Abstract
Nanoparticle-based therapeutics delivery holds great promise for the treatment of intractable diseases. The high loading of drug molecules and their precise delivery to target sites are needed to gain optimal therapeutic functions of the nanoparticle delivery system. In this communication, we highlight, among other properties of nanoparticles (e.g. size, shape, surface chemistry, and degradation), the nanoscale topography, which has recently been shown to be an important parameter, ultimately determining drug loading, cell penetration, and body clearance. This nanotopographical aspect is considered to offer a new effective strategy to the development of nanoparticles for drug and gene delivery with enhanced therapeutic outcome.
Keywords: Therapeutics, nanoparticles, topography, delivery efficiency
Delivery with nanoparticles and the parameters to consider
The delivery of therapeutics (drugs, proteins, and genetic molecules) to intracellular compartments is a critical issue in current nanomedicine. Loading efficiency, cellular uptake, targeting, and release profile are key among the considerations in the delivery process to maximize the therapeutic potential of the delivery systems. Nanoparticles (NPs) are developed to load more cargo molecules and then release sustainably;1–3 NPs are modified to target cells or subcellular components (e.g. mitochondria, nucleus);4–7 NPs are functionalized to respond specifically to environment (e.g. pH, temperature, enzyme, and electricity);8–10 and NPs are also tailored to allow imaging for tracking and diagnosis purposes.3,11–13
Over the last three decades, many NP-based delivery systems, consisting of organics (liposomes, dendrimers, and synthetic polymers), inorganics (silica, iron oxide, gold, and quantum dots), and their composites/hybrids, have been developed.14–16 Typically, NP parameters including shape, size, and surface chemistry have been carefully designed to optimize the loading and delivery capacity. For example, gold NPs with different sizes have been shown to penetrate cells at different rates.17 Among the sizes examined, 45-nm Au NPs exhibited the highest uptake which was considered optimal in minimizing cell membrane energy.17 Also, the variable shapes and sizes of gold NPs showed different cell penetration mechanisms.18 The mesoporous silica nanoparticles (MSN) with a high aspect ratio were shown to be taken up by cells more easily than those with a low aspect ratio.19,20 NPs were also often modified to have pores, channels, or internal cavity to increase the loading of therapeutic molecules.21–23 Silica NPs are the exemplar carriers that exhibit tunable pore structure and thus merit effective molecular loading.
Among other designs of NPs, the surface modification has been a powerful tool to effectively load cargo molecules, to penetrate cell membrane, to escape lysosomal degradation, and to target intracellular component space. For example, MSNs modified with poly-l-lysine (PLL) and polyethyleneimine (PEI) were reported to have improved cellular uptake efficiency.24 Also, when modified with targeting small molecules, peptides, aptamers, antibodies, and proteins, the NPs were demonstrated to follow different internalization pathways that are favorable to avoid lysosomal degradation.25–27 Furthermore, MSNs functionalized with nucleus localization signal (NLS) could reach the nucleus, ultimately leading to higher gene transfection rate.28,29
Therefore, the physical and chemical properties of NPs are of utmost importance in determining the delivery capacity of drugs and genes and their therapeutic efficacy. Among other physicochemical parameters that can affect the cellular uptake and delivery capacity of NPs (as illustrated in Figure 1 and Table 1), recently, the surface nanoscale topography of NPs has gained special attention; it is considered to control the interactions with biological entities, such as bioactive molecules and cells, in the process of molecular loading and cell penetration.30–43 While the nanotopographical impact of scaffolds and matrices on cell responses has been substantially disseminated,44–48 such aspect in the cellular interactions with NPs has recently emerged. In this short communication, we aim to highlight the nanoscale topological effects of NPs on the therapeutics loading and delivery into cells that may advance the capacity of NP-based delivery systems for the treatment of diseased tissues.
Figure 1.
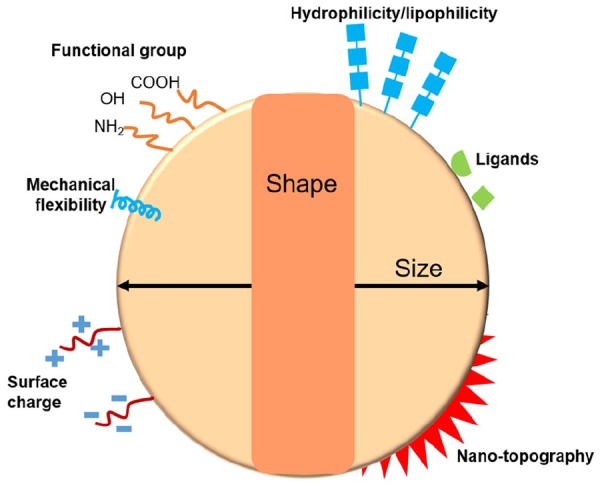
Schematic drawing of the physicochemical parameters of NPs that can affect the cellular uptake and delivery efficiency.
