Abstract
Cholera toxin B (CTB) is a subunit of cholera toxin, a bacterial enterotoxin secreted by Vibrio cholerae and also functions as an immune adjuvant. However, it remains unclear how CTB activates immune cells. We here evaluated whether or how CTB induces production of a pro-inflammatory cytokine, interleukin-1β (IL-1β). CTB induced IL-1β production not only from bone marrow-derived macrophages (BMMs) but also from resident peritoneal macrophages in synergy with O111:B4-derived lipopolysaccharide (LPS O111:B4) that can bind to CTB. Meanwhile, when prestimulated with O55:B5-derived LPS (LPS O55:B5) that fails to bind to CTB, resident peritoneal macrophages, but not BMMs, produced IL-1β in response to CTB. The CTB-induced IL-1β production in synergy with LPS in both peritoneal macrophages and BMMs was dependent on ganglioside GM1, which is required for internalization of CTB. Notably, not only the NLRP3 inflammasome but also the pyrin inflammasome were involved in CTB-induced IL-1β production from resident peritoneal macrophages, while only the NLRP3 inflammasome was involved in that from BMMs. In response to CTB, a Rho family small GTPase, RhoA, which activates pyrin inflammasome upon various kinds of biochemical modification, increased its phosphorylation at serine-188 in a GM1-dependent manner. This phosphorylation as well as CTB-induced IL-1β productions were dependent on protein kinase A (PKA), indicating critical involvement of PKA-dependent RhoA phosphorylation in CTB-induced IL-1β production. Taken together, these results suggest that CTB, incorporated through GM1, can activate resident peritoneal macrophages to produce IL-1β in synergy with LPS through novel mechanisms in which pyrin as well as NLRP3 inflammasomes are involved.
Keywords: cholera toxin B, interleukin-1β, pyrin inflammasome
CTB activates peritoneal macrophages via a novel inflammasome pathway
Introduction
Cholera toxin (CT) is a bacterial enterotoxin secreted by Vibrio cholerae and causes an acute dehydrating diarrheal disease, called cholera. CT is a holotoxin composed of one A subunit (CTA) and five B subunits (CTB). CTB binds to ganglioside GM1 on the cell surface and facilitates internalization of CT. CTA is then dissociated from CTB in the endoplasmic reticulum (ER) and translocated into the cytosol, where it catalyzes the ADP ribosylation of adenylate cyclase, leading to the increase of intracellular cAMP and diarrhea.
CT is also well known as a potent mucosal adjuvant that can induce pro-inflammatory cytokines production, generation of antigen-specific IgA- or IgG-producing plasma cells and various types of T-cell responses (1–6). Although CT shows more potent immune adjuvanticity than CTB, CTB also functions as an immune adjuvant together with several antigens (7). CTB can induce a variety of pro-inflammatory cytokines such as IL-6 (8). It, however, remains unclear how CTB exerts immune adjuvant effects.
CTB, in synergy with lipopolysaccharide (LPS), can induce production of a pro-inflammatory cytokine, interleukin-1β (IL-1β) (9). However, this induction depends on the serotype of LPS and requires the ability of LPS to bind CTB (10). This means that CTB functions as a chaperone to guide LPS into the cell. Then, intracellular LPS activates non-canonical caspases to induce IL-1β production (11, 12). These mechanisms were characterized in human cell lines or murine bone marrow (BM)-derived macrophages (BMMs), which are generated from in vitro culture of BM cells. Therefore, it is unclear whether or how CTB induces IL-1β production in tissue-resident macrophages.
In this study, we have found that CTB can induce IL-1β secretion from resident peritoneal macrophages in synergy with LPS. This synergistic induction of IL-1β by CTB was observed also with an Escherichia coli serotype, O55:B5-derived LPS (LPS O55:B5), which fails to bind to CTB. Our present results further showed involvement of the pyrin inflammasome as well as the NLRP3 inflammasome in CTB-induced IL-1β production from resident peritoneal macrophages.
Methods
Reagents
LPS O55:B5 (L2637), LPS O111:B4 (L3012), fluorescein isothiocyanate (FITC)-conjugated LPS O55:B5 (F8666) and FITC-conjugated LPS O111:B4 (F3665) were purchased from Sigma-Aldrich.
CTB (choleragenoid, 103B) and Clostridium difficle toxin B (TcdB) (6246-GT-020) were purchased from List Biological Laboratories and R&D Systems, respectively. Adenosine triphosphate (ATP) (tlrl-atp) and R848 were purchased from InvivoGen.
Mice
Eight- to 18-week-old C57BL/6 mice were purchased from CLEA Japan. β1,4-N-acetylgalactosaminyltransferase (β1,4-GalNAc-T)-deficient mice have been described previously and backcrossed more than seven times with C57BL/6 mice (13, 14). An adaptor, apoptosis-associated speck-like protein containing CARD (ASC)- and NLRP3-deficient mice have been described previously (15–17). TLR4-deficient mice were purchased from Oriental BioService. All mice were bred and maintained in the Animal Facility of Wakayama Medical University or Osaka University under specific pathogen-free conditions and were used according to the institutional guidelines of Wakayama Medical University and Osaka University. All animal experiments were approved by the animal research committees and were carried out in accordance with approved guidelines of animal care committees of Wakayama Medical University and Osaka University.
Generation of pyrin-deficient mice
Mice lacking pyrin, encoded by the Mefv gene, were generated by clustered regularly interspaced short palindromic repeats (CRIPSR)/CRISPR-associated protein 9 (Cas9)-mediated genome editing (Supplementary Figure 1). Two guide RNAs (gRNAs), gRNA-A (5′-TCCAGAGCATTCCATGGTGC-3′) and gRNA-B (5′-TGATGTAGAGAAGGGAGTAG-3′), targeting exon2 and the intron between exon2 and exon3 of Mefv, respectively, were synthesized in vitro. These two gRNAs and Cas9 mRNA were co-microinjected into single-cell stage fertilized eggs from B6C3F1 females mated with C57BL/6 males and implanted into a pseudo-pregnant mouse. Founders carrying deletion of Mefv were identified by using PCR with the following primers, Mefv-F (5′-GTGCCCAGCTCCGCAGGAATGTCAGCTCTG-3′) and Mefv-R (5′-CTCAGCCTCCTGTGCTATTACCAGAAGTC-3′), which should yield a 1188-bp product from the wild-type allele. Fourteen out of 29 offsprings showed gross deletion by this PCR and one offspring was confirmed to carry a 753-bp deletion by sequencing. The heterozygous mutant mice backcrossed with C57BL/6 mice three times were crossed to obtain homozygous mutant mice, which were utilized as pyrin-deficient mice.
