Abstract
Background:
Autologous matrix-induced chondrogenesis (AMIC) is a single-stage alternative to autologous chondrocyte implantation for treatment of localized cartilage defects of the knee. To our knowledge, no randomized controlled trial exists comparing the 2 methods.
Purpose:
To evaluate any difference in the outcome of AMIC as compared with collagen-covered autologous chondrocyte implantation (ACI-C).
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A prospective randomized controlled clinical trial was designed to assess any differences in the outcomes between ACI-C and AMIC for the treatment of ≥1 chondral or osteochondral defects of the distal femur and/or patella. The inclusion period was set to 3 years, and the aim was to include 80 patients (40 in each group). Patient inclusion was broad, with few exclusion criteria. The primary outcome was change in Knee injury and Osteoarthritis Outcome Score (KOOS) at 2 years as compared with baseline. The secondary outcomes were the number of failures in each group at 2 years and the change in KOOS subscale, Lysholm, and pain visual analog scale (VAS) scores at 2 years as compared with baseline. A 2-sample t test with a significance level of P < .05 was used to compare the change in score from baseline between groups.
Results:
A total of 41 patients over 3 years were included in the study: 21 in the ACI-C group and 20 in the AMIC group. All the patients had prior surgery to the index knee. At 2-year follow-up, the clinical scores for both groups improved significantly from baseline. No significant differences between groups were seen in the change from baseline for KOOS (AMIC, 18.1; ACI-C, 10.3), any of the KOOS subscales, the Lysholm score (AMIC, 19.7; ACI-C, 17.0), or the VAS pain score (AMIC, 30.6; ACI-C, 19.6). Two patients in the AMIC group had progressed to a total knee replacement by the 2-year follow-up as compared with none in the ACI-C group.
Conclusion:
At 2-year follow-up, no significant differences were found regarding outcomes between ACI-C and AMIC. Mid- and long-term results will be important.
Registration:
NCT01458782 (ClinicalTrials.gov identifier).
Keywords: cartilage repair, knee, autologous matrix-induced chondrogenesis, AMIC, autologous chondrocyte implantation, ACI-C, clinical outcome
Patients hampered with impaired joint function attributed to localized cartilage defects or early osteoarthritis of the knee represent a major challenge for orthopaedic surgeons. These patients have often exhausted conservative treatment options and are not eligible for definitive treatment with a total knee replacement owing to young age, participation in high-demand activities, or lack of global osteoarthritis. Defects in the hyaline cartilage may result from acute trauma, repetitive microtrauma, early osteoarthritis, or osteochondritis dissecans and can produce symptoms such as pain, catching, locking, swelling, and stiffness.
Given its avascular and aneural nature, hyaline cartilage has limited self-healing potential. Superficial defects do not cause hemorrhage or induce a local acute inflammatory response.5 Following the trauma, chondrocytes proliferate and upregulate the production of matrix molecules, but the surface is not restored.21,27 If the cartilage defect penetrates the subchondral bone plate, the highly vascularized bone marrow can aid in the healing process by forming a blood clot containing chondroprogenitor cells, bioactive molecules, and fibrin.33 This mainly produces type I collagen and therefore fibrocartilage rather than hyaline cartilage.12 Fibrocartilage lacks the specialized viscoelastic characteristics of hyaline cartilage and degenerates faster. It is commonly accepted that over time, a cartilage injury increases the risk of osteoarthritis formation regardless of depth or origin.17
Over the past 3 decades, great efforts have been made to advance the field of tissue engineering and cartilage repair around the world. Modification of old techniques and development of new methods have been conducted in search of the “holy grail”—the regeneration of hyaline cartilage with seamless integration into the cartilage defect. However, several leading researchers in the field have stated that no significant progress has been made during the past decade.17,22 Few large randomized controlled trials (RCTs) of high quality exist comparing the vast numbers of techniques. Even more worrisome is the lack of trials comparing new techniques with a nonsurgical approach. In 2017, Devitt et al9 conducted a systematic review of RCTs in search of the most appropriate surgical treatments for cartilage defects of the knee. Out of 540 articles initial identified, only 10 were found methodically sufficient to be included in the review. A most effective treatment could not be determined.
Current surgical repair strategies comprise bone marrow stimulation and transplantation of osteochondral plugs or chondrocytes. The bone marrow–stimulating procedures include drilling/chondroplasty/microfracture and modifications of these techniques, such as autologous matrix-induced chondrogenesis (AMIC). Steadman et al34,35 introduced microfracture as a modification of drilling in the 1990s. The chondral defect is stabilized and debrided, and a specially designed awl is used to perforate the subchondral bone plate, releasing bone marrow stem cells into the cartilage defect and resulting in repair tissue. A specific rehabilitation protocol with partial weightbearing and continuous passive motion is considered crucial to optimize the results after surgery. The AMIC technique was published by Benthien and Behrens3 in 2011, and it combines microfracture with a protective collagen membrane that functions both as a scaffold and as containment for the mesenchymal cells within the defect. Transplantation can be done by replacing the damaged cartilage with ≥1 osteochondral plugs (autograft or allograft) or by introducing autologous chondrocytes into the defect (autologous chondrocyte implantation [ACI]).
ACI was described by Brittberg et al4 in 1994, and it involves a 2-step procedure where a cartilage biopsy is arthroscopically harvested from a nonweightbearing area of the joint. The chondrocytes are released from the biopsy, expanded in a laboratory, and implanted with a periosteal flap to cover the cartilage defect in a second surgical procedure. The procedure has been refined with a collagen membrane (ACI-C) instead of periosteum to cover the defect, ameliorating the problem of hypertrophic repair tissue. Other modifications, such as matrix-assisted chondrocyte implantation (MACI) and characterized chondrocyte implantation (CCI), are currently being investigated.
Compared with ACI-C, the marrow stimulation procedures are technically easier and less expensive,1 do not require removal of healthy cartilage, are not dependent on laboratory facilities to expand the chondrocytes in culture, and require only 1 surgical procedure. Complementary unloading/realignment procedures for the patellofemoral joint and tibiofemoral joint have been useful and are advocated by orthopaedic surgeons if indicated.20
To our knowledge, no RCTs comparing the results of ACI-C versus AMIC currently exist. The objective of the present study was to compare ACI-C and AMIC in a randomized trial. We aimed to determine whether the cell source (bone marrow vs autologous chondrocytes) affects the outcome, given that all the other aspects of the procedures are similar. All the included patients in our study had ≥1 previous surgical procedures to the index knee, and no patients with acute injuries were recruited to the study. The results after 1 and 2 years of follow-up are presented in this article.