Table 1.
A brief summary of NP parameters (e.g. size, shape, elasticity, and chemistry) that influence the delivery of therapeutic molecules.
| Nanoparticle structure | Shape and surface | Purpose | Pathway | Remarks | References |
|---|---|---|---|---|---|
| MSN | Rod-like and rough surface | 37% higher cellular uptake and drug delivery efficacy | Nanoscale surface roughness | Xu et al.30 | |
| MSN | Virus-like | Enhanced cellular uptake | Caveolae-mediated endocytosis and macropinocytosis | Faster internalization within 5 min and higher tumor cell killing efficiency (42%) compared to conventional NPs (28%) | Wang et al.31 |
| PEI/DNA@DNPs | Virus surface-mimicking | High DOX drug-loading efficiency (97.5%) and boost the gene/chemo co-delivery | Clathrin- and caveolae-independent | Gene transfection efficiency of DNA was improved with increased roughness of decorated DNPs | Sun et al.32 |
| MSN (the core particles 200 nm with shell particles 10 nm) | Virus-mimicking rough surface | To enhance both absorptions of biomolecules and cellular uptake | Influence of nanoscale surface roughness is general, independent of surface charge and cell type | Niu et al.33 | |
| MSN | Rambutan with spike, raspberry with hemisphere, and flower-like with bowl-type | Ram-SNPs-PEI with spiky surfaces show the highest pDNA absorption capacity up to 133 ng·μg−1 and transfection efficacy of 88% | Rough surface enhanced both loading of biomolecules and cellular uptake | Song et al.34 | |
| MSN | Roughness and octadecyl hydrophobic modification (C18) MSN | Both properties (hydrophobic modification and surface roughness) enhanced protein loading capacity and sustained release | Endo/lysosomal escape | Surface roughness with higher protein adsorption capacity and sustained release efficiency | Niu et al.35 |
| Hollow silica with spikes | Pollen-like structures (R-MSHSs) and rough surface | R-MSHS particles showed four times highest lysozyme loading (270 µg mg−1) capacity | Enhanced bacterial adhesion property and antibacterial activity | Song et al.36 | |
| PEI/DNA/AuNP | Virus surface-mimicking hybrid | Enhanced uptake and consequently up to 100-fold promotion of gene transfection efficacy | Outstanding potency for the NIR photothermal therapy in cancerous cells | Hui-Zhen et al.37 | |
| Self-assembly of polyprodrug amphiphiles block copolymer NPs | Spheres, smooth disks, flower-like large compound vesicles (LCVs), and staggered lamellae with the spiked | Staggered lamellae nanoparticles showed the fastest cellular internalization rates, with LCVs the second and spheres the slowest | Clathrin- and caveolae-independent endocytosis pathway is a possibly key role for the internalization of rough nanoparticles | Nanotopography surface roughness significantly reduced repulsive interactions (hydrophilic repulsion and electrostatic), so helping adhesion and entry into cells | Hu et al.38 |
| Fe3O4@MSN microspheres | Cauliflower-like morphology | Higher cell uptake compared with smooth microspheres | Core–shell Fe3O4@MSN microspheres for fast drug delivery in cancer therapy | Yue et al.39 | |
| HPMO nanocapsules (240−310 nm) | Deformable or elastic modulus hollow periodic HPMO | 26-fold enhanced in cellular initialization and killing of cancer cells compared with solid MSN | ELASTIC modulus or deformability, as a main property prompting cellular uptake | Teng et al.40 | |
| MSNs | Asymmetrical head–tail MSNs (HTMSNs) | Higher level of uptake and in vitro maturation of immune cells | HTMSNs show superior hemocompatibility due to reduced membrane deformation of red blood cells and decreased level of reactive oxygen species | Abbaraju et al.41 | |
| Lanthanide-based core/shell/shell structured | Nanoplates | High roughness exhibits excellent performance in BBB transportation and tumor targeting | High roughness exhibits excellent uptake, bioimaging-guided PDT of the brain tumors by MRI and NIR-II FL | Wang et al.42 |
NP: nanoparticle; MSN: mesoporous silica nanoparticles; PEI: polyethyleneimine; DNA: deoxyribonucleic acid; DNP: DNA nanoparticle; DOX: doxorubicin; SNP: silica nanoparticles; R-MSHS: rough hollow silica with pollen-like structure; NIR: near-infrared; HPMO: hollow periodic mesoporous organosilica; BBB: blood–brain barrier; PDT: photodynamic therapy; MRI: magnetic resonance imaging; FL: fluorescence.
