Abstract
Objective:
This study aims to investigate the expression, role, and detailed mechanism of microRNA-204-5p and zinc finger protein 521 in gastric cancer.
Methods:
Immunohistochemistry was adopted to detect the expressions of zinc finger protein 521 in 82 cases of gastric cancer tissues. Western blot was used to detect the expressions of zinc finger protein 521 in gastric cancer cells and adjacent cells. Moreover, the correlation between zinc finger protein 521 and the prognosis of patients were also evaluated. Cell Counting Kit 8 assay and colony formation assay were performed to figure out the impact of zinc finger protein 521 on the proliferation of gastric cancer cells. By conducting flow cytometry, the effect of zinc finger protein 521 on the apoptosis of gastric cancer cells was determined. The scratch wound healing assay and transwell invasion assay were carried out to determine the effect of zinc finger protein 521 on regulating the motility and invasion of gastric cancer cells. Ultimately, the targeting relationship and interaction between microRNA-204-5p and zinc finger protein 521 were verified by real-time polymerase chain reaction, Western blot, and dual luciferase reporter gene assay.
Results:
Compared with adjacent cells, zinc finger protein 521 was highly expressed in gastric cancer cells, which was related to TNM stage (P = .0388), tumor size (P = .0168), and local lymph node metastasis (P = .0024). Overexpressed zinc finger protein 521 can promote the proliferation, migration, and invasion of gastric cancer cells and inhibit the apoptosis. Zinc finger protein 521 is a target gene of microRNA-106-5p, and there was a negative correlation between the expression of zinc finger protein 521 and microRNA-204-5p.
Conclusion:
Zinc finger protein 521 can arrest the apoptosis and enhance the proliferation, migration, and invasion of gastric cancer cells via regulating microRNA-204-5p. Our study may provide novel clues for the treatment of patients with gastric cancer.
Keywords: ZNF521, miR-204-5p, gastric cancer
Background
Gastric cancer (GC) is the leading cause of cancer-related mortality worldwide.1 The incidence and mortality of patients with GC in China account for 42.6% and 45.0% of the world, respectively.2 Although the surgery, chemotherapy, and targeted drugs offer symptomatic relief and modest improvement in survival for patients with GC, the overall survival rate remains unfavorable. Moreover, the serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen19-9, lack high sensitivity and specificity.3 Hence, an in-depth investigation into the underlying mechanism of GC and effective targets that can facilitate disease detection, staging, and prediction of therapeutic outcome are highly desirable to improve survival rate and help to determine optimized treatment for GC.
With the further study on GC pathogenesis, numerous studies have pointed out that transcription factors are abnormally expressed in GC cells, thereby affecting the tumorigenesis. For example, zinc finger protein (ZFP) family, a kind of vital transcription factors, exerts great impact on the expressions of downstream genes by binding to the downstream gene promoters.4–7 Zinc finger protein 521 (ZNF521) belongs to C2H2 ZFP family and consists of 1311 amino acid sequences. These amino acids form 30 Kruppel-like zinc finger domains interspersed in their whole amino acid sequences. Zinc finger structures, forming a total of 6 zinc finger clusters, participate in multiple signaling pathways and regulate the downstream target genes, thus affecting various biological processes such as hematopoietic differentiation, cell proliferation, and so on.8–10 In addition, mounting studies have manifested that ZNF521 is of great importance in a variety of tumors like multiple myeloma,11 pre-B-cell lymphoma,12 and so on. Nevertheless, the expression and function of ZNF 521 in GC are far from being fully elucidated.
MicroRNA (miRNA) is a class of small noncoding RNA, containing 18 to 25 nucleotides that specifically bind to the target gene 3-untranslated region (3′-UTR) to cause translation inhibition or messenger RNA (mRNA) degradation, thus regulating in gene expression.13–15 For instance, miR-196a and miR-196b were verified to be upregulated in GC tissues.16,17 MicroRNA-338 can inhibit the proliferation and migration of GC cells.18 Accumulating studies have demonstrated that miR-204-5p was aberrantly expressed in a variety of tumors such as melanoma and GC.19,20 MicroRNA-204-5p also exerts crucial effects on regulating the proliferation, migration, invasion, and other malignant biological behaviors of cancer cells, whereas its role in GC cells has not been fully clarified.
Bioinformatics suggested that ZNF521 was one of the target genes of miR-204-5p. This study aims to ascertain the relationship between the expression of ZNF521 in GC cells and the clinicopathological features as well as the prognosis of patients, and to further determine the effects of ZNF521 on the proliferation and metastasis of GC cells in vitro. Meanwhile, we also attempted to confirm the effect of miR-204-5p on targeting ZNF521, thus shed new light on the diagnosis and treatment for GC.
Materials and Methods
Tissue Collection
Cell Lines and Main Reagents
Human GC cell lines (AGS, MKN-28, SGC-823, MKN-45, and NCI-N87) and human normal gastric epithelial cells (GES-1) were provided by the Cell Center of Chinese Academy of Medical Sciences. All cells were cultured in Dulbecco Modified Eagle Medium (Hyclone, Logan, Utah) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, New York), 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37°C. The culture medium was replaced at an interval of 3 to 4 days; 0.25% trypsin (Americesco, Frramingham, Massachusetts) was used for subculture.
