Abstract
Background:
Oral lichen planus (OLP) is a chronic disease without any definitive treatment. Local corticosteroids are often prescribed, but their efficacy has been questioned by many studies. The purpose of this study was to investigate the effect of nano-based triamcinolone acetonide gel (NT) and compare it with conventional triamcinolone gel (CT) on OLP.
Materials and Methods:
In this triple-blind randomized clinical trial study, 40 patients with Erosive OLP were divided into two groups receiving (CT) and (NT). The patients were requested to apply them four times a day for 2 weeks. The severity of pain was evaluated through visual analog scale, the size of lesions was measured with paper lace, and the appearance of lesions was examined adopting Thongprasom scale . Findings will be significant via independent t-test or Chi-square test with P < 0.05.
Results:
The severity of pain in NT group was 4.9 ± 0.7 cm before the treatment and 1.5 ± 0.9 after that, whereas in CT group, it was 4.9 ± 0.8 and 1.8 ± 0.9, respectively . The mean size of the lesions in NT group was 2.1 ± 1.1 cm before the treatment and 0.8 ± 1.1 afterward, whereas in CT group, was 2.2 ± 1.1 and 1.3 ± 1.1, respectively. The OLP appearance before and after the study in NT group was 4.5 ± 0.5 and 0.8 ± 0.6, respectively, whereas in CT group was 4.6 ± 0.5 and 0.9 ± 0.7 (P = 0.3). Among these variables, only Thongprasom scale on the 6th and 14th days had a significant reduction in NT group in comparison with CT group.
Conclusion:
NT has a better impact on OLP in comparison with CT, but this difference is not statistically significant.
Key Words: Mucoadhesive, oral lichen planus, triamcinolone acetonide
INTRODUCTION
Oral lichen planus (OLP) is a chronic autoimmune disease with a prevalence of 1%–3% in various societies. OLP can occur separately or in conjunction with skin and other mucous membrane lesions.[1,2,3] The mean age of the onset is the fifth decade of life, and there is an obvious predominance among females. Although OLP may occur at any oral mucosal site, the buccal mucosa is the most common site of involvement. In contrast to cutaneous lichen planus, oral lesions have a prolonged clinical course, and the oral mucosal involvement may persist for many years. OLP can be painful, especially in the atrophic and erosive forms.[4] The exact ethiopathogenesis of OLP is still unknown. It seems that several factors, including stress, genetics, environment, and lifestyle are effective in OLP development.[5] OLP contains six clinical forms, including reticular, popular, erosive, atrophic, plaque, and bullous.[6]
No definitive treatment has yet been introduced for OLP. Local corticosteroids are considered as the preferred treatment for OLP.[7,8] Systemic corticosteroids are prescribed only in the short-term treatment of resistant cases.[7,8,9] In clinical trials, controversial results have been reported for the effectiveness of corticosteroids on OLP treatment.[10] Triamcinolone is the most prescribed topical corticosteroid for OLP.[11]
Local corticosteroids are available in the forms of ointment, cream, lotion, and gel.[10,12] Given the fact that the mucosa is covered with viscoelastic layers, topical drugs like other foreign substances do not cohere with or clog the mucus for the various mechanisms such as hydrophobic and electrostatic adhesion, hydrogen bonding, and are washed rapidly in a few seconds to a minute; accordingly, they are unable to penetrate the mucus layers and reach the epithelial surface.[13,14] For this reason, sustained and effective drug delivery to the mucosa is restricted and mucus is considered as an important barrier to the topical treatment of different oral diseases.[15]
The nano drug delivery system is a new way to cope with the rapid clearance of topical drugs of the mucus and to provide sustained and effective drug delivery to the mucosa.[15] Biodegradable nanoparticles can penetrate into the mucus layer and reach the epithelium cells.[16] In addition, some nonbiodegradable nanoparticles containing polyethylene glycol have greater adhesion to mucus, because of their hydrophilic and noncurable molecules. Therefore, they are less affected by mucus clearance in comparison with non-nano-formulated drugs.[17] Popovska et al. concluded that topic application of nano bio film (NBF) gingival gel at OLP, showed positive clinical effects in a relatively short period of time, thus avoiding application of systematic or topical steroids due to their numerous adverse effects. NBF gingival gel, with its own therapeutic modalities was recommended in the treatment of OLP.[18] In addition, Azizi et al. concluded that topical use of tacrolimus is a safe, well tolerated, and effective therapy for OLP lesions recalcitrant to traditional therapies. This drug is especially useful when lichen planus lesions are resistant to conventional treatments such as steroids.[19] In another study, Azizi et al. evaluated the evaluation of nano-based triamcinolone on improving oral OLP. Evaluated the effect of 0.1% triamcinolone containing nano-liposomal carrier on OLP lesions. At the end of 1 month, 33.3% of the subjects in the Nano group and 26.7% of the non-nano group were fully recovered.[26]
The aim of this study was to investigate the therapeutic effects of nano-triamcinolone formulation on OLP and compare it with nonnano triamcinolone formulation. The findings of this study can provide further evidence for the efficacy of triamcinolone gel with nanoparticle formulation.
