Abstract
Context
Primary macronodular adrenal hyperplasia (PMAH) is a rare type of Cushing or subclinical Cushing syndrome and is associated with bilateral multinodular formation. ARMC5 is one of the responsible genes for PMAH.
Objectives
This study was performed to identify the genotype-phenotype correlation of ARMC5 in a cohort of Japanese patients.
Patients and Methods
Fourteen patients with clinically diagnosed PMAH and family members of selected patients were studied for ARMC5 gene alteration and clinical phenotype. The associated nonadrenal tumor tissues were also studied.
Results
Of fourteen patients with PMAH, 10 had pathogenic or likely pathogenic variants of ARMC5. We found two variants. Five unrelated patients had identical variants (p.R619*). In two patients, the variant was found in offspring with the asymptomatic or presymptomatic state. Six of ten patients who tested positive for the ARMC5 pathogenic or likely pathogenic variant carried nonadrenal tumors; however, no loss of heterozygosity (LOH) or second hit of the ARMC5 gene was evident. The ARMC5 variant–positive group showed a significantly higher basal cortisol level. Furthermore, age-dependent cortisol hypersecretion was seen in the ARMC5 variant–positive group.
Conclusions
ARMC5 pathogenic variants are common (71%) in Japanese patients with PMAH. p.R619* might be a hot spot in Japanese patients with PMAH. Asymptomatic or presymptomatic pathogenic variant carriers were found among the family members of the patients. Although 50% of ARMC5 variant carriers had nonadrenal neoplastic lesions, no LOH or second hit of ARMC5 in the tumor tissues was evident. The ARMC5 variant–positive mutant group showed a higher basal cortisol level than the negative group.
Keywords: PMAH, ARMC5, variant carrier, the second hit
Primary macronodular adrenal hyperplasia (PMAH), formally called ACTH-independent macronodular adrenal hyperplasia, is one of the subtypes of Cushing syndrome (CS) or subclinical CS (SCS) and is characterized by multiple nodules in bilateral adrenal cortexes [1–3]. The prevalence of PMAH was reported to be <1% among all cases of endogenous CS [4]. Patients with PMAH display latent onset and slow progression of symptoms, leading to a late diagnosis of PMAH between the ages of 40 and 70 years old, with a high proportion of SCS [5, 6].
Although the pathophysiology of PMAH is not fully elucidated, previous studies have indicated that aberrant G-protein–coupled membrane receptors are expressed in the hyperplastic adrenal cells regulating cortisol synthesis [7]. Germline and somatic mutations of several genes were also reported to be involved in the mechanisms causing PMAH. Genes associated with PMAH include PDE11A (phosphodiesterase 11A) [8], GNAS (the stimulatory G protein alpha subunit) [9, 10], APC (adenomatous polyposis coli) [10, 11], and FH (fumarate hydratase) [10, 12], among others [13].
In 2013, ARMC5 pathogenic germline variants were first identified in 55% (18 of 33) of patients with PMAH [14]. Subsequently, several studies reported damaging variants of ARMC5 in patients with PMAH with frequencies ranging from 21% to 44% [15–19]. ARMC5 was found to be the major responsible gene in PMAH [20]. A series of clinical genome sequencing studies revealed that ARMC5 variants play an important role in adrenal tumorigenesis in PMAH [21]. ARMC5 somatic variants have been detected in hyperplastic adrenocortical nodules of patients with PMAH in addition to germline mutations, supporting a “two-hit” model for ARMC5 as a tumor suppressor gene [14, 22]. The second hit of ARMC5 has been reported to cause extra-adrenal neoplasms, such as meningioma, in patients with PMAH who carried the ARMC5 variant [16, 23]. However, the ARMC5 contribution to the formation of other neoplasms in these patients has not been reported, despite the ubiquitous expression of ARMC5 [24]. Previous studies showed that patients with PMAH and ARMC5 mutations had a higher degree of autonomous cortisol secretion and larger adrenal nodules than patients with PMAH without ARMC5 mutations [1, 17, 18]. Armc5 knockout mice mimic the phenotype of PMAH [25, 26]. However, the temporal cortisol production profile or morphological changes in adrenal glands in ARMC5 variant–positive patients had never been studied in humans.
