Abstract
Background:
The purpose of this study was to evaluate the bond strength between two porcelains (VITA VMK Master and VITA VM13) and two types of base metal alloys (Ceramill Sintron and Verabond).
Materials and Methods:
In an experimental study, 20 rectangular strips (25 mm × 4 mm × 0.5 mm) of each base metal alloy (Ceramill Sintron and Verabond) were fabricated according to the manufacturer's instructions. After sandblasting and polishing, the samples were placed in an ultrasonic device to remove surface contaminants. A surface of 8 mm × 4 mm × 1 mm of samples was veneered with Vita VM13 and VITA VMK Master. The samples were divided into four groups (n = 10 each group; VM13/Ceramill, VMK Master/Ceramill, VM13/Verabond, and VMK Master/Verabond), and bond strength was evaluated by three-point bending test with a universal testing machine. Two-way ANOVA was used for comparison in each group, post hoc Scheffe's test was used for analyzing data between groups, and the Kolmogorov–Smirnov test was used for the normality (P < 0.05).
Results:
The maximum bond strength was related to Verabond/VM13 (44.35 ± 7.9 MPa) and then the Ceramill Sintron/VM13 (39.33 ± 4.43 MPa), and the lowest was related to the Ceramill Sintron/VMK Master (29.75 ± 3.2 MPa). There was no significant difference between bond strength of VM13 with the two alloy groups (P > 0.05), and bond strength of VMK Master to Ceramill Sintron CAD/CAM alloy was less than the conventional Verabond alloy (P < 0.05); however, bond strength of all the groups was above the standard threshold (25 MPa).
Conclusion:
Based on the results of this study, the bond strength of the porcelain to Verabond was better, but the bond strength of the porcelain to Ceramill Sintron also was not less than the standard threshold; thus, this new CAD/CAM alloy can be an alternative to the conventional base metal alloys in the metal-ceramic restorations.
Key Words: Dental porcelain, dental restoration, metal-ceramic alloys
INTRODUCTION
Fixed prosthodontics and metal-ceramic restorations have a special place in dentistry.[1] Metal-ceramic restorations have been known as gold standards for fixed prosthesis and have a combination of porcelain esthetics, strength, edge alignment, and metal substructure.[1,2,3] The metals used in these restorations are alloyed and are divided into two groups: noble and base metal. Metal-ceramic restorations have some disadvantages such as the potential for allergy to metal substructure. However, this type of allergic reaction is extremely rare and is only possible when nickel-containing alloys are used.[4] Ni-based alloys contain beryllium, can release a high amount of nickel in contact with the acidic environment, leading to toxicities or allergic reactions.[5,6,7,8] In recent decades, the use of metal-based alloys has advanced to a certain extent due to advantages such as low cost and density, high strength, the possibility of providing thinner and more rigid restorations, and the formation of a stable oxide layer (which is required for the bond with porcelain), compared to noble alloys.[8] One of the most important features of metal-ceramic restorations is their resistance to break down. Ceramic bonds to base alloys are obtained by various factors, including chemical bonds, mechanical bonds, van der Waals forces, and slight mismatch between the coefficient of thermal expansion (CTE) of porcelain and metal.[4] To improve the mechanical bonding, various methods including wearing the surface of the alloy with airborne particles containing aluminum oxide, acid etching, bonding agents, laser synthesis, and laser etching are used.[9] Various factors may lead to the fracture of metal-ceramic restorations whether they are tooth supported or implant supported. Factors include technical factors, dentist-related factors, inherent material properties, direction, magnitude and frequency of applied loads, environmental factors, and mechanism of retention of implant-supported restorations and restorations with posterior cantilever.[10] One of the new ways of providing a restoration substructure is the use of CAD/CAM technology, which will save time and cost relative to conventional base alloys.[3] Previous studies have shown that the main reason for the fracture of ceramics is their inability to prevent crack growth with plastic changes in the adjacent areas of the tip of the crack under tensile force.[4] do Prado et al.[11] showed that the Noritake porcelain system with the Viron alloy had the highest resistance against shear force, while the Duceram bonded to Verabond had the lowest bond strength. Fernandes Neto et al.