MATERIALS AND METHODS
This triple-blind randomized clinical trial study (patient, examining physician, and statistic analyzer) was conducted at the Isfahan University of Medical Sciences after the approval of the Ethics Committee (Code: IR. REC. AJAUMS.1396.66) and RCT code (IRCT20181226042133N1). The population of the study was comprised 40 eligible patients with OLP who referred to the dental research center and dentistry faculty at Isfahan University of medical sciences to participate and were randomly assigned to two groups of 20 each using random number generator software (Random Allocation Software; M Saghaei, Isfahan, Iran).
The inclusion criteria for the study involved diagnosis of OLP with erosive pattern using clinical criteria and histopathologic confirmation, an age range of 16–70, absence of topical or systemic drugs for treating OLP at least 2 months before the study, severity of lesions with a score of 4 and 5 Thongprasom, and signature of written consent. Exclusion criteria included pregnancy and lactation, the use of drugs that produce lichenoid reaction such as beta blockers, immunodeficiency, the presence of any systemic disease other than lichen planus (such as viral infection and acute peptic ulcer), the presence of lesions in direct contact with the teeth treated with filling, sensitivity to corticosteroids and the use of denture. Eligible patients were matched for age, sex, and size of lesions into two groups of 20, those who were administered nano-based triamcinolone gel (NT) and the others who took conventional triamcinolone gel (CT) under the name of Kenacort by DEVA company (turkey).
Drug preparation
Preparation and evaluation of Mucoadhesive gel formulation containing 0.1% triamcinolone was performed in the pharmacy faculty of Kermanshah University of Medical Sciences, Iran. For preparation of triamcinolone gel formulation, biocompatible polymers were used through the spontaneous emulsification technique. The polymer in addition to the biocompatibility of the mucous membrane has high adhesion properties (CT). The nano composition was then added to the triamcinolone polymer gel (NT). The presence of nanoparticles, their size and morphology were controlled utilizing a scanning electron microscope.
To detect the amount of released drug from NT, a suitable amount of nanoparticles was transferred to a receptacle containing 1 ml of phosphate buffer with pH 7.7, and then the buffer and nanoparticles were separated by a dialysis membrane (Mw cutoff = 12,000-14,000 Daltons; Delchimica Scientific Glassware, Milan, Italy). The system was continuously cured at 37°C and centrifuged at 100 rpm. To prevent evaporation of the buffer, the receptacle was a sealed container. A certain amount of sample (100 λ) was removed at regular intervals from the receptacle and replaced immediately with a fresh buffer solution.
To determine the mucosal adhesion of the gel, buccal mucosa of sheep was used. The adhesion durability was calculated about 1 h. To measure the concentration of the drug in the receptor phase, ultraviolet (UV) or high-performance liquid chromatography (HPLC) was used. For this purpose, some of the drugs was dissolved in 50 ml of phosphate buffer with 6.6 pH and was shaken at 37°C. Moreover, 50 ml of methanol was added to completely dissolve the drug. Afterward, the solution's concentration was measured with UV or HPLC and compared with a standard curve. The concentration of triamcinolone was 0.1%.
