Abstract
Although bariatric surgery was developed primarily to treat morbid obesity, evidence from the earliest clinical observations to the most recent clinical trials consistently demonstrates that these procedures have substantial effects on glucose metabolism. A large base of research indicates that bariatric surgeries such as Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), and biliopancreatic diversion (BPD) improve diabetes in most patients, with effects frequently evident prior to substantial weight reduction. There is now unequivocal evidence from randomized controlled trials that the efficacy of surgery is superior to intensive life-style/medical management. Despite advances in the clinical understanding and application of bariatric surgery, there remains only limited knowledge of the mechanisms by which these procedures confer such large changes to metabolic physiology. The improvement of insulin sensitivity that occurs with weight loss (e.g., the result of diet, illness, physical training) also accompanies bariatric surgery. However, there is evidence to support specific effects of surgery on insulin clearance, hepatic glucose production, and islet function. Understanding the mechanisms by which surgery affects these parameters of glucose regulation has the potential to identify new targets for therapeutic discovery. Studies to distinguish among bariatric surgeries on key parameters of glucose metabolism are limited but would be of considerable value to assist clinicians in selecting specific procedures and investigators in delineating the resulting physiology. This review is based on literature related to factors governing glucose metabolism and insulin secretion after the commonly used RYGB and VSG, and the less frequently used BPD and adjustable gastric banding.
Essential Points
Current bariatric procedures such as Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), biliopancreatic diversion (BPD), and adjustable gastric band (AGB) modify the gastrointestinal tract in distinct ways, but all approaches improve type 2 diabetes mellitus (T2DM) in most patients
Reduction of hyperglycemia following surgery is superior to medical treatment and/or caloric restriction; many patients with diabetes achieve remission after surgery, but this effect wanes over time with a gradual return of diabetes in some patients
There is evidence for weight independent improvement in glucose control after RYGB, VSG, and BPD, which increase as weight-loss progresses; AGB improves diabetes in proportion to weight loss, similar to dietary restriction, with smaller effects than the other procedures
The effects of surgery to improve insulin secretion are readily apparent in patients with T2DM but can also be detected in nondiabetic persons when insulin sensitivity is accounted for
Stimulation of islet β-cells by enteral signals is increased after bariatric surgery; postprandial glucagon-like peptide 1 secretion is increased after RYGB or VSG and enhances insulin secretion
Preclinical data using mouse genetic models to determine mechanisms of surgery to improve glucose metabolism have not yet identified definitive factors or pathways; in total, this work suggests that the physiologic response to surgery is complex and multifactorial
This review seeks to synthesize available data regarding the effect of bariatric surgery–improved glucose control, as well as the role for surgery to enhance islet function and insulin secretion. The sources cited for this review were selected from among the enormous body of research on this topic based on the author’s view of their relevance and did not conform to a predefined search strategy. Although an attempt is made to emphasize the role of the islet β-cell, current data are often not sufficient to distinguish effects of insulin secretion from other mechanisms affecting glucose regulation. A general description of RYGB, VSG, BPD, or AGB, the principal procedures now used in clinical practice, is provided to assist the reader in interpretation of physiologic data, but the nuances of surgical technique and modifications of these and other procedures are outside the scope of this paper. Although there are few direct comparisons among this group of four surgical procedures, some inferences from the correlation of anatomy to physiology are made to raise questions and hypotheses. Data from human studies are emphasized, but a succinct description of preclinical research is included.
Overview
Bariatric surgery in the age of the diabetes epidemic
The US rate of overweight/obesity was ∼17% in 1976, grew to ∼38% in 2010, and, if current trends hold, is projected to reach upwards of 75% by 2050 (1). The prevalence of diabetes in the United States was 2.5% in 1975 (2), rose to 14% in 2014, and is projected to crest >20% by 2050 (3–5). Although there are many etiologic factors for diabetes, it seems clear that increasing rates of overweight and obesity are a major contributor to the epidemic rise in prevalence that has occurred during the past four decades. The estimated cost of treating obesity and diabetes in the United States rose from $212 billion in 2005 to $327 billion in 2017 (3), and it will invariably grow further given that adverse medical outcomes and health care costs increase exponentially with body mass index (BMI) (6). These costs are driven in great part by the fact that obesity and diabetes are largely incurable at present and must be managed as chronic diseases in most patients.
