Abstract
Background:
A carious lesion is the accumulation of numerous episodes of demineralization and remineralization, rather than a unidirectional demineralization process. Tooth destruction can be arrested or reversed by the frequent delivery of fluoride or calcium/phosphorous ions to the tooth surface. Nanohydroxyapatite particle-containing dentifrices are the newer generation of products which claim to remineralize enamel lesions effectively. The aim of this study was to evaluate and compare the remineralization ability of dentifrices containing nanohydroxyapatite, NovaMin, and amine fluoride on artificial enamel caries.
Materials and Methods:
In this in vitro study, extracted sound premolars were placed in a demineralizing solution to produce deep artificial carious lesions. The teeth were then sectioned longitudinally and divided into three groups (n = 16 in each group): Group A (nanohydroxyapatite), Group B (NovaMin), and Group C (fluoride). The sections were then subjected to pH cycling for 7 days. Polarized light microscopy was utilized to record the depth of the lesions before and after treatment with the selected dentifrices. Changes in the mean lesion depth were statistically analyzed by one-way ANOVA and t-test. The level of significance was assessed at P < 0.05.
Results:
The lesion depth decreased significantly by 10.56% in Group A, 6.73% in Group B, and 9.58% in Group C (paired t-test, P < 0.001). When comparisons were made across the groups, no statistical significance was found between the Groups A, B, and C (ANOVA test, P > 0.05).
Conclusion:
All three dentifrices were found to be effective in remineralizing artificial carious lesions. Nanohydroxyapatite dentifrice produced significantly better results compared to fluoride- and NovaMin-containing dentifrices, instigating for its use in the management of early carious lesions.
Key Words: Amine fluoride, enamel, nano-hydroxyapatite, NovaMin, remineralization
INTRODUCTION
Dental caries in enamel is unique among diseases, as enamel is both acellular and avascular, hence does not exhibit the potential to repair by a cellular mechanism of its own.[1] Therefore, the prevention and biomimetic treatment of early carious lesions in enamel, particularly in those at high risk for developing caries, has been one of the paramount challenges faced by dental professionals and public health communities.
An important concept, studied in cariology over the past decade, is demineralization and remineralization of enamel. Evidence suggests that early carious lesions can be arrested and tooth surfaces remineralized using appropriate treatment methods.[2] Thus, focus of dental research in recent times has shifted to the development of methodologies for the early detection and use of noninvasive techniques for the effective management of carious lesions.
Fluoride is a preventive agent that has mesmerized dental research with its strong cariostatic property. The decline of dental caries in many countries today can be attributed to the widespread use of toothpastes containing fluorides.[3] Reports from high-quality meta-analysis clearly demonstrate the efficacy of fluoride-containing toothpastes.[4] Hence, it is widely recommended that every effort should be made to develop affordable fluoride-containing toothpastes.[5]
The current concept on the mechanism of action of fluorides indicates that fluorides work primarily via topical mechanisms by inhibition of demineralization and enhancement of remineralization.[6] Low levels of fluoride can be found in oral fluids for several hours following brushing with fluoride toothpaste,[7,8] which is found to have a profound effect on enamel remineralization.