Key example studies are also referenced.
Effects of surface nanoscale topography
For the improved therapeutic efficacy of delivery systems several properties of the nanocarriers are important, which include (1) higher loading capacity, (2) better interaction with cell membrane, (3) better cell penetration and favorable internalization pathway, (4) better lysosomal escape, and (5) higher integration with genome (for certain genetic molecules). The topography of NPs is considered to affect the interactions with biological systems. In this part, we discuss the topography-dependent properties and the related biological responses, including biomolecules loading, cell membrane adsorption, cell uptake and pathway, and body clearance.
Biomolecular loading
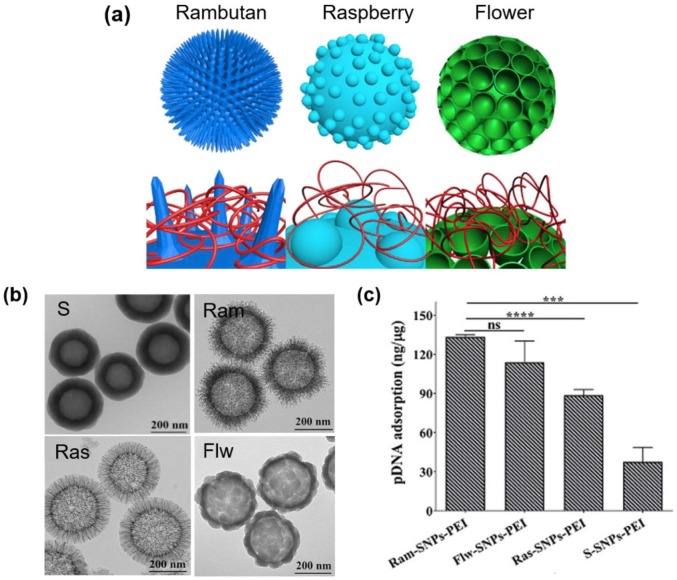
The surface nanoscale topography of particles has significant effect on the biomolecular loading. Recently, the effect of PEI-modified silica nanoparticles (SNP) with different surface roughness on pDNA binding was reported for gene delivery (Figure 2).34 Three different surfaces (rambutan with spike, raspberry with hemisphere, and flower-like with bowl-type) were designed, and in particular, the surface-roughened nanostructures could be generated by the assembly of resorcinol–formaldehyde resin and silica primary particles under Stöber synthesis condition. The outer surface was then PEI-modified. Interestingly, the rambutan-like NPs (Ram-SNPs-PEI) with spiky surface showed the highest pDNA absorption capacity (133 ng/μg) and the resultant high transfection efficacy of 88%, compared to other NPs. The unique surface nanoscale topography was illustrated to protect the DNA molecules effectively against nuclease degradation with respect to other structures. Another report showed the effect of surface roughness of SNPs with octadecyl hydrophobic modification (C18) on the protein loading.35 The rough surface NPs (C18-RSN) showed the adsorption capacity of RNase A biomolecules at significantly higher levels (~twice) than the smooth ones. Moreover, the rough surface NPs showed more sustained release profile. In another study, the rough hollow silica with pollen-like structure (R-MSHSs) was demonstrated to load lysozyme protein more (~4 times) than the smooth ones, which further exhibited sustained lysozyme release (up to 72 h) and effective antimicrobial activity toward Escherichia coli at day 3.36
Figure 2.
Effects of surface nanoscale topography on the pDNA loading efficiency: (a) schematic representation of 3D model images of nanotopography design for plasmid DNA delivery; rambutan, raspberry, and flower-like morphologies developed; (b) TEM images of different NPs; and (c) pDNA loading capacity of different NPs.
Source: Reproduced with permission from Song et al.34
*** and **** are statically significant values.
Among the nanotopological surface designs, virus-like surfaces have gained great attention as drug carriers. For example, MSNs with virus-like topography exhibited higher loading of drug compared with smooth ones and thus better therapeutic efficiency.30 The virus-like NPs loaded with doxorubicin (DOX) displayed higher tumor cell killing efficiency (42%) compared to conventional NPs (28%).31 The carrier-free nanodrug system with DNA decoration was designed to mimic virus surface.32 The nanodrug could load DOX more efficiently (97.5%), suggesting to be effective for gene/chemo codelivery system in cancer therapy. The virus mimicking surface topography was also demonstrated to enhance the adsorption of genetic molecules, including oligoDNA and siRNA,33 regardless of the surface charge and cell type. Taken together, the NP surfaces roughened at the nanoscale might have enhanced surface area that allows more interactive sites with genetic molecules and drugs, ultimately leading to increased loading capacity. In this case, the size and charge of the molecules to load should be carefully taken into account in the design of NPs.
