Abstract
Background:
Deep venous thrombosis (DVT), even though resolved, may damage the valves and may lead to chronic venous insufficiency (CVI). We designed the present study to examine the thrombotic markers or other ultrasound features in the absence of active thrombosis in patients presenting with features suggestive of CVI.
Materials and Methods:
It was a cross-sectional study of 50 DVT patients. We collected a detailed history of presenting symptoms (onset, progression, and duration) and associated history of aggravating factors. After classifying the patients, color Doppler investigation for DVT and venous incompetence and blood investigations such as Factor V, D-Dimer, total cholesterol, total triglycerides, homocysteine, high-density lipoproteins, low-density lipoproteins (LDL), and very LDL were done.
Results:
We found a raised Factor V significantly more in patients classified as severe under clinical classification compared with nonsevere (19% and 0%; P = 0.05) and in patients with a high Venous Severity Clinical Score (VSCS) compared to those with a low VSCS score (17% and 0%; P = 0.03). We also found that perforators were significantly more in patients with a high VSCS score (88% and 58%; P = 0.02), in patients with a primary venous etiology compared with those without any venous etiology (97% and 1%; P < 0.0001), in patients with obstruction/reflux compared to those without any pathology (95% and 0%; P < 0.0001), and in patients with severe clinical classification compared with nonsevere patient (95% and 55%; P = 0.002).
Conclusions:
Clinical or subclinical DVT, an important cause of CVI, may not always be seen on ultrasound, especially after resolution. However, they may have the presence of blood parameters (Factor V and hyperhomocysteinemia) suggestive of DVT; these can be used as proxy markers for the current or previous DVT.
KEY WORDS: Chronic venous insufficiency, deep venous thrombosis, serology, ultrasonography
Introduction
The risk factors of chronic venous insufficiency (CVI) have been studied for a long time. Some of these studies suggest that it can occur due to congenital valve or vessel abnormalities, but it most commonly occurs when the valves of the deep veins are damaged as a result of deep venous thrombosis (DVT).[1,2] Valves maintain the hydrostatic venous pressure in the lower extremities by preventing the reflux of venous blood when it is being pumped against gravity towards the heart. With no valves to prevent deep system reflux, the hydrostatic venous pressure in the lower extremity increases. Even after the DVT resolves the damage to the valves remains permanent, later culminating in CVI. But prior DVT can be clinical or subclinical and often not diagnosed on ultrasound after resolution.[1,2,3,4]
Studies have shown that high levels of plasma homocysteine are associated with deep-vein thrombosis, and serological markers such as D-dimer and P-selectin have been used as predictors of DVT.[5,6] Ultrasonography (USG) has also been used to assess thromboembolism.[7] Other models have suggested that ischemia and reperfusion may be associated with CVI.[8] Based on these above literature, the hypothesis that prior (clinical/subclinical) DVT is a common cause of CVI and it is not diagnosed after resolution on ultrasound is the basis of our study to find markers of thrombosis. If there is a relation, then the treating physician can manage the patients on the basis of these markers and nonthrombotic ultrasound findings to control the future CVI. Thus, we wanted to study the presence of thrombotic markers or other ultrasound features suggestive of CVI in the absence of active CVI.
Materials and Methods
The study was conducted in the dermatology outpatient department of a tertiary care hospital in Mumbai, India, catering to higher middle class and upper class patients. It was a cross-sectional study of 50 patients. All consecutive consenting patients between 18 and 65 years of age of both gender presenting with dermatological features suggestive of stasis on the feet – symptoms of pain, edema, pigmentation, distended veins, ulcers of the lower limbs, and/or with a history of past or preceding symptoms or sign of DVT were enrolled for the present study. We excluded the following patients: those with arterial diseases, those who have undergone venous surgeries, and those with arteriovenous malformations. An institutional ethics committee clearance was obtained for the study.
