Abstract
Purpose:
To analyze the correlation of hepatitis B virus reactivation with patient-related and treatment-related dose–volume factors and to describe the feasibility of hepatitis B virus reactivation analyzed by a normal tissue complication probability model for patients with hepatocellular carcinoma treated with radiotherapy.
Materials and Methods:
Ninety patients with hepatitis B virus-related hepatocellular carcinoma treated with radiotherapy were enrolled in this retrospective study and were followed from June 2009 to December 2015. Of the 90 patients, 78 had received conventional fractionation radiotherapy to a mean dose of 39.6 to 50.4 Gy and 12 patients were scheduled to receive hypofractionation. The physical doses were converted into 2 Gy equivalents for analysis. The parameters, TD50 (1), n, and m, of the Lyman-Kutcher-Burman normal tissue complication probability model were derived using maximum likelihood estimation. Bootstrap and leave-one-out were employed to against model overfitting and improve the model stability.
Results:
Radiation-induced liver diseases were 17.8%, hepatitis B virus reactivation was 22.2%, and hepatitis B virus reactivation-induced hepatitis was 21.1%, respectively. In multivariate analysis, the V 5Gy was associated with hepatitis B virus reactivation; TD50 (1), m, and n were 32.3, 0.55, and 0.71 Gy, respectively, for hepatitis B virus reactivation. Bootstrap and leave-one-out results showed that the hepatitis B virus parameter fits were extremely robust.
Conclusion:
A Lyman-Kutcher-Burman normal tissue complication probability model has been established to predict hepatitis B virus reactivation for patients with hepatocellular carcinoma who received radiotherapy.
Keywords: normal tissue complication probability, radiotherapy (RT), hepatitis B virus reactivation, radiation-induced liver diseases, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) accounts for a large proportion of all malignant tumors in East Asia,1 and approximately 85% of patients with hepatoma were caused by the infection of hepatitis B virus (HBV).2,3 Hepatitis B virus reactivation is a well-acknowledged complication in patients having HCC with chronic HBV infection undergoing antitumor therapy.4 Lacking in clarification of the HBV reactivation mechanism, complex virology, and risk factors for HBV, as well, may delay tumor treatment, affect the patients’ quality of life, or even shorten lifetime.5 The clinical implications of HBV reactivation may include asymptomatic hepatitis or severe hepatitis accompanied by impaired liver function, which can be fatal. Patients undergoing chemotherapy, particularly those with malignant lymphoma who receives chemotherapy together with rituximab, often experience the reactivation of HBV.6,7 It has been reported that the incidence of the hepatitis induced by HBV reactivation, which mostly attributed to chemotherapy, was approximately 60%.5 One hypothesis in the pathogenesis of the chemotherapy-related HBV reactivation is that chemotherapy-induced immunosuppression facilitates viral replication and subsequently restoration of the immune system facilitates destruction of infected hepatocytes. In recent years, patients with HCC,8,9 who cannot tolerate surgery, often receive 3-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT), as a result, suffer radiation-induced liver disease (RILD) as a common complication. Nevertheless, the hypothesis about immunosuppression may not be applied to the etiopathogenesis of HBV reactivation caused by radiation. It was implied by Chou et al that RT induced HBV reactivation at least partly through a bystander reaction inside the liver, indicated that both immune and nonimmune mechanisms coexist and might cooperate. Their first work proposed the in vitro HBV reactivation in irradiated HepG2.2.15 cells through the bystander effect of interleukin 6 (IL-6) released from RT-exposed endothelial cells.10 Then, in a study of transgenic mice infected with HBV, they pointed out that RT to the liver combined with IL-6 caused both in vitro and in vivo HBV replication mainly mediated through the STAT3 signaling pathway.11 Conformal radiotherapy (CRT) could induce HBV reactivation and the risk factors for HBV reactivation in patients with HCC have been reported by our previous study.12
There are many predictors for HBV reactivation, including the serum HBV deoxyribonucleic acid (DNA) level. Lau et al13 has demonstrated that a detectable HBV DNA level, as one of the significant risk factors, has value in predicting HBV reactivation, normal liver volume (NLV), and some dosimetric parameters (mean dose to normal liver [MDTNL] and V20 Gy). However, how to precisely predict the HBV reactivation by use of the abovementioned parameters after radiation therapy is still unknown. Therefore, further studies are needed for robust prediction of HBV reactivation after RT based on our previous work.
In order to predict the normal tissue complication probability (NTCP), the Lyman-Kutcher-Burman model was used to summarize the nonuniform irradiation by evaluating the equivalent dose and the corresponding volume of uniform partial organ irradiation from the dose–volume histograms (DVHs). This model assumes a sigmoid dose–response relationship between dose of uniform radiation given to a volume of an organ and the NTCP.14 The NTCP model has been widely used for research purposes, the ability of such a model to predict toxicity has been well established in the last few years.15-17 This study was aimed to evaluate whether the liver LKB model can be used as a useful tool in clinic to predict HBV reactivation for patients with HCC and, if it was, then to obtain the best estimates of the LKB model’s parameters from our patients having HCC treated with RT.
Materials and Methods
Patient Selection and Clinical Characteristics
Ninety patients with HBV-related HCC treated with RT were enrolled in this retrospective study and were followed from June 2009 to December 2015. They were all diagnosed as HCC pathologically or cytologically. The median follow-up time of all these 90 patients who met the inclusion criteria was 18.2 months. Patient and tumor characteristics are summarized in Table 1. The inclusion criteria are as follows: (1) Karnofsky Performance Status score ≥70; (2) hepatitis B surface antigen positivity with positive or negative hepatitis B e antigen (HBeAg); (3) life expectancy over 6 months; (4) grade A or B Child-Pugh classification of hepatic function; (5) patient could be subjected to RT safely without too many disseminated focuses in the liver; (6) intolerance to or disapproval with surgery; (7) favorable kidney function with serum creatinine level of <1.4 mg/dL; and (8) signed complete informed consent form. Exclusive criteria include: (1) any preemptive antiviral treatment against HBV within 6 months before the study; (2) HBV DNA level measured by quantitative polymerase chain reaction protocol in serum ≥107 copies/mL before the administration of RT; (3) chemotherapy and transhepatic arterial chemoembolization (TACE) within 1 month before the study because of insufficient recovery of hepatic function; (4) positive serum antibodies for the hepatitis C virus antigen; (5) any extrahepatic distant organ metastases; (6) previous RT for liver tumor; and (7) incapacity to confirm the tumor margin through imaging examinations. In all, 18 of the 108 patients were excluded according to the exclusion criteria.