Cell membrane adsorption
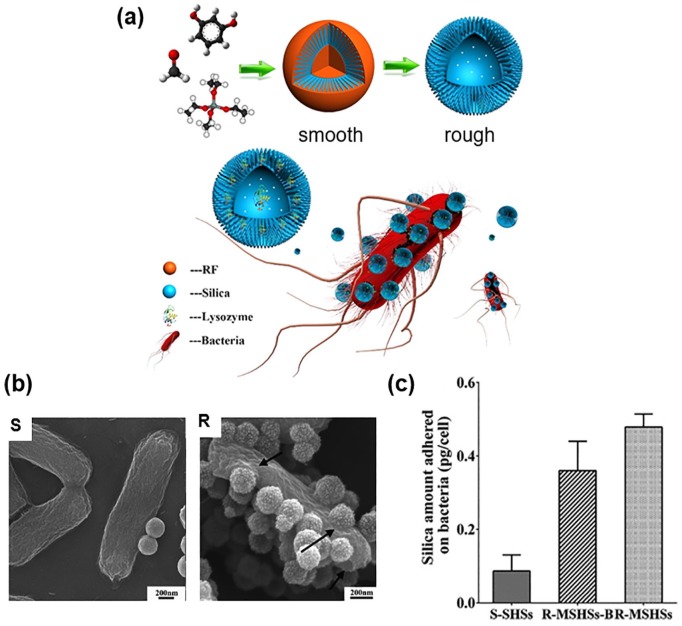
There is growing interest in how the surface topography of NPs interacts with cell membrane as the cell surface interaction is the first step for NP uptake by the cell. The physicochemical properties of NPs such as size, shape, and surface charge have been demonstrated as the typical parameters that prompt cellular interaction and adhesion. Among others, the surface nanoscale topography provides different surface area and energy to the NPs, influencing the interactions with biological entities including cell membrane molecules. In the study by Song et al.,36 the silica nanopollens with engineered rough surface were designed to enhance the bacterial adhesion (Figure 3). By introducing resorcinol, formaldehyde, and tetraethyl orthosilicate into a typical Stöber synthesis solution, a nanocomposite was assembled by selectively forming a resorcinol–formaldehyde core first, followed by a co-condensation of silica and resorcinol–formaldehyde interpenetrating shell. After calcination, silica nanopollens with accessible inner cavity and spiky surface could be obtained. The nanopollen surface was considered to have multivalent interactions induced by the surface spikes during the contact with the hairy bacteria surface (the number of particle contacts to bacteria was ~10 times higher in nanopollen surface). Because of the stronger adhesion via a large number of contacts, lysozyme was released more slowly, being enriched on the bacterial (E. coli) surface. This silica nanopollen-based lysozyme formulation exhibited potent antibacterial activity. In another report, MSNs with three different surface topologies, including smooth, wrinkle, and rough spiky surface, were developed as nanocarriers and modified with aminopropyl groups to load Ag for antibacterial application. The wrinkle and rough spiky surfaces exhibited higher antibacterial activity because of direct interaction of Ag NPs with the bacterial cell membrane.49
Figure 3.
Silica nanopollens developed for bacterial membrane adhesion and antibacterial activity: (a) schematic showing the synthesis of silica nanopollens and the lysozyme-loaded nanopollens adhesive to bacterial surface; (b) SEM images of smooth and rough NPs adhered to Escherichia coli surface; and (c) NP amount adhered to bacteria measured by ICP-OES analysis.
Source: Reproduced with permission from Song et al.36
As to the nature of the rough particle–cell membrane contact, nonspecific binding forces were proposed by Nel and colleagues.50 Based on simulation, the nanotopographical surface roughness was demonstrated to decrease repulsive interactions (hydrophilic and electrostatic) significantly, thereby promoting adhesion and cellular uptake.51 Similarly, another group reported that the surface roughness of self-assembled block copolymer NPs significantly enhanced the cell adhesion and entry into cells due to the significantly reduced repulsive interactions.38 The incorporation of cyclodextrins in poly(anhydride) NPs also increased the bio-adhesive capacity which was correlated with the enhanced surface area.52 Therefore, the increased contact of particles with cell membrane molecules is considered as the primary reason for the higher cell binding of the NPs with nano-roughened surfaces; however, the detailed binding mechanism is yet to be elucidated, which requires further investigation.