We collected a detailed history of presenting symptoms (onset, progression and duration) and associated history of aggravating factors. Local cutaneous examination and the body mass index (BMI) of the patient were taken and noted. Patients were classified using two scoring systems – Venous Severity Clinical Score (VSCS)[9] and CEAP – Clinical signs (C0-C6); etiology (primary venous etiology present or not); anatomic distribution (superficial/deep/perforator or none); and pathophysiology (reflux/obstruction/both or none).[10,11,12] In VSCS, using the seven classes of the C classification (pain, varicose veins, venous edema, skin pigmentation, inflammation, induration, ulcers, and compression therapy), a 0–3 grading scheme was assigned to clinical manifestations of each class 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. Then, the score was added and patients with VSCS score = 1–4 are classified as nonsevere and VSCS score ≥5 are classified as severe.
After classifying the patients, color Doppler investigation for DVT and venous incompetence and blood investigations such as Factor V, D-Dimer, total cholesterol, total triglycerides, homocysteine, high-density lipoproteins (HDL), low-density lipoproteins (LDL), and very LDL were done to evaluate their levels in severe and nonsevere patients and their significance as proxy markers for DVT.
All the data collected on a predetermined clinical pro forma was entered into a Microsoft Office Excel sheet which were further converted to Stata. (StataCorp©, College Station, Texas, USA). We calculated the means and the standard deviation (SD) for continuous variables and proportions for categorical variables. The means were compared using the t-test, and the proportions were compared using the Chi-square test or Fisher's exact test (for low expected cell counts). P < 0.05 was considered statistically significant.
Results
A total of 50 patients (32 males and 18 females) with CVI were evaluated. The mean age (±SD) of the males was 43.1 (±12.1) years and the mean age (±SD) of the females was 47.6 (±9.8) years respectively; the difference in the mean ages was not statistically significant (p=0.19) Patients having occupations such as kitchen managers, ward boys, and sales executive were highest (32%) followed closely by desk jobs (30%) and the most common history was of prolonged standing among our patients (76%) without any significant difference between male and female. The most frequent symptoms found in our patients were edema (88%), pigmentation (78%), and distended veins (76%) [Figure 1a and b]. The symptom seen significantly less than others was ulcer (24%). BMI of our patients was high, 32% were obese (BMI ≥30) and 22% were overweight (BMI ≥25) [Table 1].
Figure 1.
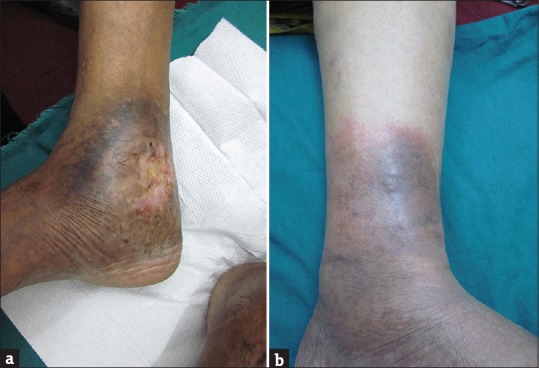
(a and b) Dermatological changes
Table 1.
Describing the occupation, symptoms, and history in our patients
| Variables | Total 50 (100 %) | Males 32 (100 %) | Females 18 (100 %) | P |
|---|---|---|---|---|
| Occupational category | ||||
| Desk job | 15 (30) | 13 (41) | 2 (11) | <0.001 |
| Labor | 6 (12) | 4 (13) | 2 (11) | |
| Homemakers | 13 (26) | 0 | 13 (100) | |
| Others (ward boys/kitchen managers/sales executive) | 16 (32) | 15 (47) | 1 (6) | |
| Associated history and symptoms | ||||
| Symptoms | ||||
| Pain | 34 (68) | 21 (65) | 13 (72) | 0.63 |
| edema | 44 (88) | 27 (84) | 17 (94) | 0.29 |
| Distended veins | 38 (76) | 22 (69) | 16 (89) | 0.11 |
| Pigmentation | 39 (78) | 27 (84) | 12 (67) | 0.15 |
| Ulcers | 12 (24) | 9 (28) | 3 (17) | 0.36 |
| History | ||||
| Prolonged standing | 38 (76) | 23 (72) | 15 (83) | 0.36 |
| Trauma | 6 (12) | 4 (13) | 2 (11) | 0.89 |
| Surgery | 7 (14) | 6 (19) | 1 (6) | 0.20 |
| Immobilization (>2 weeks) | 2 (4) | 1 (3) | 1 (6) | 0.67 |
| OC pills | 3 (6) | 0 | 3 (17) | 0.02 |
| Altered bowel habits | 24 (48) | 15 (47) | 1 (50) | 0.83 |
| Episode of DVT | 2 (4) | 2 (6) | 0 | 0.28 |
| Blood in stools | 5 (10) | 3 (9) | 2 (11) | 0.84 |
| Family history of varicose | 19 (38) | 11 (34) | 8 (44) | 0.48 |
DVT: Deep venous thrombosis, OC: Oral contraceptive pills
We found a raised Factor V significantly more in patients classified as severe under clinical classification compared with nonsevere (19% and 0%; P = 0.05) and in patients with a high VSCS score compared to those with a low VSCS score (17% and 0%; P = 0.03). No such significant difference was found when patients are classified according to etiology, anatomy, and pathology. Similarly, factors such as D-dimer, homocysteine, total cholesterol, and total triglycerides are raised, but no significant difference was found in the raised distribution between patients after classification according to VSCS and CEAP. Venereal Disease Research Laboratory test was negative in our patients.