Immunohistochemistry
Paraffin-coated GC tissues and adjacent tissues were sliced, dewaxed, and hydrated by xylene. The GC cells and matched normal cells were incubated with 0.3% H2O2 solution at room temperature for 30 minutes. Subsequently, cells were incubated with the primary antibody (anti-ZNF521 antibody-N-terminal, [Abcam, Shanghai, China], ab189956, 1:100) for 1 hour and then with the goat anti-rabbit secondary antibody (Wuhan Service Biotechnology Co, Ltd, Wuhan, China) for 20 minutes. Subsequently, slices were rinsed thoroughly with phosphate-buffered saline (PBS) solution. Then, the development was achieved using DAB (Hubei Biossci Biotechnology Co, Ltd, Wuhan, China). The scoring standard of immunohistochemistry was completed by pathologists from the department of pathology in our hospital.
Western Blot
AGS and MKN-28 cells were lysed with radioimmunoprecipitation assay lysis buffer containing protease inhibitor, the supernatant was collected after high speed centrifugation and heated in a water bath pot at 100 °C for 10 minutes to denaturalize the protein. The content of the protein was determined by bicinchoninic acid method. After that, Sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transmembrane were performed. Cells were incubated with primary antibodies at 4 °C overnight and then with the goat antirabbit secondary antibody (Wuhan Service Biotechnology Co, Ltd, GB23303, 1:2000) at room temperature for 1 hour. The primary antibodies include ZNF521 antibody (Abcam, ab189956, 1:1000), Bcl-2 antibody (Abcam, ab185002, 1:1000), BAX antibody (Abcam, ab32503, 1:1000), E-cadherin antibody (Abcam, ab194982, 1:1000), and N-cadherin antibody (Abcam, ab202030, 1:1000). Ultimately, color rendering was performed using hypersensitive enhanced chemiluminescence (ECL) (Hubei Biossci Biotechnology Co, Ltd.).
Cell Transfection
With Lipofectamine 2000 (Invitrogen, Shanghai, China), ZNF521-related plasmid was transfected into AGS transiently to establish overexpressed ZNF521 model successfully, while ZNF521 low expression model was set up by transfecting ZNF521 short hairpin RNA (shRNA) into MKN-28. We proceeded to construct AGS-ZNF521 group with overexpressed ZNF521 plasmid and AGS cell.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA from tissue and cultured cells was isolated using TRIZOL Regent (Invitrogen). Quantitative polymerase chain reaction (PCR) primers were purchased from Guangzhou Genecopoeia Company. The primer sequences are shown as follows: miR-204-p: 5′-UUCCCUUUGUCAUCCUAUGCCU-3′; U6: F: 5′-GAGGCACAGCGGAACG-3′, R: 5′-CTACCACATAGTCCAGG-3′; ZNF521, F: 5′-TGACACCTCTGAGCCTAT-3′; R: 5′-TTTCTTTTTCACGATGGCACTTTCT-3′. The results were calculated by 2−ΔΔCt method.
Cell Counting Kit 8 Assay
AGS and MKN-28 cells were harvested in logarithmic phase and trypsinized with trypsin (0.25%). Afterward, cells were cultured in 96-well plates with the density of 2 × 105/mL; 10 μL enhanced Cell Counting Kit 8 (CCK-8; Hubei Biossci Biotechnology Co, Ltd.) was added to continue the culture. After 1-hour culture, the absorbance value of each well was measured at 490 nm on the microplate reader.
Colony Formation Assay
AGS and MKN-28 cells with a concentration of 2 × 105/mL were planted in a 6-well plate. After 2 weeks of culture, the culture medium was discarded and carefully washed with PBS twice. Cells were then fixed with 10% paraformaldehyde for 10 minutes and stained with 0.1% crystal purple for 15 minutes. The number of colonies formed was recorded under a microscope.
Flow Cytometry
The transfected AGS, MKN-28 cells, and control cells were washed with cold PBS twice and then resuspended in binding buffer. The cells were then stained with FITC Annexin V apoptosis detection kit (BD Bioscience, Bedford, Massachusetts) in dark at room temperature for 30 minutes. Flow cytometry (Becton Dickinson, Mountain View, California) was performed to detect the apoptosis at 24 hours.
Scratch Wound Healing Assay
Using the tip of 200 μL aseptic pipette, the monolayer cells were scratched in a standard way to create a cell-free area. The culture medium was sucked out and replaced by a fresh complete culture medium, and then the cells were incubated at 37 °C for 48 hours. The cell motility was observed.
Transwell Assay
The serum-free medium at a density of 1 × 104 cells per well was placed into the upper chamber of transwell. After 24 hours of culture, the cells migrating to the lower chamber were fixed and counted. The steps of invasion assay were the same as the migration assay except for a layer of matrix glue (BD Biosciences, California) at the bottom of the transwell chamber.