[12] showed that bond strength of all three types of ceramics (Duceram, Williams, and Vitavmk88) varies with Ni-Cr and Co-Cr-Ti alloys and that metal-ceramic compatibility is very effective in bond strength. Stawarczyk et al.[3] showed that in REFLEX and Vita VM13 ceramics, the type of alloy does not affect bond strength, while Creation ceramic has a higher bond strength in combination with a new alloy and a laser-synthesized alloy compared to cast alloy. Furthermore, the new CAD/CAM alloy, as well as the laser-synthesized alloy with a Reflex veneer, had a bond strength less than that of with Vita VM13 veneer. Because the Ceramill Sintron alloy is a new alloy and little studies have been done on its bond strength, in this study, the metal-ceramic bond strength is compared to conventional metal-ceramic restorations with the three-point bending test and this alloy. According to previous studies, this study compared the bond strength of VITA VM13 and VITA VMK Master porcelains with two types of base metal in metal-ceramic restorations. The null hypothesis was that there is no different between the bond strength of Ceramill Sintron and the traditional base metal alloy.
MATERIALS AND METHODS
In this experimental study, a total of 20 samples were prepared from each type of alloy (Ceramill Sintron CAD/CAM alloy [AmmanGirrbach, Koblach, Austria] and Verabond traditional base metal alloy [Aalba Dent Inc., Fairfield, CA, USA]) according to the collected data from the past studies.[6,13] For Verabond samples, a plexiglass bifocal generator [Figure 1a] was prepared to make wax patterns, and a window with dimensions of 24 mm × 4 mm × 0.5 mm was considered, and the melted wax (Cavex Holland BV, Haarlem, Netherlands) was poured into it.[14] After the wax was cooled and opening the two sides of plexiglass bifocal generator, the template was removed and the thickness and diameter of it were checked with a digital caliper (Mitutoyo, Tokyo, Japan) with a precision of 0.01 mm. Considering the melting point of the alloy and according to the manufacturer's instructions, the phosphate-bonded investment (Termocast, Polidental Ltd., Paulo, SP, Brazil) was used. After the setup of the investment, during 45 min, each generator was introduced to the wax removal furnace (Onmad, Filli Manifred, and Torino, Italy). After casting (Ducatron, Ugin Dentaire, France), all samples were allowed to cool at room temperature. The samples were examined under a microscope with a magnification of ×25 to ensure that there were no defects caused by the casting process. Extracts of the compound were removed by sandblasting with 50-μ alumina particles using a Trijet machine (Labordental, Sao Paulo, SP, Brazil). The samples were processed using tungsten mill (H79 NEF, 104, 023, Brasseler USA, Savannah, GA) with a low-speed handpiece (Kavo EWL, type 4005, Leutkrich Imalgua, Germany) to remove the deflection of casting. After sandblasting, the specimens were washed with 50-μ aluminum oxide particles and placed in ultrasonic apparatus (Mini Sono Cleau CA1470, Kaigo Denki C, Ltd, Tokyo, Japan) for 10 min to remove surface contamination. For the preparation of samples of Ceramill Sintron alloys, 20 blocks were used with dimensions of 27.5 mm × 3.3 mm × 12 mm [Figure 1b]. Then, all specimens were sintered in a special furnace (Ceramill Argotherm, Amann Girrbach, Koblach, Austria) according to the manufacture's instructions. Hence, the samples of 25 mm × 4 mm × 0.5 mm were obtained.[14] Then, the samples were examined under a surgery microscope (Carl Zeiss, Oberkochen, Germany) with a magnification of 25 to ensure that no defects were observed. After sandblasting by 50-μ aluminum oxide particles, the samples were rinsed and placed in an ultrasonic apparatus for 10 min to remove surface contamination. Subsequently, using a plexiglass bifocal generator of 8 mm × 4 mm × 1 mm, the samples were veneered on an alloy with Vita VMK Master and VITA VM13 porcelains.[14] The samples were divided into four groups (VM13/Ceramill, VMK Master/Ceramill, VM13/Verabond, and VMK Master/Verabond), and bond strength was evaluated by three-point bending test with a universal testing machine (machine Zwick, 2050, Radeberg, Germany). Then, the device tip, with a diameter of 0.7 mm and a constant speed of 1 mm/min, was fed perpendicularly to the center of the samples until the porcelain was removed from the alloy. Two-way ANOVA was used for comparison, and for the normality, the Kolmogorov–Smirnov test was used. Furthermore, a comparison of the means was done using post hoc Scheffé's method (P < 0.05).