On color Doppler, it was seen that perforators were found significantly more in patients with a high VSCS score compared to patients with low VSCS score (perforators: 88% and 58%; P = 0.02), in patients with a primary venous etiology compared to those without any venous etiology (97% and 1%; P < 0.001), in patients with obstruction/reflux compared to those without any pathology (95% and 0%; P < 0.001), and in patients with severe clinical classification compared with nonsevere patients (95% and 55%; P = 0.002). Similarly, varicosity and saphenous vein incompetence findings were seen. We have described details about blood investigations and Doppler findings in Tables 2 and 3. The most common perforators were medial malleolus (74%), followed by below the knee (18%) and above the knee (2%) [Table 4].
Table 2.
Describing the serological and Doppler findings according to various types of classification
| Investigations | VSCS | Clinical classification | Etiological classification | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VSCS (≥5) 24 (100%) | VSCS (1-4) 26 (100%) | P | C4a-C6 21 (100%) | C0-C3 29 (100%) | P | Primary 36 (100%) | No venous etiology 14 (100%) | P | |
| Blood investigations | |||||||||
| Homocysteine (high) | 10 (42) | 6 (23) | 0.16 | 7 (33) | 9 (31) | 0.86 | 12 (33) | 4 (29) | 0.75 |
| D-dimer (high) | 6 (26) | 4 (15) | 0.35 | 5 (25) | 5 (17) | 0.51 | 8 (23) | 2 (14) | 0.50 |
| Factor V (+) | 4 (17) | 0 | 0.03 | 4 (19) | 0 | 0.01 | 4 (11) | 0 | 0.19 |
| Total cholesterol (high) | 5 (21) | 7 (21) | 0.61 | 5 (24) | 7 (24) | 0.98 | 8 (22) | 4 (29) | 0.64 |
| Total TG (high) | 10 (42) | 8 (31) | 0.42 | 8 (38) | 10 (35) | 0.79 | 14 (39) | 4 (29) | 0.50 |
| HDL (low) | 8 (33) | 7 (27) | 0.62 | 6 (29) | 9 (31) | 0.85 | 9 (25) | 6 (43) | 0.22 |
| VLDL (high) | 3 (13) | 1 (4) | 0.26 | 1 (5) | 3 (10) | 0.47 | 3 (8) | 1 (7) | 0.89 |
| LDL (high) | 5 (12) | 5 (19) | 0.89 | 4 (19) | 6 (21) | 0.89 | 6 (17) | 4 (29) | 0.35 |
| TG/HDL ratio (high) | 11 (46) | 9 (35) | 0.42 | 10 (48) | 10 (34) | 0.35 | 16 (44) | 4 (29) | 0.30 |
| Doppler findings | |||||||||
| Perforators (+) | 21 (88) | 15 (58) | 0.02 | 20 (95) | 16 (55) | 0.002 | 35 (97) | 1 (7) | <0.0001 |
| Great saphenous vein (+) | 11 (46) | 8 (31) | 0.27 | 13 (62) | 6 (21) | 0.003 | 18 (50) | 1 (7) | 0.005 |
| Varicosities (+) | 16 (67) | 7 (27) | 0.005 | 15 (71) | 8 (28) | 0.002 | 23 (64) | 0 | <0.0001 |
| Thrombosis (+) | 2 (8) | 0 | 0.13 | 2 (10) | 0 | 0.09 | 2 (6) | 0 | 0.37 |
HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very low-density lipoprotein, TG: Triglycerides, VSCS: Venous Severity Clinical Score
Table 3.