Cell preparation
Cells were harvested from the peritoneal cavity with phosphate-buffered saline (PBS) and used as resident peritoneal exudate cells (rPECs). To prepare BMMs, BM cells (2 × 105 cells per well) were first cultured with RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) in the presence of 20 ng ml−1 of recombinant mouse macrophage colony-stimulating factor (M-CSF) (R&D Systems) for 4 days. Then, generated cells were used as BMMs.
F4/80+ or F4/80− cells were isolated from rPECs by using magnetic-activated cell sorting (MACS). Briefly, rPECs from at least three pooled mice were blocked with anti-CD16/32 (2.4G2) for 5 min on ice, followed by incubation with biotinylated anti-F4/80 (Cl:A3-1, Serotec) for 20 min on ice. After washing cells were further incubated with streptavidin microbeads (Miltenyi Biotec) for 20 min on ice and then magnetically sorted with LS columns as per the manufacturer’s instructions (Miltenyi Biotec). The flow through cells were collected and used as F4/80− cells. Microbead conjugated cells were collected and used as F4/80+ cells. Both cells were washed by MACS buffer and re-suspended at 2 × 106 cells per ml in RPMI1640 medium.
Measurement of IL-1β production in vitro
For measuring IL-1β production in vitro, 2 × 105 cells per well in 96-well plates were first cultured for 5 h in the absence or presence of LPS O55:B5 or LPS O111:B4. Then CTB, ATP, R848 or TcdB was added at indicated concentrations and further cultured for indicated hours. A pan-caspase inhibitor, Z-VAD-FMK (4800-520, MBL), and a protein kinase A (PKA) inhibitor, H-89 (10010556, Cayman Chemical), were added 3 h before addition of CTB or ATP. Dimethyl sulfoxide (DMSO, Nacalai Tesque) was used as a solvent control for inhibitors. Culture supernatants were subjected to enzyme-linked immunosorbent assay (ELISA) for IL-1β (SMLB00C, R&D Systems). The assays were performed as recommended by the manufacturers.
Analysis for internalization of LPS
For flow cytometry analysis, BMMs or rPECs were first incubated with 5 μg ml−1 FITC-conjugated LPS O55:B5 or FITC-conjugated LPS O111:B4 for 5 h and further cultured for 19 h with or without 20 μg ml−1 CTB. Cells were then harvested, incubated with anti-CD16/32 (93, eBiosciences) to block Fc receptors and stained with biotinylated anti-mouse F4/80 (Cl:A3-1, Serotec), PerCP-Cy5.5-conjugated streptavidin (BD Biosciences) and V450-conjugated anti-CD11b (M1/70, BD Biosciences). Stained cells were analyzed on a FACS Verse and data were processed with FlowJo Version 8.8.7 software (TreeStar).
For confocal microscope analysis, CELLview cell culture dishes (627975, Greiner Bio-One International) were first treated with Cell-Tak solution (Corning) to facilitate the cell adhesion. BMMs were prepared by culturing 1 × 106 BM cells per well in treated CELLview cell culture dishes with RPMI1640 medium supplemented with 10% FBS and 20 ng ml−1 M-CSF for 4 days. F4/80+ rPECs were prepared by MACS and seeded at 4 × 105 cells per well in treated CELLview cell culture dishes. Seeded cells were incubated with 5 μg ml−1 FITC-conjugated LPS O55:B5 or FITC-conjugated LPS O111:B4 for 5 h and further cultured for 19 h with or without 20 μg ml−1 CTB. Then cells were fixed with 4% paraformaldehyde in PBS for 10 min, mounted with Vectashield containing DAPI (H1200, Vector Laboratorys Inc.) and analyzed with a confocal microscope, FV10i (Olympus).
Measurement of IL-1β production in vivo
Mice were injected intra-peritoneally (i.p.) with 100 μg LPS O55:B5 per head. Five hours later, CTB was injected i.p. at 100 μg per head. PBS was used as a control for CTB. Then further 6 h later, peritoneal lavage fluids were harvested and subjected to ELISA for IL-1β.
Quantitative real-time PCR
rPECs (4 × 105 cells) were harvested and total RNA was extracted with RNeasy micro kit (QIAGEN). Total RNAs were reverse transcribed into complementary DNA using PrimeScript RT reagent Kit (TaKaRa). Relative expression levels of RNA transcripts were determined using gene-specific primers, TB Green Premix Ex Taq II (TaKaRa) and the StepOneplus Real-Time PCR system (Applied Biosystems). Gene specific primers were Il1b: 5′-GAAATGCCACCTTTTGACAGTG-3′ and 5′-TGGATGCTCTCATCAGGACAG-3′, B4galnt1: 5′-CTGATAGC TCCCGCCAACTC-3′ and 5′-CTGTTACTTCCCCTGCCACG-3′. TaqMan probes (TaqMan Gene Expression Assay, Applied Biosystems) were used for 18S rRNA (internal control). The expression of all genes was normalized to that of 18S rRNA and is represented as the ratio to the indicated reference samples. All primers were validated for linear amplification.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris–HCl at pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and protease inhibitor cocktail (Roche). Cell lysates or cell culture supernatants were boiled in the SDS sample buffer and subjected to western blot analysis.
Membrane and cytosolic fractions from F4/80+ rPECs were prepared using the Mem-PER Plus Membrane Protein Extraction Kit (89842, Thermo Fisher Scientific). Briefly, cells were harvested and re-suspended with Permeabilization buffer containing a protease inhibitor cocktail (Roche) and incubated for 10 min on ice with constant mixing. After centrifugation at 16 000 × g for 15 min, supernatants were collected as cytosolic fractions. The pellets were re-suspended with 100 μl of Solubilization buffer containing a protease inhibitor cocktail (Roche) and incubated for 30 min on ice with constant mixing. After centrifugation at 16 000 × g for 15 min, supernatants were collected as membrane fractions. The isolated membrane and cytosolic fractions were boiled in the SDS sample buffer and subjected to western blot analysis.
In addition to streptavidin-horseradish peroxidase (HRP) (RPN1231V; 1/250 dilution, GE Healthcare), the following antibodies were used: biotinylated anti-IL-1β antibody (BAF401; 1/500 dilution, R&D Systems), anti-β-actin antibody (C4) (sc-47778; 1/1000 dilution, Santa Cruz Biotechnology), anti-RhoA antibody (26C4) (sc-418; 1/1000 dilution, Santa Cruz Biotechnology), anti-phosphorylation at serine-188 (pS188)-RhoA antibody (ab41435; 1/1000 dilution, Abcam), anti-CD107b antibody (lysosomal-associated membrane protein 2: LAMP2) (M3/84) (108501; 1/1000 dilution, BioLegend), anti-mouse IgG-HRP (NA931V; 1/1000 dilution, GE Healthcare), anti-rabbit IgG-HRP (NA934V; 1/1000 dilution, GE Healthcare) and anti-rat IgG-HRP (NA935V; 1/1000 dilution, GE Healthcare). Western Lightning Plus-ECL (PerkinElmer) was used for positive signals and the chemiluminescence was detected using a Light Capture AE-6971/2 device (ATTO).