Table 1.
Clinical Characteristics of Entire Group of Patients With HCC.
| Characteristics | Number of Patients, n = 90 (%) |
|---|---|
| Gender (male/female) | 52 (57.8)/38 (42.2) |
| Age, years (<50/≥50) | 26 (28.9)/64 (71.1) |
| Karnofsky Performance score (≤80/≥90) | 34 (37.8)/56 (62.2) |
| TNM staging, UICC 2002 | |
| T2N0M0/T3N0M0/T4N0M0 | 21 (23.3)/48 (53.4)/21 (23.3) |
| HBeAg (positive/negative) | 34 (37.8)/56 (62.2) |
| AFP, ng/mL (<400/≥400) | 48 (53.3)/42 (46.7) |
| Treated with ABC/none | 38 (42.2)/52 (57.8) |
| HBV DNA, copies/mL | |
| <1.0 × 103/1.0 × 103-105/100 × 10I5 | 34 (37.8)/33 (36.7)/23 (25.5) |
| Radiotherapy dose, Gy | |
| <50/50-60/phy | 10 (11.1)/31 (34.4)/49 (54.5) |
Abbreviations: ABC, active breathing control; AFP, α-fetoprotein; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; TNM, Tumor–node–metastasis; UICC, Union for International Cancer Control.
Computed Tomography Acquisition
The computed tomography (CT) images were acquired using a Brilliance Big Bore (Philips Health Care, Cleveland, Ohio) scanner at the following settings: 140 kVp, 500 mAs, 512 × 512 in-plane image dimensions, 1.28 × 1.28 mm2 in-plane spatial resolution, and 3-mm slice thickness.
Radiation Therapy
The details of radiation therapy had been described in our previous report.12 Among these patients, 12 patients were scheduled to receive hypofractionation, details are as follows: 45 to 54 Gy/15 to 18 fractions/3 to 3.5 weeks or 50 Gy/20 fractions/4 weeks, 78 received conventional fractionation, each patient was delivered a daily fraction of 1.8 to 2.0 Gy up to total dose of 39.6 to 74 Gy in 5 fractions per week up to 20 to 37 fractions, which were over a period of 4 to 7 weeks. The median prescription dose was 60 Gy. And there are 34 patients who underwent 3D-CRT and 56 patients with IMRT (Table 2), which was delivered with linear accelerators by use of 6 or 15-MV X-rays. In order to avoid the effect of different fractionations on results, we not only fitted the data of all 90 patients but also fitted the data of 78 patients with the 12 hypopatients removed. At the same time, the physical dose values in the dose distributions for each treatment course of all patients were converted to normalized isobiologic effective doses at 2 Gy per fraction using the linear quadratic model (α/β = 2.5 Gy) before computation of the composite dose distributions from which the DVHs were computed.18 For all patients, DVHs obtained from the computerized treatment plan of the Pinncle3 Planning System (Philips Medical Systems, Fitchburg, Wisconsin) and Concordance Correlation Coefficient (CCC) dose algorithm were used to calculate some necessary dosimetric parameters. For better tumor contouring, contrast phases of simulation CT were needed. Gross tumor volume (GTV) was defined as the hepatic tumor volume, visualized by 3-D computation of contrast CT-defined contours. Planning target volume included GTV and margins of 10 to 20 mm expanded from GTV, and an extra margin added to compensate for motion of the liver lesion caused by patient respiration. Only GTV was irradiated. Normal liver volume was defined as the total liver volume minus the GTV; V DGy was the relative volume of normal liver receiving more than a threshold dose D of radiation.
Table 2.
Univariate Analysis Results of Enumeration Data and Relatively Clinical Characteristics Associated With Hepatitis B Virus Reactivation.a
| Parameters | Total | HBV Reactivation | P Value | OR Value | 95% CI for OR |
|---|---|---|---|---|---|
| Gender, male/female | 52/38 | 10/10 | .425 | 1.5 | 1.553-4.071 |
| Karnofsky Performance score (≤80/≥90) | 34/56 | 8/12 | .816 | 0.886 | 0.32-2.452 |
| HBeAg, positive/negative | 34/56 | 7/13 | .771 | 0.858 | 0.304-2.42 |
| Treated with ABC/none | 38/52 | 9/11 | .781 | 0.8644 | 0.318-2.353 |
| PVTT/none | 56/34 | 11/9 | .450 | 1.473 | 0.538-4.033 |
| 3D-CRT/IMRT | 34/56 | 7/13 | .977 | 1.166 | 0.413-3.290 |
| 1.8 and 2Gy vs 2.5 and 3 Gy | 78/12 | 17/3 | .8086 | 0.8361 | 0.204-3.435 |
| TNM staging,T2/T3/T4 | 21/48/21 | 2/9/9 | .024 | - | - |
| Child-Pugh, A/B | 56/34 | 13/7 | .771 | 0.858 | 0.304-2.42 |
| HBV DNA, copies/mL | .0012 | 5.907 | 2.01-17.36 | ||
| <1.0 × 105 | 67 | 9 | |||
| ≥1.0 × 15 | 23 | 11 |
Abbreviations: ABC, active breathing control; CI, confidence interval; CRT, conformal radiotherapy; HBeAg, hepatitis Be antigen; IMRT, intensity-modulated radiation therapy; TNM, Tumor–node–metastasis; HBV, hepatitis B virus; PVTT, portal vein tumor thrombosis; OR, odds ratio.
a P < .05 has statistical significance.