Luciferase Reporter Gene Assay
Wild type (WT) or mutant type (MUT) ZNF521 was subcloned into psiCHECK-2 plasmids. The cells were then inoculated into a 24-well plate, 5000 cells per well. Luciferase activity was determined by dual luciferase system (Promega, Madison, Wisconsin). AGS and MKN-28 cells were cotransfected with miR-204-5p or miR-normal control and WT or MUT report vector, respectively. Luciferase activity was measured 48 hours after transfection.
Statistical Analysis
All analyses and graphs of this study were processed using GraphPad Prism 7 software. The difference of measurement data between the 2 groups was compared by t test. The χ2 test was conducted to analyze the correlation between ZNF521 expression and various pathological indexes. Kaplan-Meier method was employed to analyze survival time. Differences were considered significant with a value of P < .05.
Results
Zinc Finger Protein 521 Was Highly Expressed in GC Cells
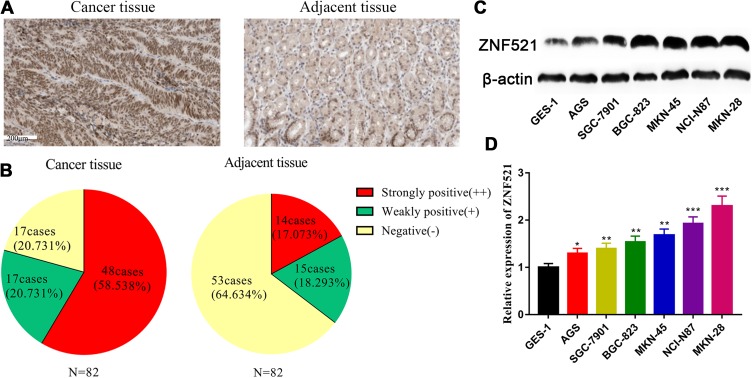
To determine the expression of ZNF521 in GC cells, ZNF521 expressions in GC tissues and adjacent tissues were detected by immunohistochemistry. The immunohistochemical images of GC tissues (left) and adjacent normal tissues (right) were represented in Figure 1A. The expressions of ZNF521 in GC tissues were significantly higher than that in adjacent tissues (P < .0001; Figure 1B). Following that, Western blot was conducted to detect the expressions of ZNF521 (Figure 1C). The results signified that compared with normal gastric epithelial cell GES-1, the expressions of ZNF521 in 6 GC cell lines were significantly higher (P < .05; Figure 1D).
Figure 1.
The expression of ZNF521 was increased in GC cells and tissues. A, Immunohistochemical images of GC tissues (left) and adjacent tissues (right). B, Immunohistochemistry was used to detect the difference of ZNF521 expressions between GC tissues and adjacent tissues (P < .001). C, Western blot was carried out to detect the expressions of ZNF521 in GC cell lines (MKN-28, SGC-7901, BGC-823, MKN-45, NCI-N87, and AGS) and normal gastric mucosal cells (GES-1). *, ** and *** refer to P < .05, P < .01, and P < .001, respectively. GC indicates gastric cancer; ZNF521, zinc finger protein 521.
The Expression of ZNF521 Was Correlated With Pathological Features in Patients With GC
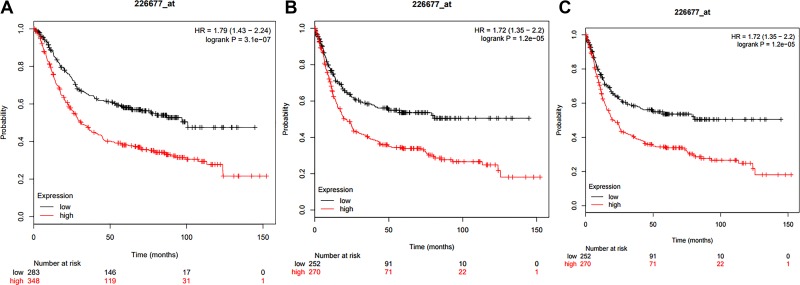
Further analyses uncovered that ZNF521 expression was correlated with tumor size (P = .017), TNM stage (P = .039), and local lymph node metastasis (P = .004) in patients with GC, while it had no relevance to age, gender, serum CEA level, and tumor differentiation (P > .05). The results suggested overexpressed ZNF521 might be involved in the proliferation and migration of GC cells (Table 1). Further review and analysis by Kaplan-Meier method (data from kmplotter.com) revealed that there was a significant correlation between the increased expression of ZNF521 and the decrease of the overall survival time (P < .001), increased first progression (P < .001), as well as shorter survival time after progression (P < .001; Figure 2). It referred that patients with higher expression of ZNF521 had worse prognosis than patients with low-expressed ZNF521, which hints its role as a potential biomarker for GC.
Table 1.
