Abstract
Background
We aimed to determine the prevalence of adverse childhood experiences (ACEs) in persons with inflammatory bowel disease (IBD) and whether having ACEs was associated with health care utilization post-IBD diagnosis.
Method
Three hundred forty-five participants from the population-based Manitoba IBD Cohort Study self-reported ACEs (ie, physical abuse, sexual abuse, death of a very close friend or family member, severe illness or injury, upheaval between parents, and any other experience thought to significantly impacts one’s life or personality) at a median of 5.3 years following IBD diagnosis. Cohort study data were linked to administrative health databases that captured use of hospitals, physician visits, and prescription drugs; use was classified as IBD-related and non-IBD-related. Mean annual estimates of health care use were produced for the 60-month period following the ACE report. Generalized linear models (GLMs) with generalized estimating equations (GEEs) with and without covariate adjustment were fit to the data.
Results
The prevalence of at least 1 ACE was 74.2%. There was no statistically significant association between having experienced an ACE and health care use. However, unadjusted mean annual non-IBD-related general practitioner visits were significantly higher for participants exposed to physical and sexual abuse than those not exposed. Selected adjusted rates of IBD-related health care use were lower for participants who reported exposure to an upheaval between parents and high perceived trauma from ACEs.
Conclusion
The estimated prevalence of at least 1 self-reported ACE in persons with diagnosed IBD was high. Health care use among those who experienced ACEs may reflect the impacts of ACE on health care anxiety.
Keywords: adverse childhood experiences, inflammatory bowel disease, population-based, cohort study, administrative health data, mental health disorders
Three quarters of adults with IBD experienced at least one adverse childhood experience. There was no significant association between having experienced an ACE and adult health care use overall. However, selected rates of IBD-related healthcare use later in life were lower for participants who reported exposure to an upheaval between parents and high perceived trauma from ACEs.
Introduction
Adverse childhood experiences (ACEs) encompass a range of child maltreatment and household challenges. Child maltreatment includes (1) physical abuse, (2) sexual abuse, (3) emotional abuse, (4) physical neglect, and (5) emotional neglect. Household upheaval includes (6) exposure to domestic violence, (7) household substance abuse, (8) household mental illness, (9) parental separation or divorce, and (10) incarceration of a household member.1 Adverse childhood experiences can be predictive of future physical and mental health outcomes.1–3 There is a known association between ACEs and the development of chronic disease; the literature indicates that a consistent relationship exists between ACEs and risk factors for chronic disease.2,4 These risk factors include alcohol and drug abuse, smoking, obesity, depression, suicide, and psychological stress.4–10 The relationship between ACEs and risk factors for chronic disease have led researchers to suggest that underlying factors (ie, psychological stress) help predict an individual’s transition from childhood adversity to chronic illness, which is the most common cause of increased health care use, disability, and early death.2,11–13
While the relationships among ACEs, risk factors, and health outcomes including individual-level health care use have been explored among a variety of disease cohorts, no studies have, to our knowledge, been conducted among individuals with inflammatory bowel disease (IBD). Although IBD is accompanied by a diverse set of physical symptoms including increased frequency and urgency of bowel movements, abdominal discomfort, and fatigue, it also shows an association with mental health outcomes including stress, anxiety, and depression.14 The association between IBD symptoms and mental health has been shown to be bidirectional. These types of mental health outcomes are also commonly associated with ACEs.6 Therefore, it is possible that ACEs could impact persons with IBD including their disease activity and overall health care use. Adverse childhood experiences were found to have a positive association with overall GI symptom activity in persons with irritable bowel syndrome (IBS).15,16 We aimed to (1) estimate the prevalence of ACEs in individuals with adult onset IBD; (2) test the association between ACEs, which included number and type and non-IBD-related and IBD-related health care use; and (3) test the association between the perceived level of trauma of ACEs and non-IBD-related and IBD-related health care use. We hypothesized that persons diagnosed with IBD who were exposed to at least 1 ACE would exhibit greater health care use than persons with IBD who were never exposed to an ACE. Furthermore, we predicted that this relationship would be influenced by the effects of demographic and behavioral risk factors, comorbid conditions, psychological stress, and IBD disease characteristics.
METHODS
Study Design and Data Source
A retrospective cohort design was adopted. The Manitoba IBD Cohort Study was a population-based prospective, longitudinal study that enrolled 388 individuals in 2002 who were at least 18 years of age and within 7 years of their IBD diagnosis.17 Study participants were surveyed semiannually and interviewed annually from 2002 to 2014 (12 years). This research includes data from all participants who completed the Childhood Traumatic Events Survey (CTES) at the 12-month cohort study survey and who provided informed consent for study researchers to access their Manitoba Health administrative data.
Manitoba Health is the provincial health insurance provider of universal health care to all residents of Manitoba. Health care utilization dating back to 1984 can be tracked for all residents through a unique personal health identification number (PHIN). Manitoba Health administrative data were linked to the the 12-month cohort study survey. To be included, participants were required to have a minimum of 5 years of health insurance coverage after completing the CTES (or 12-month cohort study survey).
Study Measures
ACEs and Perceived Level of Trauma
Information about ACEs and perceived level of trauma from these experiences was captured by the CTES, which was administered at the 12-month cohort study survey. The CTES asks about exposure to 3 commonly defined ACEs: (1) physical abuse, (2) sexual abuse, and (3) an upheaval between parents (including divorce or separation). It also asks about exposure to 3 traumatic experiences: (4) death of a very close friend or family member, (5) experience of a severe illness or injury, and (6) any other upheaval thought to significantly shape one’s life or personality. The CTES has good reliability and validity, and it has demonstrated sensitivity to clinical symptoms including post-traumatic stress disorder (PTSD).18,19 Information collected with the CTES was used to construct a binary (ie, yes/no) measure of exposure to at least 1 ACE. In addition, a count of the total number of ACEs that each cohort member was exposed to was constructed; ACES were not mutually exclusive; therefore, the count ranged from none to 6 types of ACEs. Using this count, categories were constructed (ACE Count): no ACEs, 1 ACE, 2 ACEs, and 3 or more ACEs.