1. Methods
A. Study Population
Fourteen patients with PMAH were recruited from seven endocrine facilities, without any exclusion criteria. This study included 13 patients with apparently sporadic PMAH and one patient with familial PMAH (Table 1, case 5). A diagnosis of CS was established by an expert endocrinologist at the time of presentation according to the Endocrine Society clinical guidelines for the diagnosis of CS [27]. SCS was defined as previously described [28]. Patients with apparently sporadic PMAH were defined as those without a family history based on the descriptions given by the patients. We define the presymptomatic state of PMAH as putative adrenal involvement in imaging study, without autonomous cortisol secretion.
Table 1.
Clinical Data and Genotypes of the Patients in This Study
| Case | Age (y)a | Sex | CS or SCS | Familial | Nonadrenal Tumors |
|---|---|---|---|---|---|
| 1 | 69 | F | SCS | − | Breast cancer |
| Colon cancer | |||||
| 2 | 51 | F | SCS | − | — |
| 3 | 58 | F | SCS | − | Cervical cancer |
| 4 | 64 | M | SCS | − | — |
| 5 | 71 | F | CS | + | Breast cancer |
| 6 | 44 | M | SCS | − | — |
| 7 | 53 | F | SCS | − | Parathyroid tumor |
| 8 | 47 | M | SCS | − | Pancreatic neuroendocrine tumor |
| 9 | 56 | M | SCS | − | Thyroid cancer |
| 10 | 73 | F | CS | − | Thyroid adenoma |
| 11 | 62 | M | SCS | − | — |
| 12 | 46 | M | CS | − | — |
| 13 | 59 | M | SCS | − | — |
| 14 | 46 | M | SCS | − | — |
Abbreviations: F, female; M, male.
Age at diagnosis.
We collected the clinical data and blood samples and surgically removed tumor samples for selected cases. We also studied family members of two probands (Table 1, cases 1 and 9). This study was approved by the Ethics Committee of Clinical Studies in Shizuoka General Hospital. All participants provided informed consent.
B. Hormonal Evaluation and Assays
Serum cortisol concentrations were measured via the chemiluminescence enzyme immunoassay method with an Elecsys Cortisol II (Roche Diagnostics GmbH, Mannheim, Germany) in cases 1 to 4, 6, 8, 10, 11, and 14; chemiluminescence (Immulite 2000; Siemens, Los Angeles, CA) in cases 5, 7, and 12; serum cortisol chemiluminescence enzyme immunoassay kits (Beckman Coulter, Fullerton, CA) in case 9; and chemiluminescence enzyme immunoassay (Fujifilm Corp., Tokyo, Japan) in case 13 [29–32]. Plasma ACTH levels were measured by Elecsys II in cases 1 to 6, 8 to 11, 13, and 14. Immulite (Siemens) was used in cases 7 and 12 [33, 34].
C. Genetic Analysis
Genomic DNA from the subjects was extracted from the peripheral blood or saliva with a genomic DNA purification kit (Qiagen, Hilden, Germany or DNA genoTek, Ottawa, Canada). The proband’s genomic DNA was screened for variants in ARMC5. Each protein coding exon was amplified by PCR. After the PCR products were purified with a QIAquick PCR purification kit (Qiagen), direct sequencing was performed with a 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA). Genomic DNA was also extracted from the surgically removed nonadrenal tumors for the patients who had accompanying tumors. Genomic DNA was extracted from a paraffin block with a NucleoSpin FFPE DNA kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. The variant data were evaluated with the Human Gene Mutation Database, and the pathogenicity was judged by the guidelines of the American College of Medical Genetics [35].
D. Statistical Analysis
We used t tests to compare the following hormonal parameters between patients with and without variants: basal cortisol level, late-night cortisol level, cortisol level after 1 mg of dexamethasone (DEX), and morning ACTH level. Univariate regression analysis was performed to clarify the associations between age and hormonal parameters, and slopes and P values were calculated. P < 0.05 was considered statistically significant. All statistical analyses were performed in JMP, version 13 (SAS Institute Inc., Cary, NC).
2. Results
A. Clinical Profiles and Genotypes of the Patients
Table 1 shows all the studied patients in this report. Of fourteen patients (eight men and six women), only one patient had a family history of PMAH (case 5 in Table 1). Among them, 10 patients (71%) harbored one of the five distinct genetic variants in the ARMC5 gene (Table 2). Three of the variants (p.R619*: c.1855C>T, p.R898W: c.2692C>T, and p.R654*: c.1855) have been reported as pathogenic. Two unreported variants were found in two patients: case 10 (p.R362Q; c.1085G>A) and case 13 (p.G143fs*8; c.427_454del). p.R362Q was identified as a likely pathogenic variant, and p.G143fs*8 was judged as a pathogenic variant in accordance with the guidelines of the American College of Medical Genetics. The average age at diagnosis of pathogenic or likely pathogenic variant carrier or noncarrier was 57 years old in both groups. Five unrelated patients showed identical pathogenic nonsense variants (p.R619*). In case 5, two family members who had a diagnosis of PMAH had the same variants as the proband (data not shown).