Methods
Study Design
The initial study plan was to include 80 patients over 3 years. The study was designed to be a 2-center study involving The University Hospital of North Norway, Tromsø, and 1 collaborating hospital in the region. The study site in Tromsø was expected to do the majority of surgical procedures. The collaborating site was terminated 1 year into the trial because of unforeseen practical issues.
We included patients with ≥1 chondral/osteochondral lesions of the distal femur and/or patella as identified by magnetic resonance imaging findings and/or previous arthroscopies. The inclusion of patients was broad, with few exclusion criteria (Table 1). Patients with signs of early osteoarthritis were accepted, but it was required that the surgeon had judged the main symptoms to be caused by ≥1 isolated cartilage defects suitable for biological repair. The same senior orthopaedic surgeon (G.K.), experienced in both techniques, evaluated all patients before they were included in the study and was involved in all surgical procedures.
TABLE 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age, 18-60 y Informed consent signed by patient Symptomatic cartilage defects of the knee >2 cm2 |
Inflammatory joint disease Serious illness Alcohol or drug abuse during the past 3 y Malalignment Symptomatic ligament instability |
Signed informed consent was obtained prior to surgery, and the protocol was approved by the regional ethics committee. Financial support was provided by the orthopaedic department at The University Hospital of North Norway. Patients were randomized into blocks of 10 through sealed envelopes after inclusion in the study, but before surgery.
Baseline Assessment
Baseline clinical data from the Knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm questionnaire, and visual analog scale (VAS) for pain were obtained prior to surgery. The degree of osteoarthritis at baseline was assessed by the Kellgren-Lawrence score evaluated from weightbearing radiographs of the index knee. Range of motion, alignment, and ligament status were clinically assessed.
Surgical Technique
The surgical techniques were modified to ensure that the conditions for cartilage regeneration were kept as similar as possible, with the major difference being the source of the cells introduced into the defect—namely, the cultivated autologous chondrocytes in ACI-C versus in vivo bone marrow cells in AMIC. The approach to the knee at the final operation was done by a small arthrotomy appropriate to the location of the defect. If several defects were addressed, they were accessible with the same limited arthrotomy. The defects were debrided to healthy surrounding cartilage and down to the subchondral bone plate, with removal of all the cartilage, including the calcified layer. A collagen type I/III patch (Chondro-Gide; Geistlich Pharma), exactly covering the entire defect, was sutured to the surrounding cartilage by 5.0 or 6.0 resorbable sutures and then sealed along the edges with fibrin glue (Tisseel; Baxter). The stability of the patch was assessed by flexing and extending the knee 5 times.
Autologous Matrix-Induced Chondrogenesis
A modified surgical technique was used, similar to the technique described by Benthien and Behrens.3 In contrast to the original description, we used sutures around the edge, and fibrin glue was applied only around the rim as for ACI-C. After debridement, a 1.5-mm drill was used to perforate the subchondral bone plate to a depth of 1 cm, thereby mobilizing bone marrow stem cells into the defect. Care was taken to leave areas of intact subchondral bone plate between the drill holes. The collagen patch was then sutured in place before fibrin sealant was applied.
Collagen-Covered Autologous Chondrocyte Implantation
Second-generation ACI (ACI-C) was used, similar to the technique described by Brittberg et al.4 A ∼200-mg cartilage biopsy was arthroscopically harvested from a low weightbearing area of the index knee 3 to 4 weeks prior to index surgery. Patients were instructed to unload on crutches for 2 to 4 days after the biopsy. Immediately after harvest, the cartilage biopsy was mechanically and enzymatically digested to release the chondrocytes. The chondrocytes were serially expanded to passages 2 to 3 during 3 to 4 weeks and implanted into the debrided cartilage defect. At least 1 million cells/cm2 were injected under the sutured collagen patch, and a final suture and fibrin sealant completed the transplantation. Care was taken not to induce bleeding from the bottom of the defect before transplantation.
Postoperative Rehabilitation
Continuous passive motion was performed with the Kinetec Spectra Knee for four 1-hour sessions a day during the 2- to 5-day hospital stay. Continuous passive motion was discontinued at discharge. The patients were allowed partial weightbearing (15-20 kg) with crutches for the first 6 weeks. Only patients with defects in the patellofemoral joint were issued a knee brace, restricting movement from 0° to 40° for 6 weeks. The brace was used continuously during the 6 weeks. To mimic continuous passive motion, indoor cycling was encouraged as soon as the pain and swelling allowed it. A written exercise program designed to increase range of motion and increase strength was given to all the patients before departure. At 6 weeks, the rehabilitation progress was evaluated by a physical therapist at our department. Further guided rehabilitation from a local physical therapist was issued if needed.
Follow-up
Patients were evaluated at 1 and 2 years postoperatively by 1 of the 2 surgeons involved in the study. The evaluators were not blinded to treatment rendered. The same clinical scores obtained at baseline were repeated at each follow-up. Weightbearing anteroposterior and lateral radiographs of the knee were obtained 2 years after surgery.
Outcomes
The primary outcome was defined as change in the KOOS score versus baseline at every follow-up. Secondary outcome measures were treatment failure and change from baseline in KOOS subscales, Lysholm score, and VAS pain score. Assessment of progression of osteoarthritis by change in Kellgren-Lawrence score was not done at the 2-year follow-up but is planned at the 5- and 10-year follow-up.
Treatment failures were reported as either a “hard failure” or a “clinical failure.” A hard failure was defined as the patients needing a new resurfacing procedure of the index lesion or implantation of a knee prosthesis. A clinical failure was defined as any deterioration in KOOS scores at 2-year follow-up compared with baseline. Diagnostic rearthroscopy or arthroscopy with debridement of synovia or the defect was not considered a failure.
Statistical Methods
A sample size estimation based on a similar trial performed by one of the authors (Knutsen et al24) indicated that 40 patients would be required in each group to demonstrate a difference in change between the groups of at least 0.75 SD, with a significance level of .05 and a power of 0.90. A positive change (delta) in KOOS, Lysholm, and VAS scores represented improvement as compared with baseline for all the scores. Comparison of mean delta between the groups was performed with a 2-sample t test with a significance level of P < .05. The normality assumption was assessed by descriptive statistics. Comparison between the groups was performed on the intention-to-treat (ITT) population. Missing data from patients with hard failures and patients lost to follow-up were obtained from the last observation carried forward. A noninferiority analysis comparing AMIC with ACI-C was performed by evaluation of the 95% CI of the difference in delta KOOS at 2 years. A noninferiority margin was not defined in the study protocol. Both the ITT and the per-protocol (PP) populations were used for the noninferiority analysis. In the PP population, patients with hard failure and patients lost to follow-up were excluded from the data. Bivariate correlation was examined with Pearson correlation coefficients. A sensitivity analysis comparing the difference in delta KOOS, delta Lysholm, and delta VAS was performed with the nonparametric Mann-Whitney U test. SPSS (v 25; IBM) was used for statistical analysis.
