Abstract
Traditionally, the mitochondria have been viewed as the cell’s powerhouse, producing energy in the form of ATP. As a byproduct of ATP formation, the mitochondrial electron transport chain produces substantial amounts of reactive oxygen species (ROS). First thought to be toxic, recent literature indicates an important signaling function for mitochondria-derived ROS, especially in relation to cardiovascular disease pathogenesis. This has spawned an evolution to a more contemporary view of mitochondrial function as a dynamic organelle involved in key regulatory and cell survival processes. Beyond ROS, recent studies have identified a host of mitochondria-linked factors that influence the cellular and extracellular environments, including mitochondria-derived peptides, mitochondria-localized proteins, and the mitochondrial genome itself. Interestingly, many of these factors help orchestrate ROS homeostasis and ROS-related signaling. The paradigm defining the role of mitochondria in the vasculature needs to be updated yet again to include these key signaling factors, which serves as the focus of the current review. In describing these novel signaling factors, we pay specific attention to their influence on endothelial homeostasis. Therapies targeting these pathways are discussed, as are emerging research directions.
Keywords: Mitochondria, Cardiovascular disease, Endothelium, Reactive oxygen species
Introduction
Mitochondria have historically been viewed as the battery of the cell, using oxygen to produce energy in the form of ATP, especially in highly metabolic tissue like cardiac and skeletal muscle. However, the vascular endothelium does not appear to depend on the mitochondria to meet its energy demands, instead relying primarily on glycolysis to produce ATP [17, 65]. The non-canonical function of endothelial mitochondria supports the concept that these organelles fulfill a regulatory or homeostatic, rather than an energy-producing role within this specific locale. The critical role of the mitochondria in regulating endothelial function is now clear, with particular attention having been paid to the role of reactive oxygen species (ROS) as signaling factors [26, 91].
Introduction to mitochondrial signaling
Reactive oxygen species
In the endothelium, mitochondrial ROS (mtROS) are a byproduct of the oxygen consumption required to produce ATP. High levels of ROS can promote inflammation, proliferation, and apoptosis through diverse signaling pathways. ROS production can also impact the functional status of the vasculature, and, conversely, endothelial products impact mitochondrial function. For example, endothelial nitric oxide (NO) maintains vascular dilation and suppresses production of mitochondrial ROS. NO bioavailability diminishes as rising ROS scavenge NO, leading to peroxynitrite formation and loss of mtROS suppression [38, 76]. Excessive ROS can precipitate endothelial dysfunction [10], which is characterized by an impaired dilatory response to blood flow or pharmacological agonist, and is implicated in several disease processes, including diabetes [32], hypertension [75], heart failure [43], ischemia–reperfusion injury [45], sepsis [62], and atherosclerosis [11, 18]. Endothelial dysfunction also carries prognostic significance in relation to cardiovascular outcomes [29, 68]. Therefore, clinicians and scientists have attempted to combat ROS production and improve endothelial health.
Therapeutic approaches targeting ROS in the treatment of cardiovascular diseases have been largely unsuccessful [39, 72]. The failure of several antioxidant trials aimed at globally and indiscriminately reducing ROS levels has evoked the notion that not all ROS are pathological, a narrow therapeutic window may exist when targeting ROS, and only particular cellular components may be negatively impacted by ROS. Maintaining moderate levels of ROS appears to be critical for endothelial homeostasis and signaling, and many recent reviews have addressed the idea that mitochondrial ROS production plays an important physiological role. For example, our lab showed that production of hydrogen peroxide from the mitochondria is necessary for preservation of flow-mediated dilation in human arterioles from subjects with coronary artery disease (CAD) [63]. Reports that mitochondrial ROS interact with other ROS-producing sites, such as the NADPH oxidase isoforms [19], underscore the complexity of ROS signaling and the importance of targeted antioxidants [13]. Several excellent reviews have discussed the need for and development of more specific ROS inhibitors. For example, compounds such as MitoQ [22, 25] and Nox2ds-tat [66] have been shown to improve endothelial dysfunction. These developments are promising, and further research using targeted ROS inhibitors to allow for physiological ROS signaling is necessary.
Mitochondrial signaling strategies
Within the cell, mitochondria are embedded in an interconnected signaling framework and can employ a variety of mechanisms to impact cellular dynamics. For example, retrograde signaling from the mitochondria to the nucleus provides a major intracellular communication route for mitochondria-localized factors [34, 47]. Since many mitochondrial transcription factors are encoded within the nuclear genome, such a crosstalk between these two organelles is not surprising. For instance, Hwang et al. assessed three different mitochondrial DNA (mtDNA) haplogroups and found differential expression of nuclear genes in the three groups, despite conserved mitochondrial function [33]. These findings suggest that differences in mtDNA content affect nuclear gene expression profiles.
Mitochondrial molecules also link to other organelles to regulate cellular responses through additional intracellular pathways. For example, calcium is released from the endoplasmic reticulum (ER) and can activate a wide range of cellular programs, from apoptosis to generation of NO. Intracellular mitofusin-1, a protein implicated in mitochondrial fusion, serves as a docking molecule that links the mitochondria and ER to buffer calcium excess [36, 81]. A similar calcium-buffering link has been demonstrated between the mitochondria and the Golgi apparatus and the plasma membrane [81].
In addition to subcellular signaling programs, mitochondrial factors can even be released from one cell and exert paracrine or endocrine effects on a different cell. Cellular stress can result in externalization of bits of mtDNA, and this circulating mtDNA can then invoke inflammatory receptor signaling which could ultimately precipitate systemic cardiovascular collapse [92]. Additionally, microvesicles shed from the cellular membrane, once considered to be quiescent byproducts of cell damage, are now known to participate in cell–cell communication [2, 20]. Evidence suggests that microvesicles containing mitochondrial elements can alter nearby cells [48, 80]. One such study demonstrated that release of intact, platelet-derived mitochondria within microvesicles can elicit a local inflammatory response with cellular recruitment [8]. A different report revealed that released microvesicles harboring intact mtDNA can rescue other cells with damaged mtDNA [71]. Taken together, these various strategies help to frame the mitochondria as dynamic organelles utilizing diverse intra- and extra-cellular mechanisms to impact both cellular and systemic responses.