Table 2.
Genotype and Clinical Data of the Patients
| Case | Genotype | Basal Cortisol (µg/dL) | ACTH (pg/mL) | Late-Night Cortisol (µg/dL) | Cortisol After 1 mg DEXa (µg/dL) |
|---|---|---|---|---|---|
| 1 | p.R619* (c.1855C>T) | 18.8 | 2.84 | 11 | 16.7 |
| 2 | p.R619* (c.1855C>T) | 13.7 | 7.53 | 7.3 | 6.4 |
| 3 | Wild type | 8.5 | 10.1 | 3.3 | 3.2 |
| 4 | Wild type | 7.09 | 13.3 | 6.9 | 9.25 |
| 5 | p.R898W (c.2692C>T) | 13.8 | <1.0 | 13.6 | 15.4 |
| 6 | p.R619* (c.1855C>T) | 7.8 | 8.2 | 4.9 | 5.6 |
| 7 | p.R654* (c.1855C>T) | 10.7 | 8.4 | 7.9 | 5.2 |
| 8 | Wild type | 7.4 | 7.79 | 6.1 | 5.8 |
| 9 | p.R619* (c.1855C>T) | 12.8 | <1.0 | 10.2 | 13.1 |
| 10 | p.R362Q (c.1085G>A) | 17.4 | <2.0 | 16.1 | 27.6 |
| 11 | p.R619* (c.1855C>T) | 16.6 | <2.0 | 12.4 | ND |
| 12 | p.G143Sfs*8 (c.427_454del) | 11.8 | 5.3 | 6.5 | 8.4 |
| 13 | Wild type | 8.6 | 10.1 | 5.3 | 2.1 |
| 14 | p.R654* (c.1960C>T) | 9.2 | 6.2 | 3.5 | 8.8 |
Abbreviation: ND, not done.
1 mg DEX is taken orally at 11 pm, and a single blood sample is drawn at 8 am the next morning.
B. Genotypes and Clinical Phenotypes of the Family Members
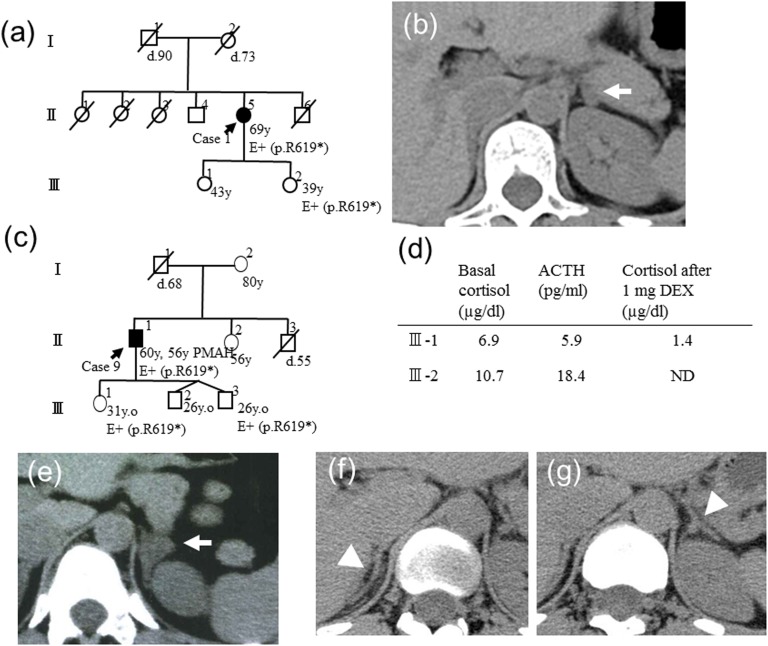
In one sporadic case (case 1), the clinical phenotype and genotype were examined for one of the daughters (age 39) with her informed consent. Her genotype of ARMC5 was identical to that of the proband [Fig. 1(a)]; however, the basal cortisol level was 7.1 μg/dL and was suppressed to 0.7 μg/dL after the 1 mg DEX administration. The CT showed a small adrenal nodule in the left adrenal gland [Fig. 1(b)]. In case 9, two of the offspring (age 26 and 31) had the same ARMC5 variant as the proband [Fig. 1(c)]. Neither of them showed basal cortisol elevation or suppression of ACTH, and III-1 showed cortisol suppression by 1 mg DEX [Fig. 1(d)]. The imaging study showed adrenal enlargement in the 31-year-old offspring but not in the 26-year-old offspring [Fig. 1(e)–1(g)].
