (4). SCr concentrations were measured every 6 h during the induction period. When the SCr index of the test dog became 1.5 times greater than the baseline level, the AKI model was considered established, and gentamicin injection was stopped in an attempt to mitigate the development of severe injury that would result in adverse clinical signs, irreversible kidney injury, or both. Between the time points of baseline and AKI establishment, 2 ml of blood was taken from a forelimb vein every 6 h and centrifuged. The samples for measurement of markers were then stored at −80°C until testing.
Detection of SCr and renal injury markers: neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (Kim-1), and liver-type fatty acid binding protein (L-FABP). The SCr concentrations of the experimental dogs were measured using a VetTest Chemistry Analyzer (IDEXX Laboratories, USA) and the plasma concentrations of NGAL, Kim-1, and L-FABP were determined by RayBiotech canine ELISA kit (RayBiotech Life, USA). The reagents and plasma samples in the kit were allowed to stand for at least 30 min at room temperature (18–25°C). First, different concentrations of standards and test samples were added to the microtitre plate. Horseradish peroxidase was added to each well, and the plate was incubated at 37°C for 60 min. Then, the solution in the microtitre plate was discarded, and the plate was rinsed thoroughly with cleaning fluid. Substrates CH4N2O3 and TMB were added to the plate, which was then incubated in the dark at 37°C for 15 min. Finally, H2SO4 was added to the plate. The absorbance (optical density) of each standard and test sample was measured within 15 min at a wavelength of 450 nm by using an enzyme-labelled instrument, and a standard curve was drawn to determine the marker concentrations in each sample.
Histopathological examination of the kidneys. One side of the kidney of two dogs was collected at the following time points: before gentamicin injection, at the earliest time when the SCr was elevated (78 μmol/L), and when AKI was established (i.e. the SCr concentration had increased to 1.5 times the baseline level). These two dogs were administered general anaesthesia. After routine surgical disinfection, samples of the kidney were fixed in 10% neutral formalin for 72 h. The samples were then dehydrated, embedded in paraffin, sectioned, and finally stained with haematoxylin-eosin (HE). The histopathological changes and extent of kidney damage were assessed under light microscopy. The degree of kidney injury was graded from 0 to 5 as follows: 0 – no injury; 1 – injury area < 10%; 2 – 10% ≤ injury area < 25%; 3 – 25% ≤ injury area < 50%; 4 – 50% ≤ injury area < 75%; and 5 – injury area ≥ 75%. Twenty fields of view (200 × high-power field) of the renal cortex and medulla were chosen for histopathological analysis. The mean scores of the lesions in all 20 visual fields were calculated, and the average renal tubular injury score of each field of view was taken as the final score. The dogs were administered postoperative analgaesia and routine treatment.
Transmission electron microscope (TEM) examination of the renal tissue. A portion of kidney tissue (2 mm × 2 mm × 2 mm) was excised and immediately fixed in a phosphate-buffered 2.5% glutaraldehyde fixative at 4°C for 24 h. The phosphate buffer was then rinsed off, and the tissue was fixed in 1% OsO4 for 1 h, dehydrated, embedded, and sectioned ultrathinly. The sections were stained by lead and uranium and photographed for assessing the ultrastructural damage to the kidney.
Data analysis. Statistical analysis was performed by using SPSS 19.0 (IBM Corp., USA). The data were computed as mean ± standard deviation (± SD) and were first tested for normality. If the data were normally distributed, the statistical analysis used one-way analysis of variance; if the data did not belong to the normal distribution, the non-parametric Kruskal– Wallis test was significant at P < 0.05. The diagnostic results and critical values were determined by receiver operating characteristic (ROC) curve analysis.
Results
Clinical findings. At about 72 h after gentamicin injection, the dogs began to show signs of depression, increased water consumption, decreased appetite, polyuria, and occasional vomiting. At about 84 h, there was no appetite, mild dehydration, and some dogs were anuric. At about 108 h after the injection, most dogs were oliguric, the number of anuric dogs was greater, and varying degrees of vomiting occurred. The colour of the vomit was yellow or white and it was viscous foamy liquid.