Results
Baseline Characteristics
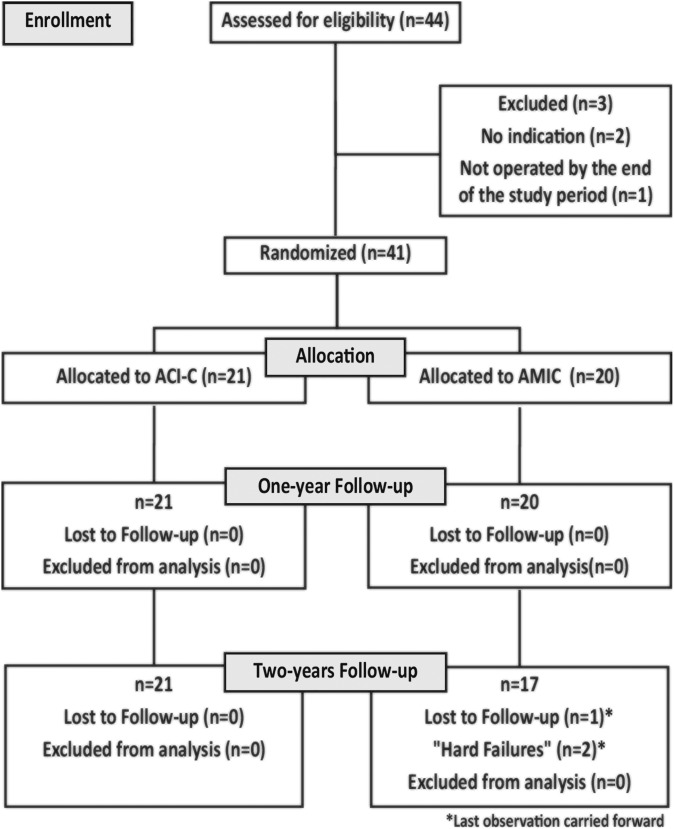
A total of 44 patients were enrolled into the study between October 2011 and November 2014. Given the technical and practical aspects, all patients were included and underwent surgery at The University Hospital of North Norway. One patient did not undergo surgery during the study period, and 2 were excluded during initial assessment owing to a lack of symptoms, thus leaving 41 patients included in the final study and representing the ITT population, where 21 patients were treated with ACI-C and 20 with AMIC (Figure 1).
Figure 1.
Trial progression flowchart. ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis.
Patient and lesion characteristics are summarized in Tables 2 and 3, respectively. The exact cause of the defect was challenging to assess given the long duration of symptoms in many of the cases. No acute traumatic defects were included in the study. All the patients had previous surgery in the index knee. These included arthroscopic debridement (33 patients), arthroscopic microfracture (21 patients), arthroscopic total and partial meniscectomy (15 patients), anterior cruciate ligament reconstruction (4 patients), drilling of osteochondritis dissecans defects (2 patients), and removal of loose bodies (3 patients). The harvest procedure in the ACI-C group was not regarded as previous surgery. Ten patients in the AMIC group and 11 in the ACI-C group had previous microfracture performed on the index knee, while the number of patients with previous microfracture on the index lesion was not recorded. Both patients with prior drilling of an osteochondritis dissecans defect were in the ACI-C group, and it was the same lesion that was addressed in our trial. In the 4 patients with anterior cruciate ligament reconstruction, the procedure had been performed 9 to 10 years prior to index surgery.
TABLE 2.
Baseline Characteristicsa (N = 41)
| ACI-C (n = 21) | AMIC (n = 20) | |
|---|---|---|
| Sex | ||
| Male | 14 (66.7) | 8 (40) |
| Female | 7 (33.3) | 12 (60) |
| Age at surgery, y | 37.2 ± 10.8 (19-55) | 38.3 ± 8.2 (24-55) |
| Body mass index, kg/m2 | 25.7 ± 4.3 | 27.9 ± 4.3 |
| Cause of defect | ||
| Chronic activity–related defect | 13 (61.9) | 13 (65.0) |
| Osteoarthritis | 0 (0) | 2 (10.0) |
| Osteochondritis dissecans | 3 (14.3) | 0 (0) |
| Other/unknown | 5 (23.8) | 5 (25.0) |
| No. of previous surgical procedures in the same knee | ||
| 1 | 6 (28.6) | 3 (15.0) |
| 2 | 6 (28.6) | 10 (50.0) |
| 3 | 7 (33.3) | 6 (30.0) |
| 4 | 1 (4.8) | 0 (0) |
| 5 | 0 (0) | 0 (0) |
| 6 | 1 (4.8) | 1 (5.0) |
| Microfracture | 11 (52.4) | 10 (50.0) |
| ACL reconstruction | 2 (9.5) | 2 (10.0) |
| Duration of symptoms, y | 9.3 ± 5.5 (1-21) | 9.4 ± 6.4 (1-23) |
| Ligament status: instability | ||
| ACL | 1 (4.8) | 1 (5.0) |
| PCL | 0 (0.0) | 2 (10.0) |
| LCL | 0 (0.0) | 1 (5.0) |
| MCL | 0 (0.0) | 1 (5.0) |
| Alignment | ||
| Normal | 17 (81.0) | 14 (70.0) |
| Varus | 3 (14.3) | 1 (5.0) |
| Valgus | 1 (4.8) | 3 (15.0) |
| Missing | 2 (10.0) | |
| Range of motion | ||
| Flexion | ||
| Full | 21 (100.0) | 13 (65.0) |
| >90° | 0 (0.0) | 6 (30.0) |
| Missing | 0 (0.0) | 1 (5.0) |
| Extension | ||
| Full | 17 (81.0) | 13 (65.0) |
| Deficit | 4 (19.0) | 6 (30.0) |
| Missing | 0 (0.0) | 1 (5.0) |
| Kellgren-Lawrence score | ||
| 0 | 7 (33.3) | 4 (20.0) |
| 1 | 9 (42.9) | 8 (40.0) |
| 2 | 2 (9.5) | 8 (40.0) |
| 3 | 3 (14.3) | 0 (0) |
| Score | ||
| KOOS | 58.5 ± 15.7 | 54.1 ± 19.2 |
| Lysholm | 52.6 ± 11.6 | 50.5 ± 18.6 |
| VAS pain | 50.0 ± 20.1 | 57.6 ± 20.6 |
aValues are presented as mean ± SD (range) or n (%). ACI-C, collagen-covered autologous chondrocyte implantation; ACL, anterior cruciate ligament; AMIC, autologous matrix-induced chondrogenesis; KOOS, Knee injury and Osteoarthritis Outcome Score; LCL, lateral collateral ligament; MCL, medial collateral ligament; PCL, posterior cruciate ligament; VAS, visual analog scale.