Intracellular uptake and pathway
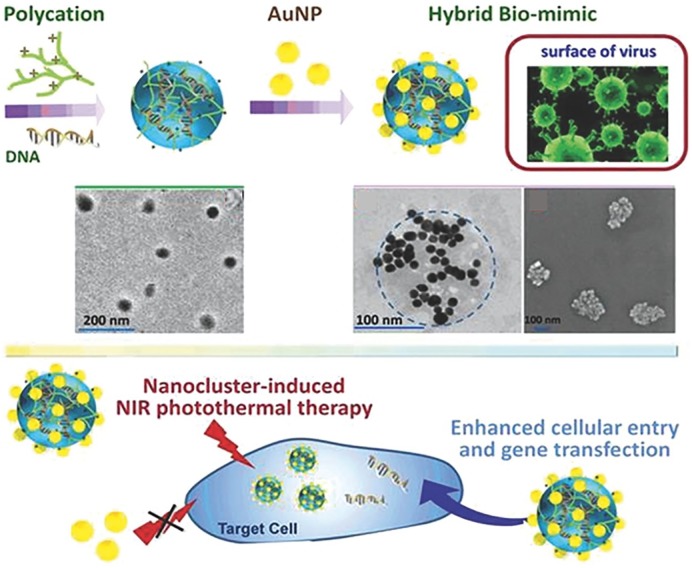
The intracellular uptake rate and the endocytic cellular pathway of NPs are affected by many physicochemical properties of NP. While studies have mainly focused on the size, shape, surface charge, functional group, and hydrophilicity of NPs, the effect of nanoscale surface topography of NPs on cellular uptake and pathway has recently been highlighted. Among the endocytosis pathways (clathrin-mediated, caveolae-mediated, clathrin/caveolae-independent, and micropinocytosis), the clathrin-mediated pathway is known to bring about ineffective drug delivery due to the lysosomal degradation of particles. Interestingly, the rough surface NPs have been shown to effectively avoid this pathway by adopting the other pathways. The MSNs designed to be virus-like displayed higher cellular internalization than the conventional MSN,31 which utilized the caveolae-mediated endocytosis and macropinocytosis, different from the conventional ones. In another study, the carrier-free nanodrug system was developed based on the virus-mimicking surface decorated with DNA-captured NPs (PEI/DNA@DNPs). The NPs were demonstrated to enhance the gene transfection efficiency,32 showing an energy-dependent process and the clathrin- and caveolae-independent internalization pathways. Recently, the DNA-loaded nanocomplexes were designed to have virus-mimic surface by decorating with Au NPs.37 The plasmid condensed with PEI (PEI800) was used as the organic core and the citrated Au NPs were electrostatically decorated to produce virus-surface mimic hybrid NPs. The designed PEI/pDNA/Au nanohybrid significantly enhanced cellular uptake and transfection efficacy (100-fold) when compared with the PEI/pDNA alone without Au NPs and ultimately increased gene/photothermal therapeutic effects (Figure 4). In another study on gene delivery with roughened NPs, the intracellular pDNA trafficking was observed by confocal microscopy.34 Among the different nanoscale topographies of MSNs, the rambutan-like spiky surface NPs enabled effective protection against nuclease degradation in cells. This work highlights the effect on degradation protection of genetic molecules implemented by the surface nanotopography. Recently, one intriguing study reported the effect on red blood cells.41 The MSNs engineered with asymmetrical head–tail morphology showed enhanced cellular uptake and higher hemocompatibility due to reduced membrane deformation of red blood cells and decreased level of reactive oxygen species. Taken the studies together, the virus-like or nanoroughened surfaces were demonstrated to significantly enhance the gene transfection efficiency either by altering the intracellular trafficking pathway or by protecting the genetic molecules from an enzymatic degradation.
Figure 4.
Virus surface-mimicking nanohybrid developed for enhanced cellular entry and gene transfection. Schematic showing the synthesis of DNA-loaded NPs decorated with AuNPs and the TEM and SEM images. Illustration showing the therapeutic efficacy via nanocluster-induced NIR photothermal therapy.