Novel mitochondrial signaling pathways
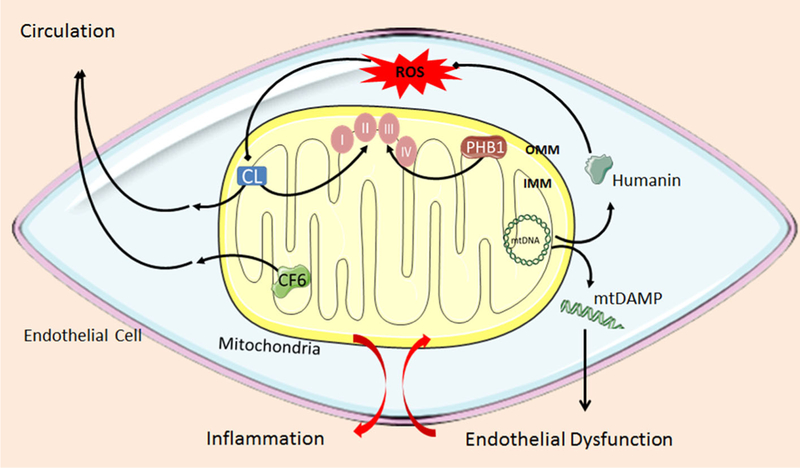
Although the investigative spotlight has largely focused on mtROS as endothelial signaling elements, other mitochondria-derived signaling molecules have been characterized. These include factors that are localized to mitochondrial compartments and, like ROS, contain the potential to influence endothelial function (Fig. 1). A growing body of literature has identified such factors, including: (1) CF6, a mitochondria-derived peptide located on the endothelial cell surface; (2) peptides derived from the 16S rRNA region of the mitochondrial genome, including humanin; (3) cardiolipin (CL), found within the inner mitochondrial membrane (IMM); and (4) circulating fragments of mtDNA (Fig. 1). These various mechanisms extend mitochondrial signaling beyond ROS, and renew our understanding of the capability of mitochondria to impact diverse processes such as energy production, apoptosis, calcium handling, and mitophagy.
Fig. 1.
Proposed common mechanism highlighting the role of these novel signaling factors in pro-inflammatory pathways within the endothelial cell. Endothelial dysfunction mobilizes pro-inflammatory mitochondrial elements and contributes to vascular disease pathogenesis. OMM outer mitochondrial membrane, IMM inner mitochondrial membrane, mtDNA mitochondrial DNA, CF6 coupling factor 6, CL cardiolipin, PHB1 prohibitin-1, mtDAMP mitochondrial damage-associated pattern
This review will discuss recent developments that characterize the role of these key new players in modulating endothelial function. We hope to demonstrate the complexity of mitochondrial signaling pathways and identify new areas and targets for further research and therapeutic intervention.
Mitochondrial signaling factors
Coupling factor 6, a mitochondrial released hormone?
CF6 is a component of mitochondrial ATP synthase, where it serves as one of four subunits of the energy-producing stalk that connects the extrinsic and intrinsic membrane domains. Curiously, CF6 is stored in the mitochondria but is also found on the vascular endothelial cell surface [56]. CF6 blocks prostacyclin synthesis and elicits frank vasoconstriction in rats [55, 57]. In agreement with the vasoconstrictor function of CF6, spontaneously hypertensive rats (SHR) were found to possess higher circulating levels of CF6 versus normotensive rats [61]. This has prompted the suggestion that CF6 is a serum marker of vascular disease.
CF6 is released from the mitochondria of vascular endothelial cells into the systemic circulation. Shear stress increases both gene expression of CF6 and release of CF6 into the medium of cultured human umbilical vein endothelial cells (HUVECs) [56]. It is fascinating to consider how this protein finds its way from the mitochondrial inner membrane to the cell surface and then into the blood. Cell damage is not the underlying mechanism by which CF6 is released from the cell. Instead, evidence in the vasculature suggests a directed mechanism exists for transport of CF6 across cell membranes and to the extracellular space, similar to that of a circulating hormone [57].
Within the mitochondria, CF6 acts on ATP synthase to increase ATP hydrolase activity. Application of ATP decreased CF6 binding to the β-subunit of ATP synthase [60]. TNF-α can stimulate the movement of CF6 from the mitochondria to the extracellular space in endothelial cells via the NF-κB pathway [67]. TNF-α has been implicated in many cardiovascular disease processes, including atherosclerosis and hypertension, and CF6 might be an overlooked component of this pro-inflammatory pathway of disease. Similar to C-reactive protein (CRP), CF6 might function as a biomarker for, or etiological factor in, cardiovascular disease. Unlike many other serum markers, CF6 is released without concurrent endothelial cell damage, positioning it as a possible marker for early-onset endothelial changes.
Several reports demonstrate that CF6 may contribute to the pathogenesis of disease processes that are characterized by inflammation. For example, Osanai et al. discovered that plasma CF6 levels were elevated in patients with hypertension and end-stage renal disease (ESRD) [58, 59], both of which are characterized by endothelial dysfunction and inflammation. In a multiple linear regression model, CF6 levels also associated with the incidence of ischemic heart disease in ESRD [58], whereas many of the traditional risk factors did not. Moreover, CF6 levels can change in response to alterations in salt load, and CF6 is increased in patients with essential hypertension relative to normotensive controls [59]. Kumagai et al. demonstrated that CF6 has a role in the pro-atherogenic pathway by downregulating PECAM-1, which is a known anti-atherogenic molecule, a mediator of NO release during shear, and a target of statins in endothelial cells. In addition to downregulating PECAM-1, application of CF6 reduced shear-induced NO release [41].
CF6 contributes to several human disease processes, and is an excellent candidate for therapeutic targeting. To that point, treatment with troglitazone, a thiazolinedione and PPAR-γ agonist, decreased CF6 levels in endothelial cells, whereas fenofibrate, a PPAR-α agonist, did not. The beneficial effects of troglitazone on CF6 levels were mediated by alterations in the NF-κB pathway [74]. Whether troglitazone or other thiazolinediones may decrease CF6 to improve blood pressure or cardiovascular outcomes in humans is unknown. The antioxidant ascorbic acid decreases CF6 in hypertensive patients, but these failed to lower blood pressure [59]. Further studies are needed to determine if directly targeting CF6 improves therapeutic outcomes.
Mitochondrial transcription
The mitochondrial genome is a double-stranded, circular structure comprised of 16,569 base pairs and 37 genes. These genes encode 13 polypeptides associated with the respiratory chain complexes, 2 rRNAs, and 22 tRNAs. The majority of genes coding for mitochondrial proteins reside in the nucleus [73]. Those peptides encoded by mitochondrial DNA (mtDNA) have some distinct properties (Fig. 1). Humanin, a 24-amino-acid peptide encoded by the 16S rRNA region of the mitochondrial genome, has received considerable attention due to its protective effects in Alzheimer’s disease [31]. Humanin is also expressed in the human vascular endothelium, but not in vascular smooth muscle cells, and plays an important role in reducing ROS in response to oxidized LDL (oxLDL) in isolated human vessels [4]. Since humanin is encoded in the mitochondrial genome, it is not surprising that it specifically affects mitochondrial ROS production. Circulating humanin levels in humans are lower in older patients and subjects with endothelial dysfunction, consistent with a role in age-related vascular disease [4, 86]. Indeed, humanin improves endothelial function and prevents progression of atherosclerosis in ApoE−/− mice [53]. Further evidence for a beneficial role in vascular disease, intraperitoneal injection of humanin reduces production of the pro-atherosclerotic endothelial sphingolipid, ceramide, and can reduce inflammatory markers associated with renal microvascular remodeling [94]. One group has reported a higher level of humanin in unstable human atherosclerotic plaques, where it may act to combat inflammation [90]. Whether humanin upregulation can serve as a therapeutic option for atherosclerosis in humans warrants further investigation.