TABLE 3.
Preoperative Lesion Characteristicsa
| ACI-C (n = 21) | AMIC (n = 20) | |
|---|---|---|
| Location of defects | ||
| Medial femoral condyle | 7 (33.3) | 7 (35.0) |
| Lateral femoral condyle | 2 (9.5) | 1 (5.0) |
| Trochlea | 7 (33.3) | 5 (25.0) |
| Patella | 1 (4.8) | 4 (20.0) |
| Trochlea and patella | 2 (9.5) | 2 (10.0) |
| Trochlea and medial femoral condyle | 2 (9.5) | 1 (5.0) |
| No. of defects | ||
| 1 | 17 (81.0) | 16 (80.0) |
| 2 | 4 (19.0) | 4 (20.0) |
| Total defect size, cm2 | 4.9 ± 4.4 (1.2-21.5) | 5.2 ± 2.4 (2.0-12.3) |
| ICRS grade | ||
| Main defect | ||
| 3 | 16 (76.2) | 17 (85.0) |
| 4 | 5 (23.8) | 3 (15.0) |
| Secondary defect | ||
| 3 | 2 (50.0) | 4 (100) |
| 4 | 2 (50.0) | 0 (0) |
aValues are presented as mean ± SD (range) or n (%). ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; ICRS, International Cartilage Repair Society.
One patient had 2 defects in the lateral femoral condyle. Four patients with a single defect had a defect size <2.0 cm2, all in the ACI-C group. Three patients with 2 addressed lesions had a main defect >2.0 cm2 but a secondary defect <2.0 cm2: 2 in the AMIC-group and 1 in the ACI-C-group. No patients had 2 defects where both were <2.0 cm2. One patient in the AMIC group with a patellar defect had an additional realignment procedure (tibial tubercle osteotomy and lateral release) at the index surgery because of habitual patellar dislocations. The rehabilitation was as for patellofemoral lesions. No other patients had realignment or unloading procedures performed.
The preoperative mean KOOS was 58.5 in the ACI-C group versus 54.1 in the AMIC group (P = .42). The mean Lysholm at baseline was 52.6 (ACI-C) versus 50.5 (AMIC) and the mean VAS, 50.0 (ACI-C) versus 57.6 (AMIC).
Clinical Outcomes at 1 and 2 Years
All 2-year follow-ups were completed by December 15, 2016. At the 1-year follow-up, there were no hard failures, and no patients were lost to follow-up. At 2 years, there were 3 failures in the ACI-C group and 5 in the AMIC group. Two patients in the AMIC group underwent total knee replacement surgery at 21 and 23 months after the primary surgery (hard failures). Their last observation data were carried forward to the 2-year follow-up. One patient in the AMIC group had missing data at 2 years and was classified as lost to follow-up at this time point. Data from the last observation were carried forward. Characteristics of the patients with failure at 2 years are summarized in Table 4.
TABLE 4.
Baseline Characteristics and KOOS at Baseline and at 2 Years of Patients With Failure at 2-Year Follow-upa
| K-L Score | Defect Size, cm2 | KOOS | |||||
|---|---|---|---|---|---|---|---|
| Surgery | Age, y | Localization | Baseline | 2 y | Comments | ||
| Clinical failure | |||||||
| ACI-C | 31 | MFC | 0 | 1.2 | 44.0 | 35.7 | Diagnosed with fibromyalgia and possible ankylosing spondylitis postsurgery. Receives disability benefits. |
| ACI-C | 33 | MFC | 0 | 3.1 | 68.5 | 47.6 | Got pregnant shortly after primary surgery. |
| ACI-C | 51 | MFC and trochlea |
3 | 4.2 and 0.8 | 82.7 | 52.4 | MRI 1 y after surgery showed major degenerative changes. |
| AMIC | 44 | Trochlea and MFC |
2 | 7.5 and 4.8 | 67.3 | 60.7b | Rearthroscopy 17 mo after surgery. Minimal regeneration cartilage and osteophyte formation in the middle of the trochlear defect. Debrided with bone cutter. Good filling of medial condyle defect. |
| AMIC | 35 | MFC | 2 | 3.8 | 97.0 | 88.7 | PCL insufficient when tested by arthroscopy at primary surgery. Not addressed. Satisfied at 1 y, but result had deteriorated at 2 y. |
| AMIC | 38 | MFC | 0 | 2.0 | 70.8 | 68.5 | Rearthroscopy within 1 y. Good filling of the defect. At 2 y, under evaluation for fibromyalgia. |
| Hard failure | |||||||
| AMIC | 55 | MFC | 2 | 6.0 | 26.2 | 26.2b | Not satisfied at last follow-up after knee replacement. |
| AMIC | 39 | Trochlea | 2 | 5.0 | 33.9 | 67.3b | Lacking information after the knee replacement. |
aACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; K-L, Kellgren Lawrence; KOOS, Knee injury and Osteoarthritis Outcome Score; MFC, medial femoral condyle; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament.
bOne-year data carried forward.