Perceived level of trauma for each type of ACEs was also assessed using a Likert-type scale, where 1 represented “not at all traumatic” and 7 represented “extremely traumatic.” If an individual experienced more than 1 type of ACE, the highest perceived level of trauma reported was used. Perceived level of trauma from ACEs was defined as a categorical variable based on the frequency of responses (median: 5): low perceived trauma (≤5) and high perceived trauma (>5).
Health care use measures
Twelve measures of health care use were defined as outcomes. These included (1) number of non-IBD-related general practitioner (GP) visits, (2) number of IBD-related GP visits, (3) number of non-IBD-related specialist (SP) visits, (4) number of IBD-related SP visits, (5) non-IBD-related inpatient hospital admission (yes/no), (6) IBD-related inpatient hospital admission (yes/no), (7) number of non-IBD-related inpatient hospital days, (8) number of IBD-related inpatient hospital days, (9) number of IBD prescription drug dispensations, (10) number of psychotropic prescription drug dispensations, (11) number of antibiotic prescription drug dispensations, and (12) number of other prescription drug dispensations.
General practitioner or specialist visits were identified by physician identifiers for every health system contact. Specialists were gastroenterologists or internists who also do endoscopy and were identified using physician specialty information provided in administrative data. All IBD-related physician visits and hospitalizations were determined using International Classification of Disease, 9th revision, Clinical Modification (ICD-9-CM) codes for all outpatient visits and all inpatient stays until 2004 and International Classification of Diseases, 10th revision, Canada (ICD-10-CA) codes for all inpatient stays from 2004 and onward.
The Drug Program Information Network provides all prescription medication dispensed for each individual dating back to 1995 and is linked to the Manitoba Health administrative database through the PHIN. Anatomical Therapeutic Chemical codes and drug identification numbers (DINs) were used to identify IBD-related medications (including 5-aminosaylicylic acid, corticosteroids, immunomodulation drugs, and biologic agents), psychotropic medications, and antibiotic medications (Appendix A). Health care use associated with pregnancy and childbirth was excluded from analyses. All health care use measures were defined for the 60-month (ie, 5-year) period after CTES completion.
Covariates
Health, demographic and behavioral risk factors, psychological stress, social functioning, and IBD disease characteristics and activity were defined for all cohort members.
Healths
Comorbidity, a common measure of health status, was captured using the Charlson Comorbidity Index (CCI).20 The CCI was defined from all ICD-9-CM and ICD-10-CA diagnoses in hospital separation abstracts and physician claims for the 365-day period before (and including) the 12-month survey completion date.
Demographic and behavioral risk factors
All measures were collected at the baseline or 12-month cohort study survey. These included sex (male, female), age (18 to 44 years, >44 years), income quintiles (<$30,000, $30,000 to 49,000, $50,000 to 69,000, $70,000 to 79,000, ≥$80,000), smoking (non-daily, daily), alcohol use (less than once per week, one to three times per week, greater than three times per week), and body mass index (BMI) (healthy weight [18.5 to 24.9] vs underweight [<18.5], overweight/obese [≥25]). Body mass index was based on measured height and weight collected at the 12-month cohort study interview.
Social functioning
This was measured as a continuous variable using the Short Form 36 item Health Survey (SF-36).21 Mean SF-36 scores were calculated from a total of 11 SF-36 scores repeatedly collected from the baseline survey through to the 60-month survey.
Psychological stress
Perceived psychological stress was assessed with the 14-item Cohen psychological stress scale (PSS-14).22 Mean PSS-14 scores were calculated from 11 PSS-14 scores repeatedly collected from the baseline survey through to the 60-month survey.
IBD disease characteristics
These characteristics included disease type (Crohn’s disease [CD] or ulcerative colitis [UC]), previous IBD-related surgeries in the year before (and including) CTES completion date (yes/no), and duration of time (years) between IBD diagnosis date and CTES completion.
IBD disease activity
The Manitoba IBD Index (MIBDI) is a single question with 6 potential responses that was designed and validated to inquire of symptom status over the prior 6 months.23 Disease activity was determined using percentage of active MIBDI scores (11 scores); less than 39% active scores indicated minimally or inactive disease, 39% to 81% active scores indicated moderately active disease, and greater than 81% active scores indicated very active disease.23
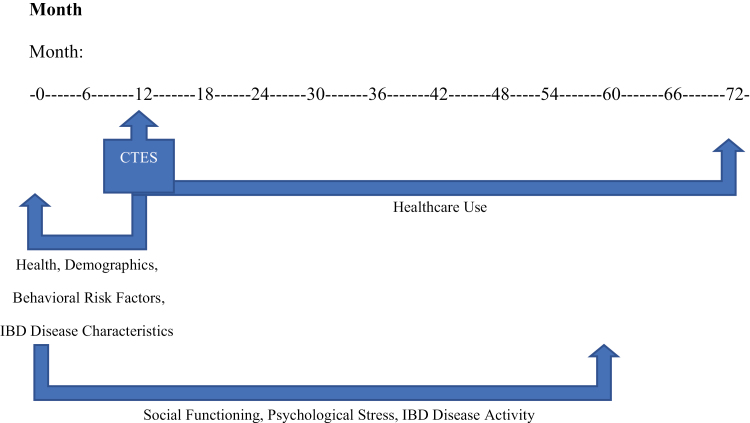
A visual aid outlining the collection of study measures is depicted in Figure 1.
Figure 1.
Chronology of study measures collected in the manitoba IBD cohort study, by month.
Statistical Analysis
We used frequencies and percentages to describe the number of individuals who were exposed to one or more ACE, each ACE Count category, each type of ACE, and high perceived trauma from ACEs. Frequencies, percentages, means, and standard deviations (SDs) were used to describe the demographic and behavioral risk factors, comorbid conditions, perceived psychological stress, and IBD disease characteristics for cohort members unexposed vs exposed to 1 or more ACE, cohort members exposed to the different ACE Count categories, and cohort members experiencing high perceived trauma vs low perceived trauma from ACEs. Differences between groups were tested using χ2 tests of independence for categorical variables and t tests and analysis of variance (ANOVA) for continuous variables.
We counted the number of outcome events in annual increments for the 60-month (ie, 5-year) period after CTES completion. The mean of the annual average estimates and its standard error were calculated for discrete health care use measures for participants: (1) unexposed vs exposed to one or more ACEs, (2) grouped by ACE Count, (3) unexposed vs exposed to each type of ACE, and (4) experiencing high perceived trauma vs low perceived trauma from ACEs. Similarly, percentages were reported for hospitalizations for each of these variable groupings. Differences between groups were tested using χ2 tests of independence for categorical variables and ANOVA for continuous variables.