Describing the serological and Doppler findings according to various types of classification and ultrasonography changes
| Investigations | Anatomic classification | Pathopysiologic classification | Ultrasonography | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Superficial, Perforator, and deep veins 38 (100%) | No venous locations 12 (100%) | P | Reflux, obstruction or both 38 (100%) | No venous pathophysiology 12 (100%) | P | Thrombosis 2 (100%) | No thrombosis 48 (100%) | P | |
| Blood investigations | |||||||||
| Homocysteine (high) | 12 (32) | 4 (33) | 0.91 | 12 (32) | 4 (33) | 0.91 | 2 (100) | 14 (29) | 0.04 |
| D-Dimer (high) | 8 (22) | 2 (17) | 0.71 | 8 (22) | 2 (17) | 0.71 | 1 (50) | 9 (19) | 0.29 |
| Factor V (+) | 4 (11) | 0 | 0.24 | 4 (11) | 0 (0) | 0.24 | 2 (100) | 2 (4) | <0.0001 |
| Total cholesterol (high) | 9 (24) | 3 (25) | 0.93 | 9 (24) | 3 (25) | 0.93 | 2 (100) | 10 (21) | 0.01 |
| Total TG (high) | 14 (37) | 4 (33) | 0.83 | 14 (37) | 4 (33) | 0.83 | 2 (100) | 16 (33) | 0.05 |
| HDL (low) | 11 (29) | 4 (33) | 0.77 | 11 (29) | 4 (33) | 0.77 | 2 (100) | 13 (27) | 0.03 |
| VLDL (high) | 3 (8) | 1 (8) | 0.96 | 3 (8) | 1 (8) | 0.96 | 0 | 4 (8) | 0.67 |
| LDL (high) | 7 (18) | 3 (25) | 0.62 | 7 (18) | 3 (25) | 0.62 | 1 (50) | 9 (19) | 0.28 |
| TG/HDL ratio (high) | 16 (42) | 4 (33) | 0.59 | 16 (42) | 4 (33) | 0.59 | 2 (100) | 18 (38) | 0.08 |
| Doppler findings | |||||||||
| Perforators (+) | 36 (95) | 0 | <0.0001 | 36 (95) | 0 | <0.0001 | |||
| Great saphenous vein (+) | 19 (50) | 0 | 0.002 | 19 (50) | 0 | 0.002 | |||
| Varicosities (+) | 23 (61) | 0 | <0.0001 | 23 (61) | 0 | <0.0001 | |||
| Thrombosis (+) | 2 (5) | 0 | 0.42 | 2 (5) | 0 | 0.42 | |||
HDL: High-density lipoprotein, LDL: Low-density lipoprotein, VLDL: Very low-density lipoprotein, TG: Triglycerides
Table 4.