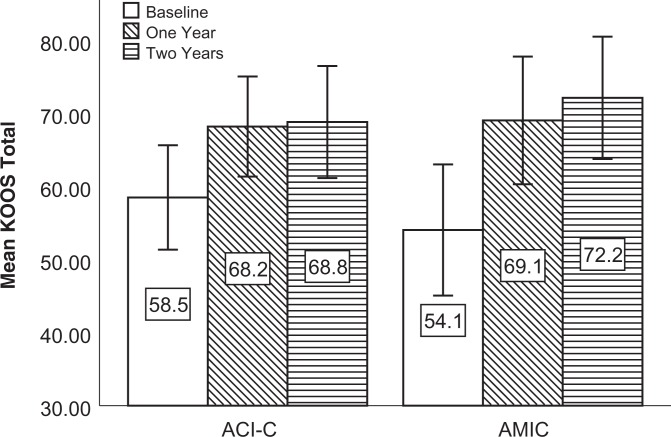
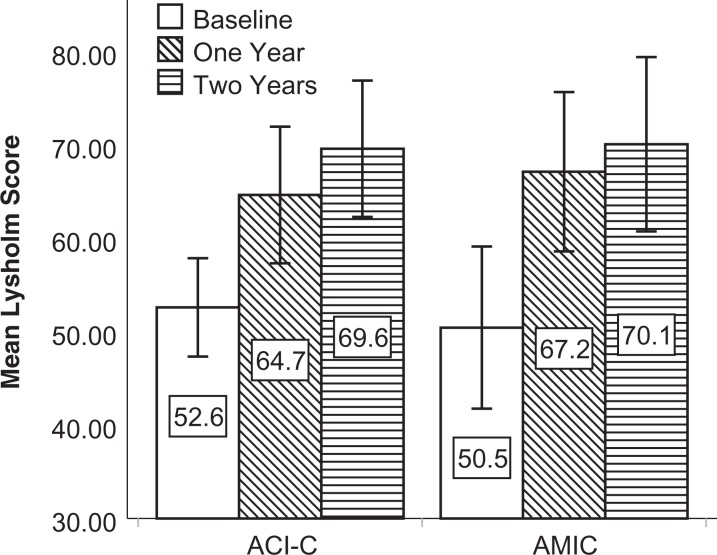
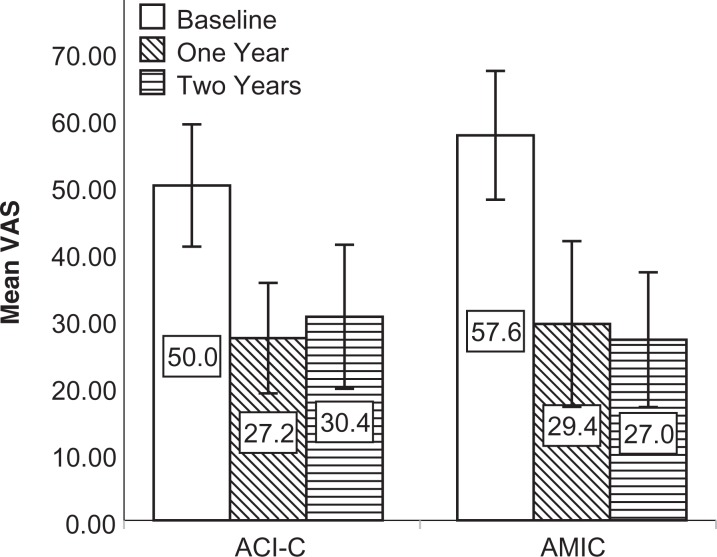
The mean KOOS, Lysholm score, and VAS score improved from baseline at both follow-ups (Figures 2 -4). Compared with baseline, the mean delta for the KOOS, Lysholm score, and VAS score was significantly higher than zero (highest P value = .018) for all patients and in both groups at both follow-ups.
Figure 2.
Mean KOOS score at baseline and follow-up for both intention-to-treat populations. Mean delta KOOS was significantly higher than zero at 1 year: 9.7 (P = .02) in the ACI-C group and 15.0 (P = .002) in the AMIC group. Mean delta KOOS was significantly higher than zero at 2 years: 10.3 (P = .008) in the ACI-C group and 18.1 (P = .001) in the AMIC group. Error bars represent 95% CIs. ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; KOOS, Knee injury and Osteoarthritis Outcome Score.
Figure 3.
Mean Lysholm score at baseline and follow-up for both intention-to-treat populations. Mean delta Lysholm score was significantly higher than zero at 1 year: 12.0 (P = .004) in the ACI-C group and 16.7 (P < .001) in the AMIC group. Mean delta Lysholm score was significantly higher than zero at 2 years: 17.0 (P < .001) in the ACI-C group and 19.7 (P = .001) in the AMIC group. Error bars represent 95% CIs. ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis.
Figure 4.
Mean VAS pain score at baseline and follow-up for both intention-to-treat populations. Mean delta VAS was significantly higher than zero at 1 year: 22.8 (P < .001) in the ACI-C group and 28.2 (P < .001) in the AMIC group. Mean delta VAS was significantly higher than zero at 2 years: 19.6 (P = .002) in the ACI-C group and 30.6 (P < .001) in the AMIC group. Error bars represent 95% CIs. ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; VAS, visual analog scale.
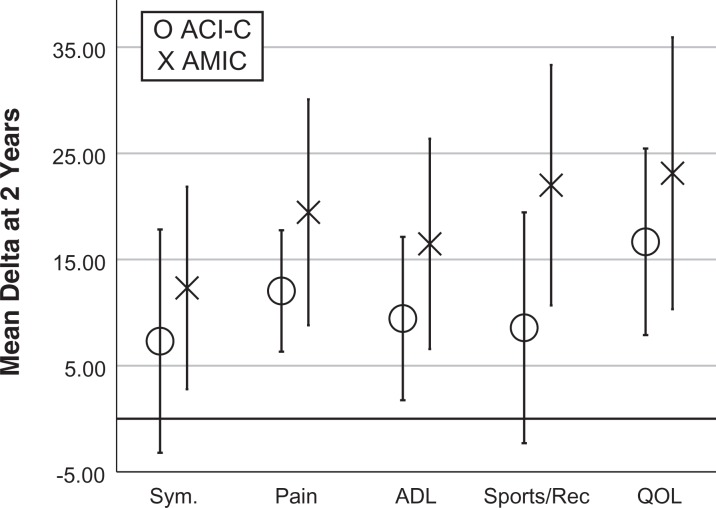
At 2 years, the primary outcome mean delta KOOS was 10.3 in the ACI-C group and 18.1 in the AMIC group (P = .17). As shown in Figure 5, the mean delta for all KOOS subscales at 2 years was higher in the AMIC group, but the difference was not statistically significant. The lowest P value was for KOOS Sports/Recreation (ACI-C, 8.6; AMIC, 22.0; P = .08). The mean delta for the KOOS Symptoms and Sports/Recreation subscales was not statistically significant higher than zero in the ACI-C group at 2 years.
Figure 5.
Comparison of mean delta for KOOS subscores in each group at 2 years. Error bars represent 95% CIs. ADL, activities of daily living; KOOS, Knee injury and Osteoarthritis Outcome Score; QOL, quality of life; Sym, Symptoms.