Figure 1.
(a) Plexiglas generators, (b) Sintron alloy blank.
RESULTS
The results showed that the highest bond strength was related to the Verabond/VM13 group (44.35 ± 7.9 MPa) and then the Cermill/VM group (39.33 ± 4.43 MPa), and the least strength was related to the Ceramill/VMK Master group (29.75 ± 3.2 MPa) [Table 1 and Figure 2]. The obtained data were normal, and there was a significant difference between groups (P < 0.05). These differences were also analyzed by the post hoc Scheffé's test [Table 2]. The bond strength difference was significant between Verabond/VM13 and Verabond/VMK Master groups (P < 0.05) and defined no significancy between other groups (P > 0.05). In the Ceramill/VMK Master group, this difference was significant with the Verabond/VMK Master group (P < 0.05). In the Verabond/VM13 group, the bond strength was significant with Ceramill/VMK Master group (P < 0.05). In the Verabond/VMK Master group, the bond strength was not significantly different with the other three groups (P > 0.05). The results also showed that the mean bond strength of the Verabond alloy group was higher (40.09 ± 7.42) [Table 3].
Table 1.
Mean±standard deviation and maximum and minimum of bond strength (MPa) of groups
| Type | Mean | n | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| VM13 and Ceramill | 39.3380 | 10 | 4.43620 | 32.24 | 46.28 |
| VMK master and Ceramill | 29.7570 | 10 | 3.25690 | 25.09 | 36.40 |
| VM13 and Verabond | 44.3520 | 10 | 7.96820 | 26.62 | 54.12 |
| VMK master and Verabond | 35.8180 | 10 | 3.50525 | 28.16 | 40.48 |
| Total | 37.3163 | 40 | 7.31062 | 25.09 | 54.12 |
SD: Standard deviation
Figure 2.
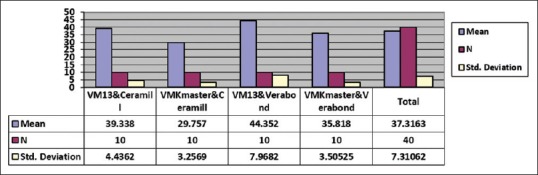
Mean and standard deviation of bond strength of the samples.
Table 2.
ANOVA test: Tests of between-subject effects
| Dependent variable: Bond strength | |||||
|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | Significant |
| Corrected model | 1129.763 | 3 | 376.588 | 14.202 | 0.000 |
| Intercept | 55,700.101 | 1 | 55,700.101 | 2100.578 | 0.000 |
| Type 1 | 820.383 | 1 | 820.383 | 30.939 | 0.000 |
| Type 2 | 306.639 | 1 | 306.639 | 11.564 | 0.002 |
| Type 1*Type 2 | 2.741 | 1 | 2.741 | 0.103 | 0.750 |
| Error | 954.596 | 36 | 26.517 | ||
| Total | 57,784.459 | 40 | |||
| Corrected total | 2084.359 | 39 | |||
*The mean difference is significant at the 0.05 level. *Type 1=VM13/VMK Master, *Type 2=Ceramill Sintron/Verabond
Table 3.