Statistical analysis
Data obtained from independent experiments (means ± SD) were analyzed using an unpaired two-tailed Student’s t-test. P-values are indicated by *P < 0.05, **P < 0.01 and ***P < 0.001. P < 0.05 was considered statistically significant.
Results
CTB can induce IL-1β production from rPECs, but not BMMs, prestimulated with LPS O55:B5
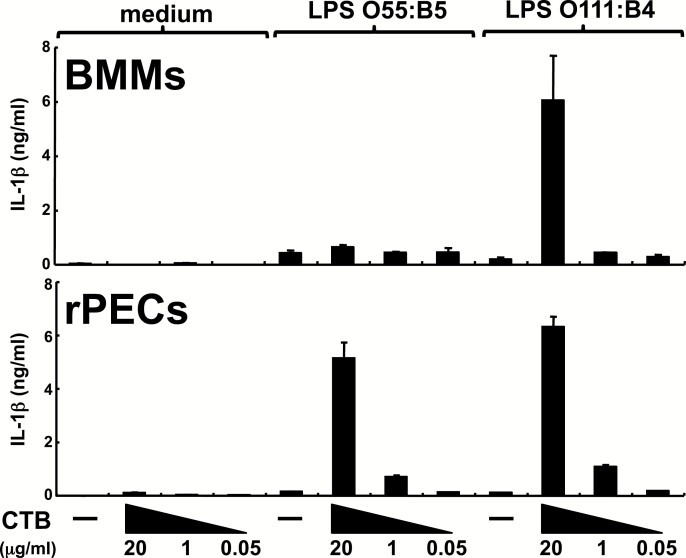
We first examined how CTB activates BMMs to produce IL-1β (Fig. 1). CTB alone or LPS alone failed to induce IL-1β production from BMMs. However, CTB could induce IL-1β production when BMMs were prestimulated with LPS. CTB showed this synergy with LPS from an E. coli serotype, O111:B4, but not from O55:B5. We then analyzed the effects of CTB on rPECs, which include resident macrophages. Similar to BMMs, rPECs produced small amounts of IL-1β in response to CTB alone or LPS alone. However, CTB could induce much higher amounts of IL-1β from rPECs prestimulated not only with LPS O111:B4 but also with LPS O55:B5 (Fig. 1).
Fig. 1.
CTB can induce IL-1β production from rPECs, but not BMMs, prestimulated with LPS O55:B5. rPECs or BMMs from wild-type C57BL/6 mice were first cultured for 5 h in the absence or presence of 500 ng ml−1 LPS O55:B5 or 500 ng ml−1 LPS O111:B4. Then CTB was added at indicated concentrations and further cultured for 19 h. IL-1β production was measured by ELISA. Data are representative of two independent experiments. The results are presented as means ± SD.
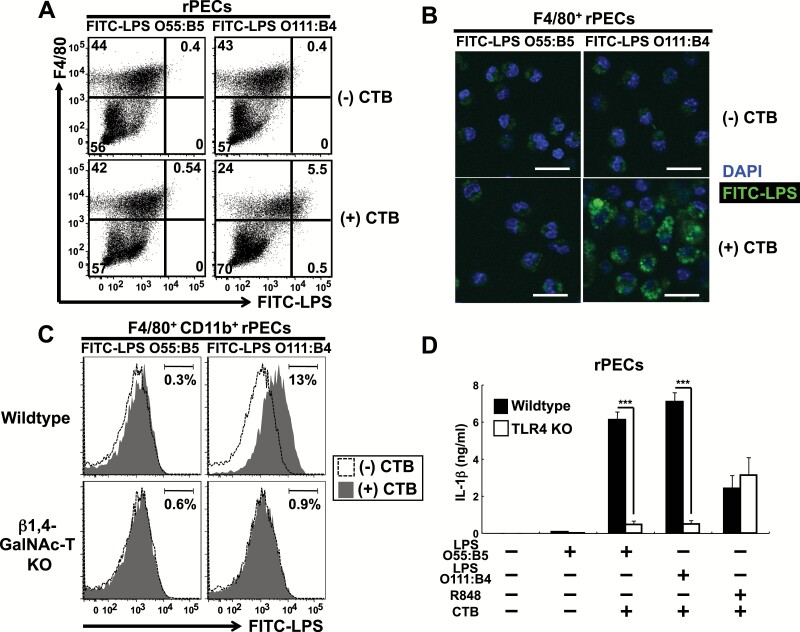
Kayagaki et al. show that CTB can bind to LPS O111:B4, but not to LPS O55:B5 and that CTB functions to incorporate LPS into the cytosol, where LPS activates an intracellular non-canonical inflammasome (10). Consistent with this report, LPS O111:B4, but not LPS O55:B5, was incorporated into the cytosol in the presence of CTB not only in BMMs but also in rPECs (Supplementary Figure 2A and B; Fig. 2A and B). CTB binds to ganglioside GM1 in the cell membrane and is incorporated into the cytosol. CTB-mediated incorporation of LPS O111:B4 in both BMMs and rPECs was abolished in β1,4-GalNAc-T-deficient mice, which lack all complex gangliosides including GM1 (13) (Supplementary Figure 2C; Fig. 2C). Because CTB induced IL-1β production from rPECs pre-treated with LPS O55:B5 that fails to enter the cytosol, a transmembrane LPS sensor, TLR4, should be involved in CTB plus LPS O55:B5-induced IL-1β production in rPECs. Consistent with this, CTB plus LPS O55:B5-induced IL-1β production was severely impaired in TLR4-deficient rPECs (Fig. 2D). Meanwhile, when pre-treated with a TLR7 agonist, R848, TLR4-deficient rPECs showed CTB-induced IL-1β production at comparable levels with wild-type rPECs (Fig. 2D). Thus, these results indicate differential responses of BMMs and rPECs to CTB plus LPS and that CTB could induce IL-1β production from rPECs prestimulated with LPS O55:B5 that fails to bind to CTB and enter into the cytosol.
Fig. 2.