Relationship Between the Expression of ZNF521 and the Clinical Features of 82 Patients With Gastric Cancer.
| Characteristics | n | Relative Expression of ZNF521 | χ2 | P | |
|---|---|---|---|---|---|
| High | Low | ||||
| Age | |||||
| ≤55 | 51 | 41 | 10 | 0.1037 | .7474 |
| >55 | 31 | 24 | 7 | ||
| Gender | |||||
| Male | 39 | 30 | 9 | 0.2489 | .6178 |
| Female | 43 | 35 | 8 | ||
| Tumor size, cm | |||||
| <5 | 24 | 15 | 9 | 5.8055 | .0159 |
| ≥5 | 58 | 50 | 8 | ||
| T stage | |||||
| T1-T2 | 33 | 22 | 11 | 5.3364 | .0209 |
| T3-T4 | 49 | 43 | 6 | ||
| CEA, μg/L | |||||
| <5 | 46 | 34 | 12 | 1.8285 | .1763 |
| ≥5 | 36 | 31 | 5 | ||
| Degree of differentiation | |||||
| High | 43 | 36 | 7 | 1.6101 | .4471 |
| Middle | 23 | 18 | 5 | ||
| Low | 16 | 11 | 5 | ||
| Lymph node metastasis | |||||
| Negative | 35 | 23 | 12 | 5.0705 | .0243 |
| Positive | 47 | 42 | 5 | ||
Abbreviations: CEA, carcinoembryonic antigen; ZNF521, zinc finger protein 521.
Figure 2.
Comparison of prognostic indications between patients with overexpression of zinc finger protein 521 (ZNF521) and those with low expression of ZNF521. A, Comparison of the overall survival (OS) between patients with overexpression of ZNF521 and those with low expression of ZNF521. B, Comparison of the first progression (FP) between patients with overexpression of ZNF521 and those with low expression of ZNF521. C, Comparison of the postprogression survival (PPS) between patients with overexpression of ZNF521 and those with low expression of ZNF521.
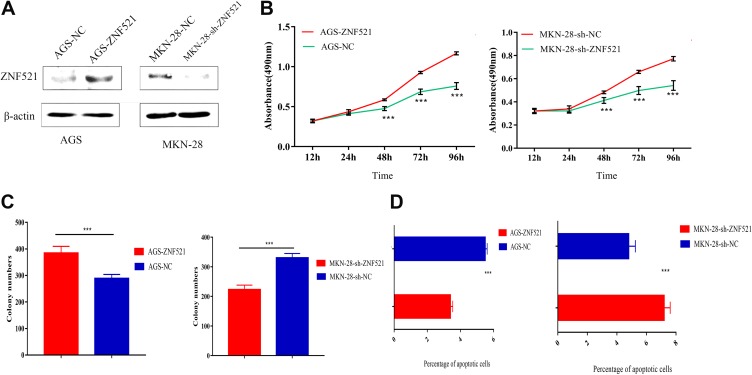
ZNF521 Can Regulate the Proliferation and Apoptosis of GC Cells
To further define the impact of ZNF521 on GC cells, ZNF521 plasmid was transfected into AGS to construct ZNF521 overexpression model, and ZNF521 shRNA was transfected into MKN-28 to establish ZNF521 low-expression model (Figure 3A). On this basis, CCK8 assay was conducted to detect the proliferation of AGS and MKN-28 cells. It turned out that AGS cells with overexpressed ZNF521 grew faster than the control one, while MKN-28 cells with knockdown ZNF521 had lower rate of proliferation (Figure 3B). Additionally, overexpressed ZNF521 group of AGS cell had larger number of cell colonies than the control group (364 ± 26 vs 312 ± 15, P < .05). In comparison, the number of colonies of MKN-28 cell with the knockdown of ZNF521 was less than the control group (256 ± 16 vs 330 ± 15, P < .05; Figure 3C). The apoptosis assay validated that the apoptosis rate of AGS with overexpressed ZNF521 in advanced stage of cancer was lower than those of the control group (P < .00). On the contrary, MKN-28 with knockdown ZNF521 had higher rate of apoptosis than the control group (P < .001; Figure 3D).
Figure 3.
Zinc finger protein 521 potentiates the proliferation but impedes the apoptosis of GC cells. A, The transfection efficiency was detected by Western blot. B, Colony formation assay was conducted to monitor the proliferation of AGS (left) with overexpressed ZNF521 and MKN-28 (right) with knockdown ZNF521. C, Flow cytometry was carried out to detect the apoptosis of AGS (left) with overexpressed ZNF521 and MKN-28 (right) with knockdown ZNF521. *** refers to P < .001. GC indicates gastric cancer; ZNF521, zinc finger protein 521.