Post-hoc Scheffe’s test results of the groups (P<0.05 is significant)
| Type (I) | Type (J) | Mean difference (I-J) | 95% CI |
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| VM13 and Ceramill | VMK Master and Ceramilla | 9.58100* | 2.8281* | 16.3339 |
| VM13 and Verabond | −5.01400 | −11.7669 | 1.7389 | |
| VMK Master and Verabond | 3.52000 | −3.2329 | 10.2729 | |
| VMK Master and Ceramill | VM13 and Ceramilla | −9.58100* | −16.3339* | −2.8281 |
| VM13 and Verabonda | −14.59500* | −21.3479* | −7.8421 | |
| VMK Master and Verabond | −6.06100 | −12.8139 | 0.6919 | |
| VM13 and Verabond | VM13 and Ceramill | 5.01400 | −1.7389 | 11.7669 |
| VMK Master and Ceramilla | 14.59500* | 7.8421* | 21.3479 | |
| VMK Master and Verabonda | 8.53400* | 1.7811* | 15.2869 | |
| VMK Master and Verabond | VM13 and Ceramill | −3.52000 | −10.2729 | 3.2329 |
| VMK Master and Ceramill | 6.06100 | −6919 | 12.8139 | |
| VM13 and Verabonda | −8.53400* | −15.2869* | −1.7811 | |
*The mean difference is significant at the 0.05 level. Significant differences showed bye letter a. CI: Confidence interval
DISCUSSION
Bond strength in all test groups was higher than the standard threshold (25 MPa). According to the results of this study, the bond strength of the cobalt-chromium alloy made with the CAD/CAM method was slightly less than the usual alloy made by casting. Therefore, the null hypothesis that the strength of the Sintron alloy bond is roughly equal with the base metal alloy is rejected. The use of base metal alloys constructed using the CAD/CAD method reduces the risk of damage or deformation occurring during the casting process.[6] Among the mechanisms for creating a metal-ceramic bond, we can mention the chemical bond, the mechanical bond, the van der Waals forces, and the bond produced by the compressive force due to the CTE difference between the metal and porcelain.[6] Factors affecting porcelain failure are mismatching of CTE of the metal and porcelain, small cracks created during the process of porcelain construction, and occlusal forces.[6] Shell and Nielsen[15] reported that chemical bonding is the most important metal-ceramic bonding mechanism, while the mechanical bond is not very important. Because the formation of the oxide layer at the metal-ceramic joint level affects the chemical bond, alloy compounds are very important. The thin oxide layer can be completely removed during porcelain baking, and the thick layer can weaken the bond due to low cohesive strength.[15] Sintron core groups exhibit higher bond strengths, when the Vita VM13 porcelain is placed on them. In the study of Lee et al.,[8] the shear bond strength of porcelain is compared to synthetic alloys and a conventional casting alloy. The bond strength of Sintron was similar to that of all cast alloys other than the Press to metal alloys with less bond strength.[8] In this study, Verabond/VM13 group bond strength was also close to that of HongandShin.[6] This group had the maximum bond strength in this study and had a higher difference with other alloy/ceramic compounds. In the Hong and Shin study,[6] the bond strength of porcelain was compared with a palladium/silver alloy and a conventional Ni-Cr alloy, using three-point bending test and it was found that the bond strength of Ni-Cr alloy was about 40.42 ± 5.72 MPa, but the stability of the palladium/silver alloy containing high percentage of gold was significantly lower due to nonconformance of CTE with porcelains in the market. Due to the complexity of metal-ceramic bonding properties, metal-ceramic bond strength can be tested with different methods such as shear bond test and three- or four-point bending tests. Anusavice et al.