Describing the perforators
| Number of perforators | Total 50 (100%) | Males 32 (100%) | Females 18 (100%) | P |
|---|---|---|---|---|
| Above knee | 0.45 | |||
| 0 | 49 (98) | 31 (97) | 18 (100) | |
| 2 | 1 (2) | 1 (3) | 0 | |
| Below knee | ||||
| 0 | 41 (82) | 26 (81) | 15 (83) | 0.88 |
| 1 | 4 (8) | 3 (9) | 1 (6) | |
| 2 | 5 (10) | 3 (9) | 2 (11) | |
| Medial malleolus | ||||
| 0 | 13 (26) | 9 (28) | 4 (22) | 0.60 |
| 1 | 17 (34) | 9 (28) | 8 (44) | |
| 2 | 14 (928) | 9 (28) | 5 (28) | |
| 3 | 3 (6) | 3 (9) | 0 (0) | |
| 4 | 3 (6) | 2 (6) | 1 (6) |
Discussion
Thus, we found patients with CVI had raised Factor V and homocysteine levels. However, none of our patients had features suggestive of active or resolved DVT on USG. We also found that these patients had a high BMI[13,14] and a history of standing for long hours due to their occupation,[15,16,17] these features have been reported in patients with CVI in the literature. Only those patients who were classified as severe cases of CVI had raised Factor V. However, both severe and nonsevere cases of patients with CVI had raised homocysteine and D-dimer. Despite these changes in the blood parameters, we did not find any signs of active DVT in any of our patients.[18]
Studies have suggested that high plasma levels of Factor V might lead to an increased prothrombinase activity causing an increased risk of thrombosis[19] and implicate that hyperhomocysteinemia (HH) promotes thrombosis by an imbalanced redox status because its sulfhydryl group is a strong reductant.[20,21] However, in a recent meta-analysis, authors have found an increased risk of thrombosis not only with high levels of Factor V but also with lower levels of this factor.[22] Although they could explain former association due to raised Factor VIII, the latter association could not be explained adequately.[22] Furthermore, it has also been reported that any mutations in Factor V genes may be associated with either thrombosis or bleeding.[23] Thus, Factor V levels should be judiciously interpreted for clinical management of patients with venous thrombosis. Studies have shown that D-dimer has a sensitivity of 91% for DVT which means patients with raised D-dimer will have a higher likelihood of DVT and both our patients with thrombosis had raised D-dimer, but other patients with raised D-dimer did not show a DVT on USG.[24,25] D-dimer has a low specificity because it is raised in other conditions also, such as bleeding, aortic dissection, and renal and liver diseases,[26] thus raised D-dimer values should be interpreted in view of the history and the overall coagulation profile.
We also found that all patients with CVI had high cholesterol and triglyceride levels. These parameters also have a role in thromboembolic events. Although the exact mechanism is unclear, it has been hypothesized that cholesterol and triglycerides may cause an impairment in coagulation through changes in blood viscosity or mediation through tissue factor pathway inhibitor.[27,28] Thus, a raised Factor V, HH, or altered lipid profile, proxy markers for DVT, may be used for diagnosis of CVI in patients without any evidence of active thrombus on USG.[29,30,31]
Once the DVT resolves, usually it cannot be seen on ultrasound. However, other features suggestive of valvular incompetence leading to CVI such as incompetent perforators, saphenous vein incompetence, and varicosities can be noted on color Doppler.[32,33] In our study, even though evidence of active DVT was not found, our patients did show some features of CVI on color Doppler.[34,35] Furthermore, all these patients were more likely to be classified as severe CVI and have a higher VSCS score.[36] Knowledge of these parameters is important in diagnosis of DVT in patients presenting with symptoms of CVI without any active thrombus.[37,38] It is quite likely that we may not find any feature suggestive of active or resolved thrombus on an ultrasound. However, even in the absence of these findings, it is essential to have a complete coagulation profile of these patients for effective diagnosis and management. It may be difficult to identify the subtle chronic changes in these veins such as eccentric or diffuse thickening of the vein wall, calcification, or partial recanalization of vein lumen with irregular channels, if the treating physician does not inform the sonologist to look for these changes due to a clinical suspicion of resolved DVT. Thus, Doppler findings such as perforator incompetence, saphenous vein incompetence, and varicosities along with blood markers of previous DVT could be a good way of diagnosing CVI.
An important limitation of our study is lack of a control group. In the absence of this, we could not estimate the predictive values of these blood parameters. Nonetheless, our study presents some important findings. As highlighted, clinical or subclinical DVT, an important cause of CVI, may not always be seen on ultrasound, especially after resolution. The presence of blood parameters suggestive of DVT such as raised Factor V/hyperhomocysteinemia/raised total triglycerides and total cholesterol may be used as proxy markers for the current or previous DVT. This needs further study to elucidate the role of ultrasound, computed tomography venogram, and a complete thrombotic profile in the evaluation of a subclinical DVT in cases of CVI.