Prescription
A total of 50 mg of the gel inside sterile white (NT) and red plastic cans (CT) was administered to the patient without their being informed of the modified formulation of the drug. For the first time, a physician applied the gels at the site of the lesion(s) to educate the patients. In this way, the oral lesion(s) were dried with sterile gas, some of the drug was removed with sterile cotton swab and placed on the lesion(s) and slightly around it. Patients were banned from receiving any food or drink for up to 30 min after dipping. Drug-using lasted four times a day for 2 weeks. Patients were requested to mark the daily timetable schedule provided to ensure regular use of the gels.
Clinical evaluation
Demographic information of the patients including age and sex was received 1 day before the study. The oral lesion variables, including the size, clinical score, and the severity of pain, as well as the healing time, were evaluated on the starting day and days 2, 4, 6, and 14 after the intervention. At each day, evaluations were conducted by a trained physician who did not know about the type of the administered drug. The size of the lesions was measured by single-use and graded paper lace. Then, the average length of the greater diameter of lesions was recorded in cm per patient (all of OLP lesions in each patient were treated). The severity of pain was measured using the visual analog scale (VAS), a 100 mm paper ruler. A higher score indicates more severity of the pain. The clinical scores of OLP were evaluated on a Thongprasom scale, which categorizes the phenotype of OPL lesions and includes 6 grades (zero = perfectly healthy, 1 = mild white lines without inflammatory regions, 2 = white lines with atrophic regions smaller than 1 cm2, 3 = white lines with atrophic regions larger than 1 cm2, 4 = white lines accompanied with erosive regions < 1 cm2, 5 = white lines with erosive areas larger than 1 cm2 or with wound injuries.[20]
Patients at grades 4 or 5 of the Thongprasom scale were included in the study. In addition, patients with lesions on both sides of their buccal mucosa were administered with both NT and CT. The success of the treatment in this study was the achievement on a 0–3 score of Thongprasom scale.[20]
Data analysis
Data were analyzed using SPSS 22. To describe the quantitative data, the mean ± standard deviation and for the qualitative data, the percentage was used. For between groups comparison, independent t-test or Chi-square test was used. Findings with P < 0.05 were reported to be significant.
RESULTS
A total of 40 eligible OLP patients were enrolled in the study. One patient in the CT group did not complete the study. The information about 39 patients are presented below. The mean age of NT group was 44.3 ± 10.3, and that of the CT group was 36.6 ± 10 (P = 0.05). In the NT and CT group, 14 (70%) and 16 (84.2%) cases were female and the rest were male (P = 0.3). The largest size of the lesions in the NT was 2.1 ± 1.1, and 2.2 ± 1.1 cm in the CT group before the study (P = 0.6). The severity of pain before the intervention in the NT and CT group was 4.9 ± 0.7 and 4.9 ± 0.8 cm, respectively (P = 0.9). Thongprasom score in the NT and CT groups was 4.5 ± 0. 5 and 4.6 ± 0.5, respectively (P = 0.6). The two groups were well matched for age, sex, the severity of pain, and the Thongprasom score.
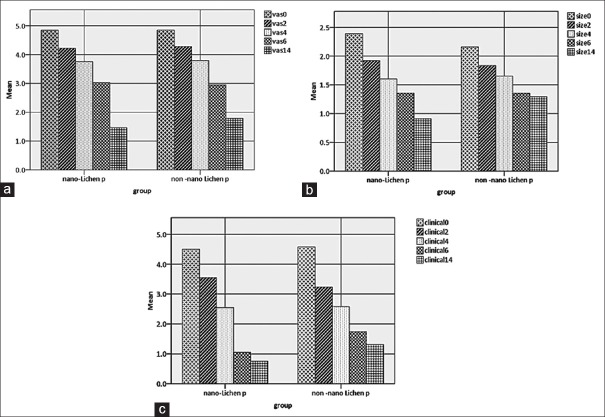
Measurement of the severity of pain before the intervention (baseline) and days 2, 4, 6, and 14 are illustrated in Table 1 and Figure 1a. In the both groups, the severity of pain showed a decreasing trend compared to the baseline. Independent t-test did not show a significant difference in pain severity between NT and CT groups, although in the NT group it was <CT.
Table 1.