When evaluating mtDNA-encoded factors, it is important to consider that the transfer of mtDNA does not follow Mendelian inheritance patterns. The notion that maternal inheritance of mtDNA might influence disease transmission to progeny is not new, but many studies focus exclusively on known maternally inherited diseases. Less frequently discussed is the maternal influence of mtDNA mutations on cardiovascular diseases that are not exclusively inherited through the maternal line. Since the mitochondrial genome is highly conserved, and both men and women inherit the same mitochondrial DNA from their mothers, it may seem counterintuitive that mtDNA could play a differential role based on sex. However, equivalent disruptions in mtDNA can impact males and females differently. For example Golob et al. studied D257A mice, which contain a mutated nuclear-encoded polymerase-γ region, rendering the mtDNA more susceptible to mutation. Male, but not female, mice bred on the same genetic background developed pulmonary and systemic hypertension [23]. Although the mtDNA content was similar between male and female mice, the response to insults to the mitochondrial genome can differ based on sex. Elucidating the mechanism for this observation may reveal the cause of known sex-driven differences in cardiovascular disease risk and progression.
Cardiolipin
Cardiolipin (CL) is a phospholipid present in both prokaryotes and eukaryotes that is synthesized and located within the inner mitochondrial membrane (Fig. 1). CL has received substantial attention for its role in cardiovascular pathologies like antiphospholipid syndrome and Barth syndrome. Elevated levels of anticardiolipin antibodies are also associated with endothelial dysfunction and atherosclerosis in humans [49]. If CL dysregulation is associated with these cardiovascular pathologies, what cellular signaling mechanisms might be at play?
Many cardiovascular diseases are associated with high levels of oxidative stress, and CL is extremely susceptible to oxidation, which leads to inactivation. This inactivation leads to disruption of mitochondrial components responsible for ATP production [12]. Oxidized CL also stimulates a proinflammatory milieu in endothelial cells by increasing 5-lipoxygenase (5-LOX) and adhesion molecules, such as ICAM-1 and VCAM-1 [82]. Inhibition of endothelial CL oxidation by the mitochondria-targeting compound SS-31 reduced inflammation and prevented microvascular rarefaction in the renal microvasculature, highlighting CL as a promising mitochondrial therapeutic target in microcirculatory disease [46]. Another signaling mechanism through which CL may impact cardiovascular homeostasis is its physical structure, with regard to its chirality as an isolated molecule, as well as its relative spatial distribution within the mitochondrial membrane. CL can move from the IMM to the OMM, resembling the movement of CF6 from the mitochondria to the cell surface and into the circulation. CL is typically distributed in an asymmetrical transmembrane fashion, and disruption of CL asymmetry or externalization of CL to the OMM can initiate a signaling response, such as activation of mitophagy, the process by which mitochondria are degraded. Pathogens appear to manipulate their own CL distribution as they invade host cells [35]. Mitochondrial stressors like rotenone can influence a similar translocation of cardiolipin within the mitochondria [12].
Aside from CL’s association with cardiovascular health, little is known about the mechanistic role of CL in contributing to both normal and pathological endothelial cell function. The pathways described above may help guide future research and serve as a starting point to decipher endothelial-specific effects of cardiolipin. Since growing attention is paid to the role of the immune system and invading pathogens as contributors to cardiovascular disease initiation and exacerbation, perhaps pathogen-related CL alterations may be one mechanism whereby normal endothelial function is affected.
Mitochondrial damage-associated molecular patterns
Damage-associated patterns (DAMPs) are non-pathogen nucleotide segments that can trigger autoimmune system activation. Recent provocative research has defined a role for mtDNA itself in impacting the vascular endothelium. Circulating mtDNA can function as an endogenous DAMP that is recognized by the Toll-like receptor 9 (TLR9), present in endothelial cells (Fig. 1). TLR9 displays specificity for unmethylated CpG regions, which are common in prokaryotic bacterial DNA but not vertebrate DNA. mtDNA contains these CpG regions, supporting the notion that they may be innate immune system activators in the same manner as bacteria, from which eukaryotic mitochondria are thought to derive. Indeed, TLR9 activation by mtDNA causes downstream activation of p38 MAP kinase and promotes IL-8 signaling [92].
Zhang et al. described a fascinating link between mtDNA relased, TLR signaling, and neutrophil recruitment in the development of systemic inflammatory response syndrome (SIRS), an event that is similar in presentation to sepsis [93]. Another study reported on the functional significance of mtDNA-TLR9 signaling in the development of heart failure through promotion of an inflammatory signaling cascade [54]. McCarthy et al. provide further evidence that supports the inflammatory role of endothelial-derived mtDNA and its association with hypertension. This group found that plasma samples from SHR contained elevated levels of the circulating mtDNA genes Cyt B and ND6, but not 16S rRNA, compared to Wistar Kyoto (WKY) rats. Treatment with hydrochlorothiazide/reserpine (HCTZ/res) returned the gene expression level to baseline values. Reduced NO bioavailability, and corresponding impaired endothelium-dependent relaxation were observed in the mesenteric arteries of SHR after TLR9 activation. TLR9 agonism by OD2395 in Sprague–Dawley (SD) rats activated COX and p38 MAPK, confirming a switch away from NO as the mediator of dilation in presence of TLR9 activation. To support a functional role for mtDNA, inhibition of TLR9 in SHR lowered systolic blood pressure, whereas TLR9 activation in SD rats raised blood pressure. Interestingly, none of the changes occurred in female rats, regardless of treatment with HCTZ/Res, providing support that mtDNA appears to not only contribute to maternal influences on cardiovascular disease across generations, but also may drive sex-related differences in cardiovascular disease manifestation [52]. Another group reported that the vascular lung injury and increase in endothelial cell leakiness after bacterial infection can be mimicked by injection of mtDNA DAMPs derived from SD rats into the pulmonary artery of isolated, perfused rat lungs [40]. Involvement of externalized mtDNA has also been suggested to contribute to the development of preeclampsia [24]. Clearly, mtDNA can exert harmful effects in the endothelium through pathological signaling.