Source: Reproduced with permission from Hui-Zhen et al.37
Blood circulation
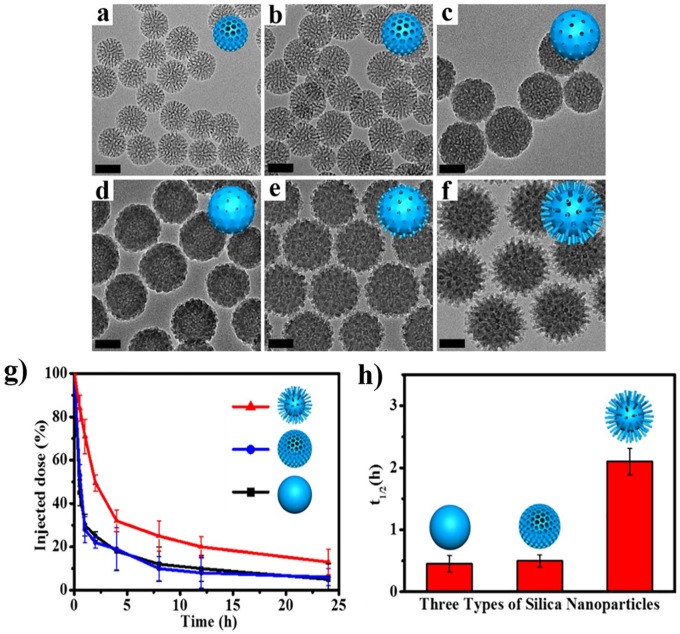
The blood circulation of NPs with different surface nanotopographies (smooth, porous, and spiky) has been reported with the MSN system.31 The surface nanotopographies could be obtained by a biphase reaction method with a low surfactant concentration, which allows the coassembly of reactants to take place at the oil–water interface for continuous interfacial/epitaxial growth. By changing the reaction time, the nanostructure was then controlled from porous to spiky surface (Figure 5). The spiky surface obtained with a longer reaction time showed a blood circulation time (t1/2 = 2.16 h) much more extended than the other smooth or porous NPs (0.45 h). In a similar study, the blood circulation was recorded to be much longer for the staggered lamellae NPs with respect to the other NPs of smooth disks and spheres.38 Such an extended blood circulation was considered to be due to a good serum stability. The carrier-free nanodrug system (PEI/DNA with DOX) also demonstrated prolonged blood circulation time for the rough surface case.32 Moreover, worm-like micelles were shown to have 1-week blood circulation time, which was approximately 10 times longer than the conventional sphere ones.53 Thus, these studies highlight the importance of surface nanoscale topography along with particle shape in the interactions with blood and circulation time, which may help designing NPs to control the accumulation and targeted disease treatment with NPs in the future studies.
Figure 5.
Controlled surface nanoscale topography of MSNs at different reaction times: (a) 6 h, (b) 12 h, (c) 18 h, (d) 24 h, (e) 36 h, and (f) 48 h. In vivo blood circulation study with different surface nanoscale topographies; after intravenous injection, (g) time-dependent level of NPs in blood and (h) blood circulation half-life (t1/2) calculated.
Source: Reproduced with permission from Wang et al.31
Concluding remarks
Here, we communicated the importance of a surface nanoscale topography of NPs in the interactions with biological entities (biomolecules and cells). Compared to other physicochemical parameters of NPs (e.g. size, shape, elasticity, and surface chemistry), the surface nanoscale topography has more recently been highlighted. As witnessed in a body of literature, the engineered nanoscale topography of NPs has significant impact on a series of biological events, such as drug/gene loading, cell surface binding, intracellular trafficking, and the stability in blood circulation, all of which are key in determining the therapeutic effects of the NP delivery system. It is thus envisaged that the engineering of NPs with unique surface nanoscale topography may offer an effective design strategy to take full advantage of the physical property of NPs, which may ultimately be helpful for the treatment of diseases.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grants from Dankook University (Priority Institute Support Program in 2019) and National Research Foundation (2018K1A4A3A01064257, 2017R1C1B1011387, and 2018R1A2B3003446), Republic of Korea.