The mean delta Lysholm score was higher in the AMIC versus the ACI-C group at 2 years (19.7 vs 17.0), but this was not significantly different (P = .66). At 2 years, the mean delta VAS was 19.6 in the ACI-C group versus 30.6 in the AMIC group, but the finding was not significant (P = .19).
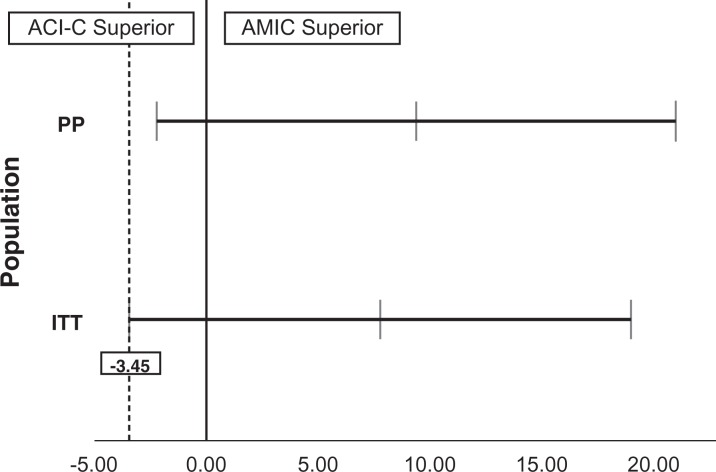
A noninferiority analysis of AMIC versus ACI-C was performed by evaluation of the 95% CI of the mean difference in delta KOOS at 2 years. As advised in a noninferiority trial, this was done for both the ITT and PP populations.6,26 As shown in Figure 6, a noninferiority margin of –3.45 would have been sufficient to statistically prove noninferiority of AMIC as compared with ACI-C with a 0.05 alpha level in the ITT population. For the PP population, the noninferiority margin would have had to be –2.22 to claim noninferiority.
Figure 6.
The mean with 95% CI for the difference between the groups in delta KOOS for the per-protocol (PP) and intention-to-treat (ITT) populations at 2 years. If the 95% CI were located entirely to the right of zero, AMIC would have been proven superior. If it were located entirely to the left of zero, ACI-C would have been proven superior. For both populations, the 95% CI crossed zero, meaning no superiority was proven. The lowest noninferiority margin (–3.45) for statistically proven noninferiority of AMIC as compared with ACI-C is shown as the vertical dotted line. ACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; KOOS, Knee injury and Osteoarthritis Outcome Score.
A sensitivity analysis with the nonparametric independent samples Mann-Whitney U test did not show any statistically significant differences between the groups with regard to delta KOOS, delta Lysholm, or delta VAS pain at either follow-up.
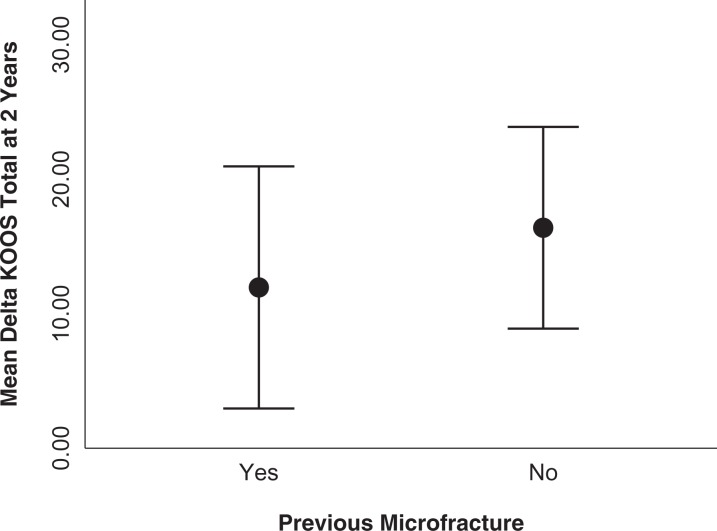
No significant correlations were found between the delta KOOS or delta Lysholm at 2 years and the total size of the defects, number of defects, age at surgery, body mass index, or sex. Patients with previous microfracture surgery to the index knee had a tendency toward a lower mean delta KOOS at 2 years, but this difference was not significant (Figure 7).
Figure 7.
Mean delta KOOS at 2 years in patients with and without previous microfracture to the index knee, P = .79. Error bars indicate 95% CI. KOOS, Knee injury and Osteoarthritis Outcome Score.
Two patients in both groups underwent rearthroscopy within the first follow-up. One of the patients in the ACI-C group underwent 2 rearthroscopies within the first year. At 2 years, 3 patients in both groups had undergone rearthroscopy since the index surgery. Two patients, 1 from each group, underwent reoperation twice within the second follow-up (Table 5).
TABLE 5.
Characteristics of Patients Who Underwent Rearthroscopy During the First 2 Years of Follow-upa
| Surgery | Time From Primary Surgery, mo | Reason for Rearthroscopy | Localization | Findings |
|---|---|---|---|---|
| ACI-C | 5; 10 | Pain; pain | Patella | Good filling of defect, scar tissue in front of and medial to the patella, debridement; still good filling of the defect, debridement |
| ACI-C | 5 | Hydrops and catching | Trochlea | Loosening of part of the collagen membrane, good filling of the defect, debridement |
| ACI-C | 16 | Pain and catching | LFC | Operated by “other” surgeon, no mentioning of the defect, synovitis, debridement |
| AMIC | 7; 24 | Acute pain and swelling; persistent pain | Trochlea | Good filling of the defect in trochlea, no filling of small defect in MFC where microfracture was performed at initial surgery, new microfracture in MFC; adequate filling of the defect, healing of defect in MFC, some general osteoarthritis |
| AMIC | 12 | Increasing pain and stiffness | MFC | Good filling of defect, some scar tissue and synovitis, debridement |
| AMIC | 16 | Swelling, pain, and extension deficit | Trochlea and MFC | Sparse regeneration cartilage in the defect with an osteophyte in the middle, removal of the osteophyte, general debridement |
aACI-C, collagen-covered autologous chondrocyte implantation; AMIC, autologous matrix-induced chondrogenesis; LFC, lateral femoral condyle; MFC, medial femoral condyle.
No major acute complications, such as deep infection, venous thrombosis, or cardiovascular events, were observed in any of the groups.