N-formyl peptides (F-MIT) are fragments of mtDNA that can also function as DAMPS to impact the vasculature. F-MIT are recognized by formyl peptide receptors (FPRs), instead of TLR9, and promote vasodilation. F-MIT transmit messages through GPCR signaling to increase Ca2+ and activate downstream MAP kinases; F-MIT also interact with chemokine signaling pathways [83]. The link between mitochondrial DAMPs and vascular function is not confined to hypertension. Like hypertension, atherosclerosis, heart failure, and sepsis are also characterized by endothelial dysfunction. The systemic cardiovascular dilation seen in septic shock is associated with documented bacteremia in only 30–50 % of patients [9]. In cases where a pathogenic cause is not discovered, septic shock may arise from endogenous factors released by host cell damage. Wenceslau et al. reported that infusion of F-MIT in Wistar rats, independent of endotoxin production, resulted in dose-dependent hypotension and increased NO production, which contrasts with the reduced NO levels after mtDNA administration and TLR9 activation. The F-MIT used contained a mitochondria NADPH dehydrogenase subunit 6 (ND6) peptide to distinguish it from the F-MIT produced by bacteria. Of note, infusion of mtDNA did not produce the same cardiovascular collapse, likely due to the opposing effects of FPR versus TLR9 activation on endothelial NO levels, emphasizing the differential cellular responses to mitochondrial DAMPs. In addition, in direct conflict with the results of the study performed by McCarthy et al., infusion of the same mtDNA biological structure mimetic and TLR9 agonist (ODN2395) did not produce a significant change in blood pressure from baseline [85]. It is intriguing that the same lab saw different responses to activators of the mtDNA pathway; however, the reason for these conflicting results was not identified. These investigators also reported a contrasting response to ODN2395 treatment between in vivo and ex vivo models, but the mechanisms for the difference were not determined. These findings highlight opportunities for clarifying the mechanism of mitochondrial signaling pathways in the vasculature, in addition to the potential for targeted manipulation of these outlined pathways.
Inflammation, the common link?
Evaluating the impact of individual mitochondrial signaling factors on endothelial homeostasis leads us to consider the question, what is the overall impact of endothelial mitochondria on cardiovascular disease? Each of the specific signaling pathways described in this review converges on inflammation as a common and unifying endothelial signaling mechanism. The influence of inflammation in cardiovascular diseases has been well documented and is now a canonical concept in vascular biology. However, the role of these specified mitochondrial factors in promoting the inflammatory milieu may be an under-recognized and under-studied component of the development of atherosclerosis and impaired tissue perfusion.
If mitochondria are symbiotic organelles derived from prokaryotic ancestors, then mechanisms likely exist to prevent immune-targeting of these “non-self” organelles. Such a phenomenon occurs on the organismal level to invoke an immunotolerant relationship between mother and fetus. Infection can precipitate an immune response that causes a breakdown of the mother–fetus tolerance, resulting in premature labor. The dysregulation of immunotolerance is also relevant to cardiovascular disease with respect to the relationship between the mitochondria and the endothelial cell. It is possible that mediators of endothelial damage—high-fat meals, excessive barotrauma, or smoking, for example—shift the relationship between mitochondria and endothelial cell from symbiotic to immunologically antagonistic. Endothelial disruption may expose mitochondrial factors that mobilize the host cell’s immune and inflammatory responses as mitochondria-derived factors, such as pieces of mtDNA or CF6, shift from the intra-mitochondrial to extra-mitochondrial environment, where they can then initiate inflammatory signaling cascades (Fig. 1). For example, it is tantalizing to observe that both a peptide synthesized by the mitochondrial genome, humanin, and the physical structure of mtDNA itself signal via the formyl peptide receptor (FPR) [30]. It is possible that these factors function similarly to, and likely synergistically with, excessive ROS through inflammatory signaling cascades, thereby contributing to cardiovascular disease development.
Transcriptional regulators that target mitochondria
These novel mitochondrial signaling molecules do not operate in isolation. A known inter-organelle signaling pathway exists between the mitochondria and nucleus. Transcriptional regulators function as one route of communication through which mitochondrial function is affected. Transcriptional regulators do not directly interact with DNA but instead recruit transcription factors and other regulatory elements to increase or decrease gene transcription. One key player in this nuclear-mitochondrial crosstalk is the nuclear protein PGC-1α (peroxisome-proliferator-activated receptor-gamma coactivator-1alpha), a master controller of mitochondrial biogenesis, and a heavily studied metabolic factor in relation to diabetes, obesity, skeletal muscle function, and neurodegeneration [64]. The role of PGC-1α in endothelial homeostasis has received less attention, but initial work has shown that PGC-1α attenuates mitochondrial ROS production in HUVECs by specific enhancement of mitochondrial antioxidant gene expression [78]. As stated above, TNF-α is a pro-inflammatory molecule implicated in the pathogenesis of atherosclerosis. Overexpression of PGC-1α stifles TNF-α-mediated increases in ROS and NF-κB expression in endothelial cells, suggesting a potential role for PGC-1α in ameliorating atherosclerosis [37]. Although PGC-1α was initially described as a nuclear protein, it is also found in the mitochondria, where it interacts with the mitochondrial transcription factor A (TFAM) of mtDNA [3]. Given both its mitochondrial localization and convergence on inflammatory pathways, PGC-1α may interact with these mitochondrial factors to regulate inflammation in the vascular endothelium. Characterizing an interaction between PGC-1α and the discussed non-ROS mitochondrial signaling factors in the vascular endothelium would identify another communication route underlying nuclear–mitochondrial crosstalk.
Telomerase also serves to link mitochondrial and nuclear signaling by virtue of the expression of its catalytic subunit, TERT, in the nucleus, where it lengthens telomeres, and the ability of TERT to translocate to the mitochondria, where it reduces mtROS production. Telomeres are nucleotide-repeat sequences located at the tips of nuclear chromosomes that are positively associated with cell survival. Decreased telomere length is associated with several cardiovascular pathologies, including hypertension, atherosclerosis, and heart failure [70]. Importantly, mtDNA does not contain telomeres, suggesting an alternative role for TERT in this location. Although TERT maintains telomere length in the nucleus, it has also evolved an independent, non-transcriptional function in the vascular endothelium outside the nucleus. Oxidative stress in endothelial cells can stimulate TERT export from the nucleus [27]. Opposite of nuclear TERT, mitochondrial localization of TERT increases in response to elevated levels of ROS [1]. Haendeler et al. extended this concept to show direct translocation of TERT from the nucleus to mitochondria. TERT has also been shown to bind to mtDNA and protect from oxidative stress [28], but the actual role of TERT in the mitochondria remains controversial. Emerging studies demonstrate roles in the mitochondria and cytosol where endothelial TERT catalytically reduces ROS generation and promotes NO formation. One recent study revealed that loss of TERT activity in healthy human vessels causes a switch from NO to pro-inflammatory hydrogen peroxide as the mediator of dilation, and restoration of telomerase activity in arterioles from human with CAD reverts the mediator of flow-induced dilation from hydrogen peroxide to NO [5]. Of note, TERT has also been shown to act downstream of PGC-1α, and upregulation of both elements prevents the development of atherosclerosis in ApoE−/− mice [87]. Therefore, PGC-1α and TERT both associate with inflammatory pathologies and appear to provide a signaling route between the nucleus and mitochondria to alter cellular and mitochondrial homeostasis.