Nevertheless, concerns have been expressed recently with the wide array of both prescription and over-the-counter fluoride products now being marketed in every country; the total fluoride intake has increased to perhaps harmful levels. It has been reported that exposure to chronic low-level fluoride can present problems in organ systems of normal individuals.[9] The prevalence of dental fluorosis, on the other hand, has increased noticeably in nonfluoridated areas and to a lesser extent in optimally fluoridated areas.[10] Although fluoride remains the cornerstone of modern noninvasive dental caries management, new and emerging methods, which can be used as alternatives to fluoride, have directed dental research to develop nontoxic anticariogenic agent that could be added to toothpaste, mouthwash, and food in an approach to lower the caries experience.[11]
Another material developed and introduced into many fields of dentistry is bioactive glass (BAG). This material has several novel features; one of the distinctive feature is, its ability to act as a biomimetic mineralizer, matching the body's own mineralizing traits.[12] Thus its development was considered a breakthrough in remineralization technology. BAG consists of minerals which occur naturally in body fluids. It reacts when it comes in contact with water, saliva, or other body fluids, releasing calcium, phosphorus, sodium, and silicon ions that results in the formation of hydroxyapatite crystals.[13] The formed hydroxyapatite crystals are chemically and structurally equivalent to naturally occurring biological apatite which is thought to aid the process of remineralization.[14]
Recently, nanotechnology has attracted a great deal of attention. Over the years, studies on nanohydroxyapatite as a biomimetic material for the reconstruction of tooth enamel suffering from mineral loss have been discussed and have received significant attention.[15,16] Hydroxyapatite is the major inorganic constituent of mineralized biological tissues. It has been used in medicine as a component of artificial bone. In dentistry, it is used in artificial roots to support implants, apatite-containing cement, and as a dental alveolar bone substitute.[17]
Dentifrices containing nanohydroxyapatite were introduced and tested as early as in the 1980s, mainly in Japan. The results from these studies, including field trials, lead to their approval as an anticaries agent by the Japanese Government in 1993. These studies, however, were mostly carried out at the manufacturer's request, and the results were published mostly in Japanese journals.[18,19] Furthermore, there exist relatively only few studies that report remineralization effects for commercially available nanohydroxyapatite-containing toothpaste.[20,21,22,23] Thus, recommendation for its effective use in remineralization is rather still limited against the superior fluoride and other nonfluoridated dentifrices.
There exist numerous experiments previously carried out to test the remineralizing efficacy of various commercially available products, individually and in combination, on extracted permanent teeth, utilizing various de- and remineralization techniques. Of this, fluorides have time and long been reported to remineralize carious lesions effectively. To corroborate this, findings from a review report the significant reduction in the incidence of caries following toothbrushing with fluoride dentifrices in 100 studies.[24] On the contrary, studies have also shown better or significant remineralization effects for nonfluoridated dentifrices compared to fluoride dentifrices.[25,26] However, with available evidence there exist limited studies to date, reporting the remineralization potential of a nanohydroxyapatite-containing dentifrice to another similar nonfluoridated and a fluoride-containing dentifrice. Hence, the aim of this in vitro study was to evaluate and compare the remineralizing potential of dentifrices containing nanohydroxyapatite, NovaMin, and amine fluoride on artificial enamel carious lesions.
MATERIALS AND METHODS
Before starting the study, the study protocol was approved by the Scientific Review Board, Saveetha University (SRB/SDMDS11PHD2).
Sample selection
This experimental in vitro study was conducted on 48 extracted sound human maxillary premolars, all of which had been obtained from extractions due to orthodontic reasons. The sample size required for the study was calculated based on the difference between two group means derived from previous studies and was estimated to be 16 per group (at 80% power and 5% alpha error).
Dentifrices used
The following three dentifrices where used for comparison:
APAGARD ROYAL (Sangi Co., Ltd., Japan) containing 10% nanohydroxyapatite
SHY-NM (Group Pharmaceuticals Ltd., India), which contained 5% calcium sodium phosphosilicate
AMFLOR (Group Pharmaceuticals Ltd., India), 1450 ppm amine fluoride-containing dentifrice.