Generalized linear models (GLMs) with generalized estimating equations (GEEs) and negative binomial distributions were used to test for differences in the health care use measures over time. For binary measures, we used GLMs with GEEs and a logit link function. Generalized estimating equations were used to account for clustering of visits within individuals over time. An autoregressive correlation structure was assumed for the repeated measurements; it allows for correlations to be highest between measurements that are closest together in time and lowest for measurements that are farther apart in time. First, unadjusted GLMs were used to test for differences in health care use measures across groups. Then, adjusted GLMs were fit to the data; they included the following covariates: sex, age group, income quintile, smoking status, alcohol use, BMI category, mean social functioning subscore, CCI score category, mean PSS score, IBD disease type, disease activity, previous IBD-related surgeries, and time (in years) between diagnosis and 12-month survey completion.
Relative rates (RRs) for count measures and odds ratios (ORs) for binary measures along with 95% confidence intervals (95% CIs) were estimated for all models. Model fit was assessed using the quasi-likelihood under the independence model criterion (QIC). When compared across models, lower QIC values indicate better fit.
This study was approved by the University of Manitoba Health Research Ethics Board. Analyses were conducted using SPSS V24 (IBM Corp., Armonk, NY, USA).
RESULTS
Objective 1: Estimate Prevalence of ACEs
A total of 345 (88.9%) participants from the Manitoba IBD Cohort Study were retained in the cohort for this research. Mean duration between diagnosis and cohort entry was 5.4 years (SD 2.1), mean age at CTES completion was 41.9 years (SD 14.5), and 60.0% of participants were female. A total of 74.2% of participants reported exposure to at least 1 ACE. For cohort study participants exposed to at least 1 ACE, mean duration between CTES completion and exposure to their first ACE was 30.5 years
In terms of the type of ACEs, the most common was death of a very close friend or family member (51.3%), followed by any other upheaval thought to significantly shape one’s life or personality (29.0%), and experience of a severe illness or injury (20.9%). Both physical abuse (12.2%) and sexual abuse (13.0%) had a similar prevalence, while upheaval between parents was experienced by one fifth (20.3%) of participants.
Cohort study participants experienced a total of 501 ACEs. For the ACE Count variable, more than one third of participants (33.6%) had experienced 1 type of ACE, more than one fifth had experienced 2 types of ACEs (21.2%), and a slightly smaller number had experienced 3 or more types of ACEs (19.4%).
The characteristics of cohort study participants by ACE Count categories are described in Table 1. More females than males were exposed to 3 or more types of ACEs (25.1% vs 10.9%, P = 0.007). Cohort study participants exposed to 3 or more ACEs exhibited a mean SF-36 score approximately 5 points lower than persons experiencing no ACEs, 1 ACE, or 2 ACEs (P < 0.0001). Cohort study participants exposed to 1 or more ACE had a mean PSS-14 score close to 3 points greater (21.8; SD 7.0) than cohort members never exposed to an ACE (19.1 ± 7.1, P = 0.002). Cohort study participants exposed to 3 or more ACEs had the highest mean PSS scores (23.9 ± 7.6 P < 0.0001). While SF-36 scores remained stable across ACE Count categories, PSS scores increased across the categories. Additionally, participants exposed to 3 or more ACEs had a higher prevalence of IBD-related surgeries in the 12 months before CTES completion than participants in all other ACE Count categories. There were no other significant differences in the characteristics of cohort study participants by ACE Count category.
Table 1.
Characteristics of Manitoba IBD Cohort Study Respondents (Stratified by ACE Count, N = 345)
| 0 ACEs | 1 ACE | 2 ACEs | 3+ ACEs | |
|---|---|---|---|---|
| n (%) | 89 (25.8) | 116 (33.6) | 73 (21.2) | 67 (19.4) |
| Sex | ||||
| Male | 43 (48.3) | 47 (40.5) | 33 (45.2) | 15 (22.4) |
| Female | 46 (51.7) | 69 (59.5) | 40 (54.8) | 52 (77.6) |
| Age (years) | ||||
| 18–44 | 50 (56.2) | 70 (60.3) | 46 (63.0) | 37 (55.2) |
| 45+ | 39 (43.8) | 46 (39.7) | 27 (37.0) | 30 (44.8) |
| Income Quintile a | ||||
| One (lowest income) | 16 (18.0) | 23 (19.8) | 19 (26.0) | 21 (31.3) |
| Two | 22 (24.7) | 21 (18.1) | 11 (15.1) | 18 (26.9) |
| Three | 17 (19.1) | 25 (21.6) | 12 (16.4) | 13 (19.4) |
| Four | 7 (7.9) | 12 (10.3) | 6 (8.2) | s |
| Five (highest income) | 19 (21.3) | 26 (22.4) | 20 (27.4) | s |
| Smoking Status | ||||
| Never/Not daily | 79 (88.8) | 103 (88.8) | 61 (83.6) | 58 (86.6) |
| Daily | 10 (11.2) | 13 (11.2) | 12 (16.4) | 9 (13.4) |
| Alcohol Use | ||||