Future directions
Other mitochondria-derived peptides
Additional less well-characterized non-ROS mitochondria-derived peptides (MDP) loom on the horizon as potential mediators of vascular mitochondrial signaling. In addition to humanin, Cobb et al. have revealed that six other small humanin-like peptides, termed SHLPs, are encoded by the 16S rRNA region. SHLP1–5 promotes cell survival in the same manner as humanin. SHLP6, however, promotes apoptosis and inhibits VEGF expression [15]. The 12S rRNA region of mtDNA is also receiving attention [21]. Lee et al. identified mitochondrial open reading frame of the 12S rRNA-c (MOTS-c) as a 16-amino-acid peptide derived from the mitochondrial genome. Treatment with MOTS-c prevented the development of obesity and improved insulin resistance after feeding a high-fat diet to mice [44]. Given the critical function of the mitochondria and MDPs in the vasculature, as well as the known association between insulin resistance and endothelial dysfunction, MOTS-c may play a key role in linking metabolic syndrome and diabetes to cardiovascular disease.
Prohibitin 1
The mitochondrial genome houses information for creating portions of the respiratory chain complexes that utilize oxygen to produce energy in the form of ATP. Once thought to be distinct enzymes, it is now recognized that these subunits can coalesce in different groupings to form supercomplexes, which are thought to improve efficiency of electron transport and ATP production, and to reduce superoxide production [79]. Prohibitin-1 (PHB1) is a protein located on the inner mitochondrial membrane that supports mitochondrial supercomplex formation by linking Complexes III and IV (Fig. 1) [50]. Downregulating PHB1 (siRNA) increased ROS production and senescence in cultured HUVECS [69]. Subsequent overexpression of PHB1 reduced levels of apoptosis. PHB1 may also be a therapeutic target. Grape seed procyanidin extract is known to have antioxidant and beneficial cardiovascular effects. A derivative, grape seed procyanidin B2 (GSPB2), was shown to increase PHB1 to attenuate HUVEC apoptosis [88].
The functional consequence of decreased PHB1 in the vascular endothelium is poorly understood. One group observed a decrease in the formation of functional blood vessels during angiogenesis after knockdown of PHB1 [69]. The increase in ROS production and impaired angiogenesis following knockdown of PHB1 is Akt-mediated, supporting a signaling role for this mitochondrial protein in the vascular endothelium. The role of PHB1 in relation to endothelial cell function is evolving with a need for studies in animals and humans.
Long non-coding RNA
The mitochondrial genome contains sections that, unlike the 16 s rRNA region, do not encode for proteins. These sections are termed long noncoding RNA (lncRNA) and occur in high abundance in the circulation (78 % mitochondrial chromosome versus 1–4 % somatic or sex chromosome in plasma samples). Circulating lncRNA’s are markers for the presence of and events related to cardiovascular disease [77]. The prototypical example is LIPCAR, which correlates with late stages of cardiac remodeling in subjects with chronic heart failure and demonstrates prognostic significance in chronic heart failure patients [42]. It remains to be determined through what mechanism LIPCAR exits the mitochondria and enters the circulation, and whether LIPCAR directly affects endothelial cell function.
Mitochondrial common deletion
In contrast to the concept that the presence of circulating pieces of mtDNA itself, independent of encoded factors, can impact the vasculature, other studies highlight that even the absence of portions of the mitochondrial genome is associated with cardiovascular complications. A 4977 base pair deletion in mtDNA, has emerged as relevant to the aging processes [16]. In patients affected by CAD, the frequency of the common deletion was reported as three times higher in subjects over 72 years of age versus younger subjects [6]. Studies have also revealed an increasing frequency of the mitochondrial common deletion in the smooth muscle cells of human aortic atherosclerotic lesions, independent of sex and oxidative DNA damage [7]. The functional significance of the mitochondrial common deletion is not immediately apparent, especially in relation to human vascular endothelial function. Future work should determine whether a mechanistic link exists between the common deletion and atherosclerosis in human subjects.
Vascular smooth muscle cells
The focus of this review is mitochondrial signaling factors in the endothelium. However, given the close functional relationship between the endothelium and vascular smooth muscle cells (VSMC) as part of the same vascular unit, it is possible that mitochondrial signaling occurs simultaneously and independently in endothelium and smooth muscle or that intercellular communication occurs via mitochondrial signaling. For example, telomerase has been shown to impact VSMC senescence [51]. CF6 also plays a role in VSMCs [61]. Enhanced CF6-mediated inhibition of prostacyclin was observed in the VSMCs of SHR. Whether the CF6 originated from endothelial cells or VSMCs is not known. One study reported that mtDNA can target VSMCs to drive atherosclerotic disease progression, independent of ROS formation, in ApoE−/− mice [89]. This same group found that human atherosclerotic plaques contained high levels of mtDNA damage. Thus, when pursuing these novel endothelial-located mitochondrial factors in future studies, it will be important to consider how they might also influence surrounding tissues.
Summary and conclusion
It is now apparent that a multitude of mitochondria-linked factors other than ROS can modulate the endothelium and are involved in signaling mechanisms in disease. An intriguing common feature of each distinct pathway is the predisposition for endothelial dysfunction via inflammatory pathways and immune system activation. Invading pathogens and subsequent activation of an immune response through interactions with pathogen- and host-associated mitochondrial signaling factors may be an underappreciated trigger for cardiovascular disease. Host mitochondrial factors released into the circulation could also function as “sterile” pathogens that initiate an inflammatory cycle within the endothelium as a result of cell damage or disruption of endothelial homeostasis. This idea has been excellently reviewed by Wenceslau et al. in relation to mtDAMPs [84], and a similar inflammatory role may exist for the additional factors in the current review.