CTB induces IL-1β production from rPECs prestimulated with LPS O55:B5, which fails to enter the cytosol of peritoneal macrophages, in a TLR4-dependent manner. (A–C) Assessment of LPS internalization in rPECs. rPECs from wild-type (A) and wild-type and β1,4-GalNAc-T-deficient (C) mice were first cultured with 5 μg ml−1 FITC-conjugated LPS O55:B5 or FITC-conjugated LPS O111:B4 for 5 h and further cultured for 19 h with or without 20 μg ml−1 CTB. Cells were then harvested, stained and subjected to flow cytometry analysis. In (A), dot plots of F4/80 versus FITC-LPS from wild-type rPECs are shown. Numbers indicate percentages of gated cells among total cells. Data are representative of three independent experiments. In (C), histograms of FITC-LPS from wild-type and β1,4-GalNAc-T-deficient F4/80+ CD11b+ cells are shown. Shaded and open histograms indicate data from cells cultured with and without CTB, respectively. Numbers indicate the percentages of gated cells among total cells. Data are representative of three independent experiments. In (B), F4/80+ rPECs cultured with or without CTB were subjected to confocal microscopic analysis. Green and blue colors represent LPS internalization and DAPI staining, respectively. The scale bars represent 20 μm. Data are representative of two independent experiments. (D) rPECs from wild-type C57BL/6 or TLR4-deficient mice were first cultured for 5 h in the absence or presence of 500 ng ml−1 LPS O55:B5, 500 ng ml−1 LPS O111:B4 or 100 nM R848. Then 20 μg ml−1 CTB was added and further cultured for 19 h. IL-1β production was measured by ELISA. Data are representative of two independent experiments. The results are presented as means ± SD. ***P < 0.001.
CTB induces IL-1β production in synergy with LPS in a GM1-dependent manner
We next investigated whether GM1 is necessary for CTB-induced IL-1β production from LPS O55:B5-prestimulated rPECs. In the presence of LPS O55:B5, ATP could induce comparable amounts of IL-1β in both wild-type and β1,4-GalNAc-T-deficient rPECs (Supplementary Figure 3A). However, CTB-induced IL-1β production was abolished in LPS O55:B5-prestimulated β1,4-GalNAc-T-deficient rPECs (Supplementary Figure 3A). We also analyzed CTB-induced IL-1β production from LPS O111:B4-prestimulated BMMs (Supplementary Figure 3B). CTB-, but not ATP-induced IL-1β production was abolished in LPS O111:B4-prestimulated β1,4-GalNAc-T-deficient BMMs (Supplementary Figure 3B). These results indicate that GM1 is essential for CTB-induced IL-1β production from rPECs or BMMs prestimulated with LPS.
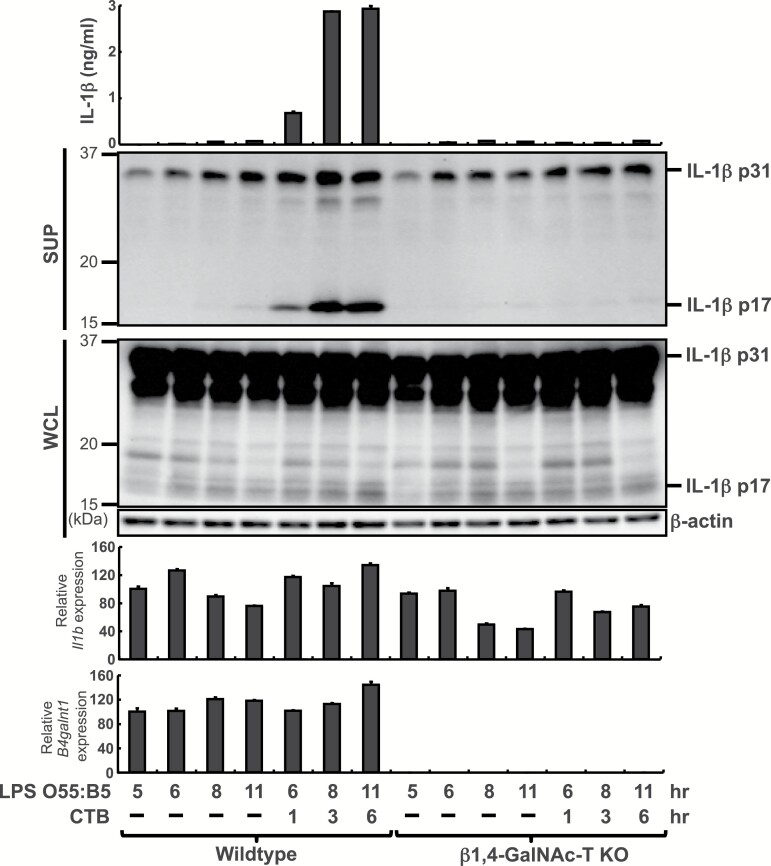
Next, we investigated how CTB augments IL-1β production from rPECs (Fig. 3). LPS O55:B5 alone did not induce secretion of mature IL-1β (p17), but further addition of CTB for 1–6 h increased the secretion. Meanwhile, CTB did not enhance Il1b transcription or amounts of pro-IL-1β (p31) protein in LPS O55:B5-prestimulated rPECs. Thus, CTB promoted processing of pro-IL-1β (p31) in LPS O55:B5-prestimulated rPECs. Generation of mature IL-1β (p17) after the addition of CTB was abolished, but both Il1b transcription and amounts of pro-IL-1β (p31) protein were retained in β1,4-GalNAc-T-deficient rPECs (Fig. 3), indicating that CTB can induce IL-1β processing in a GM1-dependent manner.
Fig. 3.
CTB induces IL-1β production from LPS O55:B5-prestimulated rPECs in a GM1-dependent manner. rPECs from wild-type or β1,4-GalNAc-T-deficient mice were first cultured for 5 h in the presence of 500 ng ml−1 LPS O55:B5. Then 20 μg ml−1 CTB was added and further cultured for indicated hours. IL-1β production was measured by ELISA. Whole cells lysate (WCL) and culture supernatants (SUP) were subjected to western blot analysis with anti-IL-1β or anti-β-actin antibodies. RNAs were subjected to quantitative real-time PCR analysis for Il1b and B4galnt1. Data are representative of at least two independent experiments. The results are presented as means ± SD.
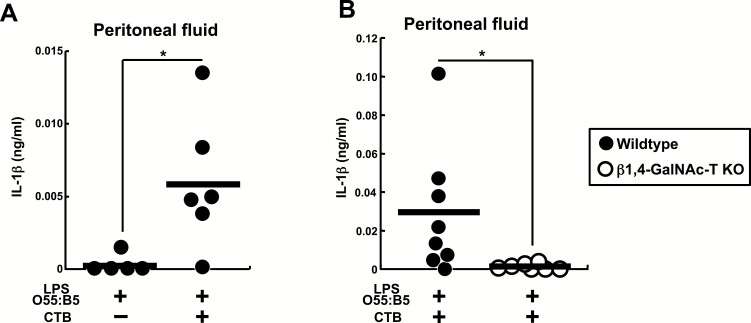
We further investigated whether CTB plus LPS O55:B5 augments in vivo IL-1β production. Injection of LPS O55:B5 did not induce peritoneal IL-1β. However, sequential injection of CTB after LPS O55:B5 significantly increased IL-1β in the peritoneal lavage fluids (Fig. 4A). This peritoneal IL-1β induction was abolished in β1,4-GalNAc-T-deficient mice (Fig. 4B). Thus, the results indicate that CTB can induce in vivo production of IL-1β in the presence of LPS O55:B5 in a GM1-dependent manner.