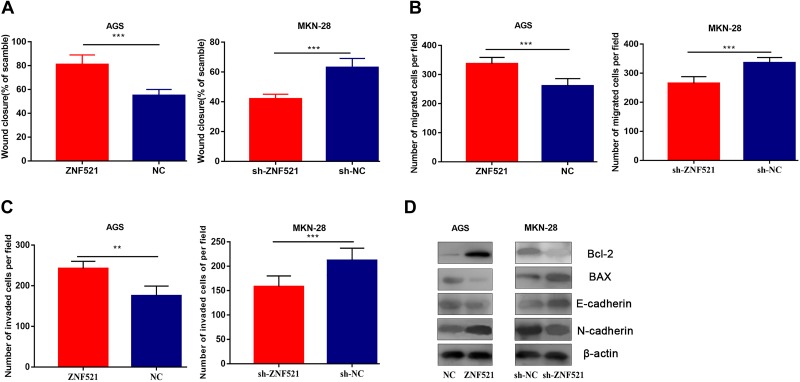
ZNF521 Facilitates the Motility, Migration, and Invasion of GC Cells
The scratch healing assay unmasked that the motility of AGS with overexpressed ZNF521 was dramatically elevated compared with the control group (P < .01). In contrast, the motility of MKN-28 cell with knockdown ZNF521 was degenerated (P < .001; Figure 4A). It was indicated that ZNF521 expedited the motility of GC cells. Apart from this, transwell assay revealed that the migration of AGS cell with overexpressed ZNF521 was significantly improved than the control group (P < .001; Figure 4B). In contrast, the migration of MKN-28 cell with knockdown ZNF521 was significantly restrained (P < .001). Besides, transwell invasion assay uncovered that the invasion of AGS with overexpressed ZNF521 was tremendously enhanced compared to the control group (P < .01), while the invasive ability of GC cells with knockdown ZNF521 was markedly impeded (P < .001). Taken together, ZNF521 promoted the invasion and migration of GC cells.
Figure 4.
Zinc finger protein 521 promotes the motility, migration, and invasion of GC cells. A, The motility of AGS (left) with overexpressed ZNF521 and MKN-28 (right) with knockdown ZNF521 detected by scratch wound healing assay. B, The migration of AGS (left) with overexpressed ZNF521 and MKN-28 (right) with knockdown ZNF521 determined by transwell assay. C, The invasion of AGS (left) with overexpressed ZNF521 and MKN-28 (right) with knockdown ZNF521 monitored by transwell assay. D, The expressions of Bcl-2, BAX, E-cadherin, and N-cadherin in GC cells were detected by Western blot. GC indicates gastric cancer; ZNF521, zinc finger protein 521.
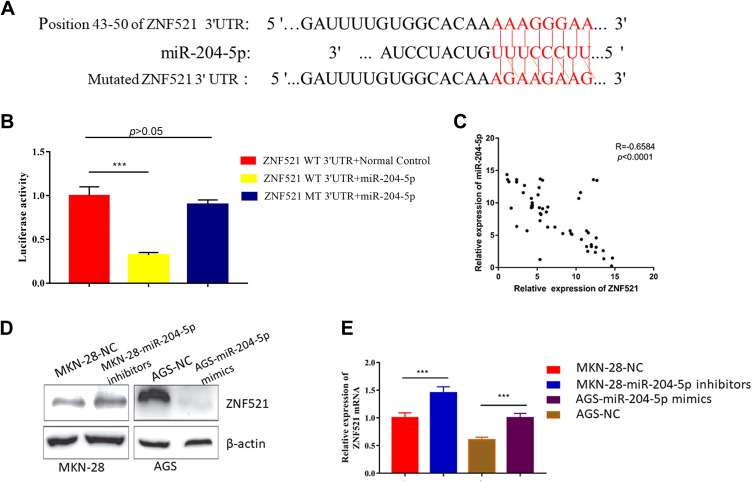
Targeting Relationship Between MiR-204-5p and ZNF521 Was Verified by Dual Luciferase Report Assay
It was predicted by TargetScan (http://www.targetscan.org) that ZNF521 was a candidate target gene for miR-204-5p (Figure 5A). Dual luciferase reporter gene assay showed that miR-204-5p could bind to 3′UTR of ZNF521 (Figure 5B), and the expression level of miR-204-5p was negatively correlated with that of ZNF521 in clinical samples (Figure 5C). Western blot exhibited that the expression of ZNF521 was significantly upregulated in the group with MKN-28-miR-204-5p inhibitors (P < .001). Meanwhile, the expression of ZNF521 was downregulated in the group of AGS-miR-204-5p mimics (P < .001; Figure 5D). Moreover, real-time PCR revealed us that the group of AGS-miR-204-5p mimics had lower expression of ZNF521 mRNA than the control group, and the group of MKN-28-miR-204-5p inhibitors had higher expression of ZNF521 mRNA than the control group (Figure 5E).
Figure 5.
Target relations between miR-204-5p and ZNF521 were verified by dual luciferase reporter gene assay. A, There was a binding site between miR-204-5p and ZNF521. B, The luciferase activity of wild type and mutant type ZNF521 was compared. C, MicroRNA-204-5p was negatively correlated with ZNF521 (Spearman correlation, P <.0001, R = −0.6584). D, The expressions of ZNF521 in AGS and MKN-28 transfected with miR-204-5p were detected by Western blot. E, The expressions of ZNF521 mRNA in AGS transfected with miR-204-5p mimics and MKN-28 transfected with miR-204-5p inhibitors were detected by PCR. *** indicates P < .001. miR-204-5p indicates microRNA-204-5p; mRNA, messenger RNA; PCR, polymerase chain reaction; ZNF521, zinc finger protein 521.