Preparation of demineralizing and remineralizing solution
The buffered de-/remineralizing solutions were prepared using analytical grade chemicals and deionized water. Demineralizing solution comprised 2.2 mM calcium chloride, 2.2 mM sodium phosphate, and 0.05 M acetic acid, with 1 M potassium hydroxide added to obtain a pH of 4.4. The remineralizing solution comprised 1.5 mM calcium chloride, 0.9 mM sodium hydrogen phosphate, and 0.15 potassium chloride at a pH adjusted to 7.0 with 1M potassium hydroxide.[27]
Enamel window and artificial carious lesion formation
The 48 intact extracted teeth collected were stored in 10% formalin solution before use. Following, the teeth were cleaned of soft tissue debris and examined for possible cracks, hypoplasia, and white spot lesions. The buccal surface of each sound tooth was then coated with acid resistant nail varnish (Revlon, USA) leaving a window of 1 mm × 1 mm wide.[20] This was done to limit the area of demineralization followed by remineralization only in the window area. Each tooth was then subjected to demineralizing solution for a period of 96 h. This was done to create artificial carious lesions of approximately 100–120 μm deep among the selected teeth. After 96 h, teeth were subjected to sectioning. The teeth were sectioned longitudinally through the lesions, using a hard tissue microtome (Leica SP 1600, Bensheim, Germany). The 48 sections were then randomly divided into three equal groups, with 16 sections in each group. The three test groups were as follows: Group A, nanohydroxyapatite dentifrice; Group B, calcium sodium phosphosilicate dentifrice; and Group C, amine fluoride dentifrice.
pH cycling model
The sections were then placed in the pH-cycling system on an orbital shaker (ThermoFisher Scientific, USA) for 7 days.[28] All solutions (demineralizing, remineralizing, and toothpaste supernatant) were freshly prepared for each cycle, and separate containers were used for each group throughout the experimental period. The pH of the demineralizing and remineralizing solutions was measured before every cycle. Each cycle involved 3 h of demineralization twice daily with 2 h of remineralization in between. Groups A, B, and C were treated for 60 s with toothpaste supernatant (5 ml/section) before the first demineralizing cycling and both before and after the second demineralizing cycles.
Evaluation technique
Polarizing light microscopy (Olympus BX51, Minneapolis, MN, USA) was used to make pre- and posttreatment records. This was accomplished by immersing the sections in water, which normally produced a clear demarcation between the sound enamel and the initial lesion. The images were captured using a 4x objective lens and a 10x eyepiece for magnification both before and after experiment. The area in microns (μm2) of the initial and final size of each lesion was carefully measured and analyzed with Image J software (NIH, Bethesda, MD, USA). The lesion depth was measured from the surface of the lesion to the depth of the lesion, at three different points: D1, D2, and D3, and the average of these three measurements was taken as the lesion depth for each specimen.[25]
Statistical analysis
Data was entered into Microsoft excel spreadsheet and analyzed using SPSS software for windows (version 23.0; IBM Corp., Armonk, NY, USA). The results were expressed as mean with standard deviation (SD) and as percentage change for changes in lesion depth. Paired t-test was used to compare the differences between the mean values in the group. One-way ANOVA was used for multiple group comparisons. For all the tests, P < 0.05 was considered statistically significant.
RESULTS
The mean (± SD) lesion depths between the groups pre- and post-pH cycling are shown in Table 1.
Table 1.
Comparison of mean lesion depth between groups at baseline and post pH cycling
| Groups | Lesion depth, mean±SD (µm) |
t | P value | |
|---|---|---|---|---|
| Baseline* | Postcycle* | |||
| Group A (n-HAP dentifrice) | 168.10±57.73 | 151.35±70.14 | 2.654 | 0.000 |
| Group B (NovaMin dentifrice) | 167.5±50.04 | 156.16±49.26 | −1.285 | 0.001 |
| Group C (amine fluoride dentifrice) | 153.53±59.76 | 135.11±59.37 | 1.835 | 0.000 |
*Paired t-test. n-HAP: Nano-hydroxyapatite; SD: Standard deviation
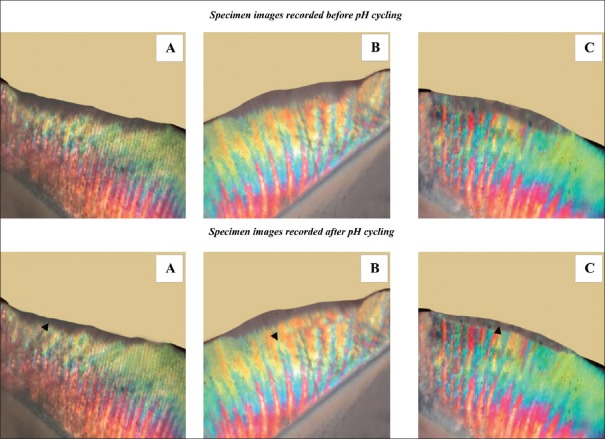
Figure 1 shows the polarized light photomicrographs of representative enamel specimens pre- and post-pH cycling for each of the groups. The mean (± SD) pretreatment lesion depth across the groups ranged from 153.58 ± 59.76 μm to 168.10 ± 57.73 μm. Comparisons between pre- and post-test lesion depths in all groups were highly significantly (P < 0.001). The reduction in mean lesion depth after pH cycling was maximum for Group C, followed by Group A and Group B.