Definitions of Terminology
The classic RILD was defined as nonmalignant ascites, and the serum alkaline phosphatase (ALP) level ≥2-fold upper limit of normal (ULN) or ALT ≥5-fold the ULN.19 Nonclassic RILD was without nonmalignant ascites.20 The imaging examinations did not show any progression of the tumor. The definition of HBV reactivation21,22 is elevated HBV DNA levels in comparison with pre-RT in serum ≥10-fold the baseline level or with HBeAg getting positive in HBeAg-negative patients. The definition of HBV reactivation-induced hepatitis was increased ALT ≥3-fold the ULN in patients with HBV reactivation or ≥100 IU/L (normal value, <33 IU/L), and hepatitis induced by tumor progression, hepatotoxic drugs, treatment-related hepatic damage, or other systemic infections were excluded.7,23 According to increased ALT levels compared with pre-RT, the hepatitis was classified into 3 grades: mild, ≤3-fold ULN; midrange, >3-fold to ≤5-fold ULN; and severe, >5-fold ULN.
Lyman-Kutcher-Burman NTCP Model for Prediction of HBV Reactivation
Data were fit to the LKB NTCP model, assuming there were a sigmoid dose–response relationship with threshold. The detailed description is given in Supplemental Appendix.
Using the LKB NTCP model, the effective volume (V eff) of the liver is:
where n represents the volume effect parameter relating the tolerance dose of uniform whole organ irradiation to uniform partial organ irradiation. The ‘j’ represents the number of dose–volume bins for each patient i and (di, vi) are the bins of a differential DVH. The V eff could convert nonuniform dose distribution into an equivalent uniform dose distribution.
Assuming a profit model for the probability of HBA reactivation of patient i:
The 3 parameters, TD50 (1), m, and n, would be adjusted to best fit the condition (with or without HBV reactivation) in each patient. By applying the maximum-likelihood method,17 the 3 parameters, TD50 (1), m, and n, would be adjusted to best adapt to each patient’s condition (whether or not with HBV reactivation). As most similar studies17,24,25 (on RILD) reported, it is associated with higher incidence when n value equals to 1. In order to observe if the model has similar results for RILD, we fit the data by fixing n = 1. We use log-likelihood and Quasi-Newton and genetic algorithm method to optimize the LKB model.26 The detailed description is given in Supplemental Appendix.
Due to the need for uniform dose–volume distributions in the LKB NTCP model, the nonuniform complex dose distribution was converted into an equivalent uniform dose distribution by using the Kutcher-Burman V eff DVH reduction scheme; V eff was defined as the NLV, if the patients were irradiated uniformly to the reference dose, they could have the possibility of developing HBV reactivation similar to the nonuniform dose distribution in actual delivery.27
Univariate and Multivariate Analyses
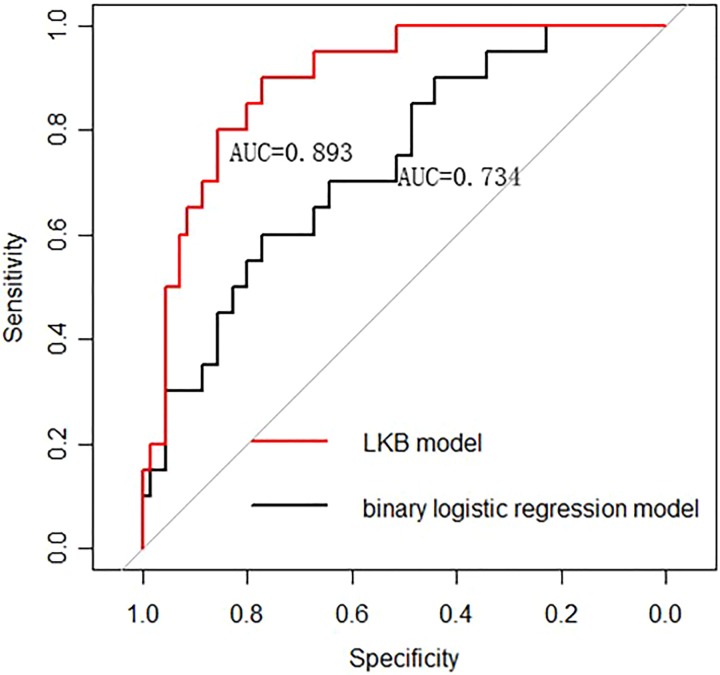
The clinical data were analyzed by R package version 3.2.3. The detailed descriptions of patients and tumor parameters are shown in Tables 2 and 3. The patient-related, treatment-related, and dosimetry-related factors were analyzed for the correlation with HBV reactivation. Levene’s test was performed on all parameters. Variance analysis or Mann-Whitney test was used to analyze the significance of differences between groups (Tables 2 and 3). Table 3 describes the average values and confidence intervals for the group with HBV reactivation and the group without HBV reactivation, respectively. Multivariate analysis was carried out by use of the forward stepwise procedure of the binary logistic regression model, which contained all statistically significant variables in univariate analysis (P ≤ .05). Receiver operating characteristic (ROC) curves have been used to identify discriminate threshold, the discriminative power of the model was assessed by calculating the area under the curve (AUC) of the ROC, and the AUC was optimized from a bootstrap sampling procedure and leave-one-out cross-validation test (Figure 1).28
Table 3.