Discussion
This study is to our knowledge the first head-to-head comparison of the clinical outcomes for ACI-C versus AMIC. Both groups significantly improved as compared with baseline despite having ≥1 previous surgical procedures to the index knee. AMIC patients had a higher mean improvement in all clinical scores at 1 and 2 years as compared with ACI-C. According to the primary outcome (mean delta KOOS), our RCT showed no statistically significant superiority of either ACI-C or AMIC at 2 years for treatment of cartilage defects of the knee. This conclusion was supported by the secondary outcome measures. However, we had 2 hard failures in the AMIC group at the 2-year mark, as opposed to none in the ACI-C group.
Microfracture is widely accepted as a first-line treatment option for small- to medium-sized cartilage defects of the knee.29,37 For larger defects, many clinicians prefer osteochondral plugs or a cartilage-regenerative technique, such as ACI-C or MACI, because of concern about the efficacy of microfracture to treat larger noncontained defects. Four high-quality RCTs comparing microfracture and cartilage regenerative techniques with follow-up of 2 to 5 years were identified in the review article by Devitt et al.9 Vanlauwe et al36 (microfracture vs CCI) and Knutsen et al23,24 (microfracture vs ACI-C) reported no difference between the groups at 5 years. In the early treatment group (symptoms <3 years), Vanlauwe et al detected a favorable result for CCI. Saris et al32 and Crawford et al7 compared microfracture with MACI and found superior results in favor of MACI in some of their outcomes at 2 years. In 2016, Knutsen et al22 published long-term data from a trial comparing microfracture and ACI-C. No difference was found between the “survivors.” Several studies have reported favorable short-time results for the microfracture-based AMIC procedure.2,13,25 High-quality studies comparing the outcomes of ACI-C and AMIC are, however, lacking.
In our trial, patients treated with AMIC reported higher mean delta in all outcomes examined as compared with ACI-C, but given the low power following inclusion of fewer patients than initially planned, no statistical superiority could be shown. As a consequence, we performed a noninferiority analysis of AMIC versus ACI-C. Interpreting a superiority trial as a noninferiority trial may be feasible given a set of conditions.6 In our trial, a noninferiority test with a noninferiority margin of –3.45 would have yielded a statistically significant noninferiority of AMIC as compared with ACI-C for mean delta KOOS at 2 years. Since our trial was designed as a superiority trial, no noninferiority margin was set in the protocol, and the noninferiority analysis must be considered a sensitivity analysis. Improvement or decline of 10 points has been suggested as a cutoff representing a minimal clinically important difference in the KOOS score.31 A posttrial noninferiority margin of –5 therefore seems like a conservative estimate to statistically prove that AMIC is noninferior to ACI-C with a significance level of P < .05.
In the review article by Devitt et al,9 no significant difference was found in the failure rates of various techniques in any trial up to 5 years. Knutsen et al22 found no significant difference in treatment failure comparing ACI-C and microfracture after 15 years of follow-up. A major limitation when comparing the results of different cartilage repair techniques is the diverging definition of failure. Filardo et al11 recommended that a failure be regarded as patient-reported knee scores that fail to remain improved (>10 points) from baseline or revision surgery with active manipulation at the index lesion. In our trial, failure was defined as any deterioration of the KOOS score and/or a resurfacing/joint replacement procedure. This definition led to 5 failures in the AMIC group and 3 in the ACI-C group at 2 years. Two patients in the AMIC group became hard failures when they underwent a knee replacement operation within 2 years; both patients had a Kellgren-Lawrence score of 2 prior to surgery. The relative number of patients with failure was higher in the AMIC group at 2 years. Most cases classified as failure had some factors that could have contributed to the unfavorable outcome (see Table 4). In the future, we should aim toward a uniform definition of failure to be used in RCTs comparing different techniques for cartilage repair.
The assumed cell source differs fundamentally between the marrow stimulation techniques and the chondrocyte transplantation techniques. Chondrocytes and mesenchymal stem cells both demonstrate a potential to build hyaline-like cartilage in laboratory models,10,19 but in clinical trials, the resulting repair tissue is often a mix of fibrocartilage and hyaline cartilage. In their histological examination of 67 biopsies from patients treated with ACI-C (n = 32) or microfracture (n = 35), Knutsen et al24 demonstrated a trend toward more hyaline-like cartilage in patients treated with ACI, but the difference was not significant. The highest-quality predominantly hyaline cartilage was produced in 6 (19%) patients with ACI and 4 (11%) with microfracture. Saris et al32 presented microscopically similar repair tissue quality when comparing MACI and microfracture. In addition, several studies have shown no correlation between the quality of the regeneration tissue and the clinical outcome.23 The same conclusion was made in a study from our group, in which the surplus chondrocytes from the ACI-C group in this trial were used to investigate any correlation between in vitro chondrogenic potential and clinical outcome.18
The exact mechanism behind the formation of repair tissue is poorly understood for both marrow stimulation and chondrocyte transplantation. Conflicting evidence exists about the fate of the transplanted chondrocytes and how they contribute to cartilage regeneration. Grande et al15 found only 8% of the repair cells to be transplanted chondrocytes in a rabbit model. Mierisch et al28 were able to detect transplanted transgenic chondrocytes labeled with enhanced green fluorescent protein in the defect, but they did not appear to form repair tissue, and they decreased in number with time. Hirschmann et al16 and Dell’Accio et al8 found, using different labeling techniques, that the transplanted chondrocytes could persist in the defect and become a part of the repair tissue. Elvenes et al10 demonstrated in 2009 that the bone marrow collected from a single “microfracture hole” in the middle of a cartilage defect contained mesenchymal progenitor cells with the potential to transform into cartilage-forming cells.
It seems as though the microfracture technique is capable of generating repair tissue of the same quality as the cell transplantation techniques, but the risk of fibrocartilage formation is higher. This could be from the lack of scaffolding and containment. One could even argue that the marrow stimulation and cell transplantation techniques, from a biological and biomechanical point of view, are basically the same.17 During preparation of the lesion prior to chondrocyte transplantation, some degree of bleeding from the floor of the defect usually occurs, thereby inducing a spontaneous repair response from the bone marrow compartment. From our trial, it seems as if the source of the cells plays an inferior role with regard to short-term clinical outcome.
The main limitation of our trial is the small number of patients in each group. After 3 years, we had included 41 patients. We estimate that at least 3 more years would be needed to reach the 80 patients called for by the power calculation, and we decided to end the inclusion of patients for economic and practical reasons.