Multiple randomized clinical trials demonstrate that intensive dietary, behavioral, and/or exercise interventions result in ≤15% of patients with obesity achieving and maintaining long-term body weight goals (7). Similarly, intensive medical and behavioral measures lead to only a small minority of patients with type 2 diabetes mellitus (T2DM) reaching and holding glycated Hb (HbA1c) targets (8–12). In contrast, there are now several clinical trials demonstrating that, depending on surgical intervention and postoperative time course, between 25% and 95% of patients receiving bariatric surgery achieve substantial, long-term body weight reduction (8–11). Moreover, improvement in glucose control among patients with diabetes having surgery is almost uniform, with nearly half maintaining nondiabetic glycated Hb (HbA1c) without requiring diabetes medications. Notably, there has been an accumulation of evidence that some of the improvements in glucose regulation occur soon after surgery and are independent of weight loss. Bariatric procedures also have positive outcomes on hyperlipidemia, hypertension, and sleep apnea (13), addressing many of the major comorbidities that contribute to early death in patients with diabetes.
Historic development of bariatric procedures
The detailed history of surgical intervention for weight loss has been reviewed elsewhere (14, 15), but it is worth noting the progression of thought regarding the effects of bariatric interventions on glucose homeostasis. Although surgical interventions have been used to treat obesity since the 10th century (16, 17), the true precursors to modern bariatric surgery were actually developed to treat peptic ulcer disease and gastric cancer (18, 19). The weight-reducing effects of these interventions became clear as early as the 1940s, with reports that ∼90% of patients experienced significant weight reduction, an observation initially attributed to diarrhea or loss of appetite (20). Although the focus in gastric surgery remained the treatment of peptic ulcer disease through the 1960s and 1970s (21), this period also saw some of the initial steps toward procedures developed specifically for weight loss. The advent of effective medical treatment of peptic ulcers in the late 1970s completely changed the clinical paradigm for treating this condition, at roughly the same time the average BMI of the American population began an exponential rise (22–24). These two events focused the attention of surgeons and other physicians on the treatment of obesity and comorbid conditions coincident with the development of several effective bariatric procedures. The growth in bariatric surgery as a therapeutic modality and topic for clinical research started in the 1980s, with greater attention on the development of new procedures, refined operative techniques, and more comprehensive outcome assessment.
Explanations for changes in glucose regulation following gastrointestinal surgery
Although the effects of gastrointestinal (GI) surgery on weight loss were the primary goal in developing bariatric surgery, the extraordinary effects these procedures have on glucose regulation can be gleaned even from early literature. Preclinical physiology laid the groundwork for understanding the potential for intestinal resection, or bypass, to reduce nutrient absorption and cause weight loss (25). Multiple case reports indicate that jejunoileal bypass improved glucose tolerance in conjunction with massive weight loss (26, 27). For example, it was noted that intestinal bypass reduced glucose excursion during an oral glucose tolerance test (OGTT) in the immediate postoperative time frame, prior to weight loss, a finding the authors attributed to poor glucose absorption (28). The role of the reservoir function of the stomach in glycemic regulation was noted in papers from the 1950s (29) and 1960s (30), with the potential for gastrectomy to improve diabetes noted by two groups (28, 31). The usual explanation for these findings was that gastrectomy, unlike jejunoileal bypass, accelerated nutrient absorption due to the lack of gastric capacity for temporary nutrient storage, precipitating acute enterally-driven hyperglycemia that caused high rates of insulin secretion (28, 32, 33). The simplicity of this model was questioned because absolute levels of glycemia or insulinemia were not always predictive of lower blood glucose (28), but work during this period established rapid gastric emptying after surgery as having a significant influence on glucose homeostasis. These studies were also foundational in defining the incretin effect, that is, the greater insulin secretion following oral compared with parenteral glucose (20, 34). Despite demonstrations that gastric resection and intestinal bypass modified glucose metabolism in patients, the lack of procedural uniformity, the morbidity of the patients treated with surgery, and the wide use of vagotomy, with its myriad effects on GI function, confounded the interpretation of the glycemic effects of early bariatric surgical procedures (35, 36).