Figure 1.
Polarized light photomicrographs of representative enamel specimen from each group (A-C corresponds to the groups in the study).
There was no significant differential change in mean lesion depth across various groups pre- and post-pH cycling (one-way ANOVA), though a considerable decrease in lesion depth was observed across all the groups. Figure 2 indicates reduction in lesion depth post pH cycling indicating effective remineralization.
Figure 2.
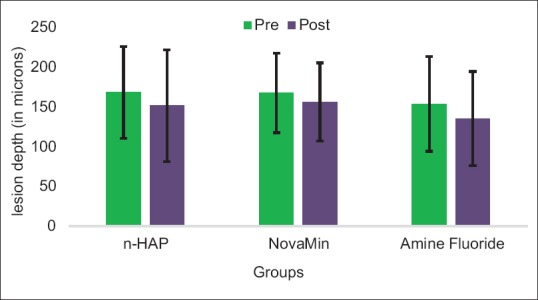
Comparison of change in mean lesion depth among various groups pre- and post-pH cycling.
The maximum decrease in lesion depth expressed as percentage change [Figure 3] was seen in group treated with nanohydroxyapatite dentifrice (−10.56 ± 13.63), followed closely by amine fluoride (−9.58 ± 29.77) and calcium sodium phosphosilicate containing dentifrice (−6.73 ± 20.21).
Figure 3.
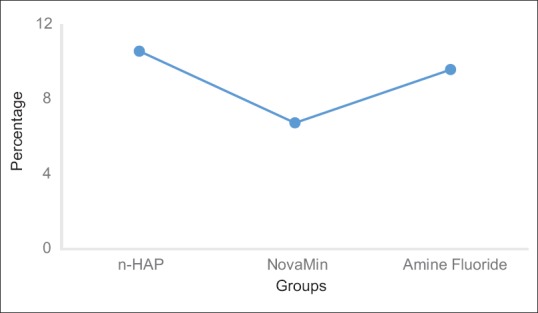
Percentage change of lesion depth among various groups post pH cycling.
DISCUSSION
When tooth erupts into the oral cavity, the hypomineralized enamel encounters a highly complex ecology. Imbalance in this complex environment holds the clue to the origin of the unique disease called dental caries. The onset of dental caries requires the establishments of necessary physiochemical conditions for mineral dissolution.[29] Certain chemical agents have the potential to modify this mineral loss caused by organic acids.