Conclusion
Thus, it is imperative that all treating physicians become aware of USG findings as well as communicate with the sonologist to actively look for these changes. Even if these findings are missing (as was the case in our study), their blood profile should be examined to look for any alteration in the coagulation parameters suggestive of predilection to thrombosis, and plan the treatment protocol accordingly. These patients should also be regularly followed for evidence of any recurrence of thrombotic episodes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Scott THE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: A dual case-control study. J Vasc Surg. 1995;22:622–8. doi: 10.1016/s0741-5214(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 2.Weiss R. Venous Insufficiency. 2017. [Last accessed on 2018 Jun 02]. Available from: https://www.emedicine.medscape.com/article/1085412-overview .
- 3.Baliyan V, Tajmir S, Hedgire SS, Ganguli S, Prabhakar AM. Lower extremity venous reflux. Cardiovasc Diagn Ther. 2016;6:533–43. doi: 10.21037/cdt.2016.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissner MH. Lower extremity venous anatomy. Semin Intervent Radiol. 2005;22:147–56. doi: 10.1055/s-2005-921948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Heijer M, Koster T, Blom HJ, Bos GM, Briet E, Reitsma PH, et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–62. doi: 10.1056/NEJM199603213341203. [DOI] [PubMed] [Google Scholar]
- 6.Rectenwald JE, Myers DD, Jr, Hawley AE, Longo C, Henke PK, Guire KE, et al. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–7. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 7.Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R, Slosman D, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353:190–5. doi: 10.1016/S0140-6736(98)05248-9. [DOI] [PubMed] [Google Scholar]
- 8.Korthuis RJ, Unthank JL. Experimental models to investigate inflammatory processes in chronic venous insufficiency. Microcirculation. 2000;7:S13–22. [PubMed] [Google Scholar]
- 9.Rutherford RB, Padberg FT, Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL, et al. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–12. doi: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- 10.Reporting Standards in venous disease. Prepared by the Subcommittee on Reporting Standards in Venous Disease, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1988;8:172–81. [PubMed] [Google Scholar]
- 11.Carpentier PH, Cornu-Thénard A, Uhl JF, Partsch H, Antignani PL Société Française de Médecine Vasculaire. Appraisal of the information content of the C classes of CEAP clinical classification of chronic venous disorders: A multicenter evaluation of 872 patients. J Vasc Surg. 2003;37:827–33. doi: 10.1067/mva.2003.147. [DOI] [PubMed] [Google Scholar]
- 12.Porter JM, Moneta GL. Reporting standards in venous disease: An update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–45. doi: 10.1016/s0741-5214(95)70195-8. [DOI] [PubMed] [Google Scholar]
- 13.Danielsson G, Eklof B, Grandinetti A, Kistner RL. The influence of obesity on chronic venous disease. Vasc Endovascular Surg. 2002;36:271–6. doi: 10.1177/153857440203600404. [DOI] [PubMed] [Google Scholar]
- 14.Oganov RG, Savel'ev VS, Shal'nova SA, Kirienko AI, Zolotukhin IA. Risk factors of chronic venous insufficiency of the lower extremities and possibilities of its medication in therapeutic practice. Ter Arkh. 2006;78:68–72. [PubMed] [Google Scholar]
- 15.Shai A, Karakis I, Shemesh D. Possible ramifications of prolonged standing at the workplace and its association with the development of chronic venous insufficiency. Harefuah. 2007;146:677–85. [PubMed] [Google Scholar]
- 16.Stvrtinová V, Kolesár J, Wimmer G. Prevalence of varicose veins of the lower limbs in the women working at a department store. Int Angiol. 1991;10:2–5. [PubMed] [Google Scholar]
- 17.Tüchsen F, Krause N, Hannerz H, Burr H, Kristensen TS. Standing at work and varicose veins. Scand J Work Environ Health. 2000;26:414–20. doi: 10.5271/sjweh.562. [DOI] [PubMed] [Google Scholar]