Discussion
Gastric cancer is a multifactor, multistep, and progressive disease.21–26 Mounting evidences supported that the gene dysregulation including oncogenes and tumor suppressor were implicated in the tumorigenesis of GC. For instance, some scholars pointed out that P53 and PTEN conduct an inhibitory role in malignant biological behaviors.27–30 It has also been well-documented that targeted regulation of oncogene or tumor suppressor can be considered as an effective treatment for malignant tumors. Hence, it can shed new light on the targeting therapy for GC.31 From this study, it was found that the high expression of ZNF521 in GC was associated with tumor volume, TNM stage, and local lymph node metastasis. In addition, Kaplan-Meier Plotter database signified that patients with highly expressed ZNF521 had shorter overall survival, postprogression survival, and faster first progression than patients with the low expression of ZNF521. Therefore, it can be concluded that ZNF521 might play a carcinogenic role in GC cells.
Zinc finger protein family has been well-documented to modulate the tumorigenesis and progression of diverse cancers.32–36 For instance, ZNF139, a transcription factor, inhibits apoptosis by regulating the expression of B-cell lymphoma 237; ZNF280B was revealed to induce the expression of murine double mimute 2 and promoted GC growth via controlling the subcellular localization and stability of p53.38 Zinc finger protein 545 can impede the proliferation and facilitate apoptosis via nuclear factor κB and activator protein 1 signaling pathway.39 Studies have demonstrated that ZNF521 played a regulatory role in acute myeloid leukemia and arrested the activation of downstream specific erythroid differentiation genes by binding to GATA binding protein 1, thus inhibiting the differentiation of erythroid cells and the division of immature red blood cells.11 In addition, overexpressed ZNF521 can accelerate the proliferation of tumor cells by increasing the expression of proto-oncogene c-Myc.40 In this study, the expression of ZNF521 was significantly upregulated in GC cells when compared with the gastric epithelial cells. Further, by upregulating the expression of ZNF521, the malignant behaviors of GC cells, such as proliferation, migration, and invasion, were significantly facilitated than that of the control group. After downregulating the expression of ZNF521 in GC cells, the above biological ability of GC cells was markedly suppressed. Collectively, ZNF521 was validated to serve as an oncogene to notably accelerate the progression of GC.
Emerging evidences have proved that miRNA was intensively involved in the tumorigenesis and played a crucial part in the diagnosis and treatment of tumors.41–44 MicroRNA-204-5p is an important member of miRNA family, and various evidences demonstrated that miRNA-204-5p modulated the progression of cancers. For example, miR-204-5p regulates the expression of cytokines in tumor cells by changing the number of myeloid cells and lymphocytes and modulates the immune microenvironment, thus inhibiting the biological behaviors of breast cancer.45,46 MicroRNA-204-5p can also restrain the proliferation of hepatoma cells by directly regulating the expression of SIX homeobox 1.47 In addition, miRNA-204-5p restrained the proliferation of GC cells by downregulating ubiquitin-specific peptidase 47 and RAB22A.48 These studies have proved that miR-204-5p played an antitumor role in tumor cells. In this study, it was found that miR-204-5p might bind to 3′-UTR of ZNF521. Further, dual luciferase reporter gene assay demonstrated that there was a regulatory relationship between ZNF521 and miR-204-5p. It was confirmed that low expression of miRNA-204-5p can contribute to the proliferation, migration, and invasion of GC cells by upregulating ZNF521.
To sum up, this study verified that ZNF521 can facilitate the proliferation, migration, and invasion of the GC cells via miR-204-5p. Accordingly, ZNF521 can be an outstanding reference indicator to evaluate the clinical progression and prognosis of patients with GC. On top of that, the animal tests and larger number of specimen are required for the further validation.
Supplemental Materials
Supplement_Materials for Zinc Finger Protein 521, Negatively Regulated by MicroRNA-204-5p, Promotes Proliferation, Motility and Invasion of Gastric Cancer Cells by Chen Huan, Cai Xiaoxu and Ren Xifang in Technology in Cancer Research & Treatment
Abbreviations
- 3′-UTR
3-untranslated region
- CCK-8
Cell Counting Kit 8
- CEA
carcinoembryonic antigen
- GC
gastric cancer
- miRNA
microRNA
- mRNA
messenger RNA
- MUT
mutant type
- PCR
polymerase chain reaction
- PBS
phosphate-buffered saline
- WT
wild type
- ZFP
zinc finger protein
- ZNF521
zinc finger protein 521.
Footnotes
Authors’ Note: All patients involved gave informed consent to the study and signed a written consent form; 82 cases (49 males and 33 females) of GC tissues and matched nontumor tissues were collected under the approval of Ethics Review Committee of the First Hospital of Yichang (ethical approval number: YC2017006). The resected tissue was immediately frozen in liquid nitrogen and stored at −196 °C for further analysis. All cancer tissues were pathologically confirmed as GC and no other malignant tumors were found. No patients received neoadjuvant therapy (chemotherapy or radiotherapy) before operation.
Author Contributions: CH and RXF conceived and designed the experiments; CH, CXX, and RXF performed the experiments; CH performed statistical analysis; CH and RXF wrote the paper. All authors read and approved the final manuscript.