Univariate Analysis of Measurement Data Associated with Hepatitis B Virus Reactivation.
| Parameters | Reactivation | Inactivation | P Value | ||
|---|---|---|---|---|---|
| Age, years | 57.30 | 52.90-61.60 | 55.90 | 53.31-58.50 | .89 |
| AFP, μF/mL | 553.02 | 17.62-872.18 | .99 | ||
| ALT, IU/mL | 24.90 | 22.40-28.50 | 26.80 | 22.80-27.90 | .65 |
| ALP, IU/mL | 136.50 | 82.50-162.50 | 130.80 | 92.30-180.20 | .77 |
| Radiotherapy dose, Gy | 57.60 | 54.30-60.90 | 57.94 | 56.22-59.66 | .84 |
| GTV, cm3 | 195.47 | 119.91-271.02 | 173.56 | 115.81-231.31 | .37 |
| PTV, cm3 | 451.45 | 332.03-570.86 | 373.32 | 294.61-452.04 | .11 |
| NLV, cm3 | 1260.63 | 990.62-1500.42 | 1680.32 | 1220.34-2010.25 | .02 |
| D max, Gy | 68.69 | 64.64-72.73 | 70.35 | 68.20-72.49 | .58 |
| MDTNL, Gy | 18.65 | 15.84-21.46 | 15.13 | 13.68-16.58 | .03 |
| V 5 (%) | 59.94 | 52.96-66.93 | 49.32 | 45.11-53.53 | .02 |
| V 10 (%) | 52.35 | 45.75-58.96 | 41.87 | 38.04-45.70 | .02 |
| V 15 (%) | 44.75 | 37.88-51.62 | 34.91 | 31.60-38.21 | .02 |
| V 20 (%) | 38.26 | 31.55-44.96 | 29.16 | 26.25-32.08 | .02 |
| V 25 (%) | 31.67 | 25.40-37.94 | 23.82 | 21.39-26.25 | .03 |
| V 30 (%) | 26.75 | 21.02-32.47 | 19.56 | 17.39-21.72 | .02 |
| V 35 (%) | 21.02 | 16.75-25.29 | 15.82 | 13.93-17.71 | .02 |
| V 40 (%) | 16.25 | 12.93-19.57 | 12.49 | 10.86-14.12 | .02 |
| V 45 (%) | 11.67 | 8.25-15.08 | 9.70 | 8.30-11.11 | .03 |
Abbreviations: AFP, α-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; CI, confidence interval; GTV, gross tumor volume; HBV, hepatitis B virus; PLV, plan liver volume; NLV, normal liver volume; OR, odds ratio.
Note: In the table, the boldface values were all less than .05, which meant that the corresponding variables were significant.
Figure 1.
The LKB model has better predictive ability than the multivariate logistic regression analysis (AUC: 0.893 vs 0.734). AUC indicates area under the curve; LKB, Lyman-Kutcher-Burman.
Results
Among 18 excluded patients from all of the 108 patients, 6 underwent lamivudine antiviral therapy in 6 months, 7 underwent chemotherapy and transcatheter hepatic arterial chemoembolization therapy in 4 weeks, 4 had HBV DNA assay in serum of ≥107 copies/mL, and 1 was hepatitis C virus antigen-positive.
Incidence Rate and Sequelae of RILD
The RILD incidence rate was 17.8% (16/90), with 10% (9/90) diagnosed as classic RILD and 7.8% (7/90) as nonclassic RILD. The RILD was more likely to happen at 8 weeks after the RT, which was 6.7% (6/90). The cumulative RILD rates at 4, 8, 12, and 16 weeks after the RT were 4.4% (4/90), 11.1% (10/90), 15.6% (14/90), and 17.8% (16/90), respectively. Among 16 patients with RILD, although 9 patients, who lived for up to the follow-up end point, had efficient responses to protection treatment of liver function, 7 patients had inefficient responses, and died of liver function failure 4 months after the RT (5 patients with classic RILD and 2 patients with nonclassic RILD).
Incidence Rate of HBV Reactivation
The occurrence rates of HBV reactivation were 4.4% (4/90) at 4 weeks, 11.1% (10/90) at 8 weeks, and 6.7% (6/90) at 12 weeks after the RT, totally 22.2% (20/90). The cumulative HBV reactivation rates were 4.4%, 15.6%, 22.2%, and 22.2%, respectively, at 4, 8, 12, and 16 weeks after the RT.
Incidence Rate and Sequelae of HBV Reactivation-Induced Hepatitis
According to their diagnostic criteria, 5 patients were not diagnosed as RILD or HBV reactivation, although they had elevated HBV DNA or/and ALT levels compared with pre-RT, concerning that these increased parameters might be induced by chronic hepatitis. The hepatitis rate, which was induced by HBV reactivation, was 21.1% (19/90). Among these 19 patients, 11 had moderate hepatitis and 8 had severe hepatitis. The HBV reactivation-related hepatitis was extremely likely to occur at 8 weeks after the RT, whose probability was 7.8%. The cumulative rates at 4, 8, 12, and 16 weeks after the RT were 4.3%, 12.2%, 21.1%, and 21.1%, respectively.
Treatment of HBV Reactivation
The patients with HBV reactivation all accepted antiviral therapy with lamivudine or entecavir (100 mg/d) on time. The median antiviral therapy time was 14 weeks (range, 2-30 weeks). The HBV DNA and ALT levels in serum both descended to normal levels in 9 patients 3 weeks after antiviral therapy. The HBV DNA levels in 3 patients changed to normal, but ALT was still higher than the baseline level. But, 3 patients were insensitive to antiviral therapy and died of liver function failure with a mortality rate of 15.8%.