A lingering question is whether the mitochondria-linked inflammation occurs prior to, and thus drives overt endothelial dysfunction, or if these listed mitochondrial factors are released as a result of pre-existing endothelial dysfunction and act to exacerbate the vascular disease phenotype. Although either scenario is possible, the authors suggest that the presented data demonstrate that the mobilization of these factors is the result of endothelial dysfunction. It appears that an inciting event is required to shift these factors outside of the mitochondria—such as TNF-α [67], high salt, or ROS [59] stimulating release of CF6 or cell damage leading to release of mtDAMPs [84]—where they can then stimulate pro-inflammatory pathways. Moreover, in normotensive human subjects, a high salt load did not elicit release of CF6, whereas, in hypertensive human subjects, salt loading did cause release of CF6, suggesting that some pre-existing, underlying endothelial dysfunction from HTN allows for these factors to contribute to and worsen the disease process [59]. One possibility is that an insult induces damage, recruiting repair mechanisms, and if repair is inadequate, the resulting endothelial dysfunction stimulates release of these mitochondrial factors. One way to investigate this question might be the utilization of a time-course model, determining whether circulating levels of these factors rise before or after experimental endothelial dysfunction is observed. An alternative approach could involve initiating a stressor that impairs endothelial function and determining if inhibition of these factors—such as CF6 or mtDAMPs—is able to improve the endothelial dysfunction.
It is important to note that the mitochondrial signaling factors in this review demonstrate a link with ROS production. The inability of several antioxidant trials to uncover a clear benefit of targeting ROS likely arises from the complexity and diversity of these outlined inflammatory signaling pathways in the mitochondria. It is possible that strategies that utilize a more comprehensive therapeutic approach, including targeting these mitochondria-associated regulatory molecules, might improve study outcomes, particularly in diabetes mellitus and other conditions in which mitochondrial dysfunction is centrally involved. A single magic bullet against vascular disease is unlikely, given the complexity of regulating mitochondrial signaling in the vascular endothelium, but manipulating multiple pathways could achieve better outcomes. Identifying a regulatory element that inhibits several of these pro-inflammatory mitochondrial signaling factors, such as microRNAs, a broad-acting pharmacologic agent, or a common mediator of inflammatory signaling pathways, is a compelling next step. One intriguing possibility is the supplement lipoic acid, an antioxidant undergoing clinical testing that has been shown to have beneficial effects on a host of the outlined factors, including oxidized CL, PGC-1α, and TERT [14, 87]. Much work is needed to elucidate the relationships among these factors, their involvement in disease, and their ability to serve as therapeutic targets.
Acknowledgments
We thank Dr. Michael Widlansky, Dr. David Zhang, Dr. Neil Hogg, and Dawid Chabowski for providing excellent critical input on this manuscript.
Footnotes
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G (2008) Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121:1046–1053. doi: 10.1242/jcs.019372 [DOI] [PubMed] [Google Scholar]
- 2.Antonyak MA, Cerione RA (2014) Microvesicles as mediators of intercellular communication in cancer. Methods Mol Biol 1165:147–173. doi: 10.1007/978-1-4939-0856-1_11 [DOI] [PubMed] [Google Scholar]
- 3.Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR (2010) Peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem 285:21590–21599. doi: 10.1074/jbc.M109.070169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A (2010) Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res 88:360–366. doi: 10.1093/cvr/cvq191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparii M, Rokkas CK, Santos JH, Priel E, Gutterman DD (2015) Critical role for telomerase in the mechanism of flow mediated dilation in the human microcirculation. Circ Res. doi: 10.1161/circresaha.115.307918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogliolo M, Izzotti A, De Flora S, Carli C, Abbondandolo A, Degan P (1999) Detection of the ‘4977 bp’ mitochondrial DNA deletion in human atherosclerotic lesions. Mutagenesis 14:77–82. doi: 10.1093/mutage/14.1.77 [DOI] [PubMed] [Google Scholar]
- 7.Botto N, Berti S, Manfredi S, Al-Jabri A, Federici C, Clerico A, Ciofini E, Biagini A, Andreassi MG (2005) Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat Res 570:81–88. doi: 10.1016/j.mrfmmm.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 8.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Pare A, Rousseau M, Naika GS, Levesque T, Laflamme C, Marcoux G, Lambeau G, Farndale RW, Pouliot M, Hamzeh-Cognasse H, Cognasse F, Garraud O, Nigrovic PA, Guderley H, Lacroix S, Thibault L, Semple JW, Gelb MH, Boilard E (2014) Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124:2173–2183. doi: 10.1182/blood-2014-05-573543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B (1995) Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 274:968–974. doi: 10.1001/jama.1995.03530120060042 [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844. doi: 10.1161/01.RES.87.10.840 [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen K, Gooch V, Sullivan I, Lloyd J, Deanfield J, Spiegelhalter D (1992) Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340:1111–1115. doi: 10.1016/0140-6736(92)93147-F [DOI] [PubMed] [Google Scholar]
- 12.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15:1197–1205. doi: 10.1038/ncb2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cifuentes-Pagano E, Meijles DN, Pagano PJ (2014) The quest for selective nox inhibitors and therapeutics: challenges, triumphs and pitfalls. Antioxid Redox Signal 20:2741–2754. doi: 10.1089/ars.2013.5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cimolai MC, Vanasco V, Marchini T, Magnani ND, Evelson P, Alvarez S (2014) alpha-Lipoic acid protects kidney from oxidative stress and mitochondrial dysfunction associated to inflammatory conditions. Food Funct 5:3143–3150. doi: 10.1039/c4fo00489b [DOI] [PubMed] [Google Scholar]
- 15.Cobb LJ, Nakamura H, Cohen P (2011) Abstract 2848: sHLP6: a naturally occurring mitochondrial-derived peptide has therapeutic potential in prostate cancer. Cancer Res 71:2848. doi: 10.1158/1538-7445.am2011-284821467168 [DOI] [Google Scholar]
- 16.Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC (1992) Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res 275:169–180. doi: 10.1016/0921-8734(92)90021-G [DOI] [PubMed] [Google Scholar]
- 17.Culic O, Gruwel ML, Schrader J (1997) Energy turnover of vascular endothelial cells. Am J Physiol 273:C205–C213 [DOI] [PubMed] [Google Scholar]
- 18.Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109:III-27–III-32. doi: 10.1161/01.CIR.0000131515.03336.f8 [DOI] [PubMed] [Google Scholar]