Data Availability Statement: The data used to support the findings of this study are available from the corresponding author upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statement: Our study was approved by Medical College Review Board of the First People’s Hospital of Yichang.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by Natural Science Foundation of First People’s Hospital of Yichang.
ORCID iD: Ren Xifang  https://orcid.org/0000-0002-1145-2593
https://orcid.org/0000-0002-1145-2593
Supplemental Materials: Supplemental material for this article is available online.
References
- 1. Gullo I, Oliveira P, Athelogou M, et al. New insights into the inflamed tumor immune microenvironment of gastric cancer with lymphoid stroma: from morphology and digital analysis to gene expression. Gastric Cancer. 2019;22(1):77–90. [DOI] [PubMed] [Google Scholar]
- 2. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22(4):2311–2316. [PubMed] [Google Scholar]
- 4. Fan Y, Zhan Q, Xu H, et al. Epigenetic identification of ZNF545 as a functional tumor suppressor in multiple myeloma via activation of p53 signaling pathway. Biochem Biophys Res Commun. 2016;474(4):660–666. [DOI] [PubMed] [Google Scholar]
- 5. Nie H, Mu J, Wang J, Li Y. MIR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol Rep. 2018;40(3):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shahi P, Wang CY, Lawson DA, et al. A1ZNF503/Zpo2 drives aggressive breast cancer progression by down-regulation of GATA3 expression. Proc Natl Acad Sci U S A. 2017;114(12):3169–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuki R, Aoyama K, Kubota S, et al. Overexpression of zinc-finger protein 777 (ZNF777) inhibits proliferation at low cell density through down-regulation of FAM129A. J Cell Biochem. 2015;116(6):954–968. [DOI] [PubMed] [Google Scholar]
- 8. Fleenor CJ, Arends T, Lei H, et al. Zinc finger protein 521 regulates early hematopoiesis through cell extrinsic mechanisms in the bone marrow microenvironment. Mol Cell Biol. 2018;38(17):pii: e00603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiarella E, Aloisio A, Codispoti B, et al. ZNF521 has an inhibitory effect on the adipogenic differentiation of human adipose-derived mesenchymal stem cells. Stem Cell Rev. 2018;14(6):901–914. [DOI] [PubMed] [Google Scholar]
- 10. Mesuraca M, Chiarella E, Scicchitano S, et al. ZNF423 and ZNF521: EBF1 antagonists of potential relevance in B-lymphoid malignancies. Biomed Res Int. 2015;2015:165–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Germano G, Morello G, Aveic S, et al. ZNF521 sustains the differentiation block in MLL-rearranged acute myeloid leukemia. Oncotarget. 2017;8(16):26129–26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiratsuka T, Takei Y, Ohmori R, et al. ZFP521 contributes to pre-B-cell lymphomagenesis through modulation of the pre-B-cell receptor signaling pathway. Oncogene. 2016;35(25):3227–3238. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Shen B, Cui Y. Ago HITS-CLIP expands microRNA-mRNA interactions in nucleus and cytoplasm of gastric cancer cells. BMC Cancer. 2019;19(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang R, Sun Y, Yu W, et al. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J Exp Clin Cancer Res. 2019;38(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi Y, Huang X, Chen G, et al. miR-632 promotes gastric cancer progression by accelerating angiogenesis in a TFF1-dependent manner. BMC Cancer. 2019;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun M, Liu XH, Li JH, et al. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27kip1. Mol Cancer Ther. 2012;11(4):842–852. [DOI] [PubMed] [Google Scholar]
- 17. Tsai KW, Hu LY, Wu CW, et al. Epigenetic regulation of miR-196b expression in gastric cancer. Genes Chromosomes Cancer. 2010;49(11):969–980. [DOI] [PubMed] [Google Scholar]
- 18. Song B, Lin HX, Dong LL, Ma JJ, Jiang ZG. MicroRNA-338 inhibits proliferation, migration, and invasion of gastric cancer cells by the Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(5):1290–1296. [DOI] [PubMed] [Google Scholar]
- 19. Palkina N, Komina A, Aksenenko M, Moshev A, Savchenko A, Ruksha T. MiR-204-5p and miR-3065-5p exert antitumor effects on melanoma cells. Oncol Lett. 2018;15(6):8269–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Zhang H, Ge S. Effects of miR-138-5p and miR-204-5p on the migration and proliferation of gastric cancer cells by targeting EGFR. Oncol Rep. 2018;39(6):2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Zhou ML, Li GC, Yang W. Adjuvant chemoradiotherapy versus adjuvant chemotherapy for R1 resected gastric cancer: a retrospective cohort study. Br J Radiol. 2018;91(1089):20180276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Q, Chen B, Liu P, Yang J. XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185. J Cell Biochem. 2018;119(3):2787–2796. [DOI] [PubMed] [Google Scholar]
- 23. Li D, Lo W, Rudloff U. Merging perspectives: genotype-directed molecular therapy for hereditary diffuse gastric cancer (HDGC) and E-cadherin–EGFR crosstalk. Clin Transl Med. 2018;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim HJ, Oh SC. Novel systemic therapies for advanced gastric cancer. J Gastric Cancer. 2018;18(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang Y, Wenbo LI, Jun Lu, Xin Zhao, Liang Li. Association betweenPRKAA1rs13361707 T>C polymorphism and gastric cancer risk. Medicine (Baltimore). 2018;97(14):e0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for helicobacter pylori: a population-based study. Gut. 2018;67(1):28–35. [DOI] [PubMed] [Google Scholar]
- 27. Cheng Y, Song Y, Qu J, et al. The chemokine receptor CXCR4 and c-MET cooperatively promote epithelial-mesenchymal transition in gastric cancer cells. Transl Oncol. 2018;11(2):487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polom K, Das K, Marrelli D, et al. K-RAS mutation in gastric cancer and its link with microsatellite instability status. Pathol Oncol Res. 2019;25(1):333–340. [DOI] [PubMed] [Google Scholar]
- 29. Li L, Pang X, Zhu Z, et al. GTPBP4 promotes gastric cancer progression via regulating P53 activity. Cell Physiol Biochem. 2018;45(2):667–676. [DOI] [PubMed] [Google Scholar]
- 30. Pan H, Li T, Jiang Y, et al. Overexpression of circular RNA ciRS-7 abrogates the tumor suppressive effect of miR-7 on gastric cancer via PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 2018;119(1):440–446. [DOI] [PubMed] [Google Scholar]
- 31. Chan SL, So JB. Gastric cancer therapy. Enc Cancer. 2016.