Another limitation of the study design is the broad inclusion criteria used. This led to heterogeneity regarding the location of defects, number of defects, etiology of the defects, duration of symptoms, and age for the group as a whole. A difference in sex distribution was observed, with a higher percentage of women in the AMIC group. In a study from 2010, Gille et al14 reported a significantly higher clinical score in males treated with AMIC than in females. If female patients have a poorer outcome than male patients, this would have affected our results for the AMIC group. However, other trials,13 including our own, have not shown the same correlation. Four patients in the ACI-C group were, at the time of final surgery, shown to have a total defect size smaller than the size described in the inclusion criteria. This was due to overestimation of the nondebrided defect size during arthroscopy. These patients were not excluded from the study, and their results could have inflated the outcome of the ACI-C group. Many patients had signs of early osteoarthritis, even at baseline, but no patients were included who were clear candidates for unicompartmental/total knee replacement or osteotomy. Unloading braces were not used for lesions in the femorotibial articulation, but any negative effect that this practice could have on the outcome scores would be applicable to both groups.
The broad inclusion of patients might have led to the inclusion of patients with some degree of a chronic pain condition. No acute lesions were included in the trial, and this could have had a negative impact on the outcome scores in both groups.36 Previous microfracture is known to negatively affect the outcome of subsequent cartilage regeneration procedures.30 Although we saw the same tendency in our study, the disadvantage was equally distributed between the groups. Since the aim of the trial was to compare the results of AMIC versus ACI-C and not to evaluate the effectiveness of the treatments, we do not consider the broad inclusion criteria a major limitation of the trial’s conclusion. The heterogeneity of the population and the chronicity of the defects make it more relevant to extrapolate the results of our study to the typical group of patients seen in a clinical setting.
Conclusion
This RCT comparing ACI-C and AMIC as a treatment for cartilage defects of the knee indicated that the 2 treatments result in similar clinical outcomes at 2-year follow-up. The patients in this study are scheduled for 5- and 10-year follow-ups. If the conclusion of the present study stands and is confirmed by further clinical trials, AMIC could be considered an equal alternative to techniques based on chondrocyte transplantation for treatment of cartilage defects of the knee. If considering that AMIC is a less expensive 1-step procedure, one could even argue that AMIC should be preferred. Further basic and clinical research is needed in this field, as all available surgical methods today are imperfect for cartilage repair.
Footnotes
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Regional Ethics Committee (2011/1159).
References
- 1. Aae TF, Randsborg PH, Luras H, Aroen A, Lian OB. Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC(R)) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–1319. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. [DOI] [PubMed] [Google Scholar]
- 5. Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. [DOI] [PubMed] [Google Scholar]
- 6. Committee for Proprietary Medicinal Products. Points to consider on switching between superiority and non-inferiority. Br J Clin Pharmacol. 2001;52(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford DC, DeBerardino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94(11):979–989. [DOI] [PubMed] [Google Scholar]
- 8. Dell’Accio F, Vanlauwe J, Bellemans J, Neys J, De Bari C, Luyten FP. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21(1):123–131. [DOI] [PubMed] [Google Scholar]
- 9. Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2017;24(3):508–517. [DOI] [PubMed] [Google Scholar]
- 10. Elvenes J, Knutsen G, Johansen O, Moe BT, Martinez I. Development of a new method to harvest chondroprogenitor cells from underneath cartilage defects in the knees. J Orthop Sci. 2009;14(4):410–417. [DOI] [PubMed] [Google Scholar]
- 11. Filardo G, Andriolo L, Balboni F, Marcacci M, Kon E. Cartilage failures: systematic literature review, critical survey analysis, and definition. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3660–3669. [DOI] [PubMed] [Google Scholar]
- 12. Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79–89. [PubMed] [Google Scholar]
- 13. Gille J, Behrens P, Volpi P, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456–1464. [DOI] [PubMed] [Google Scholar]
- 15. Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7(2):208–218. [DOI] [PubMed] [Google Scholar]
- 16. Hirschmann F, Verhoeyen E, Wirth D, Bauwens S, Hauser H, Rudert M. Vital marking of articular chondrocytes by retroviral infection using green fluorescence protein. Osteoarthritis Cartilage. 2002;10(2):109–118. [DOI] [PubMed] [Google Scholar]
- 17. Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts and practice—myths and misconceptions—progress and prospects. Osteoarthritis Cartilage. 2015;23(3):334–350. [DOI] [PubMed] [Google Scholar]
- 18. Islam A, Fossum V, Hansen AK, Urbarova I, Knutsen G, Martinez-Zubiaurre I. In vitro chondrogenic potency of surplus chondrocytes from autologous transplantation procedures does not predict short-term clinical outcomes. BMC Musculoskelet Disord. 2019;20(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Islam A, Hansen AK, Mennan C, Martinez-Zubiaurre I. Mesenchymal stromal cells from human umbilical cords display poor chondrogenic potential in scaffold-free three dimensional cultures. Eur Cell Mater. 2016;31:407–424. [DOI] [PubMed] [Google Scholar]
- 20. Kahlenberg CA, Nwachukwu BU, Hamid KS, Steinhaus ME, Williams RJ., 3rd Analysis of outcomes for high tibial osteotomies performed with cartilage restoration techniques. Arthroscopy. 2017;33(2):486–492. [DOI] [PubMed] [Google Scholar]
- 21. Kim HK, Moran ME, Salter RB. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion: an experimental investigation in rabbits. J Bone Joint Surg Am. 1991;73(9):1301–1315. [PubMed] [Google Scholar]
- 22. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am. 2016;98(16):1332–1339. [DOI] [PubMed] [Google Scholar]
- 23. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. [DOI] [PubMed] [Google Scholar]
- 24. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86(3):455–464. [DOI] [PubMed] [Google Scholar]
- 25. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109–2115. [DOI] [PubMed] [Google Scholar]
- 26. Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66(2):150–154. [PubMed] [Google Scholar]
- 27. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–466. [PubMed] [Google Scholar]
- 28. Mierisch CM, Wilson HA, Turner MA, et al. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85(9):1757–1767. [DOI] [PubMed] [Google Scholar]
- 29. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 30. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325–331. [DOI] [PubMed] [Google Scholar]
- 31. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saris D, Price A, Widuchowski W, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42(6):1384–1394. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–553. [DOI] [PubMed] [Google Scholar]
- 34. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369. [DOI] [PubMed] [Google Scholar]
- 35. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7(4):300–304. [Google Scholar]
- 36. Vanlauwe J, Saris DB, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566–2574. [DOI] [PubMed] [Google Scholar]
- 37. Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instr Course Lect. 2007;56:419–428. [PubMed] [Google Scholar]