- 19.Dikalov S (2011) Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM (2013) Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci 7:182. doi: 10.3389/fncel.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuku N, Pareja-Galeano H, Zempo H, Alis R, Arai Y, Lucia A, Hirose N (2015) The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell 14:921–923. doi: 10.1111/acel.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR (2014) Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592:2549–2561. doi: 10.1113/jphysiol.2013.268680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golob MJ, Tian L, Wang Z, Zimmerman TA, Caneba CA, Hacker TA, Song G, Chesler NC (2015) Mitochondria DNA mutations cause sex-dependent development of hypertension and alterations in cardiovascular function. J Biomech 48:405–412. doi: 10.1016/j.jbiomech.2014.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC (2012) Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 123:429–435. doi: 10.1042/cs20120130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF (2009) Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54:322–328. doi: 10.1161/hypertensionaha.109.130351 [DOI] [PubMed] [Google Scholar]
- 26.Gutterman DD (2005) Mitochondria and reactive oxygen species: an evolution in function. Circ Res 97:302–304. doi: 10.1161/01.RES.0000179773.18195.12 [DOI] [PubMed] [Google Scholar]
- 27.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S (2003) Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phospho-rylation of tyrosine 707. Mol Cell Biol 23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S (2009) Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol 29:929–935. doi: 10.1161/atvbaha.109.185546 [DOI] [PubMed] [Google Scholar]
- 29.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA (2002) Prognostic value of coronary vascular endothelial dysfunction. Circulation 106:653–658. doi: 10.1161/01.CIR.0000025404.78001.D8 [DOI] [PubMed] [Google Scholar]
- 30.Harada M, Habata Y, Hosoya M, Nishi K, Fujii R, Kobayashi M, Hinuma S (2004) N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun 324:255–261. doi: 10.1016/j.bbrc.2004.09.046 [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I (2001) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98:6336–6341. doi: 10.1073/pnas.101133498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:e14–e22. doi: 10.1161/01.RES.88.2.e14 [DOI] [PubMed] [Google Scholar]
- 33.Hwang S, Kwak SH, Bhak J, Kang HS, Lee YR, Koo BK, Park KS, Lee HK, Cho YM (2011) Gene expression pattern in transmitochondrial cytoplasmic hybrid cells harboring type 2 diabetes-associated mitochondrial DNA haplogroups. PLoS One 6:e22116. doi: 10.1371/journal.pone.0022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jazwinski SM (2013) The retrograde response: when mitochondrial quality control is not enough. Biochim Biophys Acta 1833:400–409. doi: 10.1016/j.bbamcr.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan VE, Chu CT, Tyurina YY, Cheikhi A, Bayir H (2014) Cardiolipin asymmetry, oxidation and signaling. Chem Phys Lipids 179:64–69. doi: 10.1016/j.chemphyslip.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman RJ, Malhotra JD (2014) Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 1843:2233–2239. doi: 10.1016/j.bbamcr.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK (2007) Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal 9:301–307. doi: 10.1089/ars.2006.1456 [DOI] [PubMed] [Google Scholar]
- 38.Kojda G, Harrison D (1999) Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43:652–671. doi: 10.1016/S0008-6363(99)00169-8 [DOI] [PubMed] [Google Scholar]
- 39.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL, Nutrition Committee of the American Heart Association Council on Nutrition PA and Metabolism (2004) Antioxidant vitamin supplements and cardiovascular disease. Circulation 110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1 [DOI] [PubMed] [Google Scholar]
- 40.Kuck JL, Obiako BO, Gorodnya OM, Pastukh VM, Kua J, Simmons JD, Gillespie MN (2015) Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 308:L1078–L1085. doi: 10.1152/ajplung.00015.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumagai A, Osanai T, Katoh C, Tanaka M, Tomita H, Morimoto T, Murakami R, Magota K, Okumura K (2008) Coupling factor 6 downregulates platelet endothelial cell adhesion molecule-1 via c-Src activation and acts as a proatherogenic molecule. Atherosclerosis 200:45–50. doi: 10.1016/j.atherosclerosis.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 42.Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T (2014) Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 114:1569–1575. doi: 10.1161/circresaha.114.303915 [DOI] [PubMed] [Google Scholar]
- 43.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H (2002) Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106:3073–3078. doi: 10.1161/01.CIR.0000041431.57222.A [DOI] [PubMed] [Google Scholar]
- 44.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21:443–454. doi: 10.1016/j.cmet.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefer AM, Tsao PS, Lefer DJ, Ma X (1991) Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J 5:2029–2034 [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Soong Y, Seshan SV, Szeto HH (2014) Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol 306:F970–F980. doi: 10.1152/ajprenal.00697.2013 [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Butow RA (2006) Mitochondrial retrograde signaling. Annu Rev Genet 40:159–185. doi: 10.1146/annurev.genet.40.110405.090613 [DOI] [PubMed] [Google Scholar]
- 48.Losche W, Scholz T, Temmler U, Oberle V, Claus RA (2004) Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets 15:109–115. doi: 10.1080/09537100310001649885 [DOI] [PubMed] [Google Scholar]
- 49.Marai I, Shechter M, Langevitz P, Gilburd B, Rubenstein A, Matssura E, Sherer Y, Shoenfeld Y (2008) Anti-cardiolipin antibodies and endothelial function in patients with coronary artery disease. Am J Cardiol 101:1094–1097. doi: 10.1016/j.amjcard.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 50.Marques I, Dencher NA, Videira A, Krause F (2007) Supramolecular organization of the respiratory chain in Neurospora crassa mitochondria. Eukaryot Cell 6:2391–2405. doi: 10.1128/ec.00149-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc [DOI] [PubMed] [Google Scholar]
- 52.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC (2015) Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res 107:119–130. doi: 10.1093/cvr/cvv137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, Lerman LO, Cohen P, Lerman A (2011) Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis 219:65–73. doi: 10.1016/j.atherosclerosis.2011.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485:251–255. doi: 10.1038/nature10992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osanai T, Kamada T, Fujiwara N, Katoh T, Takahashi K, Kimura M, Satoh K, Magota K, Kodama S, Tanaka T, Okumura K (1998) A novel inhibitory effect on prostacyclin synthesis of coupling factor 6 extracted from the heart of spontaneously hypertensive rats. J Biol Chem 273:31778–31783. doi: 10.1074/jbc.273.48.31778 [DOI] [PubMed] [Google Scholar]
- 56.Osanai T, Okada S, Sirato K, Nakano T, Saitoh M, Magota K, Okumura K (2001) Mitochondrial coupling factor 6 is present on the surface of human vascular endothelial cells and is released by shear stress. Circulation 104:3132–3136. doi: 10.1161/hc5001.100832 [DOI] [PubMed] [Google Scholar]
- 57.Osanai T, Tanaka M, Kamada T, Nakano T, Takahashi K, Okada S, Sirato K, Magota K, Kodama S, Okumura K (2001) Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor. J Clin Invest 108:1023–1030. doi: 10.1172/jci11076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osanai T, Nakamura M, Sasaki S, Tomita H, Saitoh M, Osawa H, Yamabe H, Murakami S, Magota K, Okumura K (2003) Plasma concentration of coupling factor 6 and cardiovascular events in patients with end-stage renal disease. Kidney Int 64:2291–2297. doi: 10.1046/j.1523-1755.2003.00334.x [DOI] [PubMed] [Google Scholar]