Figure 1.
The genotype-phenotype relationship of the family members of cases 1 and 4. (a) The pedigree of case 1. (b) III-2 had p.R619* and showed a small adrenal nodule on the left adrenal gland. (c) The pedigree of case 4. The ARMC5 genotypes of III-1 and III-3 were analyzed, and p.R619* was found in both individuals. (d) The clinical data of III-1 and III-3 are shown. The imaging findings of (e) III-1 and (f) III-3 right adrenal gland and (g) left adrenal gland are shown. The arrows indicate the nodule or enlarged adrenal gland. The arrowheads indicate the normal adrenal glands.
C. ARMC5 Gene in the Nonadrenal Tumors of Patients With PMAH
Among the 10 ARMC5 variant carriers, five patients (50%) had accompanying nonadrenal tumors (Table 1). No loss of heterozygosity (LOH) or second hit of the ARMC5 gene was demonstrated in any of the tumors examined in these five patients (cases 1, 5, 7, 9, and 10) who had nonadrenal tumors (cases 1 and 5, breast cancer; case 7, parathyroid tumor; case 9, thyroid cancer; and case 10, thyroid adenoma) (data not shown).
D. Endocrinological Characteristics of ARMC5 Pathogenic or Likely Pathogenic Variants
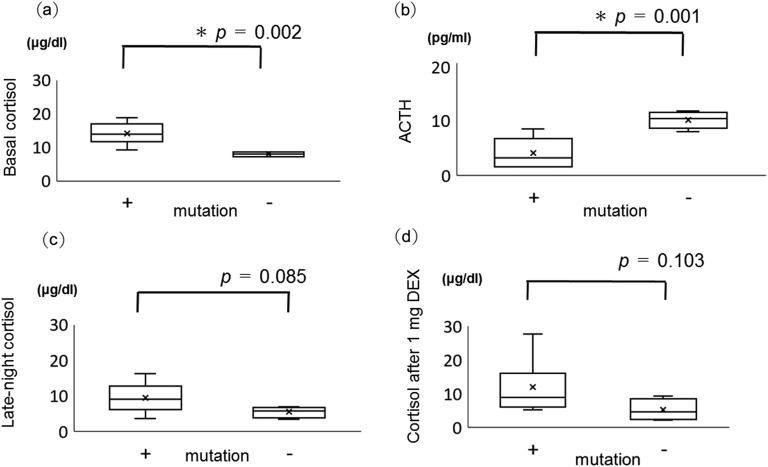
The ARMC5 pathogenic or likely pathogenic variant–positive group showed significantly higher basal cortisol and lower serum ACTH levels compared with the ARMC5 variant–negative group [Fig. 2(a), 2(b)]. Both late-night cortisol and cortisol after 1 mg DEX administration were higher in the ARMC5 pathogenic or likely variant–positive group but with no statistical significance [Fig. 2(c), 2(d)].
Figure 2.
(a) Basal cortisol level, (b) plasma ACTH level, (c) late-night cortisol level, and (d) cortisol level after 1 mg DEX in ARMC5 pathogenic or likely pathogenic variant–positive patients (+) and negative patients (−). Box plots show median (interior line), minimum and maximum (whiskers), and average (x). *P < 0.05.