Detection of SCr, NGAL, Kim-1, and L-FABP concentrations. As can be seen from the results shown in Table 1, the plasma concentrations of Kim-1 and NGAL gradually increased with treatment time and were well correlated with the degree of renal injury. At 18 and 30 h, the plasma concentrations of NGAL and Kim-1 were significantly higher than their baseline concentrations, and this increase occurred significantly earlier than that in SCr concentration. The SCr concentration at 78 h after injection was significantly higher than that before injection. The plasma concentration of L-FABP rose irregularly and was not correlated with the time of gentamicin administration.
Table 1.
Changes in plasma concentrations of renal injury markers before and after gentamicin administration
| Time (h) | NGAL content (ng/mL) | Kim-1 content (pg/mL) | L-FABP content (pg/mL) | SCr content (μmol/L) |
|---|---|---|---|---|
| 0 | 16.42 ± 2.55 | 29.35 ± 2.82 | 161.70 ± 9.77 | 72.50 ± 9.10 |
| 6 | 16.70 ± 2.83 | 27.97 ± 2.15 | 151.85 ± 18.24 | 71.29 ± 6.47 |
| 12 | 18.32 ± 2.27 | 29.51 ± 4.20 | 203.78 ± 43.31* | 71.79 ± 5.96 |
| 18 | 21.81 ± 2.86*** | 33.24 ± 5.60 | 204.86 ± 44.98* | 73.79 ± 5.49 |
| 24 | 24.43 ± 4.02*** | 31.11 ± 6.27 | 197.70 ± 55.87* | 71.07 ± 8.47 |
| 30 | 22.39 ± 3.88*** | 33.44 ± 6.47* | 158.91 ± 38.40 | 75.79 ± 6.17 |
| 36 | 22.11 ± 3.79*** | 39.04 ± 5.86*** | 191.15 ± 51.00 | 73.36 ± 4.03 |
| 42 | 22.13 ± 3.64*** | 51.64 ± 7.99*** | 192.49 ± 52.17 | 74.50 ± 11.80 |
| 50 | 22.89 ± 6.19*** | 57.95 ± 3.32*** | 179.70 ± 18.36 | 78.36 ± 8.08 |
| 56 | 20.85 ± 3.26** | 58.14 ± 4.56*** | 195.61 ± 60.94* | 74.93 ± 7.99 |
| 62 | 23.63 ± 3.17*** | 58.38 ± 5.07*** | 168.15 ± 42.62 | 76.50 ± 6.42 |
| 68 | 22.36 ± 2.37*** | 58.99 ± 6.45*** | 163.16 ± 33.00 | 75.29 ± 6.37 |
| 72 | 25.80 ± 2.69*** | 59.20 ± 6.22*** | 130.70 ± 33.27* | 78.50 ± 5.95 |
| 78 | 30.41 ± 4.60*** | 58.96 ± 5.46*** | 148.56 ± 37.39 | 82.00 ± 4.41* |
| 84 | 32.88 ± 3.63*** | 67.49 ± 8.58*** | 161.701 ± 9.77 | 87.00 ± 4.63* |
| 90 | 33.36 ± 7.26*** | 69.81 ± 8.73*** | 151.85 ± 18.24 | 84.92 ± 8.84* |
| 96 | 33.56 ± 7.80*** | 85.95 ± 9.20*** | 203.78 ± 43.31* | 93.75 ± 8.31*** |
| 102 | 37.89 ± 10.99*** | 90.51 ± 10.84*** | 204.86 ± 44.98* | 115.08 ± 16.75*** |
| 108 | 36.74 ± 3.39*** | 113.89 ± 28.50*** | 197.70 ± 55.87* | 129.00 ± 19.42*** |
* P < 0.05, ** P < 0.01, and *** P < 0.001, compared with the levels before injection
Histopathological findings. As can be seen in Fig. 1, at the earliest time when the SCr was elevated, the renal tubular epithelial cells showed a small extent of damage due to mild oedema, degeneration, and vacuolar changes (Fig. 1b). At this time, the difference in SCr concentration was not significant, whereas NGAL and Kim-1 concentrations were significantly increased. At the time point when AKI was established, the cortical renal tubules showed obvious expansion, interstitial widening, and severe damage (Fig. 1c). At the same time point, the concentrations of SCr, NGAL, and Kim-1 were significantly increased. It can be seen from Table 2 that the damage caused by gentamicin was mainly in the renal cortex, and the degree of kidney damage gradually increased with gentamicin administration time.