- 59.Osanai T, Sasaki S, Kamada T, Fujiwara N, Nakano T, Tomita H, Matsunaga T, Magota K, Okumura K (2003) Circulating coupling factor 6 in human hypertension: role of reactive oxygen species. J Hypertens 21:2323–2328. doi: 10.1097/01.hjh.0000098161.70956.08 [DOI] [PubMed] [Google Scholar]
- 60.Osanai T, Magota K, Tanaka M, Shimada M, Murakami R, Sasaki S, Tomita H, Maeda N, Okumura K (2005) Intracellular signaling for vasoconstrictor coupling factor 6: novel function of beta-subunit of ATP synthase as receptor. Hypertension 46:1140–1146. doi: 10.1161/01.hyp.0000186483.86750.85 [DOI] [PubMed] [Google Scholar]
- 61.Osanai T, Tomita H, Yamada M, Tanaka M, Ashitate T, Echizen T, Katoh C, Magota K, Okumura K (2009) Coupling factor 6-induced prostacyclin inhibition is enhanced in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens 27:1823–1828. doi: 10.1097/HJH.0b013e32832d4b05 [DOI] [PubMed] [Google Scholar]
- 62.Peters K, Unger RE, Brunner J, Kirkpatrick CJ (2003) Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res 60:49–57. doi: 10.1016/S0008-6363(03)00397-3 [DOI] [PubMed] [Google Scholar]
- 63.Phillips SA, Hatoum OA, Gutterman DD (2007) The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292:H93–H100. doi: 10.1152/ajpheart.00819.2006 [DOI] [PubMed] [Google Scholar]
- 64.Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90. doi: 10.1210/er.2002-0012 [DOI] [PubMed] [Google Scholar]
- 65.Quintero M, Colombo SL, Godfrey A, Moncada S (2006) Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci 103:5379–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ (2001) Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(–) and systolic blood pressure in mice. Circ Res 89:408–414 [DOI] [PubMed] [Google Scholar]
- 67.Sasaki S, Osanai T, Tomita H, Matsunaga T, Magota K, Okumura K (2004) Tumor necrosis factor alpha as an endogenous stimulator for circulating coupling factor 6. Cardiovasc Res 62:578–586. doi: 10.1016/j.cardiores.2004.01.031 [DOI] [PubMed] [Google Scholar]
- 68.Schächinger V, Britten MB, Zeiher AM (2000) Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101:1899–1906. doi: 10.1161/01.CIR.101.16.1899 [DOI] [PubMed] [Google Scholar]
- 69.Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC (2008) Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol 180:101–112. doi: 10.1083/jcb.200706072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serrano AL, Andres V (2004) Telomeres and cardiovascular disease: does size matter? Circ Res 94:575–584. doi: 10.1161/01.res.0000122141.18795.9c [DOI] [PubMed] [Google Scholar]
- 71.Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103:1283–1288. doi: 10.1073/pnas.0510511103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinhubl SR (2008) Why have antioxidants failed in clinical trials? Am J Cardiol 101:S14–S19. doi: 10.1016/j.amjcard.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 73.Taanman JW (1999) The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta 1410:103–123. doi: 10.1016/S0005-2728(98)00161-3 [DOI] [PubMed] [Google Scholar]
- 74.Tomita H, Osanai T, Toki T, Sasaki S, Maeda N, Murakami R, Magota K, Yasujima M, Okumura K (2005) Troglitazone and 15-deoxy-delta(12,14)-prostaglandin J2 inhibit shear-induced coupling factor 6 release in endothelial cells. Cardiovasc Res 67:134–141. doi: 10.1016/j.cardiores.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 75.Touyz RM (2004) Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension what is the clinical significance? Hypertension 44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d [DOI] [PubMed] [Google Scholar]
- 76.Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344. doi: 10.1111/j.1469-7793.2003.00335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uchida S, Dimmeler S (2015) Long noncoding RNAs in cardiovascular diseases. Circ Res 116:737–750. doi: 10.1161/circresaha.116.302521 [DOI] [PubMed] [Google Scholar]
- 78.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M (2005) PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 66:562–573. doi: 10.1016/j.cardiores.2005.01.026 [DOI] [PubMed] [Google Scholar]
- 79.Vartak R, Porras CA, Bai Y (2013) Respiratory supercomplexes: structure, function and assembly. Protein Cell 4:582–590. doi: 10.1007/s13238-013-3032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waldenstrom A, Genneback N, Hellman U, Ronquist G (2012) Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7:e34653. doi: 10.1371/journal.pone.0034653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A (2009) Modulation of calcium signalling by mitochondria. Biochim Biophys Acta 1787:1374–1382. doi: 10.1016/j.bbabio.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 82.Wan M, Hua X, Su J, Thiagarajan D, Frostegard AG, Haeggstrom JZ, Frostegard J (2014) Oxidized but not native cardiolipin has pro-inflammatory effects, which are inhibited by Annexin A5. Atherosclerosis 235:592–598. doi: 10.1016/j.atherosclerosis.2014.05.913 [DOI] [PubMed] [Google Scholar]
- 83.Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, Webb RC (2013) Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses 81:532–535. doi: 10.1016/j.mehy.2013.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC, Working Group on DiCD (2014) Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 35:1172–1177. doi: 10.1093/eurheartj/ehu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, Webb RC (2015) Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol 308:H768–H777. doi: 10.1152/ajpheart.00779.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A (2013) Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304:H393–H397. doi: 10.1152/ajpheart.00765.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong S, Patrushev N, Forouzandeh F, Hilenski L, Alexander RW (2015) PGC-1alpha modulates telomere function and DNA damage in protecting against aging-related chronic diseases. Cell Rep 12:1391–1399. doi: 10.1016/j.celrep.2015.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yin W, Li B, Li X, Yu F, Cai Q, Zhang Z, Wang J, Zhang J, Zhou R, Cheng M, Gao H (2015) Critical role of prohibitin in endothelial cell apoptosis caused by glycated low-density lipoproteins and protective effects of grape seed procyanidin B2. J Cardiovasc Pharmacol 65:13–21. doi: 10.1097/fjc.0000000000000157 [DOI] [PubMed] [Google Scholar]
- 89.Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, Kumar S, Wang JC, Hurst LA, Obaid DR, Logan A, West NEJ, Clarke MCH, Vidal-Puig A, Murphy MP, Bennett MR (2013) Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation 128:702–712. doi: 10.1161/circulationaha.113.002271 [DOI] [PubMed] [Google Scholar]
- 90.Zacharias DG, Kim SG, Massat AE, Bachar AR, Oh YK, Herrmann J, Rodriguez-Porcel M, Cohen P, Lerman LO, Lerman A (2012) Humanin, a cytoprotective peptide, is expressed in carotid atherosclerotic [corrected] plaques in humans. PLoS One 7:e31065. doi: 10.1371/journal.pone.0031065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang DX, Gutterman DD (2007) Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006 [DOI] [PubMed] [Google Scholar]
- 92.Zhang Q, Itagaki K, Hauser CJ (2010) Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08 [DOI] [PubMed] [Google Scholar]
- 93.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107. doi: 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO (2012) Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91:199–206. doi: 10.1016/j.lfs.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]