E. Correlations Between Age and Endocrinological Profiles of Patients With ARMC5 Pathogenic or Likely Pathogenic Variants
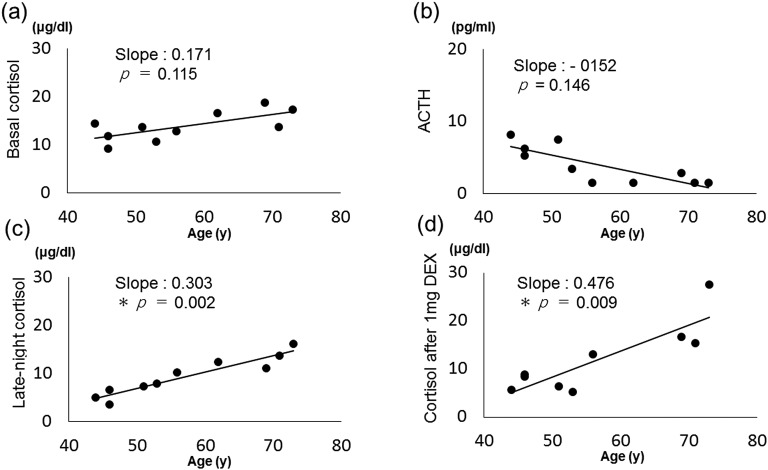
Figure 3 shows the results of morning basal cortisol level, morning ACTH level, late-night cortisol level, and cortisol level after 1 mg DEX (d) by age of the patients in the ARMC5 pathogenic or likely pathogenic variant–positive group. The late-night cortisol level and cortisol level after dexamethasone administration were positively correlated with patient age [Fig. 3(c), 3(d)]. The basal cortisol level showed an age-dependent increase, and the ACTH level tended to decrease in an age-dependent manner, but both were not significant [Fig. 3(a), 3(b)].
Figure 3.
Correlations of age with (a) basal cortisol level, (b) plasma ACTH, (c) late-night cortisol level, and (d) cortisol level after 1 mg DEX in ARMC5 pathogenic or likely pathogenic variant–positive patients. *P < 0.05.
Discussion
In the current study, the prevalence of ARMC5 pathogenic or likely pathogenic variants was detected in 10 of 14 (71%) of all studied patients and 9 of 13 (69%) in patients with apparently sporadic PMAH. Although this case study included a small sample, the frequency of variant-positive patients with PMAH was higher than that in previous reports [14, 15, 17–19, 36]. ARMC5 gene alteration might play a major role in the pathogenicity of PMAH in the Japanese population. We identified two ARMC5 variants, p.R362Q and p.G143Sfs*8. The p.R362Q variant was in the sixth ARM-repeat domain and was judged as likely pathogenic. The p.G143Sfs*8 variant was in the first ARM-repeat domain and was judged as pathogenic. These variants might be responsible for the PMAH phenotype in each patient and suggest a wider spectrum of variants in the ARMC5 gene. c.1855C>T (p.R619*) was identified in five unrelated PMAH probands from distinct regions in Japan, suggesting that p.R619* might be a hot spot in Japanese patients with PMAH.
In this study, we described asymptomatic or presymptomatic AMRC5 pathogenic variants in the offsprings of cases 1 and 9. Two out of three offspring, aged 39 and 31, showed a putative early stage of an adrenal lesion of PMAH in the imaging study, although they did not show autonomous cortisol secretion. One family member with an ARMC5 pathogenic variant, who did not show adrenal enlargement in the imaging study, was younger than the above two offspring, at the age of 26. It might be that ARMC5 variant carriers develop the clinical phenotype in an age-dependent manner [37]. In addition, these findings suggest that morphological changes in the adrenal glands might proceed to endocrinological alterations in ARMC5 pathogenic variant carriers. It is well known that in some families, there is a high penetrance of the PMAH phenotype [38–40]. However, the majority of patients with PMAH usually do not reveal a family history of the disease, as in this study. The penetrance of ARMC5 pathogenic variants has not been clarified. Close and continuous clinical examination including imaging studies might increase the detected frequency of familial PMAH, because the diagnosis is based on endocrinological and imaging studies, which are not routinely carried out in the family members of patients with PMAH. A follow-up study of these patients might give us useful information on this issue.
Because the ARMC5 gene is regarded as a tumor suppressor gene, a second hit is necessary for tumorigenesis in adrenal nodule formation [14]. The ARMC5 variants were also demonstrated in extra-adrenal tumors, such as meningioma. Therefore, ARMC5 pathogenic variants cause genetically defined multiorgan tumors [23]. In the current study, 50% of patients who had ARMC5 pathogenic or likely pathogenic variants had accompanying nonadrenal tumors, such as breast cancer, thyroid cancer, parathyroid tumor, and thyroid adenoma, suggesting a role of ARMC5 gene alteration in these nonadrenal tumorigeneses. However, neither LOH nor a second hit of the ARMC5 gene was evident in this study. The role of ARMC5 gene alteration in nonadrenal neoplasms was not clarified in this study. Promoter and intron domains and the copy number of the ARMC5 gene were not examined, so the second hit may have been missed by methodological problems. Furthermore, haploinsufficiency was not completely ruled out in these tumors [25, 41, 42].