Experimental models based on the formation of lesions in in vitro systems can be used to understand the effects of such agents on carious processes. These in vitro systems are at the forefront of caries research, because they are less expensive and time consuming than other testing methods.[30] Another major advantage of the former system is the ability to perform single variable experiments in a controlled environment.[31] This study made use of an in vitro pH-cycling model to test the remineralization effects of three dentifrices containing nanohydroxyapatite, calcium sodium phosphosilicate, and amine fluoride on artificial carious lesions on extracted sound permanent human teeth. There exists substantial evidence on the reliability of pH cycling model for the evaluation of lesion progression and mineral changes of artificial enamel carious lesions as it simulates to a major extent the in vivo conditions leading to the process of caries.[32]
In the present study, artificial carious lesions were produced using standardized demineralizing solutions.[27] Artificial carious lesions are considered to be more reproducible than natural carious lesions, thus making the experimental model more reliable.[33] They facilitate the testing of multiple areas in any lesions at different time intervals, to assess the remineralizing phenomena. The artificial caries such as lesions created for use in this study had lesions depths of 100–120 μm; this replicates a natural carious lesion that has been active for 12 months in the oral cavity.[34]
A single section technique was used for preparing the samples in this study. The reason is the possibility to measure the mineral changes after multiple periods of exposure to the test agents at the same site and it avoids the difficulties if other models that are associated with reintroducing sections into the medium for further evaluation. Although the use of the single section technique is tedious, it can provide the exact dose response of the test agent as well as a measure of de/remineralization.[35]
In the interest of standardization, all of the specimens in the present study were subjected to 7 days of pH cycling which involved 3 h of demineralization twice a day, with 2 h of remineralization in between. During the pH cycle, the specimens were treated thrice daily with respective dentifrices to replicate daily brushing patterns. This helps to mimic the real life situations to a greater extend that occur in-vivo.
Three types of dentifrices were used in the present study: nanohydroxyapatite, calcium sodium phosphosilicate, and amine fluoride containing. Although their mechanisms of action are different, the results obtained indicate all the dentifrices contributed substantially to enamel remineralization. Statistically significant differences were seen in lesion depths in the three groups when comparisons were made pre- and post-treatment.
Hydroxyapatite-containing dentifrices are the newer generation products available in the market showcasing its effective remineralizing capacity. The results from the present study were comparable to other in vitro studies, where remineralization was evident on using dentifrices containing nanohydroxyapatite.[20,21,22,23,36,37] Lesion depth decreased significantly (10.56%) following pH cycling in the group treated with nanohydroxyapatite dentifrice. This was comparatively higher when compared to another study where the decrease in lesion depth was only 5%.[38] This difference could be due to the forms of nanohydroxyapatite dentifrice used (supernatant to slurry form).
Most studies in literature provide evidence of NovaMin (calcium sodium phosphosilicate) as a successful desensitizing agent, with only limited studies understanding the remineralizing potential of NovaMin on enamel.[39,40] In the present study, the group treated with NovaMin depicted a reduction in lesion depth. The results were in agreement to previous studies.[40,41,42,43,44] However, in the current study, the effect proved to be less significant in comparison to nanohydroxyapatite and amine fluoride dentifrice. Findings from a previous study report that NovaMin can be an effective adjunct along with fluoride therapy but not an alternative for potential remineralization.[37] To elucidate this finding, little or no remineralization occurred when nanoparticulate BAG was used to remineralize dentin.[45]
For over 40 years, numerous fluoride preparations have been available to members of the public to inhibit caries and promote remineralization. There is no doubt that fluoride has played a major role in the decline of dental caries. For the caries preventive effect, the bioavailability of fluoride is of importance, and this is dependent from the solubility of the fluoride-containing compound and from the adhesion of the fluoride compound to the surface. In dentifrices, different fluoride formulations are used as carrier for fluoride ions of which the most frequent are sodium fluoride, sodium monofluorophosphate, and amine fluoride.