Correlation Factors of HBV Reactivation
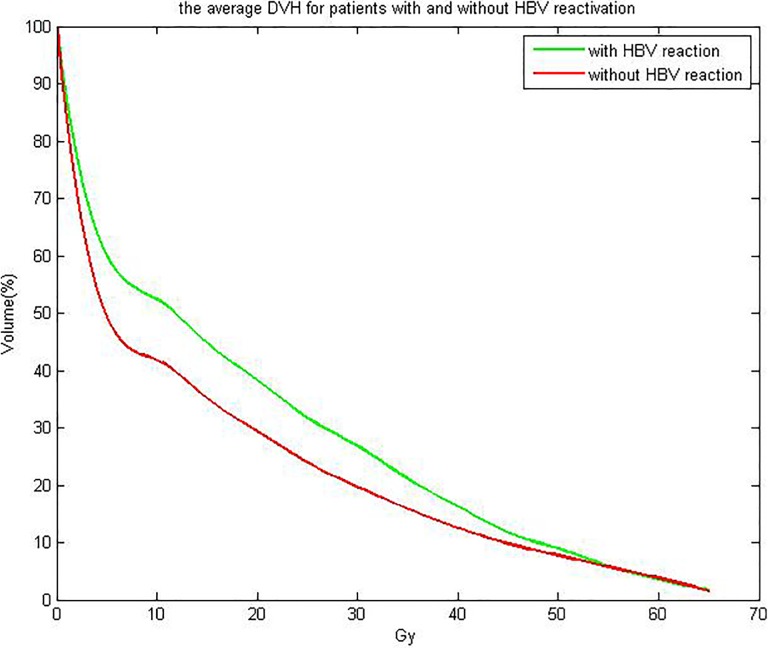
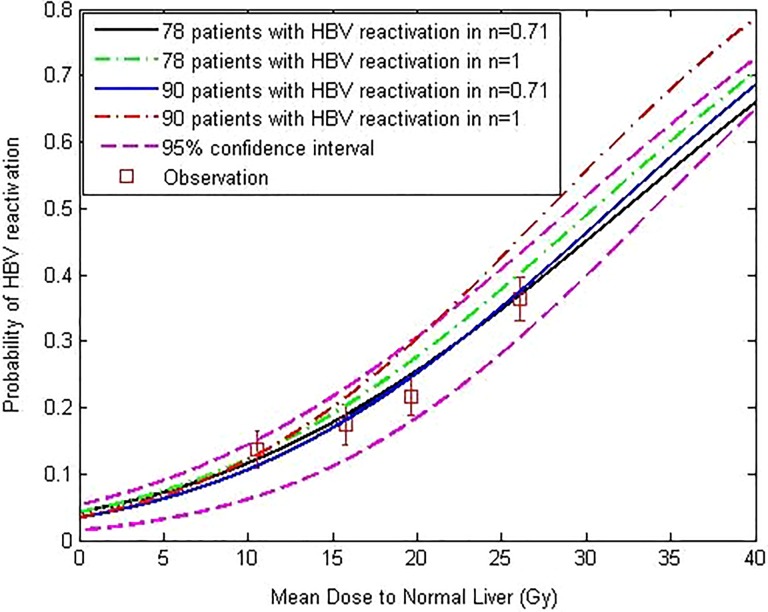
Tables 2 and 3 showed the univariate analysis results about HBV reactivation with clinical data and dosimetric parameters. From Table 2, we can see that the variable Tumor–node–metastasis (TNM) staging and HBV DNA copies are significant at univariate analysis. From Table 3, we can see that MDTNL, V 5Gy, V 10Gy, V 15Gy, V 20Gy, V 25Gy, V 30Gy, V 35Gy, V 40Gy, and V 45Gy parameters are significantly correlated with the analyzed outcome. Figure 2 shows the average DVH for patients with and without HBV reactivation. Among the 90 patients, 20 patients developed HBV reactivation but 70 patients did not. Figure 3 demonstrates the predicted and observed NTCP values for HBV reactivation in the 90 patients with HCC, by the use of the identified parameters of the LKB NTCP model. The study pooled together a large majority of conventionally treated patients with 12 hypopatients. For more clarity, we not only fitted the data of 78 patients with the 12 hypopatients removed but fitted the data of all 90 patients. For all 90 patients, the fitting results are as follows: m = 0.55, n = 0.71, TD50 (1) = 32.3 Gy. As 12 hypopatients excluded, the fitting results were TD50 = 32.8, n = 0.71, and m = 0.58. The results indicate that the differences between the 2 fitting results were not obvious. The way of hypofractionation did not have a great influence on the final fitting results. As well, the data of it reflect the whole data results very well. Above this, we found that the differences between the 2 fitting results were not obvious. Four observation points were collected from patients with different dose-threshold groups and distributed through the NTCP curve. Both methods (χ2 and “lillietest” function) of assessing results showed that these 4 viewpoints are subject to normal distribution and the appropriate parameters could be used to describe the results of NTCP by the code developed by Matlab (χ2 = 5.82 and P < .1).29 From Figure 1, we can see that AUC value for LKB model is 0.893 (95% confidence interval [CI]: 0.812-0.921) and AUC value for binary logistic regression model is 0.734 (95% CI: 0.663-0.882). The Z statistics test value is 3.976, and P value is .0002. The difference in the diagnostic value of the 2 models is statistically significant, and the predictive value of LKB model is higher than the binary logistic regression model.
Figure 2.
The average DVH for patients with and without HBV reactivation. The green line represents the average DVH with HBV reactivation and the red line represents the average DVH without HBV reactivation. DVH indicates dose–volume histogram; HBV, hepatitis B virus.
Figure 3.
Prediction of probability of radiation-induced HBV reactivation by the LKB model. Normal tissue complication probability (NTCP) curves of 4 treatment groups of patients (all 90 patients with n = 1, all 90 patients with n = 0.71, the patients excluding 12 hypopatients with n = 1, and the patients excluding 12 hypopatients with n = 0.71). The squares on the figure are the observed incidences of HBV reactivation. The results are as follows: TD50 = 32.3 (95% CI: 28.7-36.4 Gy), n = 0.71 (95% CI: 0.62-0.83), m = 0.55 (95% CI: 0.48-0.67), which derived from the data of all 90 patients; as 12 hypopatients excluded, the fitting results are TD50 = 32.8, n = 0.71, m = 0.58. CI indicates confidence interval; LKB, Lyman-Kutcher-Burman; HBV, hepatitis B virus.
Multivariate Analysis
As shown in Table 4, we applied a logistic multivariate analysis to analyze the risk factors, including V 45Gy, V 5Gy, and NLV, of HBV reactivation. We observed that the main factors of HBV reactivation were V 5Gy (P < .05) as continuous variable (odds ratio = 1.053, 95% CI: 1.0094-1.098, P = .017). However, neither V 45Gy or NLV contribute to the incidence of HBV reactivation.
Table 4.
Logistic Regression of Risk Factors Associated With HBV Reactivation.
| Parameters | Coefficient | SE | Sig | OR | 95% CI for OR |
|---|---|---|---|---|---|
| Constant | −5.212 | ||||
| HBV DNA level | 0.142 | 0.038 | 0.021 | 1.365 | 1.163-1.625 |
| V 45 (%) | 0.022 | 0.052 | 0.8934 | 1.0123 | 0.932-1.213 |
| V 5 (%) | 0.0523 | 0.0241 | 0.0183 | 1.121 | 0.992-1.221 |
| NLV,cm3 | −0.012 | 0.0022 | 0.9623 | 0.988 | 0.962-1.113 |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; NLV, normal liver volume; SE, standard error; Sig, significance.