- 32. Wei S, Wang L, Zhang L, et al. ZNF143 enhances metastasis of gastric cancer by promoting the process of EMT through PI3K/AKT signaling pathway. Tumour Biol. 2016;37(9):12813–12821. [DOI] [PubMed] [Google Scholar]
- 33. Liu X, Ge X, Zhang Z, et al. MicroRNA-940 promotes tumor cell invasion and metastasis by downregulating ZNF24 in gastric cancer. Oncotarget. 2015;6(28):25418–25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang G, Ma F, Zhong M, et al. ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol Rep. 2014;31(4):1877–1882. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Tan BB, Zhao Q, Fan LQ, Wang D, Liu Y. ZNF139 promotes tumor metastasis by increasing migration and invasion in human gastric cancer cells. Neoplasma. 2014;61(3):291–298. [DOI] [PubMed] [Google Scholar]
- 36. Song IS, Oh NS, Kim HT, et al. Human ZNF312b promotes the progression of gastric cancer by transcriptional activation of the K-ras gene. Cancer Res. 2009;69(7):3131–3139. [DOI] [PubMed] [Google Scholar]
- 37. Fan L, Tan B, Li Y, et al. Silencing of ZNF139-siRNA induces apoptosis in human gastric cancer cell line BGC823. Int J Clin Exp Pathol. 2015;8(10):12428–12436. [PMC free article] [PubMed] [Google Scholar]
- 38. Zhai J, Yang Z, Cai X, et al. ZNF280B promotes the growth of gastric cancer in vitro and in vivo. Oncol Lett. 2018;15(4):5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang W, Yang S, Zhang M, Gao D, He T, Guo M. ZNF545 suppresses human hepatocellular carcinoma growth by inhibiting NF-kB signaling. Genes Cancer. 2017;8(3-4):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffman B, Amanullah A, Shafarenko M, Liebermann DA. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene. 2002;21(21):3414–3421. [DOI] [PubMed] [Google Scholar]
- 41. Basati G, Mohammadpour H, Emami Razavi A. Association of high expression levels of SOX2, NANOG, and OCT4 in gastric cancer tumor tissues with progression and poor prognosis [published online ahead of print January 9, 2019]. J Gastrointest Cancer. 2019. doi: 10.1007/s12029-018-00200-x. [DOI] [PubMed] [Google Scholar]
- 42. Chi SW, Zang JB, Mele A, Darnell RB. Ago HITS-CLIP decodes miRNA-mRNA interaction maps. Nature. 2009;460(7254):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dong J, Jilian LI, Huang J, Wu J. Identification of suitable reference genes for miRNA quantitation in bumblebee (hymenoptera: apidae) response to reproduction. Apidologie. 2019;50(1):40–50. [Google Scholar]
- 44. Fan S, Tian T, Chen W, et al. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. P Canres.2019;79(6):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeng J, Wei M, Shi R, et al. MiR-204-5p/Six1 feedback loop promotes epithelial-mesenchymal transition in breast cancer. Tumour Biol. 2016;37(2):2729–2735. [DOI] [PubMed] [Google Scholar]
- 46. Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7(6):3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 47. Chu Y, Jiang M, Du F, et al. Mir-204-5p suppresses hepatocellular cancer proliferation by regulating homeoprotein SIX1 expression. FEBS Open Bio. 2018;8(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang B, Yin Y, Hu Y, et al. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med Oncol. 2015;32(1):331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement_Materials for Zinc Finger Protein 521, Negatively Regulated by MicroRNA-204-5p, Promotes Proliferation, Motility and Invasion of Gastric Cancer Cells by Chen Huan, Cai Xiaoxu and Ren Xifang in Technology in Cancer Research & Treatment