Fig. 1.
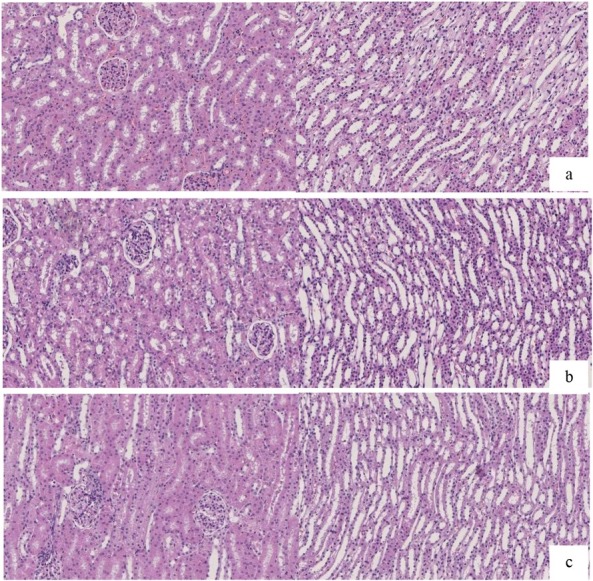
Pathological changes in canine kidneys before and after gentamicin administration (HE, 20 ×); a – control kidney tissue; b – appearance of the kidney tissue at the earliest SCr elevation; c – appearance of the kidney tissue after acute kidney injury was established
Table 2.
Tubular injury score (n = 2)
| Group | Cortical area (score) | Medulla area (score) |
|---|---|---|
| Normal control | 0.20 ± 0.41 | 0.25 ± 0.44 |
| Earliest SCr elevation | 1.50 ± 0.51 | 0.70 ± 0.46 |
| Day of acute kidney injury establishment | 4.95 ± 0.22 | 0.73 ± 0.45 |
TEM findings. As shown in Fig. 2, at the earliest time when the SCr was elevated, the TEM findings revealed no pyknosis in the nucleus and mild oedema in the cytoplasm and mitochondria; in addition, the mitochondria were still present, and the lysosomal number and volume were both lower than those observed when the SCr concentration had increased to 1.5 times the baseline level. The difference in SCr concentration was not significant then, whereas NGAL and Kim-1 concentrations were significantly increased. When AKI was established, the mitochondria were found to be obviously swollen and round, and their structure was fuzzy, the sputum was disordered, the mitochondrial ridge was fractured, some of them showed vacuolar degeneration, they were partially disintegrated, the nuclear structure was loose, and the nuclear chromatin was light. The SCr, NGAL, and Kim-1 concentrations were significantly increased at this time.
Fig. 2.