Distribution of pain intensity based on 10 cm visual analogue scale (independent t-test)
| Time (day) | Group | n | Mean±SD | P |
|---|---|---|---|---|
| 0 | NT | 20 | 4.9±0.7 | 0.9 |
| CT | 19 | 4.9±0.8 | ||
| 2 | NT | 20 | 4.2±0.8 | 0.8 |
| CT | 19 | 4.3±0.7 | ||
| 4 | NT | 20 | 3.8±0.9 | 0.9 |
| CT | 19 | 3.8±0.7 | ||
| 6 | NT | 20 | 3±0.9 | 0.7 |
| CT | 19 | 2.9±0.7 | ||
| 14 | NT | 20 | 1.5±0.9 | 0.2 |
| CT | 19 | 1.8±0.9 |
SD: Standard deviation; CT: Conventional triamcinolone gel; NT: Nano-based triamcinolone acetonide gel
Figure 1.
Mean changes in pain intensity (cm, a), diameter of the lesions (cm, b), and clinical score of the form (c) on the base days and after the intervention.
In both groups, the size of lesions had a decreasing trend. In the NT group, size of the lesions in the baseline was 2.1 ± 1.1 and 2.2 ± 1.1 cm in the CT groups, which did not show significant differences between the two groups (P = 0.6). There was no significant difference between the two groups with independent t-test, taking into account the corresponding days [Table 2 and Figure 1b]. At the end of the 2 weeks of intervention in the NT group, 7 cases (35%), and in the CT group 4 cases (21.05%), recovered, which were not significantly different from each other (P = 0.3).
Table 2.
Changes in ulcer size per cm (independent t)
| Time (day) | Group | n | Mean±SD | P |
|---|---|---|---|---|
| 0 | NT | 20 | 2.1±1.1 | 0.8 |
| CT | 19 | 2.2±1.1 | ||
| 2 | NT | 20 | 1.8±1.1 | 0.8 |
| CT | 19 | 1.9±1.1 | ||
| 4 | NT | 20 | 1.4±1.3 | 0.6 |
| CT | 19 | 1.5±1.1 | ||
| 6 | NT | 20 | 1.2±1.2 | 0.7 |
| CT | 19 | 1.3±1 | ||
| 14 | NT | 20 | 0.8±1.1 | 0.02 |
| CT | 19 | 1.3±1.1 |
SD: Standard deviation; CT: Conventional triamcinolone gel; NT: Nano-based triamcinolone acetonide gel
The distribution of the clinical score (Thongprasom) is presented in Table 3 and Figure 1c. As indicated, the score was significantly lower in the NT group on the 6th and 14th days in comparison to the CT group that indicates a better effect on the appearance improvement. At the end of the study, 20 (100%) of NT cases and 19 (100%) of CT cases had thongprasom scores of 0–3, according to which all subjects received clinical improvement.
Table 3.
Distribution of clinical index (ulcer appearance based on thongprasom 6-point scale, independent t-test)
| Time (day) | Group | n | Mean±SD | P |
|---|---|---|---|---|
| 0 | NT | 20 | 4.5±0.5 | 0.6 |
| CT | 19 | 4.6±0.5 | ||
| 2 | NT | 20 | 3.6±0.5 | 0.3 |
| CT | 19 | 3.2±1.2 | ||
| 4 | NT | 20 | 2.6±0.5 | 0.9 |
| CT | 19 | 2.6±0.5 | ||
| 6 | NT | 20 | 1.1±0.8 | 0.007 |
| CT | 19 | 1.7±0.8 | ||
| 14 | NT | 20 | 0.8±0.6 | 0.02 |
| CT | 19 | 0.9±0.7 |
SD: Standard deviation; CT: Conventional triamcinolone gel; NT: Nano-based triamcinolone acetonide gel
DISCUSSION
The aim of this study was to evaluate the effectiveness of triamcinolone acetonide gel (orabase) with and without nanoparticle combinations on OLP. The findings showed that although nano-triamcinolone increases the rate of pain relief, and reduces the size of lesions, its difference overall was not significant compared to non-nano triamcinolone orabase gel.
In the present study, triamcinolone acetonide was polymerized and contained nanoparticles. Thus, with polymer, more adhesion to mucus, and with nanoparticles, accumulation and gradual release of the drug at the mucosal surface was achieved.[16,17] However, it seems that the addition of the nanoparticles only accelerated the recovery and decreased symptoms, as a result.