Note: In the table, the boldface values were all less than .05, which meant that the corresponding variables were significant.
Discussion
Nowadays, RT becomes an important treatment method of the comprehensive therapy to treat HCC in China. Three-dimensional conformal radiotherapy and IMRT have been widely and increasingly applied in patients with HCC to prolong survival time.8,9 Although dose escalation was proven to be beneficial in HCC control and even improve survival,11 it also increases the risk of RILD. In East Asia, HBV infection rate is relatively high, in China, with 7.18% of the overall population chronic carriers in 2006.30 The HBV reactivation, which is induced by cytotoxicity of chemotherapy, as a well-recognized complication, has been increasingly observed in patients having HCC infected with HBV.20,31-33 However, there are few studies in analyzing the risk factors for HBV reactivation after the CRT, and models to predict the risk of HBV reactivation after the CRT were even fewer. Kim et al34 performed a retrospective study with 48 patients with unresectable HCC who underwent 3D-CRT, which indicated that the HBV reactivation was 21.8% in the group who did not receive antiviral therapy. However, they find that HBV reactivation had no correlation with radiation dose or modified Union for International Cancer Control (UICC) stage (P > .05). The main reason for the negative finding may include that the study was retrospective, patient sample capacity was in small size, the identification criteria of HBV reactivation was strict, and the use of a relatively insensitive solution-hybridization assay for HBV DNA. Cheng35 also pointed out another reason about Kim’s negative results, demonstrating that the prescribed radiation dose, which was homogeneous in these patients (53.9 ± 0.5 Gy), was the only dosimetric factor included. Several effective dosimetric parameters in prediction of RILD were not evaluated in Kim’s study. Our study also showed that the incidence of HBV reactivation in patients with HCC after the RT was relatively high, NLV and some normal liver-related dosimetric parameters, including V 5Gy and MDTNL, were the prognosis factors for HBV reactivation and should be carefully considered before RT.12
Radiation-induced liver disease is the main adverse reaction of patients having HCC treated with RT. Of the 90 patients, the RILD incidence rate was 17.8% (16/90), with 10% (9/90) diagnosed as classic RILD and 7.8% (7/90) as nonclassic RILD. The incidence of RILD is closely related to radiation dose and liver function. In our study, ALT levels and HBV DNA levels were elevated in 5 patients, consistent with the diagnostic criteria for hepatitis caused by HBV reactivation. After 4 weeks of antiviral treatment, their serum HBV DNA levels returned to normal, but ALT levels did not return to the baseline level, which may be caused by overlapping diseases and should be determined by liver biopsy. However, these patients often have cirrhosis, liver insufficiency, and coagulation disorders, which liver biopsies cannot tolerate. Due to the limitations of this technique, it is still difficult to distinguish hepatitis in patients with HCC induced by RILD and HBV reactivation using imaging methods, especially hepatitis induced by nonclassical RILD and severe HBV reactivation. Some patients may have overlapping diseases.
In addition to the dosimetric parameters mentioned earlier, as well, there are other mathematical models available to predict the probability of HBV reactivation in the literature.36,37 But the model is far from satisfactory because HBV reactivation is a complex process involving multivariate factors in its occurrence. In the literature, although the LKB NTCP model has been widely used to describe the volume dependence of radiation toxicity in normal tissues, its evaluated power of predicting the incidence of HBV reactivation is rarely reported. According to the above issues, we conducted this retrospective study to analyze the risk factors for HBV reactivation after the RT and use an LKB NTCP model to predict the occurrence for HBV reactivation. In the current study, the reasons of radiation-induced HBV reactivation were analyzed with dose-volumetric parameters in detail. However, the multivariate logistic regression analysis of our study showed that only V 5Gy was significantly related to HBV reactivation and could be regarded as a prognostic factor for HBV reactivation. Perhaps V 5Gy, as a low-dose factor, is related to the HBV reactivation. And V 5Gy was generated from the dose–volume data of liver, in contrast to the prescribed dose to the tumor. The LKB NTCP models have been tested under this goal. Nevertheless, there were some uncertainties appeared in NTCP models and some authors have challenged their application in the prediction of HBV reactivation.38,39
In the current study, the reasons of radiation-induced HBV reactivation were analyzed with dose-volumetric parameters in detail. All these DVH parameters were generated from the dose–volume data of liver, in contrast to the prescribed dose to the tumor. As well, whether dose-fractionation is related to HBV reactivation has not been validated in this study. Because in this study, 12 patients underwent hypofractionation with 2 HBV reactivation and 78 received conventional fractionation with 18 HBV reactivation. Hypofractionation did not lead to a rise in the new reactivation rate, while it may be related to a small sample size. The above defects can be corrected by the subsequent expanded capacity of the sample and the LKB NTCP model. Thus, clinical studies needed to be carried to confirm the ability of NTCP model to predict toxicity. In our study, we were able to identify parameters of the NTCP model which could predict HBV reactivation in RT for patients with HCC. Using ROC analysis, Figure 1 shows the LKB model has better predictive ability than the multivariate logistic regression analysis (AUC: 0.893 vs 0.734).