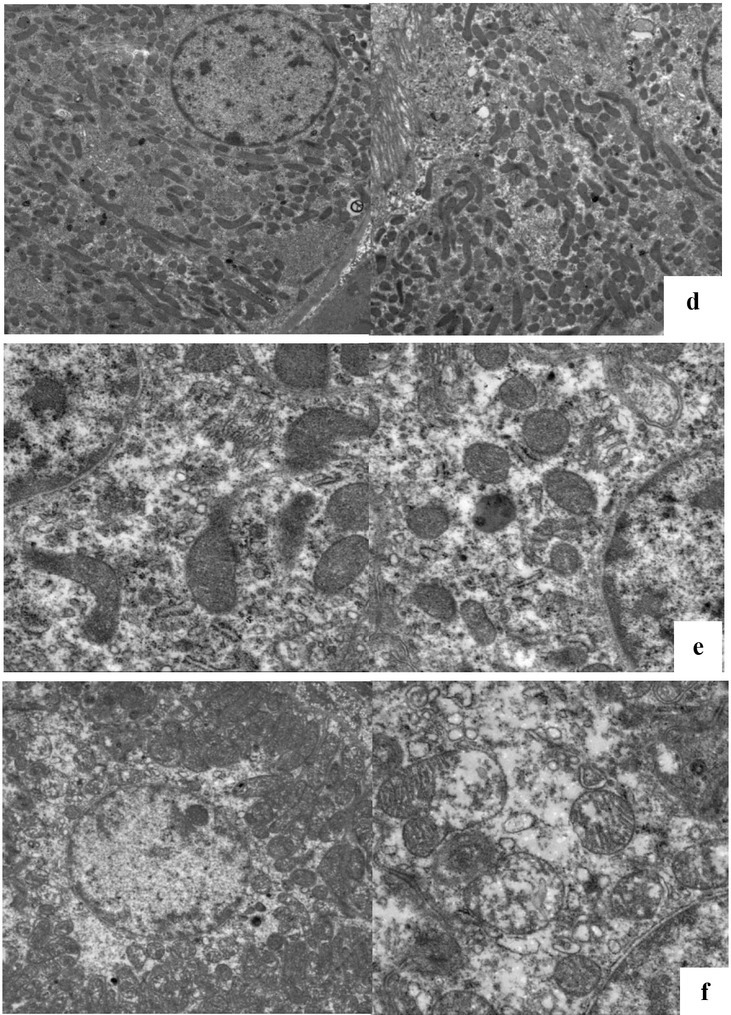
Ultrastructural changes in canine kidney tissues before and after gentamicin administration (1,700 ×) d – control kidney tissue; e – appearance of the kidney tissue at the earliest SCr elevation; f – appearance of the kidney tissue after acute kidney injury was established
ROC analysis findings. At the earliest time when the SCr was elevated, the pathological examination showed mild renal injury. Renal pathology showed renal tubular degeneration and necrosis as the gold standard signs. The pathological change was positive, and did not appear as the negative group. The concentrations of the two renal injury markers NGAL and Kim-1 were evaluated by ROC analysis. Upon calculating the Youden index, the optimal cutoff plasma concentration of NGAL was found to be > 25.31 ng/mL (sensitivity 0.68, specificity 0.825), while that of Kim-1 was > 48.52 pg/mL (sensitivity 0.992, specificity 0.802).
Table 3.
Cutoff concentrations, sensitivity, specificity, and area under the ROC curve of NGAL and Kim-1
| Variables | Area under the curve (AUC) | 95% confidence interval | Standard error |
|---|---|---|---|
| NGAL | 0.84 | 0.793–0.887 | 0.031 |
| Kim-1 | 0.954 | 0.941–0.977 | 0.028 |
Fig. 3.
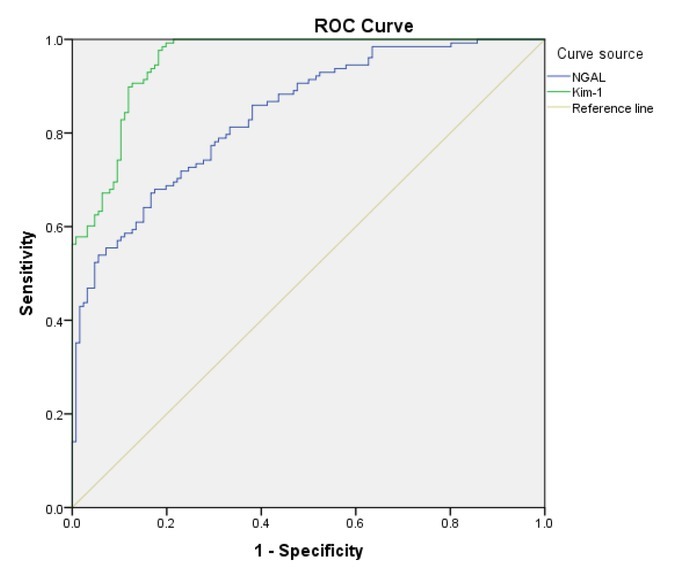
Area under the ROC curve for NGAL and Kim-1
Discussion
NGAL, a component of special neutrophil particles, was discovered by Kjeldsen et al. (7) in 1993. It is closely related to matrix metalloproteinase-9. NGAL is expressed in renal tubules, where it can bind and transport iron ions and promote the release of chemokines and interleukins, which play an important role in regeneration after renal injury (2).