ORCID iD: Hae-Won Kim  https://orcid.org/0000-0001-6400-6100
https://orcid.org/0000-0001-6400-6100
References
- 1. Jafari S, Derakhshankhah H, Alaei L, et al. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother 2019; 109: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 2. Vallet-Regí M, Colilla M, Izquierdo-Barba I, et al. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules 2017; 23: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh RK, Patel KD, Mahapatra C, et al. C-dot generated bioactive organosilica nanospheres in theranostics: multicolor luminescent and photothermal properties combined with drug delivery capacity. ACS Appl Mater Inter 2016; 8: 24433–24444. [DOI] [PubMed] [Google Scholar]
- 4. Hu J-J, Xiao D, Zhang X-Z. Advances in peptide functionalization on mesoporous silica nanoparticles for controlled drug release. Small 2016; 12: 3344–3359. [DOI] [PubMed] [Google Scholar]
- 5. Zhang C, Wu W, Li R-Q, et al. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv Funct Mater 2018; 28: 1804492. [Google Scholar]
- 6. Qin S-Y, Zhang A-Q, Zhang X-Z. Recent advances in targeted tumor chemotherapy based on smart nanomedicines. Small 2018; 14: 1802417. [DOI] [PubMed] [Google Scholar]
- 7. Chen W-H, Luo G-F, Zhang X-Z. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv Mater 2019; 31: 1802725. [DOI] [PubMed] [Google Scholar]
- 8. Baeza A, Colilla M, Vallet-Regí M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin Drug Del 2015; 12: 319–337. [DOI] [PubMed] [Google Scholar]
- 9. Baek S, Singh RK, Khanal D, et al. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015; 7: 14191–14216. [DOI] [PubMed] [Google Scholar]
- 10. Singh RK, Patel KD, Mahapatra C, et al. Combinatory cancer therapeutics with nanoceria-capped mesoporous silica nanocarriers through pH-triggered drug release and redox activity. ACS Appl Mater Inter 2019; 11: 288–299. [DOI] [PubMed] [Google Scholar]
- 11. Baek S, Singh RK, Kim T-H, et al. Triple hit with drug carriers: pH- and temperature-responsive theranostics for multimodal chemo- and photothermal therapy and diagnostic applications. ACS Appl Mater Inter 2016; 8: 8967–8979. [DOI] [PubMed] [Google Scholar]
- 12. Singh RK, Patel KD, Leong KW, et al. Progress in nanotheranostics based on mesoporous silica nanomaterial platforms. ACS Appl Mater Inter 2017; 9: 10309–10337. [DOI] [PubMed] [Google Scholar]
- 13. Patel KD, Singh RK, Kim H-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater Horiz 2019; 6: 434–469. [Google Scholar]
- 14. Naz S, Shamoon M, Wang R, et al. Advances in therapeutic implications of inorganic drug delivery nano-platforms for cancer. Int J Mol Sci 2019; 20: 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008; 2: 889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater 2019; 2019: 3702518. [Google Scholar]
- 17. Wang S-H, Lee C-W, Chiou A, et al. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnol 2010; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding L, Yao C, Yin X, et al. Size, shape, and protein corona determine cellular uptake and removal mechanisms of gold nanoparticles. Small 2018; 14: 1801451. [DOI] [PubMed] [Google Scholar]
- 19. Hao N, Yang H, Li L, et al. The shape effect of mesoporous silica nanoparticles on intracellular reactive oxygen species in A375 cells. New J Chem 2014; 38: 4258–4266. [Google Scholar]
- 20. Huang X, Teng X, Chen D, et al. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010; 31: 438–448. [DOI] [PubMed] [Google Scholar]
- 21. Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 2012; 24: 1504–1534. [DOI] [PubMed] [Google Scholar]
- 22. Perez RA, Singh RK, Kim T-H, et al. Silica-based multifunctional nanodelivery systems toward regenerative medicine. Mater Horiz 2017; 4: 772–799. [Google Scholar]
- 23. He Q, Shi J. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J Mater Chem 2011; 21: 5845–5855. [Google Scholar]
- 24. Hom C, Lu J, Tamanoi F. Silica nanoparticles as a delivery system for nucleic acid-based reagents. J Mater Chem 2009; 19: 6308–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chompoosor A, Saha K, Ghosh PS, et al. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small 2010; 6: 2246–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small 2010; 6: 12–21. [DOI] [PubMed] [Google Scholar]
- 27. Li Z, Zhang Y, Zhu D, et al. Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways. Drug Deliv 2017; 24: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan L, He Q, Liu J, et al. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J Am Chem Soc 2012; 134: 5722–5725. [DOI] [PubMed] [Google Scholar]