The basal cortisol levels were significantly higher in the ARMC5 pathogenic or likely pathogenic variant group than in the nonvariant group. In contrast, the ACTH levels were significantly lower in the ARMC5 pathogenic or likely pathogenic variant group than in the nonvariant group. These observations were consistent with previous reports [15, 17]. The late-night cortisol levels and cortisol levels after 1 mg DEX were significantly higher in the older patients in the ARMC5 pathogenic or likely pathogenic variant group, which was consistent with the phenotype of the knockout mouse [25, 26]. Altogether, these findings suggest that ARMC5 pathogenic or likely pathogenic variant carriers might be asymptomatic until an older age. The penetrance of ARMC5-related PMAH might be underestimated because of the time course of clinical phenotypes and the lack of a survey program for the family members of the proband. These considerations should be taken into account when investigating the penetrance of ARMC5 pathogenic variants.
In conclusion, our results suggest that ARMC5 pathogenic germline variants are common in Japanese patients with PMAH despite the lack of family history. The pathogenic variant carriers develop a putative early stage of PMAH in an age-dependent manner, suggesting a higher penetrance of ARMC5-related PMAH than previously estimated. Our results demonstrate the importance of ARMC5 screening for PMAH family members to detect insidious PMAH.
Acknowledgments
We are grateful to Dr. Eiji Nakatani, Research Support Center, Shizuoka General Hospital, for statistical analysis.
Financial Support: This work is a part of the Medical Research Support Project of the Shizuoka Prefectural Hospital Organization.
Glossary
Abbreviations:
- CS
Cushing syndrome
- DEX
dexamethasone
- LOH
loss of heterozygosity
- PMAH
primary macronodular adrenal hyperplasia
- SCS
subclinical Cushing syndrome
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Kirschner MA, Powell RD Jr, Lipsett MB. Cushing’s syndrome: nodular cortical hyperplasia of adrenal glands with clinical and pathological features suggesting adrenocortical tumor. J Clin Endocrinol Metab. 1964;24(10):947–955. [DOI] [PubMed] [Google Scholar]
- 2. Aiba M, Hirayama A, Iri H, Ito Y, Fujimoto Y, Mabuchi G, Murai M, Tazaki H, Maruyama H, Saruta T, Suda T, Demura H. Adrenocorticotropic hormone–independent bilateral adrenocortical macronodular hyperplasia as a distinct subtype of Cushing’s syndrome. Enzyme histochemical and ultrastructural study of four cases with a review of the literature. Am J Clin Pathol. 1991;96(3):334–340. [DOI] [PubMed] [Google Scholar]
- 3. Lieberman SA, Eccleshall TR, Feldman D. ACTH-independent massive bilateral adrenal disease (AIMBAD): a subtype of Cushing’s syndrome with major diagnostic and therapeutic implications. Eur J Endocrinol. 1994;131(1):67–73. [DOI] [PubMed] [Google Scholar]
- 4. Lacroix A. ACTH-independent macronodular adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):245–259. [DOI] [PubMed] [Google Scholar]
- 5. Swain JM, Grant CS, Schlinkert RT, Thompson GB, vanHeerden JA, Lloyd RV, Young WF. Corticotropin-independent macronodular adrenal hyperplasia: a clinicopathologic correlation. Arch Surg. 1998;133(5):541–545, discussion 545–546. [DOI] [PubMed] [Google Scholar]
- 6. Ohashi A, Yamada Y, Sakaguchi K, Inoue T, Kubo M, Fushimi H. A natural history of adrenocorticotropin-independent bilateral adrenal macronodular hyperplasia (AIMAH) from preclinical to clinically overt Cushing’s syndrome. Endocr J. 2001;48(6):677–683. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr Rev. 2001;22(1):75–110. [DOI] [PubMed] [Google Scholar]