- 18.Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost. 2005;3:401–2. doi: 10.1111/j.1538-7836.2004.01106.x. [DOI] [PubMed] [Google Scholar]
- 19.Kamphuisen PW, Rosendaal FR, Eikenboom JC, Bos R, Bertina RM. Factor V antigen levels and venous thrombosis: Risk profile, interaction with factor V leiden, and relation with factor VIII antigen levels. Arterioscler Thromb Vasc Biol. 2000;20:1382–6. doi: 10.1161/01.atv.20.5.1382. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Dabiri G, Damstetter E, Baiyee Ebot E, Powers JG, Phillips T, et al. Coagulation disorders and their cutaneous presentations: Pathophysiology. J Am Acad Dermatol. 2016;74:783–92. doi: 10.1016/j.jaad.2015.08.072. [DOI] [PubMed] [Google Scholar]
- 21.Coppola A, Davi G, De Stefano V, Mancini FP, Cerbone AM, Di Minno G. Homocysteine, coagulation, platelet function, and thrombosis. Semin Thromb Hemost. 2000;26:243–54. doi: 10.1055/s-2000-8469. [DOI] [PubMed] [Google Scholar]
- 22.Rietveld IM, Bos MH, Lijfering WM, Li-Gao R, Rosendaal FR, Reitsma PH, et al. Factor V levels and risk of venous thrombosis: The MEGA case-control study. Res Pract Thromb Haemost. 2018;2:320–6. doi: 10.1002/rth2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38(Suppl 1):4–11. doi: 10.1111/ijlh.12508. [DOI] [PubMed] [Google Scholar]
- 24.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, et al. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337:196–200. doi: 10.1016/0140-6736(91)92158-x. [DOI] [PubMed] [Google Scholar]
- 25.Turkstra F, van Beek EJ, Büller HR. Observer and biological variation of a rapid whole blood D-dimer test. Thromb Haemost. 1998;79:91–3. [PubMed] [Google Scholar]
- 26.Koracevic GP. Pragmatic classification of the causes of high D-dimer. Am J Emerg Med. 2009;27:1016.e5–7. doi: 10.1016/j.ajem.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Belaj K, Hackl G, Rief P, Eller P, Brodmann M, Gary T. Changes in lipid metabolism and extension of venous thromboembolism. Ann Nutr Metab. 2014;64:122–6. doi: 10.1159/000360484. [DOI] [PubMed] [Google Scholar]
- 28.Vayá A, Mira Y, Ferrando F, Contreras M, Estelles A, España F, et al. Hyperlipidaemia and venous thromboembolism in patients lacking thrombophilic risk factors. Br J Haematol. 2002;118:255–9. doi: 10.1046/j.1365-2141.2002.03563.x. [DOI] [PubMed] [Google Scholar]
- 29.Monkovic DD, Tracy PB. Activation of human factor V by factor Xa and thrombin. Biochemistry. 1990;29:1118–28. doi: 10.1021/bi00457a004. [DOI] [PubMed] [Google Scholar]
- 30.Kane WH, Majerus PW. Purification and characterization of human coagulation factor V. J Biol Chem. 1981;256:1002–7. [PubMed] [Google Scholar]
- 31.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 32.Chander R, Monahan T. Ultrasound assessment of great saphenous vein insufficiency. J Vascul Diagn Interv. 2015;2015(3):25–31. [Google Scholar]
- 33.Choudhary VA, Patil SS. Role of Doppler ultrasound in lower limb chronic venous insufficiency. Indian J Appl Res. 2016;6:400–1. [Google Scholar]
- 34.Sarin S, Sommerville K, Farrah J, Scurr JH, Coleridge Smith PD. Duplex ultrasonography for assessment of venous valvular function of the lower limb. Br J Surg. 1994;81:1591–5. doi: 10.1002/bjs.1800811108. [DOI] [PubMed] [Google Scholar]
- 35.Folse R, Alexander RH. Directional flow detection for localizing venous valvular incompetency. Surgery. 1970;67:114–21. [PubMed] [Google Scholar]
- 36.Passman MA, McLafferty RB, Lentz MF, Nagre SB, Iafrati MD, Bohannon WT, et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. J Vasc Surg. 2011;54:2S–9S. doi: 10.1016/j.jvs.2011.05.117. [DOI] [PubMed] [Google Scholar]
- 37.Rosing J, Tans G, Govers-Riemslag JW, Zwaal RF, Hemker HC. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980;255:274–83. [PubMed] [Google Scholar]
- 38.Esmon CT, Owen WG, Jackson CM. A plausible mechanism for prothrombin activation by factor Xa, factor Va, phospholipid, and calcium ions. J Biol Chem. 1974;249:8045–7. [PubMed] [Google Scholar]