The findings from the present study reconfirm the remineralizing potential of amine fluoride as previously reported by a number of investigators.[46,47,48] Interestingly, in the present study, the remineralizing potential of amine fluoride was lesser compared to nanohydroxyapatite, but greater to NovaMin, though not statistically significant. This was in contrast to studies where the findings were comparable.[20,21]
Inevitably, certain limitations are associated with in vitro studies such as the present study. This include the lack of saliva, plaque, and salivary pellicle which would be present in the oral cavity. These variations in the characteristics and quantities of these factors, which vary between individuals, need equalization in in vitro studies. Another may be that although the samples were randomly divided into the three treatment groups, some teeth might have greater susceptibility than others to demineralization due to the age of the donor and exposure to environmental factors such as fluoride. Furthermore, the specimens in the pH cycle were subjected to repeated cycles of remineralization and demineralization which is more aggressive than the acid attacks that a tooth is exposed to, on a daily basis in the oral cavity. Hence, it is apparent that no in vitro model can be a realistic substitute for the conditions that prevail in the oral cavity. However, these models offer valuable means for testing of new products, which are intended to produce remineralization before they are tested in human subjects. It is also recommended that further controlled in vivo studies and clinical trials are needed to ascertain the true clinical efficacy of dentifrices containing nanohydroxyapatite.
CONCLUSION
Based on the results, it can be concluded that all three dentifrices had the ability to reduce the progress of demineralization, while simultaneously enhancing remineralization process on artificial carious lesions. However, within the limitations of this in vitro study, it was seen that nanohydroxyapatite dentifrice produced better effects compared to fluoride- and NovaMin-containing dentifrices, and hence could be considered for use as a potential dentifrice in remineralization of early carious lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declared that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgments
The authors would like to thank Dr. Sri Sakthi, Reader, Department of Public Health Dentistry, Saveetha Dental College and Hospital, for her valuable support and guidance during the entire study. In addition, the authors would also like to acknowledge the Department of Oral Pathology, Saveetha Dental College and Hospital, for assisting with specimen preparation.
REFERENCES
- 1.Abou Neel EA, Aljabo A, Strange A, Ibrahim S, Coathup M, Young AM, et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743–63. doi: 10.2147/IJN.S107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaechi BT. Remineralization therapies for initial caries lesions. Curr Oral Health Rep. 2015;2:95–101. [Google Scholar]
- 3.Petersen PE, Ogawa H. Prevention of dental caries through the use of fluoride – The WHO approach. Community Dent Health. 2016;33:66–8. [PubMed] [Google Scholar]
- 4.Marinho VC, Higgins JP, Logan S, Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2003;4:CD002782. doi: 10.1002/14651858.CD002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman AS, Yee R, Holmgren CJ, Benzian H. Global affordability of fluoride toothpaste. Global Health. 2008;4:7. doi: 10.1186/1744-8603-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–9. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth RM, Morgan SN. Oral fluoride retention after use of fluoride dentifrices. Caries Res. 1991;25:123–9. doi: 10.1159/000261354. [DOI] [PubMed] [Google Scholar]
- 8.Vale GC, Cruz PF, Bohn AC, de Moura MS. Salivary fluoride levels after use of high-fluoride dentifrice. ScientificWorldJournal. 2015;2015:302717. doi: 10.1155/2015/302717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanduti D, Sterbenk P, Artnik B. Fluoride: A review of use and effects on health. Mater Sociomed. 2016;28:133–7. doi: 10.5455/msm.2016.28.133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascarenhas AK. Risk factors for dental fluorosis: A review of the recent literature. Pediatr Dent. 2000;22:269–77. [PubMed] [Google Scholar]