- 8. Vezzosi D, Libé R, Baudry C, Rizk-Rabin M, Horvath A, Levy I, René-Corail F, Ragazzon B, Stratakis CA, Vandecasteele G, Bertherat J. Phosphodiesterase 11A (PDE11A) gene defects in patients with ACTH-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors. J Clin Endocrinol Metab. 2012;97(11):E2063–E2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fragoso MC, Domenice S, Latronico AC, Martin RM, Pereira MA, Zerbini MC, Lucon AM, Mendonca BB. Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J Clin Endocrinol Metab. 2003;88(5):2147–2151. [DOI] [PubMed] [Google Scholar]
- 10. Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94(8):2930–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamakita N, Murai T, Ito Y, Miura K, Ikeda T, Miyamoto K, Onami S, Yoshida T. Adrenocorticotropin-independent macronodular adrenocortical hyperplasia associated with multiple colon adenomas/carcinomas which showed a point mutation in the APC gene. Intern Med. 1997;36(8):536–542. [DOI] [PubMed] [Google Scholar]
- 12. Matyakhina L, Freedman RJ, Bourdeau I, Wei M-H, Stergiopoulos SG, Chidakel A, Walther M, Abu-Asab M, Tsokos M, Keil M, Toro J, Linehan WM, Stratakis CA. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90(6):3773–3779. [DOI] [PubMed] [Google Scholar]
- 13. Faillot S, Assie G. Endocrine tumours: the genomics of adrenocortical tumors. Eur J Endocrinol. 2016;174(6):R249–R265. [DOI] [PubMed] [Google Scholar]
- 14. Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefèvre L, Sibony M, Guignat L, Rodriguez S, Perlemoine K, René-Corail F, Letourneur F, Trabulsi B, Poussier A, Chabbert-Buffet N, Borson-Chazot F, Groussin L, Bertagna X, Stratakis CA, Ragazzon B, Bertherat J. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albiger NM, Regazzo D, Rubin B, Ferrara AM, Rizzati S, Taschin E, Ceccato F, Arnaldi G, Pecori Giraldi F, Stigliano A, Cerquetti L, Grimaldi F, De Menis E, Boscaro M, Iacobone M, Occhi G, Scaroni C. A multicenter experience on the prevalence of ARMC5 mutations in patients with primary bilateral macronodular adrenal hyperplasia: from genetic characterization to clinical phenotype. Endocrine. 2017;55(3):959–968. [DOI] [PubMed] [Google Scholar]
- 16. Alencar GA, Lerario AM, Nishi MY, Mariani BM, Almeida MQ, Tremblay J, Hamet P, Bourdeau I, Zerbini MC, Pereira MA, Gomes GC, Rocha MS, Chambo JL, Lacroix A, Mendonca BB, Fragoso MC. ARMC5 mutations are a frequent cause of primary macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99(8):E1501–E1509. [DOI] [PubMed] [Google Scholar]
- 17. Espiard S, Drougat L, Libé R, Assié G, Perlemoine K, Guignat L, Barrande G, Brucker-Davis F, Doullay F, Lopez S, Sonnet E, Torremocha F, Pinsard D, Chabbert-Buffet N, Raffin-Sanson ML, Groussin L, Borson-Chazot F, Coste J, Bertagna X, Stratakis CA, Beuschlein F, Ragazzon B, Bertherat J. ARMC5 mutations in a large cohort of primary macronodular adrenal hyperplasia: clinical and functional consequences. J Clin Endocrinol Metab. 2015;100(6):E926–E935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, Berthon A, Libé R, Assié G, Espiard S, Drougat L, Ragazzon B, Bertherat J, Stratakis CA. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J Clin Endocrinol Metab. 2014;99(6):E1113–E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu L, Zhang J, Guo X, Chen X, He Z, He Q. ARMC5 mutations in familial and sporadic primary bilateral macronodular adrenal hyperplasia. PLoS One. 2018;13(1):e0191602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr Relat Cancer. 2018;25(3):R131–R152. [DOI] [PubMed] [Google Scholar]
- 21. Lodish M, Stratakis CA. A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat Rev Endocrinol. 2016;12(5):255–262. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Q, Cui L, Gao JP, Yan WH, Jin N, Chen K, Zang L, Du J, Wang XL, Guo QH, Yang GQ, Yang LJ, Ba JM, Gu WJ, Lv ZH, Dou JT, Mu YM, Lu JM. Whole-genome sequencing revealed armadillo repeat containing 5 (ARMC5) mutation in a Chinese family with ACTH-independent macronodular adrenal hyperplasia. Endocr J. 2018;65(3):269–279. [DOI] [PubMed] [Google Scholar]