Hepatic toxicity after irradiation has been routinely reported in the radiation series,40,41 it was sometimes difficult to define the cause of abnormal liver function tests between various treatment-related toxicity and disease progression. Kim et al34 reported that 3D-CRT increased HBV reactivation rate (21.8%) compared with the control group and increased HBV DNA at least 3-fold more than baseline level of control group. But the study did not find the high-risk factors for dosimetric parameters for HBV reactivation. Jun et al42 and Chou et al10 also reported that HBV reactivation can occur after RT. Combination treatment of RT with TACE and nonantiviral treatment are major risk factors for HBV reactivation during or after RT. The study also did not carefully analyze whether the dose of normal liver and dosimetric parameters in RT had an effect on the HBV reactivation. We think that it is most likely these abnormal presentations were from the direct radiation injury of liver and poor compensation of hepatic function. Our previous study also has reported that CRT could induce HBV reactivation and the dosimetric parameters risk factors for HBV reactivation in patients with HCC. But there is no individualized model to guide treatment and explain the nature of the effects of dosimetric parameters.12 The fundamental purpose of this study was to evaluate the ability of the LKB NTCP model to describe the probability of radiation-related HBV reactivation following HCC irradiation, by analyzing crucial improved model parameters, which may mean the dosimetric parameters only. As far as we know, this is the first study to provide specific values of the parameters m, n, and TD50 (1) for HBV reactivation in patients with HCC (m = 0.55 [95% CI: 0.48-0.67], n = 0.71 [95% CI: 0.62-0.83], TD50 (1) = 32.3 Gy [95% CI: 28.7Gy-36.4 Gy]). The value of m for HBV reactivation was found in the current study (= 0.55). According to the definition of m, the complications we are currently investigating could be expected to occur in a relatively wide range of dose around TD50 (1), or alternately, that the distribution of complications versus dose for uniform partial volume liver irradiation in a population of patients would have an extensive spread. In the LKB NTCP model, n represented the volume effect. The large value for n meant great volume effect and the small value for n meant less volume effect. The n value obtained in our series is 0.71, which implies the tolerance to liver irradiation was significantly associated with the liver volume irradiated for HCC reactivation. Out the other side, liver is a parallel organ with volume effect in general. All of these factors make accurate estimation of liver irradiation tolerance very difficult. Based on this conclusion, in China, radiation oncologist and physicist should focus on mean doses or large liver volumes for patients with HCC.
TD50 (1) indicated the irradiation tolerance, that the TD50 (1) value for acute HBV reactivation in this study (=32.3 Gy) was a risk dose which represented the tolerance of the whole liver to irradiation. According to our clinical experiences, HBV reactivation often appears during 4 to 8 weeks after the end of RT. So, the tolerance of irradiation about the liver with HBV was much poorer. Although the NTCP may be useful in estimating the risk of HBV reactivation, there are limitations to using these factors as predictors. The LKB NTCP model does not take that the volume thresholds for HBV reactivation into consideration, and they continually penalize (increase the NTCP prediction) for even very small volumes irradiated to high doses. This conclusion suggests patients having HCC with HBV infection can tolerate irradiation in lower dose and deserves more attention.
Conclusion
In conclusion, HBV reactivation is considered as a noteworthy clinical risk factor for patients with HCC who experienced RT. The HBV DNA level in serum and some dosimetric parameters (V 5Gy) should be considered carefully before RT since they were the prognosis factors for HBV reactivation. The TD50 value for HBV reactivation was 32.3 Gy in the current study, implying a threshold of doses may exist and lower dose of irradiation is less likely to induce HBV reactivation. At the same time, further studies are needed to be implemented to clarify the optimum time to start antiviral therapy in order to prevent/reduce HBV reactivation during RT and to find a reliable method to measure the probability of HBV reactivation for patients who are undergoing RT. Nevertheless, our LKB model for HBV reactivation should be put into use with special attention because it was obtained from only a small sample capacity of patients, and large-scale hepatic-irradiation data should be collected to verify the outcome.
Supplemental Material
Supplementary_material for Analysis of Hepatitis B Virus Reactivation After Radiotherapy in Patients With Hepatocellular Carcinoma Using the Lyman NTCP Model by Zhenjiang Li, Yinping Dong, Min Fan, Yong Yin, Jian Zhu, Baosheng Li and Wei Huang in Technology in Cancer Research & Treatment
Abbreviations
- ALP
alkaline phosphatase
- AUC
area under the curve
- CI
confidence interval
- CRT
conformal radiotherapy
- CT
computed tomography
- 3D-CRT
3-dimensional conformal radiotherapy
- DNA
deoxyribonucleic acid
- DVH
dose–volume histogram
- GTV
gross tumor volume
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IMRT
intensity-modulated radiotherapy
- MDTNL
mean dose to normal liver
- LKB
Lyman-Kutcher-Burman
- NLV
normal liver volume
- NTCP
normal tissue complication probability
- RILD
radiation-induced liver disease
- ROC
receiver operating characteristic
- RT
radiotherapy
- TACE
transhepatic arterial chemoembolization
- ULN
upper limit of normal
Footnotes
Authors’ Note: The authors Zhenjiang Li and Yinping Dong contributed equally to this work. And all authors have contributed significantly. All authors read and approved the final manuscript.
Availability of Data and Material: The results reported in the article was supported by the data collected from Shandong Cancer Hospital & Institute. All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate: Our study include a statement on ethics approval and consent, which is approved by the Ethics Committee of Shandong Cancer Hospital & Institute. The committee’s reference number is SDTHEC 20140304.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This work was supported by the National Natural Science Foundation of China [grant numbers 81773232, 81402538, 61375013, 81530060, 81671785], the Taishan Scholars Program of Shandong Province, China [grant numbers ts20120505], and Natural Science Foundation of Shandong Provice [grant number ZR2018BH028].