- 11.Kalra DD, Kalra RD, Kini PV, Prabhu CA. Nonfluoride remineralization: An evidence-based review of contemporary technologies. J Dent Allied Sci. 2014;3:24. [Google Scholar]
- 12.Reynolds EC. Calcium phosphate-based remineralization systems: Scientific evidence? Aust Dent J. 2008;53:268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 13.Taha AA, Patel MP, Hill RG, Fleming PS. The effect of bioactive glasses on enamel remineralization: A systematic review. J Dent. 2017;67:9–17. doi: 10.1016/j.jdent.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Ramashetty Prabhakar A, Arali V. Comparison of the remineralizing effects of sodium fluoride and bioactive glass using bioerodible gel systems. J Dent Res Dent Clin Dent Prospects. 2009;3:117–21. doi: 10.5681/joddd.2009.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santosh KP, Chu MC, Balakrishnan A, Lee YJ, Kim TN, Cho SJ. Synthesis of nano hydroxyapatite powder that simulate teeth particle morphology and composition. Curr Appl Phys. 2009;9:1459–62. [Google Scholar]
- 16.Melo MA, Guedes SF, Xu HH, Rodrigues LK. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31:459–67. doi: 10.1016/j.tibtech.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proussaefs P, Kan J, Lozada J, Kleinman A, Farnos A. Effects of immediate loading with threaded hydroxyapatite-coated root-form implants on single premolar replacements: A preliminary report. Int J Oral Maxillofac Implants. 2002;17:567–72. [PubMed] [Google Scholar]
- 18.Kani T, Kani M, Isozaki A, Kato H, Fukuoka Y, Omashi T, et al. The effect of apatite-containing dentifrices on artificial caries lesions. Journal of the Japanese Society of Dental Health. 1988;38:364–5. [Google Scholar]
- 19.Kani T, Kani M, Isozaki A, Shintani H, Ohashi T, Tokumoto T. Effect of apatite-containing toothpastes on dental caries in school children. J Dent Health. 1989;39:104–9. [Google Scholar]
- 20.Itthagarun A, King NM, Cheung YM. The effect of nano-hydroxyapatite toothpaste on artificial enamel carious lesion progression: An in vitro pH-cycling study. Hong Kong Dent J. 2010;7:61–6. [Google Scholar]
- 21.Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–7. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011;45:460–8. doi: 10.1159/000331207. [DOI] [PubMed] [Google Scholar]
- 23.Ebadifar A, Nomani M, Fatemi SA. Effect of nano-hydroxyapatite toothpaste on microhardness of artificial carious lesions created on extracted teeth. J Dent Res Dent Clin Dent Prospects. 2017;11:14–7. doi: 10.15171/joddd.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt RD, Murray JJ. Developments in fluoride toothpastes – An overview. Community Dent Health. 1997;14:4–10. [PubMed] [Google Scholar]
- 25.Rirattanapong P, Smutkeeree A, Surarit R, Saendsirinavin C, Kunanantsak V. Effects of fluoride dentifrice on remineralization of demineralized primary enamel. Southeast Asian J Trop Med Public Health. 2010;41:243–9. [PubMed] [Google Scholar]
- 26.Guclu ZA, Alacam A, Coleman NJ. A 12-week assessment of the treatment of white spot lesions with CCP-ACP paste and/or fluoride varnish. Biomed Res Int. 2016;2016:8357621. doi: 10.1155/2016/8357621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–10. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 28.Heilman JR, Wefel JS, Pyrz JW, Faller RV. Development of a root caries pH cycling model. J Dent Res. 1991;71:201. [Google Scholar]
- 29.Advani S, Sogi S, Hugar S, Indushekar KR, Kiran K, Hallikerimath S, et al. Remineralization effects of two pediatric dentifrices and one regular dentifrice on artificial carious lesion in primary teeth: An in vitro study. J Int Soc Prev Community Dent. 2014;4:96–102. doi: 10.4103/2231-0762.137627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu OY, Zhao IS, Mei ML, Lo EC, Chu CH. A review of the common models used in mechanistic studies on demineralization-remineralization for cariology research. Dent J (Basel) 2017;5:pii: E20. doi: 10.3390/dj5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salli KM, Ouwehand AC. The use of in vitro model systems to study dental biofilms associated with caries: A short review. J Oral Microbiol. 