Fig. 4.
CTB induces in vivo IL-1β production in synergy with LPS O55:B5. (A) Wild-type C57BL/6 mice were first injected i.p. with 100 μg per head of LPS O55:B5. Five hours later PBS or 100 μg per head of CTB was injected i.p. Then after a further 6 h, peritoneal lavage fluids were harvested and subjected to ELISA for IL-1β. (B) Wild-type or β1,4-GalNAc-T-deficient mice were injected i.p. with LPS and CTB as in (A) and peritoneal lavage fluids were harvested and subjected to ELISA for IL-1β. (A and B) Each symbol represents the data from one mouse and the bars indicate the means. The results are presented as means ± SD. *P < 0.05.
CTB-induced IL-1β production from LPS-prestimulated peritoneal macrophages depends on pyrin as well as NLRP3 inflammasomes
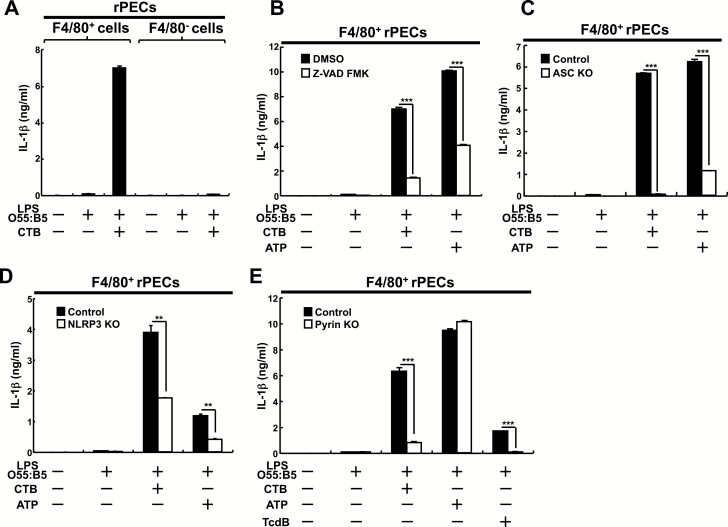
rPECs are heterogeneous and about 20% of them are macrophages, which can be defined by surface expression of F4/80. We then separated rPECs into F4/80+ and F4/80− cells (Supplementary Figure 4) and analyzed the effects of CTB on those cells (Fig. 5A). CTB could induce IL-1β production from peritoneal F4/80+, but not F4/80− cells prestimulated with LPS O55:B5. The results indicate that F4/80+, but not F4/80− cells are responsible for CTB-induced IL-1β production from rPECs prestimulated with LPS O55:B5.
Fig. 5.
CTB-induced IL-1β production from LPS O55:B5-prestimulated peritoneal macrophages depends on pyrin as well as NLRP3 inflammasomes. (A and B) F4/80+ or F4/80− rPECs from wild-type C57BL/6 mice were prepared by MACS and cultured for 5 h in the absence or presence of 500 ng ml−1 LPS O55:B5. Then 20 μg ml−1 CTB or 1 mM ATP (B) was added and further cultured for 19 h. In (B), pan-caspase inhibitor Z-VAD-FMK (10 μM) or DMSO as control was added 3 h before addition of CTB or ATP. Data are representative of two independent experiments. (C–E) MACS-purified F4/80+ rPECs from control or mutant mice lacking ASC (C), NLRP3 (D) or pyrin (E) were cultured for 5 h in the absence or presence of 500 ng ml−1 LPS O55:B5. Then 20 μg ml−1 CTB, 1 mM ATP or 5 μg ml−1 TcdB (E) was added and further cultured for 19 h. As control mice, littermate ASC heterozygous mutant (C), littermate NLRP3 heterozygous mutant (D) or littermate wild-type mice (E) were used. IL-1β production was measured by ELISA. Data are representative of at least three independent experiments. The results are presented as means ± SD. **P < 0.01, ***P < 0.001.
Caspases are generally involved in IL-1β production. To clarify whether caspases are required for CTB-induced IL-1β production, we have investigated the effect of a pan-caspase inhibitor, Z-VAD-FMK. In the presence of Z-VAD-FMK, both CTB and ATP-induced IL-1β production were impaired in F4/80+ rPECs (Fig. 5B). Activation of caspases depends on an inflammasome complex, which comprises a sensor, a protease precursor, pro-caspase-1, and an adaptor, ASC (18). The sensors include nucleotide-binding oligomerization domain-like receptors (NLRs) such as NLRP3, absent in melanoma 2 (AIM2) or pyrin (19–21). We then investigated which inflammasome is involved in CTB-induced IL-1β production from F4/80+ rPECs. CTB-induced IL-1β production was abolished in ASC-deficient F4/80+ rPECs and significantly impaired, although almost half was retained, in NLRP3-deficient F4/80+ rPECs (Fig. 5C and D). Meanwhile, ATP-induced IL-1β production, which depends on the NLRP3 inflammasome (16), was severely impaired in both ASC- and NLRP3-deficient F4/80+ rPECs (Fig. 5C and D). These results indicate that CTB-induced IL-1β production from LPS O55:B5-prestimulated F4/80+ rPECs requires an NLRP3-independent pathway.
Then we analyzed F4/80+ rPECs from mutant mice lacking pyrin, which is required for IL-1β production induced by TcdB or Clostridium botulinum ADP-ribosylating C3 toxin (22). Although ATP-induced IL-1β production was retained, TcdB-induced IL-1β production was abolished and CTB-induced IL-1β induction was severely impaired in LPS O55:B5-prestimulated pyrin-deficient F4/80+ rPECs (Fig. 5E). These results indicate that pyrin as well as the NLRP3 inflammasome is involved in CTB-induced IL-1β production from LPS O55:B5-prestimulated F4/80+ rPECs.