| Never/Less than once a week | 45 (50.6) | 63 (54.3) | 47 (64.4) | 41 (61.2) |
| 1–3 times per week | 25 (28.1) | 29 (25.0) | s | s |
| >3 times per week | 10 (11.2) | 8 (6.9) | s | s |
| No response | 9 (10.1) | 16 (13.8) | 8 (11.0) | 7 (10.4) |
| Body Mass Index a | ||||
| Healthy weight | 37 (41.6) | 48 (41.4) | 34 (46.6) | 25 (37.3) |
| Underweight/Overweight/Obese | 50 (56.2) | 65 (56.0) | 37 (50.7) | 42 (62.7) |
| Social Functioning | 48.7 (SD: 7.6) | 48.4 (SD: 7.6) | 48.6 (SD: 7.0) | 43.8 (SD: 9.8) |
| Comorbidity | ||||
| 0 | s | 103 (88.8) | s | 58 (86.6) |
| 1 or more | s | 13 (11.2) | s | 9 (13.4) |
| Perceived Psychological Stress | 19.1 (SD: 7.1) | 20.7 (SD: 6.6) | 21.4 (SD: 6.7) | 23.9 (SD: 7.6) |
| IBD Disease Type | ||||
| Crohn’s Disease | 39 (43.8) | 55 (47.4) | 40 (54.8) | 33 (49.3) |
| Ulcerative/Indeterminate colitis | 50 (56.2) | 61 (52.6) | 33 (45.2) | 34 (50.7) |
| Disease Activity a | ||||
| Minimally/Inactive disease | 34 (38.2) | 42 (36.2) | 22 (30.1) | 16 (23.9) |
| Moderately active disease | 25 (28.1) | 30 (25.9) | 27 (37.0) | 18 (26.9) |
| Very active disease | 30 (33.7%) | 44 (37.9%) | 23 (31.5%) | 33 (49.2%) |
| Previous IBD-related Surgery | ||||
| Yes | s | s | s | 9 (13.4) |
| No | s | s | s | 58 (86.6) |
| Time between diagnosis and 12m survey completion (years) | 5.7 (SD: 1.9) | 5.5 (SD: 2.1) | 5.2 (SD: 2.1) | 5.1 (SD: 2.1) |
aSome persons were missing data; therefore, column percentages do not sum to 100
s Indicates suppression due to small cell sizes
Bold values indicate a statistically significant difference between persons exposed to no ACEs, one ACE, two ACEs, and three or more ACEs at α = 0.05
Objective 2: The Association Between ACEs and Health Care Use
On average, cohort study participants who were exposed to physical abuse visited their GP (mean 7.3, SD 7.0, median 4.9) and SP (mean 7.7, SD 11.2, median 4.1) for non-IBD-related reasons more times per year than participants never exposed to abuse (GP, mean 4.9, SD 4.5, median 3.8, P = 0.004; SP, mean 4.7, SD 7.3, median 2.6, P = 0.020). On average, cohort study participants who were exposed to sexual abuse also visited their GP for non-IBD-related reasons more times per year (6.7 ± 7.3, median 4.0) than participants who were not exposed to sexual abuse (5.0 ± 4.5, median 4.0, P = 0.036). After adjustment for model covariates (Table 2), physical and sexual abuse did not associate with health care use rates.
Table 2.
Rate Ratio and Odds Ratio Estimates (95% CIs) of Health Care Use for Study Respondents Exposed to Physical and Sexual Abuse
| Exposed to Physical Abuse (95% CI) | Exposed to Sexual Abuse (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| GP visits (non-IBD) | 1.37 (1.01–1.86) | 1.20 (0.84–1.72) | 1.26 (0.91–1.75) | 1.06 (0.77–1.44) |
| GP visits (IBD) | 0.62 (0.34–1.13) | 0.64 (0.38–1.08) | 1.14 (0.50–2.61) | 0.81 (0.41–1.63) |
| SP visits (non-IBD) | 1.36 (0.93–1.99) | 1.36 (0.70–2.66) | 1.07 (0.73–1.58) | 0.82 (0.60–1.13) |
| SP visits (IBD) | 0.86 (0.55–1.36) | 0.86 (0.57–1.32) | 0.75 (0.48–1.17) | 0.66 (0.44–1.01) |
| Hospitalized (non-IBD) | 0.88a (0.45–1.73) | 0.76a (0.44–1.29) | 0.90a (0.53–1.51) | 0.89a (0.53–1.50) |
| Hospitalized (IBD) | 0.70a (0.33–1.50) | 0.74a (0.43–1.27) | 0.95a (0.60–1.50) | 0.77a (0.45–1.31) |
| Hospital days (non-IBD) | 1.05 (0.76–1.47) | 0.68 (0.33–1.39) | 0.78 (0.40–1.53) | 0.60 (0.27–1.32) |
| Hospital days (IBD) | 1.58 (0.89–2.82) | 0.82 (0.39–2.75) | 0.50 (0.22–1.11) | 0.45 (0.19–1.03) |
| IBD drugs | 1.08 (0.65–1.80) | 1.13 (0.80–1.60) | 1.06 (0.79–1.42) | 1.15 (0.83–1.59) |
| Psychotropic drugs | 1.30 (0.87–1.94) | 1.40 (0.65–3.01) | 1.55 (0.89–2.72) | 1.33 (0.60–2.96) |
| Antibiotic drugs | 0.88 (0.45–1.73) | 0.74 (0.47–1.18) | 1.13 (0.72–1.77) | 0.89 (0.62–1.29) |
| Other drugs | 0.70 (0.33–1.50) | 1.17 (0.78–1.77) | 1.30 (0.84–2.01) | 0.93 (0.62–1.38) |
aIndicate Odds Ratio; Note bold-face estimates indicate a statistically significant association at α = 0.05.
Cohort study participants who were exposed to an upheaval between parents had an increased annual estimate for non-IBD-related SP visits (mean 7.4, SD 14.6, median 2.4) compared with members who were not exposed (mean 4.7, SD 5.8, median 2.8, P = 0.019). Additionally, participants who were exposed to an upheaval between parents were hospitalized less over 60 months (52.9%) for IBD-related reasons than members who were not exposed (68.1%, P = 0.017). After adjustment for model covariates, participants exposed to an upheaval between parents had a 38% lower rate (P = 0.005) for IBD-related SP visits when compared with unexposed participants (Table 3). Additionally, exposure to an upheaval between parents resulted in a 37% lower odds (P = 0.036) of hospital admission and a 40% lower rate (P = 0.006) of hospital days for IBD-related reasons relative to exposed participants.
Table 3.