2015;7:26149. doi: 10.3402/jom.v7.26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buzalaf MA, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem AC, et al. PH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: Strengths and limitations. J Appl Oral Sci. 2010;18:316–34. doi: 10.1590/S1678-77572010000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang B, Flaim G, Dickens SH. Remineralization of human natural caries and artificial caries-like lesions with an experimental whisker-reinforced ART composite. Acta Biomater. 2011;7:2303–9. doi: 10.1016/j.actbio.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White DJ, Faller RV, Bowman WD. demineralization and remineralization evaluation technique added consideration. J Dent Res. 1992;71:929–933. doi: 10.1177/002203459207100S28. [DOI] [PubMed] [Google Scholar]
- 35.Wefel JS, Jensen ME. An intra-oral single section mineralization/remineralization model. J Dent Res. 1992;71:860–3. doi: 10.1177/002203459207100S14. [DOI] [PubMed] [Google Scholar]
- 36.Jeong SH, Hong SJ, Choi CH, Kim BI. Effect of new dentifrice containing nano-sized carbonated apatite on enamel remineralization. Key Eng Mater. 2007;330-332:291–94. [Google Scholar]
- 37.Vyavhare S, Sharma DS, Kulkarni VK. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J Clin Pediatr Dent. 2015;39:149–60. doi: 10.17796/jcpd.39.2.yn2r54nw24l03741. [DOI] [PubMed] [Google Scholar]
- 38.Najibfard K, Ramalingam K, Chedjieu I, Amaechi BT. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J Clin Dent. 2011;22:139–43. [PubMed] [Google Scholar]
- 39.Diamanti I, Koletsi-Kounari H, Mamai-Homata E, Vougiouklakis G. Effect of fluoride and of calcium sodium phosphosilicate toothpastes on pre-softened dentin demineralization and remineralization in vitro. J Dent. 2010;38:671–7. doi: 10.1016/j.jdent.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Vahid Golpayegani M, Sohrabi A, Biria M, Ansari G. Remineralization effect of topical NovaMin versus sodium fluoride (1.1%) on caries-like lesions in permanent teeth. J Dent (Tehran) 2012;9:68–75. [PMC free article] [PubMed] [Google Scholar]
- 41.Gjorgievska ES, Nicholson JW. A preliminary study of enamel remineralization by dentifrices based on Recalden (CPP-ACP) and NovaMin (calcium-sodium-phosphosilicate) Acta Odontol Latinoam. 2010;23:234–9. [PubMed] [Google Scholar]
- 42.Preethee T, Kandaswamy D, Rosaline H, Arathi G. Comparing the remineralizing potential of NovaMin and casein phosphopeptide-amorphous calcium phosphate using quantitative light induced florescence. Amrita J Med. 2011;7:1–44. [Google Scholar]
- 43.Palaniswamy UK, Prashar N, Kaushik M, Lakkam SR, Arya S, Pebbeti S, et al. A comparative evaluation of remineralizing ability of bioactive glass and amorphous calcium phosphate casein phosphopeptide on early enamel lesion. Dent Res J (Isfahan) 2016;13:297–302. doi: 10.4103/1735-3327.187872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Mei L, Gong L, Li J, He S, Ji Y, et al. Remineralization of early enamel caries lesions using different bioactive elements containing toothpastes: An in vitro study. Technol Health Care. 2016;24:701–11. doi: 10.3233/THC-161221. [DOI] [PubMed] [Google Scholar]
- 45.Vollenweider M, Brunner TJ, Knecht S, Grass RN, Zehnder M, Imfeld T, et al. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007;3:936–43. doi: 10.1016/j.actbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Petersson LG, Hakestam U, Baigi A, Lynch E. Remineralization of primary root caries lesions using an amine fluoride rinse and dentifrice twice a day. Am J Dent. 2007;20:93–6. [PubMed] [Google Scholar]
- 47.Arnold WH, Haase A, Hacklaender J, Gintner Z, Bánóczy J, Gaengler P, et al. Effect of pH of amine fluoride containing toothpastes on enamel remineralization in vitro. BMC Oral Health. 2007;7:14. doi: 10.1186/1472-6831-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naumova EA, Niemann N, Aretz L, Arnold WH. Effects of different amine fluoride concentrations on enamel remineralization. J Dent. 2012;40:750–5. doi: 10.1016/j.jdent.2012.05.006. [DOI] [PubMed] [Google Scholar]