[4] concluded that there is no ideal test method because the samples have different patterns of stress distribution, which could lead to different bond strengths. Papazoglou and Brantley[16] showed that there is no consistent agreement between the results of various tests. According to the results of Hammad and Talic[17] on different bond strength tests (shear, tensile, bending, and stretch tests) of metal-ceramic systems, the shear bond strength test with flat-shared surface can only measure the forces that are applied on the metal-ceramic joint and cannot evaluate the metal modulus elastic to evaluate the bending test. Some studies consider the shear test to be more reliable due to the joint power, but Hammad and Talic[17] have stated that the best way to evaluate the metal-ceramic bond is when the least variables exist and the remaining stresses at the metal-ceramic joint are minimal and the best time is when the break down is cohesive. In the present study, the metal-ceramic bond strength was in the range of 29.75–44.35 MPa. The Verabond/VM13 group had the highest metal-ceramic bond strength (44.35 MPa), after that Ceramill/VM13 (39.33 MPa) and then Verabond/VMK Master (35.81 MPa), and finally, the minimum bond strength was related to the Ceramill/VMK Master group (29.75 MPa) that was less than Lee's study (34 MPa).[8] This may be due to the lack of control of the thickness of the oxide layer or the different bond strength test types. This difference indicates that if using a Ceramill Sintron alloy, it is better to use the Vita VM13 porcelain instead of the Vita VMK Master although both bond strengths are higher than the standard threshold. Metal/ceramic thermal matching is very important during restoration. The difference between the CTE of the alloy and porcelain is generally recommended to be 0.5–1 × 10−6/°C.[8] The CTE of the alloys used in this study corresponded to the used porcelains (for Ceramill equal to 14.5 × 10−6/K and for Verabond equal to 14 × 10−6/K), because according to the manufacture's data, these two porcelains were compatible with alloys with CTE ranged from 13.8 to 15.2 × 10−6/K. However, it should be kept in mind that not only the thermal expansion characteristic but also other mechanical and chemical properties can affect the bond strength. The maximum break down strength in a three-point bending test is not likely to be equal to bond strength. Wood et al.[18] have stated that plastic break down occurs in the metal-ceramic sample. The maximum registered force is not equal to the metal-ceramic debonding initiation force, and the initiating force is considerably less than the maximum force in the curve resulting from the machine's calculations. In this study, because the maximum force is considered for calculating bond strength, there is a possibility that the bond strength is too high.
CONCLUSION
The metal-ceramic bond strength for the new Ceramill Sintron alloy is above the standard threshold. CAD/CAM blanks of this alloy can easily be rasped in the laboratory and can be used in dental laboratories instead of cast alloys. It is better to veneer Sintron alloy with VITA VM13 porcelain instead of VITA VMK porcelain. The Verabond alloy with VITA VM13 porcelain has a higher bond strength than with the VITA VMK Master.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declared that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgment
This article is based on thesis from the Dental School of Ahvaz Jundishapur University of Science with approval code of 80-36-MP-D.