CTB-induced IL-1β production from LPS-prestimulated BMMs depends on NLRP3, but not on pyrin inflammasomes
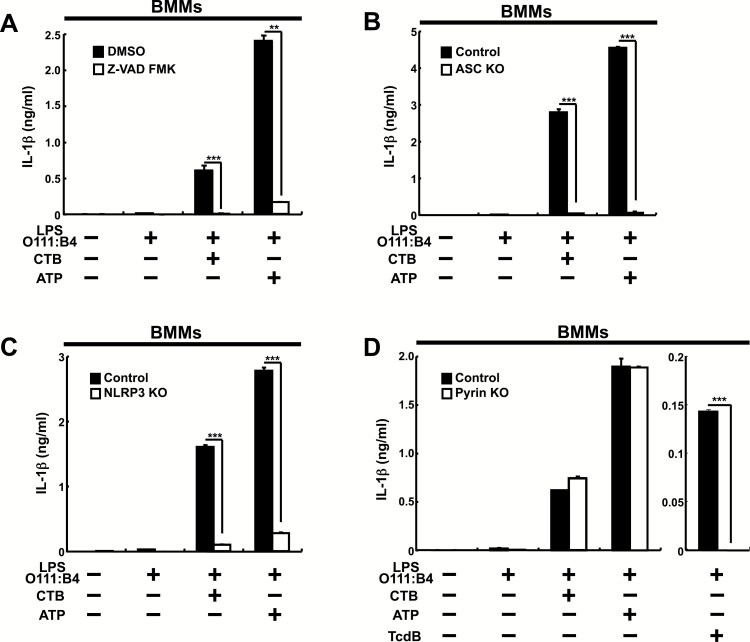
We then analyzed how inflammasomes are involved in IL-1β production from LPS O111:B4-prestimulated BMMs (Fig. 6). In the presence of Z-VAD-FMK, both CTB and ATP-induced IL-1β production were impaired in BMMs (Fig. 6A). We then investigated which inflammasome is involved in CTB-induced IL-1β production from BMMs (Fig. 6B–D). Both CTB and ATP-induced IL-1β production were abolished in both ASC- and NLRP3-deficient BMMs, but retained in pyrin-deficient BMMs, which did not produce IL-1β in response to TcdB. The results indicate that NLRP3, but not pyrin is indispensable for both CTB- and ATP-induced IL-1β production, consistent with the previous finding (9) and that CTB induces IL-1β production in a cell type-specific manner.
Fig. 6.
CTB-induced IL-1β production from LPS O111:B4-prestimulated BMMs depends on NLRP3, but not on pyrin inflammasome. (A) BMMs from wild-type C57BL/6 mice were cultured as described in Fig. 5(B) legend. (B–D) BMMs from control or mutant mice lacking ASC (B), NLRP3 (C) or pyrin (D) were cultured as described in Fig. 5(C–E) legends. LPS O111:B4 instead of LPS O55:B5 was used. IL-1β production was measured by ELISA. Data are representative of at least three independent experiments. The results are presented as means ± SD. **P < 0.01, ***P < 0.001.
CTB increases pS188 of RhoA in the cytosol of peritoneal macrophages
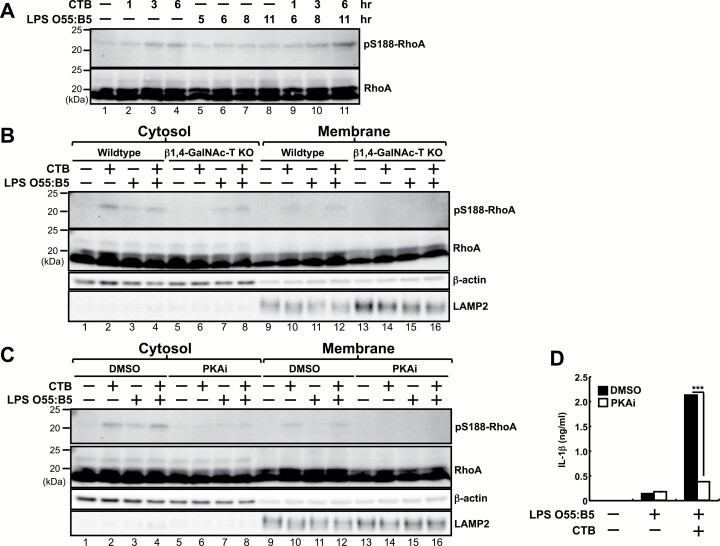
In unstimulated cells, a Rho family GTPase, RhoA, is localized with GTP as an active form in the plasma membrane. Upon stimulation with pyrin inflammasome activators such as TcdB or C3 toxin, RhoA is inactivated by various kinds of biochemical modification such as deamidation, glucosylation, adenylylation or ADP-ribosylation on various residues of RhoA, leading to activation of the pyrin inflammasome (22–24). RhoA is also inactivated, in response to an increase of intracellular cAMP, by PKA-dependent pS188 (25–27). Then this phosphorylation leads to translocation of RhoA from the membrane to the cytosol and subsequent activation of the pyrin inflammasome (24).
We then examined how RhoA is modified by CTB in resident peritoneal macrophages. RhoA protein levels were not increased or decreased in response to CTB in the presence or absence of LPS O55:B5 (Fig. 7A). Meanwhile, pS188 of RhoA was significantly increased at 1–6 h in response to CTB (Fig. 7A, lanes 2–4; Supplementary Figure 5). LPS O55:B5 slightly induced pS188 of RhoA (Fig. 7A, lanes 5–8; Supplementary Figure 5) and further stimulation with CTB enhanced the phosphorylation (Fig. 7A, lanes 9–11; Supplementary Figure 5). We then analyzed subcellular fractions of wild-type and β1,4-GalNAc-T-deficient peritoneal macrophages. In wild-type peritoneal macrophages, CTB alone increased pS188 of RhoA in the cytosol (Fig. 7B, lane 2; Supplementary Figure 5) more prominently than in the membrane (Fig. 7B, lane 10; Supplementary Figure 5). This CTB-induced pS188 of RhoA in the cytosol was abolished in β1,4-GalNAc-T-deficient peritoneal macrophages (Fig. 7B, lane 6; Supplementary Figure 5). Meanwhile, LPS O55:B5 alone-induced pS188 of RhoA in the cytosol was comparable between wild-type and β1,4-GalNAc-T-deficient peritoneal macrophages (Fig. 7B, lanes 3 and 7; Supplementary Figure 5).
Fig. 7.
CTB increases pS188 of RhoA in the cytosol of peritoneal macrophages. (A–D) F4/80+ rPECs from wild-type C57BL/6 (A-D) or β1,4-GalNAc-T-deficient (B) mice were first cultured for 5 h in the absence or presence of 500 ng ml−1 LPS O55:B5. Then 20 μg ml−1 CTB was added and further cultured for indicated (A) or 6 (B–D) h. In (C) and (D), cells were treated with a PKA inhibitor (PKAi), H-89 (40 μM) or DMSO as control. Whole cell lysates (A) or cell fractions (B and C) were subjected to western blot analysis for antibodies against pS188-RhoA, RhoA, β-actin or LAMP2. β-actin and LAMP2 were used as the cytosol and membrane markers, respectively. IL-1β production was measured by ELISA (D). Data are representative of two independent experiments. The results are presented as means ± SD. ***P < 0.001.
We then investigated the effect of a PKA inhibitor, H-89, on pS188 of RhoA. In the presence of H-89, both CTB- and LPS O55:B5-induced pS188 of RhoA in the cytosol were severely impaired (Fig. 7C, lanes 6–8; Supplementary Figure 5). CTB-induced IL-1β production from LPS O55:B5-prestimulated peritoneal macrophages was also inhibited by H-89, although small, but significant, amounts of IL-1β production induced by LPS O55:B5 alone were not inhibited (Fig. 7D). These results suggest that CTB, incorporated by GM1, can induce pS188 of RhoA through PKA and trigger activation of the pyrin inflammasome, leading to IL-1β production in LPS O55:B5-prestimulated peritoneal macrophages.