Kim-1 has low basal expression in healthy kidneys. However, after hypoxia, a large number of epithelial tissues of renal proximal convoluted tubules can be found by urine test. This elevated Kim-1 expression is an effective indicator for renal ischaemia and hypoxia injury (5).
L-FABP belongs to a family of 15-kDa cytoplasmic proteins. L-FABP, which is involved in the conversion of long-chain amino acids into cells, can selectively bind the products of lipid peroxidation and decrease their toxicity. Therefore, its protective effect can serve as a potential biomarker for cell damage (6).
In the present study, canine kidney tissues showed mild renal injury at the earliest time when the SCr concentration was increased, and severe injury was observed when AKI was established. The SCr concentrations did not begin to increase significantly until 78 h, at which point the kidneys were already severely damaged. It can be seen that the traditional diagnostic indicator SCr was only significantly increased in case of severe kidney injury, because of which the disease would be diagnosed at a late stage in real veterinary practice, which diminishes the opportunity for positive treatment and intervention. Creatinine is a protein metabolite excreted by the kidneys. Its concentration is affected by non-renal factors such as protein and water intake and muscle mass. The limitations of these influencing factors preclude creatinine’s being an ideal diagnostic indicator for AKI.
In this study, we found that changes in plasma L-FABP concentrations before and after gentamicin injection were not associated with kidney damage. Plasma L-FABP concentration was not associated with the degree of renal injury. Therefore, this protein cannot be used as a diagnostic index for AKI.
The plasma concentrations of NGAL and Kim-1 were significantly increased at 18 and 30 h after gentamicin injection, respectively. At these time points, the results of HE staining and TEM showed mild renal damage, which suggested that NGAL and Kim-1 were synthesised and secreted at an early stage of AKI when the pathological changes were only mild. Compared with SCr concentration, NGAL and Kim-1 concentrations provided a quicker and more accurate indication of AKI. The changes in NGAL and Kim-1 concentrations were directly proportional to the extent of renal injury, suggesting that the elevated concentrations were an indication of the prognosis of AKI, which was consistent with the reports in literature (10, 14).
In the present study, plasma NGAL and Kim-1 concentrations were elevated in the AKI model. The specific mechanism for this increase might be related to the upregulation of NGAL expression in the kidneys; NGAL is then secreted into urine and freely filtered in the glomerulus, and reabsorbed through macrophage-dependent endocytosis in the proximal tubule; however, elevated plasma NGAL may be due to damage-related tubular leak-back or reduced glomerular filtration (13).
After renal injury, the part of Kim-1 located outside the cell membrane can be lysed, allowing it to rapidly enter the small lumen and thus become detectable in urine. The secretion of Kim-1 into urine is closely related to its concentration in plasma. As a marker of early renal injury, plasma Kim-1 concentration is not affected by hepatic toxicity and can, therefore, reflect the damage to proximal renal tubular epithelial cells (11).