- 29. Li Z-Y, Liu Y, Hu J-J, et al. Stepwise-acid-active multifunctional mesoporous silica nanoparticles for tumor-specific nucleus-targeted drug delivery. ACS Appl Mater Inter 2014; 6: 14568–14575. [DOI] [PubMed] [Google Scholar]
- 30. Xu C, Niu Y, Popat A, et al. Rod-like mesoporous silica nanoparticles with rough surfaces for enhanced cellular delivery. J Mater Chem B 2014; 2: 253–256. [DOI] [PubMed] [Google Scholar]
- 31. Wang W, Wang P, Tang X, et al. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent Sci 2017; 3: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun X, Li M, Yang Y, et al. Carrier-free nanodrug-based virus-surface-mimicking nanosystems for efficient drug/gene co-delivery. Biomater Sci 2018; 6: 3300–3308. [DOI] [PubMed] [Google Scholar]
- 33. Niu Y, Yu M, Hartono SB, et al. Nanoparticles mimicking viral surface topography for enhanced cellular delivery. Adv Mater 2013; 25: 6233–6237. [DOI] [PubMed] [Google Scholar]
- 34. Song H, Yu M, Lu Y, et al. Plasmid DNA delivery: nanotopography matters. J Am Chem Soc 2017; 139: 18247–18254. [DOI] [PubMed] [Google Scholar]
- 35. Niu Y, Yu M, Meka A, et al. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J Mater Chem B 2016; 4: 212–219. [DOI] [PubMed] [Google Scholar]
- 36. Song H, Ahmad Nor Y, Yu M, et al. Silica nanopollens enhance adhesion for long-term bacterial inhibition. J Am Chem Soc 2016; 138: 6455–6462. [DOI] [PubMed] [Google Scholar]
- 37. Hui-Zhen J, Wei-Hai C, Xuli W, et al. Virus-surface-mimicking surface clustering of AuNPs onto DNA-entrapped polymeric nanoparticle for enhanced cellular internalization and nanocluster-induced NIR photothermal therapy. Adv Sci 2015; 2: 1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu X, Hu J, Tian J, et al. Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J Am Chem Soc 2013; 135: 17617–17629. [DOI] [PubMed] [Google Scholar]
- 39. Yue Q, Zhang Y, Jiang Y, et al. Nanoengineering of core–shell magnetic mesoporous microspheres with tunable surface roughness. J Am Chem Soc 2017; 139: 4954–4961. [DOI] [PubMed] [Google Scholar]
- 40. Teng Z, Wang C, Tang Y, et al. Deformable hollow periodic mesoporous organosilica nanocapsules for significantly improved cellular uptake. J Am Chem Soc 2018; 140: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 41. Abbaraju PL, Meka AK, Song H, et al. Asymmetric silica nanoparticles with tunable head–tail structures enhance hemocompatibility and maturation of immune cells. J Am Chem Soc 2017; 139: 6321–6328. [DOI] [PubMed] [Google Scholar]
- 42. Wang P, Wang C, Lu L, et al. Kinetics-mediate fabrication of multi-model bioimaging lanthanide nanoplates with controllable surface roughness for blood brain barrier transportation. Biomaterials 2017; 141: 223–232. [DOI] [PubMed] [Google Scholar]
- 43. Han K, Zhang J, Zhang W, et al. Tumor-triggered geometrical shape switch of chimeric peptide for enhanced in vivo tumor internalization and photodynamic therapy. ACS Nano 2017; 11: 3178–3188. [DOI] [PubMed] [Google Scholar]
- 44. Allan C, Ker A, Smith CA. Osteoblast response to disordered nanotopography. J Tissue Eng 2018; 9: 2041731418784098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Curtis A, Wilkinson C. Topographical control of cells. Biomaterials 1997; 18: 1573–1583. [DOI] [PubMed] [Google Scholar]
- 46. Damiati L, Eales MG, Nobbs AH. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J Tissue Eng 2018; 9: 2041731418790694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Costa E, Gonzalez-Garcia C, Gomez Ribelles JL, et al. Maintenance of chondrocyte phenotype during expansion on PLLA microtopographies. J Tissue Eng 2018; 9: 2041731418789829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007; 6: 997–1003. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Wang Y, Su L, et al. Effect of surface topology morphologies of silica nanocarriers on the loading of Ag nanoparticles and antibacterial performance. J Alloy Compd 2019; 783: 136–144. [Google Scholar]
- 50. Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 2009; 8: 543–557. [DOI] [PubMed] [Google Scholar]
- 51. Hoek EMV, Agarwal GK. Extended DLVO interactions between spherical particles and rough surfaces. J Colloid Interf Sci 2006; 298: 50–58. [DOI] [PubMed] [Google Scholar]
- 52. Agüeros M, Areses P, Campanero MA, et al. Bioadhesive properties and biodistribution of cyclodextrin–poly(anhydride) nanoparticles. Eur J Pharm Sci 2009; 37: 231–240. [DOI] [PubMed] [Google Scholar]
- 53. Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2007; 2: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]