Discussion
In contrast to BMMs, CTB could induce IL-1β production from resident peritoneal macrophages prestimulated with LPS O55:B5, which is not incorporated into the cytosol (Fig. 2B). The IL-1β production depended on internalization of CTB, because it was abolished in the absence of ganglioside GM1, which is required for CTB to translocate into the cells. The CTB-induced IL-1β production depended not only on the NLRP3 but also on the pyrin inflammasome.
It so far remained unclear whether CTB itself is involved in IL-1β production. CTB could induce IL-1β production from LPS-prestimulated BMMs. However, CTB exerts this synergy only with LPS O111:B4 that can bind to CTB and is incorporated into the cytosol (Supplementary Figure 2B), indicating that CTB functions as a chaperone to escort LPS into cells. Our present results are consistent with previous findings on BMMs, showing the selective synergy of CTB with LPS O111:B4 (10) and involvement of ASC and NLRP3 in CTB-induced IL-1β production (9). In this study, we have first characterized how resident peritoneal macrophages respond to CTB and revealed that CTB induces IL-1β production in the presence of LPS in a cell type-dependent manner. In both peritoneal macrophages and BMMs, CTB-induced IL-1β production in the presence of LPS required GM1 (Fig. 3; Supplementary Figure 3). Therefore, responses to CTB should be differentiated at the downstream of CTB internalization. Gene expression of pyrin was more than 10 times higher in BMMs than in rPECs (Supplementary Figure 6). So the pyrin expression level alone cannot account for differential involvement of the pyrin inflammasome pathway between BMMs and rPECs. TcdB-induced IL-1β production was lower in BMMs than in resident peritoneal macrophages (Figs 5E and 6D), indicating that pyrin inflammasome activity might be low or repressed in BMMs, although this possibility should be further examined. At present, the detailed molecular mechanisms by which resident peritoneal macrophages and BMMs differentially respond to CTB remain unclear.
It should be noted that CTB was identified as a novel activator for the pyrin inflammasome. So far bacterial toxins such as TcdB and C3 toxins and a Yersinia outer protein T (YopT) are known to activate the pyrin inflammasome. These substances first inactivate a GTPase, RhoA through various modifications. TcdB and C3 toxins induce glucosylation and ADP-ribosylation in the switch I region of RhoA, respectively (22, 23). YopT, which functions as a protease, cleaves the carboxyl terminus of RhoA. Then inactivated or cleaved RhoA leads to activation of the pyrin inflammasome (28–30). In resident peritoneal macrophages, upon stimulation with CTB, pS188 of RhoA was increased in the cytosol in resident peritoneal macrophages in a GM1-dependent manner (Fig. 7). Previous reports showed that RhoA is phosphorylated at this serine by cAMP through PKA and translocated from the membrane to the cytosol (25, 27), thereby potentiating IL-1β production from murine cells harboring the mutant pyrin with constitutive activation (24). Not only CTB-induced pS188 of RhoA but also CTB-induced IL-1β production were abolished by a PKA inhibitor, suggesting that pS188 of RhoA be involved in CTB-induced activation of the pyrin inflammasome.
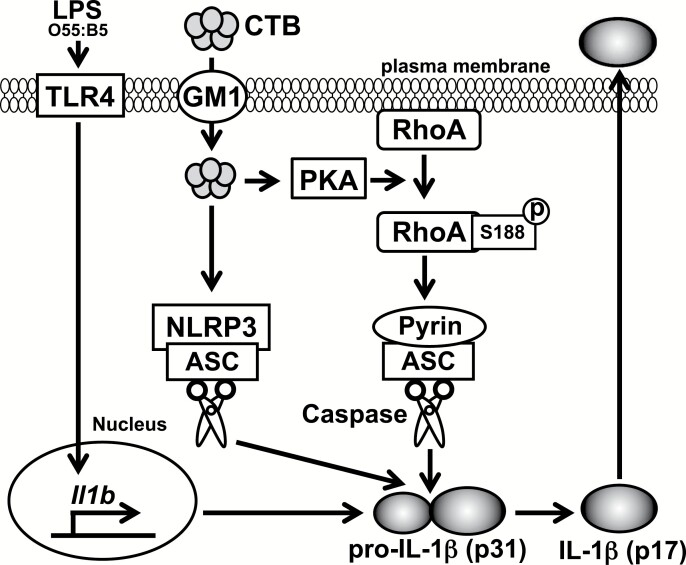
Taking our present results together, molecular mechanisms for CTB-induced IL-1β production in peritoneal macrophages can be speculated upon as follows (Fig. 8). First, LPS induces expression of Il1b through TLR4. It can be assumed that CTB can induce IL-1β production from rPECs in synergy with any stimuli that can induce expression of Il1b. Consistent with this, in addition to LPS, a TLR7 agonist, R848, a TLR2 agonist, Pam3CSK4 and a TLR3 and RIG-I-like receptor (RLR) agonist, polyinosinic:polycytidylic acids [poly(I:C)], induced IL-1β production from rPECs in synergy with CTB (Fig. 2D; Supplementary Figure 7). CTB is internalized through GM1 and induces pS188 of RhoA through PKA, thereby activating the pyrin inflammasome. In parallel with or subsequently to pyrin inflammasome activation, the NLRP3 inflammasome is also activated. Finally, activation of these inflammasomes leads to production of IL-1β. It requires further study to clarify whether CTB also induces other types of biochemical modification of RhoA or whether pS188 of RhoA is solely responsible for CTB-induced activation of the pyrin inflammasome.
Fig. 8.
A hypothetical model of CTB-induced IL-1β production in peritoneal macrophages.
IL-1β induction is often accompanied by cell death such as pyroptosis. CTB induced cell death in both F4/80+ and F4/80− rPECs prestimulated with LPS O55:B5 (Supplementary Figure 8A and B). The cell death was abolished in β1,4-GalNAc-T-deficient rPECs (Supplementary Figure 8A and B). ATP induced cell death more prominently than CTB in both F4/80+ and F4/80− rPECs prestimulated with LPS O55:B5 and the cell death was independent of GM1 (Supplementary Figure 8A and B). Thus, CTB induced weak, but significant levels of cell death in rPECs after its internalization through GM1. Further studies are necessary to examine whether or how the cell death contributes to CTB-induced IL-1β production.
We here clarified novel mechanisms for CTB-induced IL-1β production in resident peritoneal macrophages. Our findings should contribute to further understanding of the mechanisms and making the best use of CTB-induced immune adjuvant effects.