Rate Ratio and Odds Ratio Estimates (95% CI) of Health Care Use for Study Respondents Exposed to Death of a Close Friend/Family Member, Severe Illness/Injury, and Upheaval Between Parents
| Exposed to Death of Family/Friend (95% CI) | Exposed to Illness/Injury (95% CI) | Exposed to Parent Upheaval (95% CI) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| GP visits (non-IBD) | 1.09 (0.90–1.33) | 1.02 (0.84–1.23) | 0.91 (0.72–1.15) | 0.88 (0.70–1.11) | 1.00 (0.75–1.34) | 0.94 (0.70–1.26) |
| GP visits (IBD) | 1.09 (0.72–1.66) | 1.10 (0.76–1.59) | 0.87 (0.54–1.39) | 0.84 (0.57–1.22) | 0.88 (0.55–1.40) | 0.78 (0.51–1.19) |
| SP visits (non-IBD) | 0.99 (0.73–1.34) | 1.02 (0.79–1.32) | 1.02 (0.73–1.42) | 0.99 (0.73–1.34) | 1.21 (0.75–1.95) | 1.16 (0.70–1.92) |
| SP visits (IBD) | 1.29 (0.90–1.83) | 1.09 (0.83–1.43) | 1.27 (0.73–2.21) | 1.09 (0.76–1.55) | 0.77 (0.54–1.09) | 0.62 (0.45–0.86) |
| Hospitalization (non-IBD) | 1.22a (0.861.72) | 1.38a (0.96–1.97) | 0.91a (0.59–1.40) | 1.01a (0.66–1.54) | 0.98a (0.63–1.57) | 0.99a (0.64–1.53) |
| Hospitalization (IBD) | 1.13a (0.84–1.50) | 1.13a (0.83–1.55) | 1.20a (0.82–1.76) | 1.17a (0.77–1.76) | 0.69a (0.46–1.05) | 0.63 a (0.41–0.97) |
| Hospital days (non-IBD) | 1.10 (0.61–1.99) | 1.28 (0.79–2.06) | 1.27 (0.53–3.00) | 1.23 (0.65–2.32) | 0.82 (0.44–1.51) | 0.65 (0.35–1.22) |
| Hospital days (IBD) | 1.51 (0.75–3.02) | 1.36 (0.78–2.36) | 1.63 (0.52–5.14) | 1.28 (0.68–2.39) | 0.57 (0.28–1.16) | 0.40 (0.21–0.77) |
| IBD drugs | 0.94 (0.75–1.17) | 0.89 (0.69–1.13) | 1.09 (0.85–1.41) | 1.13 (0.86–1.49) | 0.92 (0.67–1.25) | 0.94 (0.66–1.34) |
| Psychotropic drugs | 1.15 (0.69–1.92) | 0.93 (0.56–1.54) | 1.53 (0.87–2.67) | 1.70 (0.93–3.11) | 0.88 (0.47–1.67) | 0.69 (0.32–1.49) |
| Antibiotic drugs | 1.17 (0.88–1.55) | 1.08 (0.82–1.43) | 0.87 (0.61–1.22) | 0.78 (0.58–1.03) | 1.10 (0.74–1.65) | 0.91 (0.66–1.26) |
| Other drugs | 1.23 (0.90–1.69) | 1.03 (0.80–1.32) | 0.99 (0.70–1.38) | 1.05 (0.78–1.42) | 0.86 (0.60–1.25) | 0.90 (0.66–1.21) |
aIndicate Odds Ratio; Bold estimates indicate a statistically significant association at α = 0.05
Objective 3: The Association Between Perceived Level of Trauma of ACEs and Health Care Use
A total of 253 cohort study participants were exposed to an ACE and completed the 12-month CTES questions pertaining to perceived level of trauma for ACEs. Approximately half of these study participants (48.6%) report a high perceived level of trauma. The characteristics of cohort study participants who experienced a high level of trauma in relation to their ACEs and participants who experienced a low level of trauma in relation to their ACEs are displayed in Table 4. The table shows that significantly more females report a high level of trauma compared with males (55.3% vs 37.2%, P = 0.005). Although less than half (48.5%) of participants reporting a low level of trauma were characterized as either underweight, overweight, or obese, close to two thirds (64.2%, P = 0.019) of participants reporting a high level of trauma fell into this underweight/overweight/obese category. Higher levels of reported trauma (ie, score >5) were associated with both lower SF-36 scores (mean 45.6, SD ± 9.0 vs mean 48.8, SD 7.3; P = 0.002) and higher PSS scores (mean 22.7, SD 7.4 vs mean 21.0, SD 6.6; P = 0.049) than lower levels of reported trauma (ie, score ≤ 5). Additionally, more participants who had reported a high trauma ACE (7.3%) underwent an IBD-related surgery in the year before CTES completion than cohort members who had experienced a low trauma ACE (1.5%, P = 0.024) and underwent an IBD-related surgery in the year before CTES completion. There were no other significant differences between participants experiencing at least 1 ACE who report a high perceived level of trauma and participants experiencing at least 1 ACE who report a low perceived level of trauma.
Table 4.
Characteristics of Manitoba IBD Cohort Study Respondents, Stratified by Perceived Level of ACE Trauma, N = 253
| High Perceived Trauma | Low Perceived Trauma | |
|---|---|---|
| n (%) | 123 (48.6) | 130 (51.4) |
| Sex | ||
| Male | 35 (28.5) | 59 (45.4) |
| Female | 88 (71.5) | 71 (54.6) |
| Age (years) | ||
| 18–44 | 70 (56.9) | 83 (63.8) |
| 45+ | 53 (43.1) | 47 (36.2) |
| Income Quintile a | ||
| One (lowest) | 30 (24.4) | 31 (23.8) |
| Two | 30 (24.4) | 19 (14.6) |
| Three | 23 (18.7) | 27 (20.8) |
| Four | 10 (8.1) | 10 (7.7) |
| Five (highest) | 20 (16.3) | 37 (28.5) |
| Smoking Status | ||
| Never/Not daily | 103 (83.7) | 116 (89.2) |
| Daily | 20 (16.3) | 14 (10.8) |
| Alcohol Use | ||
| Never/Less than once a week | 73 (59.3) | 77 (59.2) |
| 1–3 times per week | 29 (23.6) | 34 (26.2) |
| >3 times per week | 6 (4.9) | 5 (3.8) |
| No response | 15 (12.2) | 14 (10.8) |
| Body Mass Index a | ||
| Healthy weight | 43 (35.0) | 63 (48.5) |
| Underweight/Overweight/Obese | 79 (64.2) | 63 (48.5) |
| Social Functioning | 45.6 (SD: 9.0) | 48.8 (SD: 7.3) |
| Comorbidity | ||
| 0 | 108 (87.8) | 121 (93.1) |
| 1 or more | 15 (12.2) | 9 (6.9) |
| Perceived Psychological Stress | 22.7 (SD: 7.4) | 21.0 (SD: 6.6) |
| IBD Disease Type | ||
| Crohn’s Disease | 67 (54.5) | 60 (46.2) |
| Ulcerative/indeterminate colitis | 56 (45.5) | 70 (53.8) |
| Disease Activity a | ||
| Minimally/inactive disease | 36 (29.3) | 44 (33.8) |
| Moderately active disease | 32 (26.0) | 40 (30.8) |
| Very active disease | 54 (43.9) | 46 (35.4) |
| Previous IBD-related Surgery | ||
| Yes | 9 (7.3) | 2 (1.5) |
| No | 114 (92.7) | 128 (98.5) |
| Time between diagnosis and 12m survey completion (years) | 5.3 (SD: 2.1) | 5.3 (SD: 2.1) |
aSome cohort members were missing data; therefore, column percentages do not sum to 100
Bold values indicate a statistically significant difference between cohort members reporting high perceived trauma and cohort members reporting low perceived trauma at α = 0.05
On average, cohort study participants who report high perceived trauma saw their GP for non-IBD-related reasons more times per year than cohort members who report low perceived trauma (mean 6.1, SD 6.0 vs mean 4.6, SD 3.9; P = 0.023). After adjustment for model covariates, there were no significant difference in the rates of non-IBD-related GP visits between cohort study participants who report high perceived trauma and participants who report low perceived trauma. However, the adjusted results do reveal that report of high ACE trauma predicted a 49% decrease in the RR (P = 0.000) of IBD-related GP visits and a 52% decrease in the rate (P = 0.041) of IBD-related hospital days (Table 5).