Triamcinolone acetonide (ointment, gel, and mouthwash) has been applied locally with a good therapeutic effect on OLP.[21,22,23] Furthermore, changes in corticosteroid compounds can improve their effectiveness on OLP. Campisi et al. compared to the new formulation of clobetasol 17-propionate (containing 0.25% lipid microspheres) and its common formulation on OLP. Their findings showed that the new compound has a better effect on pain intensity (VAS), clinical score, and patient satisfaction.[24] Cilurzo et al. compared the slow-release tablet of clobetasol (17 propionates in combination with polymer) with conventional clobetasol ointment and placebo tablet on OLP. At the end of the study, the number of asymptomatic individuals in the polymer group was 13 out of 16, in the ointment group, 11 out of 16 cases and in the placebo group 3 out of 16 cases. In the ointment group, two cases of candidiasis and four cases with changes in the taste sensation occurred. They conclude that using the polymerized clobetasol is effective on OPL with fewer side effects.[25]
Limited studies have been conducted on the evaluation of nano-based triamcinolone on improving oral OLP. Azizi et al. evaluated the effect of 0.1% triamcinolone containing nano-liposomal carrier on OLP lesions. Sixty patients were divided into two groups as follows: triamcinolone acetonide and nano triamcinolone. The medications continued topically three times daily for 1 month. The severity of pain was measured by VAS and the size of the lesions with paper lace. Their findings showed that both drugs reduced the severity of pain and the size of the lesions during the study. However, patients in the Nano group were significantly better in the 2nd and 4th weeks. At the end of 1 month, 33.3% of the patients in the nano group and 26.7% of the non-nano group were fully recovered.[26] In the present study, 6 (30%) patients recovered from OLP after 2 weeks of NT intervention. This amount was 3 (15.8%) cases in the CT, which is close to Azizi et al.'s findings.
CONCLUSION
The findings of this randomized clinical trial study showed that nano-based triamcinolone gel accelerates the recovery of OLP in comparison to non-nano-based triamcinolone, but the observed difference is not statistically significant.
Financial support and sponsorship
Vice Chancellor for Research and Technology, Aja University of Medical Sciences.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Shulman JD, Beach MM, Rivera-Hidalgo F. The prevalence of oral mucosal lesions in U.S. adults: Data from the third national health and nutrition examination survey, 1988-1994. J Am Dent Assoc. 2004;135:1279–86. doi: 10.14219/jada.archive.2004.0403. [DOI] [PubMed] [Google Scholar]
- 2.Mansour Ghanaei F, Joukar F, Rabiei M, Dadashzadeh A, Kord Valeshabad A. Prevalence of oral mucosal lesions in an adult Iranian population. Iran Red Crescent Med J. 2013;15:600–4. doi: 10.5812/ircmj.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew AL, Pai KM, Sholapurkar AA, Vengal M. The prevalence of oral mucosal lesions in patients visiting a dental school in Southern India. Indian J Dent Res. 2008;19:99–103. doi: 10.4103/0970-9290.40461. [DOI] [PubMed] [Google Scholar]
- 4.Agha-Hosseini F, Khalili M, Rohani B. Immunohistochemistry analysis of P53 and Ki-67 proteins in oral lichen planus and normal oral mucosa. Iran J Public Health. 2009;38:37–43. [Google Scholar]
- 5.Agha-Hosseini F, Rohani B. Evaluation of the effects of dental implants on oral lesions. J Contemp Dent Pract. 2015;16:400–6. doi: 10.5005/jp-journals-10024-1697. [DOI] [PubMed] [Google Scholar]
- 6.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 7.Ho JK, Hantash BM. Systematic review of current systemic treatment options for erosive lichen planus. Expert Rev Dermatol. 2012;7:269–82. [Google Scholar]
- 8.McBride DR. Management of aphthous ulcers. Am Fam Physician. 2000;62:149–54, 160. [PubMed] [Google Scholar]
- 9.Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371–8. doi: 10.4103/0378-6323.178903. [DOI] [PubMed] [Google Scholar]