In summary, in the presence of AKI, the plasma concentrations of NGAL and Kim-1 were elevated earlier than those of the conventional index SCr. This indicated that plasma NGAL and Kim-1 levels can be used as early-warning indicators of gentamicin-induced canine AKI. We speculate that respective plasma NGAL and Kim-1 concentrations > 25.31 ng/mL and > 48.52 pg/mL have predictive value for the occurrence of gentamicin-induced AKI.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This research was supported by the China Postdoctoral Science Foundation, China, (2018M641889) and Heilongjiang Bayi Agricultural University Research Project, China, (XDB201804).
Animal Rights Statement: The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of Heilongjiang Bayi Agricultural University, Daqing, China. All beagle experiments were performed in accordance with the regulations for the Administration of Affairs Concerning Experimental Animals approved by the School Council of Heilongjiang Bayi Agricultural University.
References
- 1.Cowgill L.D., Langston C. Bartges J., Polzin D. Nephrology and Urology of Small Animals. John Wiley & Sons; New Jersey: 2011. Acute kidney insufficiency; pp. 472–523. edited by. [Google Scholar]
- 2.Devarajan P.. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest. 2008;68:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster D., Lauver L.S.. When a diabetic foot ulcer results in amputation: a qualitative study of the lived experience of 15 patients. Ostomy Wound Manag. 2014;60:16–22. [PubMed] [Google Scholar]
- 4.Grauer G.F., Greco D.S., Behrend E.N., Fettman M.J., Jaenke R.S., Allen T.A.. Effects of dietary protein conditioning on gentamicin-induced nephrotoxicosis in healthy male dogs. Am J Vet Res. 1994;55:90–97. [PubMed] [Google Scholar]
- 5.Han W.K., Bailly V., Abichandani R., Thadhani R., Bonventre J.V.. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt S.M., Dear J., Star R.A.. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- 7.Kjeldsen L., Johnsen A.H., Sengeløv H., Borregaard N.. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 8.Langston C.E. Ettinger S.J., Feldman E.C., Saunders W.B. Textbook of Veterinary Internal Medicine. Philadelphia: 2010. Acute uremia; pp. 1955–2115. edited by. [Google Scholar]
- 9.Maisel A.S., Katz N., Hillege H.L., Shaw A., Zanco P., Bellomo R., Anand I., Anker S.D., Aspromonte N., Bagshaw S.M., Berl T., Bobek I., Cruz D.N., Daliento L., Davenport A., Haapio M., House A.A., Mankad S., McCullough P., Mebazaa A., Palazzuoli A., Ponikowski P., Ronco F., Sheinfeld G., Soni S., Vescovo G., Zamperetti N., Ronco C.. Biomarkers in kidney and heart disease. Nephrol Dial Transplant. 2011;26:62–74. doi: 10.1093/ndt/gfq647. [DOI] [PubMed] [Google Scholar]
- 10.Rached E., Hoffmann D., Blumbach K., Weber K., Dekant W., Mally A.. Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin a in vivo and in vitro. Toxicol Sci. 2008;103:371–381. doi: 10.1093/toxsci/kfn040. [DOI] [PubMed] [Google Scholar]
- 11.Sabbisetti V.S., Waikar S.S., Antoine D.J., Smiles A., Wang C., Ravisankar A., Ito K., Sharma S., Ramadesikan S., Lee M., Briskin R., De Jager P.L., Ngo T.T., Radlinski M., Dear J.W., Park K.B., Betensky R., Krolewski A.S., Bonventre J.V.. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki D., Yamada A., Umeno H., Kurihara H., Nakatsuji S., Fujihira S., Tsubota K., Ono M., Moriguchi A., Watanabe K., Seki J.. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16:553–566. doi: 10.3109/1354750X.2011.613123. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Ott K.M.. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury––where do we stand today? Nephrol Dial Transplant. 2011;26:762–764. doi: 10.1093/ndt/gfr006. [DOI] [PubMed] [Google Scholar]
- 14.Wasilewska A., Taranta-Janusz K., Dębek W., Zoch-Zwierz W., Kuroczycka-Saniutycz E.. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatric Nephrol. 2011;26:579–586. doi: 10.1007/s00467-011-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