Table 5.
Rate Ratio and Odds Ratio Estimates of Health Care Use for Study Respondents Reporting High Perceived ACE Trauma
| Unadjusted Estimate (95% CI) | Adjusted Estimate (95% CI) | |
|---|---|---|
| GP visits (non-IBD) | 1.28 (1.02–1.61) | 1.04 (0.83–1.31) |
| GP visits (IBD) | 0.70 (0.45–1.11) | 0.51 (0.35–0.74) |
| SP visits (non-IBD) | 1.19 (0.81–1.76) | 0.97 (0.71–1.34) |
| SP visits (IBD) | 0.91 (0.56–1.46) | 0.77 (0.55–1.10) |
| Hospitalization (non-IBD) | 1.47a (0.96–2.25) | 1.20a (0.75–1.92) |
| Hospitalization (IBD) | 0.86a (0.61–1.22) | 0.70a (0.46–1.05) |
| Hospital days (non-IBD) | 1.36 (0.63–2.91) | 1.01 (0.35–2.94) |
| Hospital days (IBD) | 0.68 (0.27–1.71) | 0.48 (0.24–0.97) |
| IBD drugs | 0.79 (0.62–1.02) | 0.81 (0.61–1.06) |
| Psychotropic drugs | 1.05 (0.59–1.89) | 0.82 (0.40–1.72) |
| Antibiotic drugs | 1.20 (0.87–1.67) | 0.96 (0.70–1.33) |
| Other drugs | 1.30 (0.88–1.93) | 0.89 (0.65–1.22) |
a Indicate Odds Ratio; Bold estimates are statistically significant at α = 0.05
Discussion
In summary, we found that almost 75% of the cohort was exposed to 1 or more ACE. Exposure to 1 or more ACE was not associated with various measures of health care use over a 5-year period. Overall, 12% of the cohort was exposed to physical abuse, 13% to sexual abuse, and 20% to an upheaval between parents. Physical and sexual abuse were associated with higher mean annual estimates for non-IBD related GP visits. Similarly, exposure to physical abuse and parental upheavals was associated with increased mean annual estimates for non-IBD-related SP visits. However after covariate adjustment, exposure to physical and sexual abuse and parental upheavals was not associated with increased health care use. In contrast, exposure to an upheaval between parents resulted in decreased odds of hospital admission and decreased rates of SP visits and hospital days for IBD reasons relative to unexposed cohort members. High perceived trauma from ACEs was also associated with increased mean annual estimates for non-IBD-related GP visits. However after covariate adjustment no significant increases were observed. After covariate adjustment, high perceived trauma from ACEs was associated with decreased IBD-related health care use including rates of GP visits and hospital days.
The number of individuals exposed to 1 or more ACEs in this study was higher (74%) than previously reported in other US and Canadian population-based studies (55%–60%).24,25 This higher prevalence may result from the different types of ACEs included in this study: experience of illness or injury, death of a close friend or family member, and any other upheaval thought to significantly shape one’s life or personality. Moreover, exposure to any other upheaval thought to significantly shape one’s life or personality is likely to include a wide variety of experiences not captured in other ACE measures. When population estimates are compared with IBD cohort estimates, similar results are produced for 2 commonly defined ACEs—sexual abuse and parental upheavals. Statistics Canada reported the prevalence of sexual abuse at 10.0% in the general population, and it was 13.0% in the Manitoba IBD Cohort Study.26 Likewise, population data from the US ACE Study reported the prevalence of parental upheavals at 23.3%, whereas it was 20.3% in the Manitoba IBD Cohort Study.24 However, the prevalence of physical abuse in the Manitoba IBD Cohort Study was low compared with Canadian estimates reported by Statistics Canada (12.2% vs 26.0%).26 The prevalence from the Manitoba IBD Cohort Study was similar to the estimate reported by the Alberta ACE Study for the general population (11.0%).25
Adverse childhood experiences have been associated with the evolution of psychological stress; therefore, we expected PSS to be higher among participants exposed to 1 or more ACE compared with those never exposed to an ACE.27 Our findings were consistent with previous research on psychological stress and ACEs; perceived psychological stress increased with exposure to more types of ACEs.