ORCID iD: Wei Huang  https://orcid.org/0000-0002-2495-5747
https://orcid.org/0000-0002-2495-5747
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Cook G. Hepatocellular carcinoma: one of the world’s most common malignancies. QJM. 1985. [PubMed] [Google Scholar]
- 2. Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28(10):1660–1665. [DOI] [PubMed] [Google Scholar]
- 3. Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol. 2011;23(8):695–700. [DOI] [PubMed] [Google Scholar]
- 4. Yeo W, Chan P, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62(3):299–307. [DOI] [PubMed] [Google Scholar]
- 5. Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15(11):1661–1666. [DOI] [PubMed] [Google Scholar]
- 6. Aksoy S, Harputluoglu H, Kilickap S, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48(7):1307–1312. [DOI] [PubMed] [Google Scholar]
- 7. Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37(6):1320–1328. [DOI] [PubMed] [Google Scholar]
- 8. Kang MK, Kim MS, Kim SK, et al. High-dose radiotherapy with intensity-modulated radiation therapy for advanced hepatocellular carcinoma. Tumori. 2011;97(6):724–731. [DOI] [PubMed] [Google Scholar]
- 9. Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42(8):721–729. [DOI] [PubMed] [Google Scholar]
- 10. Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res. 2007;13(3):851–857. [DOI] [PubMed] [Google Scholar]
- 11. Chou CH, Chen PJ, Jeng YM, Cheng AL, Huang LR, Cheng JC. Synergistic effect of radiation and interleukin-6 on hepatitis B virus reactivation in liver through STAT3 signaling pathway. Int J Radiat Oncol Biol Phys. 2009;75(5):1545–1552. [DOI] [PubMed] [Google Scholar]
- 12. Huang W, Zhang W, Fan M, et al. Risk factors for hepatitis B virus reactivation after conformal radiotherapy in patients with hepatocellular carcinoma. Cancer sci. 2014;105(6):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau GK, Leung YH, Fong DY. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99(7):2324–2330. [DOI] [PubMed] [Google Scholar]
- 14. Lyman JT. Complication Probability as Assessed from Dose-Volume Histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 15. Seppenwoolde Y, Lebesque JV, De Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2003;55(3):724–735. [DOI] [PubMed] [Google Scholar]
- 16. Semenenko VA, Li XA. Lyman-Kutcher-Burman NTCP model parameters for radiation pneumonitis and xerostomia based on combined analysis of published clinical data. Phys Med Biol. 2008;53(3):737–755. [DOI] [PubMed] [Google Scholar]
- 17. Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. [DOI] [PubMed] [Google Scholar]
- 18. Toramatsu C, Katoh N, Shimizu S, et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat Oncol. 2013;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence TS, Kessler ML, Lavigne ML, Lyman JT, Ten Haken RK. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23(4):781–788. [DOI] [PubMed] [Google Scholar]
- 20. Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47(1):13–47. [DOI] [PubMed] [Google Scholar]
- 21. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43(2):209–220. [DOI] [PubMed] [Google Scholar]
- 22. Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol. 2009;90(1):13–23. [DOI] [PubMed] [Google Scholar]
- 23. Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43(2):233–240. [DOI] [PubMed] [Google Scholar]
- 24. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31(5):1237–1248. [DOI] [PubMed] [Google Scholar]
- 25. Xu ZY, Liang SX, Zhu J, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65(1):189–195. [DOI] [PubMed] [Google Scholar]
- 26. Roberts SA, Hendry JH. The delay before onset of accelerated tumour cell repopulation during radiotherapy: a direct maximum-likelihood analysis of a collection of worldwide tumour-control data. Radiother Oncol. 1993;29(1):69–74. [DOI] [PubMed] [Google Scholar]
- 27. Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16(6):1623–1630. [DOI] [PubMed] [Google Scholar]
- 28. Steyerberg EW, Harrell FE, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF. Internal validation of predictive models. J Clin Epidemiol. 2001;54(8):774–781. [DOI] [PubMed] [Google Scholar]
- 29. Zhu J, Zhang ZC, Li BS, et al. Analysis of acute radiation-induced esophagitis in non-small-cell lung cancer patients using the Lyman NTCP model. Radiother Oncol.2010;97(3):449–454. [DOI] [PubMed] [Google Scholar]
- 30. Lu FM, Zhuang H. Management of hepatitis B in china. Chin Med J (Engl). 2009;122(1):3–4. [PubMed] [Google Scholar]
- 31. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605–611. [DOI] [PubMed] [Google Scholar]
- 32. Koo YX, Tay M, Teh YE, et al. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol. 2011;90(10):1219–1223. [DOI] [PubMed] [Google Scholar]
- 33. Koo YX, Tan DS, Tan IB, Tao M, Chow WC, Lim ST. Hepatitis B virus reactivation and role of antiviral prophylaxis in lymphoma patients with past hepatitis B virus infection who are receiving chemoimmunotherapy. Cancer. 2010;116(1):115–121. [DOI] [PubMed] [Google Scholar]
- 34. Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.2007;69(3):813–819. [DOI] [PubMed] [Google Scholar]
- 35. Cheng JC. Essential dosimetric parameters of liver for the association with radiation-induced liver disease and virus reactivation: in regard to Kim et al. (Int J Radiat Oncol Biol Phys 2007;69:813-819). Int J Radiat Oncol Biol Phys. 2008;71(3):961–962. [DOI] [PubMed] [Google Scholar]
- 36. Yeo W, Zee B, Zhong S, et al. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer.2004;90(7):1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeo W, Chan PK, Ho WM, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22(5):927–934. [DOI] [PubMed] [Google Scholar]
- 38. Langer M, Morrill SS, Lane RA. Test of the claim that plan rankings are determined by relative complication and tumor-control probabilities. Int J Radiat Oncol Biol Phys. 1998;41(2):451–457. [DOI] [PubMed] [Google Scholar]
- 39. Brenner DJ, Sachs RK. A more robust biologically based ranking criterion for treatment plans. Int J Radiat Oncol Biol Phys. 1999;43(3):697–698. [PubMed] [Google Scholar]
- 40. Robertson JM, Lawrence TS, Dworzanin LM, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11(7):1286–1293. [DOI] [PubMed] [Google Scholar]
- 41. Seong J, Keum KC, Han KH, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(2):393–397. [DOI] [PubMed] [Google Scholar]
- 42. Jun BG, Kim YD, Kim SG, et al. Hepatitis B virus reactivation after radiotherapy for hepatocellular carcinoma and efficacy of antiviral treatment: a multicenter study.PLoS One. 2018;13(7):e0201316 PMID: 30059513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for Analysis of Hepatitis B Virus Reactivation After Radiotherapy in Patients With Hepatocellular Carcinoma Using the Lyman NTCP Model by Zhenjiang Li, Yinping Dong, Min Fan, Yong Yin, Jian Zhu, Baosheng Li and Wei Huang in Technology in Cancer Research & Treatment