Funding
This work was supported by Grants-in-Aids for Scientific Research (B) (grant 17H04088 and 26293106 to T.K.), for Scientific Research (C) (grant 15K08431 and 18K07071 to H.H.), for Scientific Research on Innovative Areas (grant 17H05799 to T.K.), for Exploratory Research (grant 17K19568 to T.K.), for Young Scientists (B) (grant 15K19077 and 25870401 to I.S., grant 16K19585 to Y.F.-O.) and for JSPS fellows (to T.Oh.) from the Japan Society for the Promotion of Science, the Uehara Memorial Foundation (to T.K. and I.S.), Takeda Science Foundation (to T.K., H.H. and I.S.), AMED under grant number JP19ek0109199 (to T.K. and H.H.), the Ichiro Kanehara Foundation for the promotion of Medical Sciences and Medical care (to H.H.), the Inamori Foundation (to I.S.) and 2017 Wakayama Medical Award for Young Researchers (to I.S.). This work was also supported in part by the Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University, a Cooperative Research Grant from the Institute for Enzyme Research, Joint Usage/Research Center, Tokushima University, the Grant for Joint Research Program of the Institute for Genetic Medicine Hokkaido University, the Grant for Joint Research Project of the Institute of Medical Science, the University of Tokyo and Research Grant on Priority Areas from Wakayama Medical University.
Supplementary Material
Acknowledgements
We thank Ms. Aoi Tawaki-Matsumura and Ms. Akane Nishiwaki for secretarial assistance, Ms. Ikuko Hattori, Ms. Naoko Nishiyama and Ms. Chihiro Nakai for technical assistance. T.Or., I.S., H.H., T.Oz., Y.F.-O., T.Oh., M.M., M.K. and T.Y. performed the experiments and analyzed the data; Y.S., T.T., K.H., K.K., S.F., K.M., M.Y. and T.S. provided technical support and assisted in setting up the experiments and analyzing the data; K.F. provided β1,4-GalNAc-T-deficient mice and assisted in analyzing the data; E.K. and K.J.I. provided the NLRP3- and ASC-deficient mice; T.Or., I.S., K.T. and T.K. designed the study and wrote the manuscript; K.T. and T.K. supervised the research. All authors participated in discussion of the results, critically polished and approved the final draft.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Elson C. O. and Ealding W. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736. [PubMed] [Google Scholar]
- 2. Xu-Amano J.,, Kiyono H.,, Jackson R. J., et al. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marinaro M.,, Staats H. F.,, Hiroi T., et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621. [PubMed] [Google Scholar]
- 4. Wakabayashi A.,Nakagawa Y.,Shimizu M.,Moriya K.,Nishiyama Y. and Takahashi H. 2008. Suppression of an already established tumor growing through activated mucosal CTLs induced by oral administration of tumor antigen with cholera toxin. J. Immunol. 180:4000. [DOI] [PubMed] [Google Scholar]
- 5. Yuki Y.,, Tokuhara D.,, Nochi T., et al. 2009. Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine 27:5982. [DOI] [PubMed] [Google Scholar]
- 6. Datta S. K., Sabet M., Nguyen K. P.et al. 2010. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl Acad. Sci. USA 107:10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiedinger K.,Pinho D. and Bitsaktsis C. 2017. Utilization of cholera toxin B as a mucosal adjuvant elicits antibody-mediated protection against S. pneumoniae infection in mice. Ther. Adv. Vaccines 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagatomo D.,, Taniai M.,, Ariyasu H., et al. 2015. Cholesteryl pullulan encapsulated TNF-α nanoparticles are an effective mucosal vaccine adjuvant against influenza virus. Biomed. Res. Int. 2015:471468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kayagaki N.,, Warming S.,, Lamkanfi M., et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117. [DOI] [PubMed] [Google Scholar]
- 10. Kayagaki N.,, Wong M. T.,, Stowe I. B., et al. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246. [DOI] [PubMed] [Google Scholar]
- 11. Hagar J. A.,Powell D. A.,Aachoui Y.,Ernst R. K. and Miao E. A. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi J.,, Zhao Y.,, Wang Y., et al. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187. [DOI] [PubMed] [Google Scholar]
- 13. Takamiya K., Yamamoto A., Furukawa K.et al. 1996. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl Acad. Sci. USA 93:10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawamura Y. I.,, Kawashima R.,, Shirai Y., et al. 2003. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur. J. Immunol. 33:3205. [DOI] [PubMed] [Google Scholar]
- 15. Mariathasan S.,, Newton K.,, Monack D. M., et al. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213. [DOI] [PubMed] [Google Scholar]
- 16. Mariathasan S.,, Weiss D. S.,, Newton K., et al. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228. [DOI] [PubMed] [Google Scholar]
- 17. Kuroda E.,, Ozasa K.,, Temizoz B., et al. 2016. Inhaled fine particles induce alveolar macrophage death and interleukin-1α release to promote inducible bronchus-associated lymphoid tissue formation. Immunity 45:1299. [DOI] [PubMed] [Google Scholar]
- 18. Latz E.,Xiao T. S. and Stutz A. 2013. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Man S. M. and Kanneganti T. D. 2016. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma D. and Kanneganti T. D. 2016. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J. Cell Biol. 213:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Torre-Minguela C.,Mesa Del Castillo P. and Pelegrín P. 2017. The NLRP3 and pyrin inflammasomes: implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu H.,, Yang J.,, Gao W., et al. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 513:237. [DOI] [PubMed] [Google Scholar]
- 23. Visvikis O.,Maddugoda M. P. and Lemichez E. 2010. Direct modifications of Rho proteins: deconstructing GTPase regulation. Biol. Cell 102:377. [DOI] [PubMed] [Google Scholar]
- 24. Park Y. H.,Wood G.,Kastner D. L. and Chae J. J. 2016. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 17:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang P.,Gesbert F.,Delespine-Carmagnat M.,Stancou R.,Pouchelet M. and Bertoglio J. 1996. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 15:510. [PMC free article] [PubMed] [Google Scholar]
- 26. Tamma G.,Klussmann E.,Procino G.,Svelto M.,Rosenthal W. and Valenti G. 2003. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J. Cell Sci. 116:1519. [DOI] [PubMed] [Google Scholar]
- 27. Aburima A.,Wraith K. S.,Raslan Z.,Law R.,Magwenzi S. and Naseem K. M. 2013. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood 122:3533. [DOI] [PubMed] [Google Scholar]
- 28. Chung L. K.,, Park Y. H.,, Zheng Y., et al. 2016. The Yersinia virulence factor YopM hijacks host kinases to inhibit type III effector-triggered activation of the pyrin inflammasome. Cell Host Microbe 20:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ratner D.,, Orning M. P.,, Proulx M. K., et al. 2016. The Yersinia pestis effector YopM inhibits pyrin inflammasome activation. PLoS Pathog. 12:e1006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heilig R. and Broz P. 2018. Function and mechanism of the pyrin inflammasome. Eur. J. Immunol. 48:230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.