Although previous research suggests that exposure to ACEs is associated with increased health care use, after consideration of other covariates, any number of ACEs was not predictive of increased non-IBD-related or IBD-related health care use in our study.28–31 Additionally, reports of higher perceived trauma from ACE exposure were not associated with increased non-IBD-related or IBD-related health care use. It is possible that the weak associations were the result of the study’s temporal characteristics. Cohort members were exposed to their ACEs before the age of 17; this was characteristic of the CTES used to measure ACE exposure. Health care use was assessed in the 5 years following CTES completion, not exposure to the ACE. For cohort study participants exposed to at least 1 ACE, the mean number of years between CTES completion and exposure to their first ACE was 30.5 years. While this factor has not been recognized by previous ACE research, it is possible that the long period of time between ACE exposure and health care use measurement may have contributed to the null findings. Even though this study found that the number of ACEs, different types of ACEs, and higher perceived trauma from ACEs did not predict overall increased health care use in persons with IBD, ACEs may still have a significant impact on persons with IBD. Mean annual estimates for non-IBD-related health care use including GP and SP visits indicate that persons with IBD who were exposed to physical abuse, sexual abuse, and parental upheavals are utilizing more health care than persons with IBD who were not exposed to these ACEs. While GLMs indicate that this increase in utilization cannot be attributed to ACE exposure, other confounding covariates, (eg, social functioning, comorbidity, and disease activity) may play a mediating role between ACE exposure and health care utilization.
It was unexpected that exposure to parental upheavals would be associated with decreased IBD-related health care use, including SP visits, hospitalizations, and hospital days. It was also unexpected that high levels of perceived trauma from ACEs would be associated with decreased IBD-related health care use including GP visits and hospital days. There is no previous literature or evidence to explain why these trends may exist; however, we can speculate that reduced social functioning and uneasiness around authoritative figures may play an important role. Persons exposed to ACEs and trauma may have been taken to health care providers by their parent/guardians less often than persons not exposed to ACEs; this pattern of seeking health care less often may persist into adulthood. It is important to acknowledge that ACE exposure and higher levels of perceived ACE trauma may inhibit persons with IBD from accessing the IBD-related health care services they require to properly manage their disease.
Our main finding was that persons with IBD may have a higher prevalence of ACEs than previously reported population estimates, although the size of the estimate may be influenced by the method of measurement. Even though the number, type, and perceived level of ACE trauma did not predict worse outcomes for persons with IBD as measured by health care utilization, other outcomes should be explored. Descriptive statistics suggest that perceived psychological stress and social functioning may be significantly associated with ACE exposure.32 These are important outcomes to consider as people with IBD often report that their symptoms increase during periods of high stress. An increase in symptoms can have a significant impact on an individual’s life.17, 33 As exposure to parental upheavals and higher levels of perceived ACE trauma were predictive of decreased IBD-related health care use, clinicians should consider inquiry into ACEs as a component of IBD care. Inquiry into ACEs may allow clinicians to better counsel their IBD patients in terms of managing psychosocial factors, including stress, anxiety, and depression, and seeking out the appropriate supports.34 While psychosocial screening in patients with IBD is not a new phenomenon, inquiry into ACEs has surfaced as a controversial topic.35
Screening tools have been used as successful indicators for social support, depression, anxiety, distress, and interest in receiving psychological care for persons with IBD.34, 36–38 Low screening rates for ACEs can be partially attributed to a lack of appropriate assessment tools and competing opinions.35, 39 Although some believe that ACE screening may itself provide opportunity for acknowledgement, reflection, and therapeutic benefit, critics of ACE screening argue that it may be disruptive, adding a sense of stigma and harming health care relationships.
Our study has some limitations. We did not measure the 10 commonly defined ACEs. Although we only measured 3 commonly defined ACEs (physical abuse, sexual abuse, and parental upheavals), our study included experiences other studies have not assessed (severe illness/injury and death of a close friend/family member). As ACEs may have occurred years earlier, it is possible that participants were unable to recall exposure or the associated trauma. The small sample size resulted in insufficient power to analyze potential mediating effects; however, the sample was large enough to find differences between the groups we sought to analyze. To ensure that including 12 interrelated measures of health care use in a small sample size was not problematic, a composite health care use measure was created. This measure compiled all physician and hospital visits regardless of specialty or relationship to IBD. No associations were found between ACE exposure or perceived level of trauma and the composite measure.
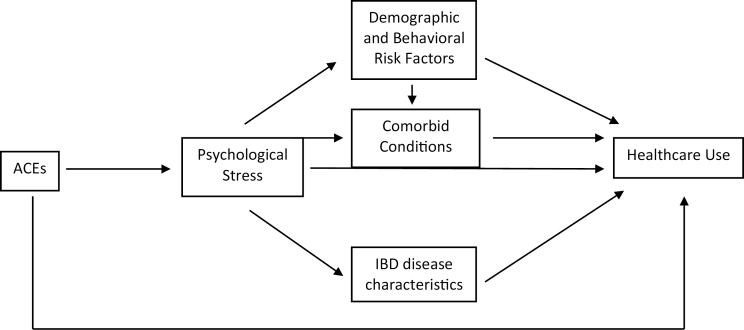
The relationship between ACE and IBD warrants further investigation. It may be beneficial to test if demographic and behavioral risk factors, comorbid conditions, perceived psychological stress, and IBD disease characteristics play a mediating role in the relationship between ACEs and health care use. This is possible using structural equation modelling (SEM), although a larger sample size would be required to test this model (Fig. 2).
Figure 2.
Suggested causal pathway for structural equation modeling.
Moreover, it is possible that exposure to an ACE can serve as an important environmental trigger or risk factor for the development of IBD.33, 40 While we do not have a matched control group for our Manitoba IBD Cohort Study to optimally interpret this finding, the high prevalence of ACEs among this IBD population does provide a rationale for more study in this area, preferably of a prospective nature.
Conflicts of Interest: CB has served on advisory boards of Abbvie Canada, Ferring Canada, Janssen Canada, Napo Pharmaceuticals, Pfizer Canada, Shire Canada, Takeda Canada, and has consulted to 4D Pharma and Mylan Pharmaceuticals. He has received unrestricted educational grants from Abbvie Canada, Janssen Canada, Shire Canada, and Takeda Canada. He has been on the speaker’s bureau of Ferring Canada Medtronic Canada, Takeda Canada, and Shire Canada. No other authors have conflicts to declare.
Supported by: This study was funded in part by a grant from the Canadian Institutes of Health Research. CB is funded in part by the Bingham Chair in Gastroenterology. LL is supported by a Tier I Canada Research Chair.
Author Contribution: KMW, LML, JRW, KAS, and CNB contributed to the study concept and design. KMW, LML, TA, JRW, KAS, and CNB contributed to the analysis and interpretation of data. KMW contributed to the drafting of the manuscript. KMW, LML, TA, JRW, KAS, and CNB contributed to the critical revision of the manuscript for important intellectual content. KMW, LML, and KAS contributed to the statistical analysis. LML, TA, JRW, KAS, ZN, CH, and CNB contributed to the technical or material support.