REFERENCES
- 1.Koodaryan R, Sazgara H, Seyedan K, Hafezeqoran A. Comparing the effect of porcelain firing cycles on marginal adaptation of procera all-ceramic restorations with metal ceramics. J Islamic Dent Assoc IRAN. 2010;22:80–6. [Google Scholar]
- 2.Koosha S, Pourmahdi Broujeni M, Amirpoor S. Spectrophotometric comparison of the influence of porcelain types on shade of metal-ceramic restorations. J Dent Sch Shahid Beheshti Univ Med Sci. 2011;29:171–7. [Google Scholar]
- 3.Stawarczyk B, Eichberger M, Hoffmann R, Noack F, Schweiger J, Edelhoff D, et al. A novel CAD/CAM base metal compared to conventional coCrMo alloys: An in vitro study of the long-term metal-ceramic bond strength. Oral Health Dent Manag. 2014;13:446–52. [PubMed] [Google Scholar]
- 4.Anusavice KJ, Shen C, Rawls HR. Phillips' Science of Dental Materials. St.Louis, Missouri: Elsevier Health Sciences; 2013. [Google Scholar]
- 5.Kelly M, Asgar K, O'Brien WJ. Tensile strength determination of the interface between porcelain fused to gold. J Biomed Mater Res. 1969;3:403–8. doi: 10.1002/jbm.820030302. [DOI] [PubMed] [Google Scholar]
- 6.Hong JT, Shin SY. A comparative study on the bond strength of porcelain to the millingable Pd-Ag alloy. J Adv Prosthodont. 2014;6:372–8. doi: 10.4047/jap.2014.6.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar SM, Pereira S, Ccahuana VV, Passos SP, Vanderlei AD, Pavanelli CA, et al. Shear bond strength between metal alloy and a ceramic system, submitted to different thermocycling immersion times. Acta Odontol Latinoam. 2007;20:97–102. [PubMed] [Google Scholar]
- 8.Lee DH, Lee BJ, Kim SH, Lee KB. Shear bond strength of porcelain to a new millable alloy and a conventional castable alloy. J Prosthet Dent. 2015;113:329–35. doi: 10.1016/j.prosdent.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Deepak K, Ahila SC, Muthukumar B, Vasanthkumar M. Comparative evaluation of effect of laser on shear bond strength of ceramic bonded with two base metal alloys: An in vitro study. Indian J Dent Res. 2013;24:610–5. doi: 10.4103/0970-9290.123396. [DOI] [PubMed] [Google Scholar]
- 10.Shadid R, Sadaqah N, Abu Naba'a L. Porcelain fracture of metal-ceramic tooth-supported and implant-supported restorations: A review. OJST. 2013;3:411–8. [Google Scholar]
- 11.do Prado RA, Panzeri H, Fernandes Neto AJ, das Neves FD, da Silva MR, Mendonça G, et al. Shear bond strength of dental porcelains to nickel-chromium alloys. Braz Dent J. 2005;16:202–6. doi: 10.1590/s0103-64402005000300006. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes Neto AJ, Panzeri H, Neves FD, Prado RA, Mendonça G. Bond strength of three dental porcelains to Ni-Cr and Co-Cr-Ti alloys. Braz Dent J. 2006;17:24–8. doi: 10.1590/s0103-64402006000100006. [DOI] [PubMed] [Google Scholar]
- 13.Gola R, Frizzas D, Rodrigues RC, Ribeiro R. Shear bond strength of dental ceramics to cast commercially pure titanium. Braz J Oral Sci. 2010;9:362–5. [Google Scholar]
- 14.International Organization for Standardization. Metal-Ceramic Dental Restorative System. International Organization for Standardization 2012 (ISO 2012) [Google Scholar]
- 15.Shell JS, Nielsen JP. Study of the bond between gold alloys and porcelain. J Dent Res. 1962;41:1424–37. doi: 10.1177/00220345620410062101. [DOI] [PubMed] [Google Scholar]
- 16.Papazoglou E, Brantley WA. Porcelain adherence vs. force to failure for palladium-gallium alloys: A critique of metal-ceramic bond testing. Dent Mater. 1998;14:112–9. doi: 10.1016/s0109-5641(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 17.Hammad IA, Talic YF. Designs of bond strength tests for metal-ceramic complexes: Review of the literature. J Prosthet Dent. 1996;75:602–8. doi: 10.1016/s0022-3913(96)90244-9. [DOI] [PubMed] [Google Scholar]
- 18.Wood MC, Thompson GA, Agar JR. A comparison of debonding strengths of four metal-ceramic systems with and without opaque porcelain. J Prosthet Dent. 2007;97:141–9. doi: 10.1016/j.prosdent.2006.12.013. [DOI] [PubMed] [Google Scholar]