- 10.Suresh SS, Chokshi K, Desai S, Malu R, Chokshi A. Medical management of oral lichen planus: A systematic review. J Clin Diagn Res. 2016;10:ZE10–5. doi: 10.7860/JCDR/2016/16715.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamishehkar H, Nokhodchi A, Ghanbarzadeh S, Kouhsoltani M. Triamcinolone acetonide oromucoadhesive paste for treatment of aphthous stomatitis. Adv Pharm Bull. 2015;5:277–82. doi: 10.15171/apb.2015.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra AK, Khaitan BK, Sethuraman G, Sharma VK. Betamethasone oral mini-pulse therapy compared with topical triamcinolone acetonide (0.1%) paste in oral lichen planus: A randomized comparative study. J Am Acad Dermatol. 2008;58:596–602. doi: 10.1016/j.jaad.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 13.González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Patol Oral Cir Bucal. 2010;15:e827–31. [PubMed] [Google Scholar]
- 14.Stone OJ. Aphthous stomatitis (canker sores): A consequence of high oral submucosal viscosity (the role of extracellular matrix and the possible role of lectins) Med Hypotheses. 1991;36:341–4. doi: 10.1016/0306-9877(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 15.Laffleur F, Bernkop-Schnürch A. Strategies for improving mucosal drug delivery. Nanomedicine (Lond) 2013;8:2061–75. doi: 10.2217/nnm.13.178. [DOI] [PubMed] [Google Scholar]
- 16.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci U S A. 2009;106:19268–73. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–7. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popovska M, Fidovski J, Mindova S, Dirjanska K, Ristoska S, Stefanovska E, et al. The effects of NBF gingival gel application in the treatment of the erosive lichen planus: Case report. Open Access Maced J Med Sci. 2016;4:158–63. doi: 10.3889/oamjms.2016.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azizi A, Lawaf S. The comparison of efficacy of adcortyl ointment and topical tacrolimus in treatment of erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2007;1:99–102. doi: 10.5681/joddd.2007.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thongprasom K, Luangjarmekorn L, Sererat T, Taweesap W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J Oral Pathol Med. 1992;21:456–8. doi: 10.1111/j.1600-0714.1992.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 21.Miles DA, Bricker SL, Razmus TF, Potter RH. Triamcinolone acetonide versus chlorhexidine for treatment of recurrent stomatitis. Oral Surg Oral Med Oral Pathol. 1993;75:397–402. doi: 10.1016/0030-4220(93)90158-z. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Shin SY, Kim SW, Eun YG. Intralesional injection versus mouth rinse of triamcinolone acetonide in oral lichen planus: A randomized controlled study. Otolaryngol Head Neck Surg. 2013;148:443–9. doi: 10.1177/0194599812473237. [DOI] [PubMed] [Google Scholar]
- 23.Siponen M, Huuskonen L, Kallio-Pulkkinen S, Nieminen P, Salo T. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: A pilot randomized controlled trial. Oral Dis. 2017;23:660–8. doi: 10.1111/odi.12653. [DOI] [PubMed] [Google Scholar]
- 24.Campisi G, Giandalia G, De Caro V, Di Liberto C, Aricò P, Giannola LI, et al. A new delivery system of clobetasol-17-propionate (lipid-loaded microspheres 0.025%) compared with a conventional formulation (lipophilic ointment in a hydrophilic phase 0.025%) in topical treatment of atrophic/erosive oral lichen planus. A phase IV, randomized, observer-blinded, parallel group clinical trial. Br J Dermatol. 2004;150:984–90. doi: 10.1111/j.1365-2133.2004.05943.x. [DOI] [PubMed] [Google Scholar]
- 25.Cilurzo F, Gennari CG, Selmin F, Epstein JB, Gaeta GM, Colella G, et al. A new mucoadhesive dosage form for the management of oral lichen planus: Formulation study and clinical study. Eur J Pharm Biopharm. 2010;76:437–42. doi: 10.1016/j.ejpb.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Azizi A, Dadras OG, Jafari M, Moezi Ghadim N, Lawaf S, Sadri D. Efficacy of 0.1% triamcinolone with nanoliposomal carrier formulation in orabase for oral lichen planus patients: A clinical trial. Eur J Integr Med. 2016;8:275–80. [Google Scholar]