References
- 1. Anda RF, Butchart A, Felitti VJ, et al. . Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39:93–98. [DOI] [PubMed] [Google Scholar]
- 2. Felitti VJ, Anda RF, Nordenberg D, et al. . Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert LK, Breiding MJ, Merrick MT, et al. . Childhood adversity and adult chronic disease: an update from ten states and the district of Columbia, 2010. Am J Prev Med. 2015;48:345–349. [DOI] [PubMed] [Google Scholar]
- 4. Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med. 2016;50:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anda RF, Croft JB, Felitti VJ, et al. . Adverse childhood experiences and smoking during adolescence and adulthood. Jama. 1999;282:1652–1658. [DOI] [PubMed] [Google Scholar]
- 6. Chapman DP, Whitfield CL, Felitti VJ, et al. . Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. [DOI] [PubMed] [Google Scholar]
- 7. Danese A, Moffitt TE, Harrington H, et al. . Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dube SR, Anda RF, Felitti VJ, et al. . Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the adverse childhood experiences study. JAMA. 2001;286:3089–3096. [DOI] [PubMed] [Google Scholar]
- 9. Dube SR, Felitti VJ, Dong M, et al. . Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. [DOI] [PubMed] [Google Scholar]
- 10. Shonkoff JP, Garner AS; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. [DOI] [PubMed] [Google Scholar]
- 11. Chung MC, Chen ZS. Child abuse and psychiatric co-morbidity among chinese adolescents: emotional processing as mediator and PTSD from past trauma as moderator. Child Psychiatry Hum Dev. 2017;48:610–618. [DOI] [PubMed] [Google Scholar]
- 12. Huh HJ, Kim KH, Lee HK, et al. . The relationship between childhood trauma and the severity of adulthood depression and anxiety symptoms in a clinical sample: the mediating role of cognitive emotion regulation strategies. J Affect Disord. 2017;213:44–50. [DOI] [PubMed] [Google Scholar]
- 13. Schüssler-Fiorenza Rose SM, Xie D, Stineman M. Adverse childhood experiences and disability in U.S. adults. Pm R. 2014;6:670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein MT, Targownik LE, Sexton KA, et al. . Assessing the relationship between sources of stress and symptom changes among persons with IBD over time: a prospective study. Can J Gastroenterol Hepatol. 2016;2016:1681507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drossman DA, Leserman J, Nachman G, et al. . Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. [DOI] [PubMed] [Google Scholar]
- 16. Park SH, Videlock EJ, Shih W, et al. . Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;28:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lix LM, Graff LA, Walker JR, et al. . Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575–1584. [DOI] [PubMed] [Google Scholar]
- 18. Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26:327–332. [DOI] [PubMed] [Google Scholar]
- 19. Scheller-Gilkey G, Moynes K, Cooper I, et al. . Early life stress and PTSD symptoms in patients with comorbid schizophrenia and substance abuse. Schizophr Res. 2004;69:167–174. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. [DOI] [PubMed] [Google Scholar]
- 22. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 23. Clara I, Lix LM, Walker JR, et al. . The manitoba IBD index: evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009;104:1754–1763. [DOI] [PubMed] [Google Scholar]
- 24. Metzler M, Merrick MT, Klevens J, et al. . Adverse childhood experiences and life opportunities: shifting the narrative. Child. Youth Serv. Rev. 2017;72:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDonald S, Kingston D, Bayrampour H, et al. . Adverse childhood experiences in Alberta, Canada: a population based study. Medical Research Archives. 2015;2. ISSN 2375–1924. [Google Scholar]
- 26. Afifi TO, MacMillan HL, Boyle M, et al. . Child abuse and mental disorders in Canada. CMAJ. 2014;186:E324–E332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nurius PS, Green S, Logan-Greene P, et al. . Life course pathways of adverse childhood experiences toward adult psychological well-being: a stress process analysis. Child Abuse Negl. 2015;45:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anda RF, Brown DW, Felitti VJ, et al. . Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health. 2008;8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellis M, Hughes K, Hardcastle K, et al. . The impact of adverse childhood experiences on health service use across the life course using a retrospective cohort study. J Health Serv Res Policy. 2017;22:168–177. [Google Scholar]
- 30. Bonomi AE, Anderson ML, Rivara FP, et al. . Health care utilization and costs associated with childhood abuse. J Gen Intern Med. 2008;23:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koskenvuo K, Koskenvuo M. Childhood adversities predict strongly the use of psychotropic drugs in adulthood: a population-based cohort study of 24,284 finns. J Epidemiol Community Health. 2015;69:354–360. [DOI] [PubMed] [Google Scholar]
- 32. Reiser SJ, McMillan KA, Wright KD, et al. . Adverse childhood experiences and health anxiety in adulthood. Child Abuse Negl. 2014;38:407–413. [DOI] [PubMed] [Google Scholar]
- 33. Bernstein CN, Singh S, Graff LA, et al. . A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 34. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–1118. [DOI] [PubMed] [Google Scholar]
- 35. Finkelhor D. Screening for adverse childhood experiences (aces): cautions and suggestions. Child Abuse Negl. 2018;85:174–179. [DOI] [PubMed] [Google Scholar]
- 36. Kunzendorf S, Jantschek G, Straubinger K, et al. . The luebeck interview for psychosocial screening in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:33–41. [DOI] [PubMed] [Google Scholar]
- 37. Means-Christensen AJ, Sherbourne CD, Roy-Byrne PP, et al. . Using five questions to screen for five common mental disorders in primary care: diagnostic accuracy of the anxiety and depression detector. Gen Hosp Psychiatry. 2006;28:108–118. [DOI] [PubMed] [Google Scholar]
- 38. Mulrow CD, Williams JW Jr, Gerety MB, et al. . Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–921. [DOI] [PubMed] [Google Scholar]
- 39. Denton R, Frogley C, Jackson S, et al. . The assessment of developmental trauma in children and adolescents: a systematic review. Clin Child Psychol Psychiatry. 2017;22:260–287. [DOI] [PubMed] [Google Scholar]
- 40. Bernstein CN. Psychological stress and depression: risk factors for IBD? Dig Dis. 2016;34:58–63. [DOI] [PubMed] [Google Scholar]