- 23. Elbelt U, Trovato A, Kloth M, Gentz E, Finke R, Spranger J, Galas D, Weber S, Wolf C, König K, Arlt W, Büttner R, May P, Allolio B, Schneider JG. Molecular and clinical evidence for an ARMC5 tumor syndrome: concurrent inactivating germline and somatic mutations are associated with both primary macronodular adrenal hyperplasia and meningioma. J Clin Endocrinol Metab. 2015;100(1):E119–E128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthon A, Faucz F, Bertherat J, Stratakis CA. Analysis of ARMC5 expression in human tissues. Mol Cell Endocrinol. 2017;441:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berthon A, Faucz FR, Espiard S, Drougat L, Bertherat J, Stratakis CA. Age-dependent effects of Armc5 haploinsufficiency on adrenocortical function. Hum Mol Genet. 2017;26(18):3495–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Y, Lao L, Mao J, Jin W, Luo H, Charpentier T, Qi S, Peng J, Hu B, Marcinkiewicz MM, Lamarre A, Wu J. Armc5 deletion causes developmental defects and compromises T-cell immune responses. Nat Commun. 2017;8(1):13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanase T, Oki Y, Katabami T, Otsuki M, Kageyama K, Tanaka T, Kawate H, Tanabe M, Doi M, Akehi Y, Ichijo T. New diagnostic criteria of adrenal subclinical Cushing’s syndrome: opinion from the Japan Endocrine Society. Endocr J. 2018;65(4):383–393. [DOI] [PubMed] [Google Scholar]
- 29. RRID:AB_2802131, http://antibodyregistry.org/AB_2802131.
- 30. RRID:AB_2810257, http://antibodyregistry.org/AB_2810257.
- 31. RRID:AB_2802133, http://antibodyregistry.org/AB_2802133.
- 32. RRID:AB_2810227, http://antibodyregistry.org/AB_2810227.
- 33. RRID:AB_2783634, http://antibodyregistry.org/AB_2783634.
- 34. RRID:AB_2783635, http://antibodyregistry.org/AB_2783635.
- 35. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gagliardi L, Schreiber AW, Hahn CN, Feng J, Cranston T, Boon H, Hotu C, Oftedal BE, Cutfield R, Adelson DL, Braund WJ, Gordon RD, Rees DA, Grossman AB, Torpy DJ, Scott HS. ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99(9):E1784–E1792. [DOI] [PubMed] [Google Scholar]
- 37. Emms H, Tsirou I, Cranston T, Tsagarakis S, Grossman AB. Do patients with incidentally discovered bilateral adrenal nodules represent an early form of ARMC5-mediated bilateral macronodular hyperplasia? Endocrine. 2016;53(3):801–808. [DOI] [PubMed] [Google Scholar]
- 38. Gagliardi L, Hotu C, Casey G, Braund WJ, Ling KH, Dodd T, Manavis J, Devitt PG, Cutfield R, Rudzki Z, Scott HS, Torpy DJ. Familial vasopressin-sensitive ACTH-independent macronodular adrenal hyperplasia (VPs-AIMAH): clinical studies of three kindreds. Clin Endocrinol (Oxf). 2009;70(6):883–891. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki S, Tatsuno I, Oohara E, Nakayama A, Komai E, Shiga A, Kono T, Takiguchi T, Higuchi S, Sakuma I, Nagano H, Hashimoto N, Mayama T, Koide H, Sasano H, Nakatani Y, Imamoto T, Ichikawa T, Yokote K, Tanaka T. Germline deletion of Armc5 in familial primary macronodular adrenal hyperplasia. Endocr Pract. 2015;21(10):1152–1160. [DOI] [PubMed] [Google Scholar]
- 40. Zhu J, Cui L, Wang W, Hang XY, Xu AX, Yang SX, Dou JT, Mu YM, Zhang X, Gao JP. Whole exome sequencing identifies mutation of EDNRA involved in ACTH-independent macronodular adrenal hyperplasia. Fam Cancer. 2013;12(4):657–667. [DOI] [PubMed] [Google Scholar]
- 41. Correa R, Zilbermint M, Berthon A, Espiard S, Batsis M, Papadakis GZ, Xekouki P, Lodish MB, Bertherat J, Faucz FR, Stratakis CA. The ARMC5 gene shows extensive genetic variance in primary macronodular adrenocortical hyperplasia. Eur J Endocrinol. 2015;173(4):435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paige AJ. Redefining tumour suppressor genes: exceptions to the two-hit hypothesis. Cell Mol Life Sci. 2003;60(10):2147–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]