With an increased focus on using GI surgery for weight loss, surgical methodology was refined through the 1980s and 1990s. In this setting the often-dramatic shifts in glucose homeostasis in the early postoperative course became more apparent and compelled the search for mechanisms by which this occurred. Concurrent advances in understanding insulin action, with the widespread use of glucose clamps in clinical research and advances in molecular understanding of the insulin signaling pathway in laboratory science, led to the observation that large-scale weight loss after surgery increased insulin sensitivity (7, 37). This observation fit with the then popular notion that T2DM was primarily a disease of insulin resistance (38), and it provided a model for the emerging benefits of weight loss surgery on diabetes.
Just as insulin resistance was demonstrated to be only a part of the pathophysiologic mechanism underlying T2DM (39), the notion that reduced insulin resistance after bariatric surgery explained all the changes in glucose metabolism was also challenged. The “rediscovery” of the incretin effect in the 1990s, as well as evidence that Roux-en-Y gastric bypass (RYGB) was associated with increased insulinotropic hormone secretion (40, 41), a finding initially reported many decades earlier (20), changed the consensus view of how bariatric surgery affected glucose metabolism. New findings suggested that bariatric procedures had broader effects on metabolic regulation through the stimulation of intestinal hormones that enhanced insulin secretion; the benefits of surgery to improve diabetes was reconceptualized to include an endocrine component that paralleled the use of incretin-based drugs in patient care (42).
The last decade has seen a profusion of human studies characterizing aspects of metabolic physiology after bariatric surgery that have identified other potential mechanisms that contribute to the changes in glycemia. Insulin secretion is generally improved, and this may occur independent of the incretin effect in T2DM patients, whereas effects in nondiabetic persons are subtler. Increased insulin clearance is one of the more proximate changes following RYGB that has been noted repeatedly, although how this is regulated and whether it is related to insulin secretion and insulin action are not yet known. Studies have increasingly focused on distinctions between hepatic and peripheral insulin sensitivity in explaining greater insulin action following surgery. However, despite considerable advances in experimental evidence and increasing sophistication in conceptual models, there remain major gaps in understanding the profound metabolic changes that follow surgical procedures.
Current implementation of bariatric procedures
Starting in the 1950s, surgery began to be conceptualized as a means to treat obesity and was put into practice by a small number of surgeons with special interest in the area. Most early procedures involved variations on intestinal bypass to promote some degree of enteral caloric wasting (43–46). These procedures were effective for weight loss and lowering circulating glucose and lipids (47), but over time they fell out of use due to a range of side effects that ranged from bothersome to morbid (36, 44, 48), as well as the increasing availability of safer, more effective procedures. The dawn of modern bariatric surgery can be traced to two influential papers by Mason. In the first, Mason and Ito (49) described the forerunner of the current RYGB, and in the second, Mason (50) described a vertical gastroplasty that standardized gastric restrictive procedures and presaged the eventual development of vertical sleeve gastrectomy (VSG). Refinement of the RYGB and its broader application to weight loss in diabetic and nondiabetic subjects became more common in the 1980s, exemplified by the efforts of Pories and colleagues (40, 51, 52). This laid the groundwork for greater acceptance of bariatric surgery in the treatment of metabolic disease (13, 53). Scopinaro et al. (54–56) described biliopancreatic diversion (BPD) in 1976 as a safer version of jejunal bypass, and clinical development of this procedure proceeded in parallel to work on RYGB, albeit at fewer surgical centers. Subsequent advances that spurred development in the field include: the addition of a sleeve gastrectomy to the BPD, termed the duodenal switch (BPD-DS) (57); introduction of the adjustable gastric band (AGB) (58) as a refined gastric restrictive procedure for mechanically limiting meal size; application of laparoscopic methods to perform bariatric procedures (59), which heralded a steady decline in surgical complications; and the discovery that sleeve gastrectomy, as the first step in staged bypass procedures for the very obese, caused significant weight loss as a stand-alone surgery (60).
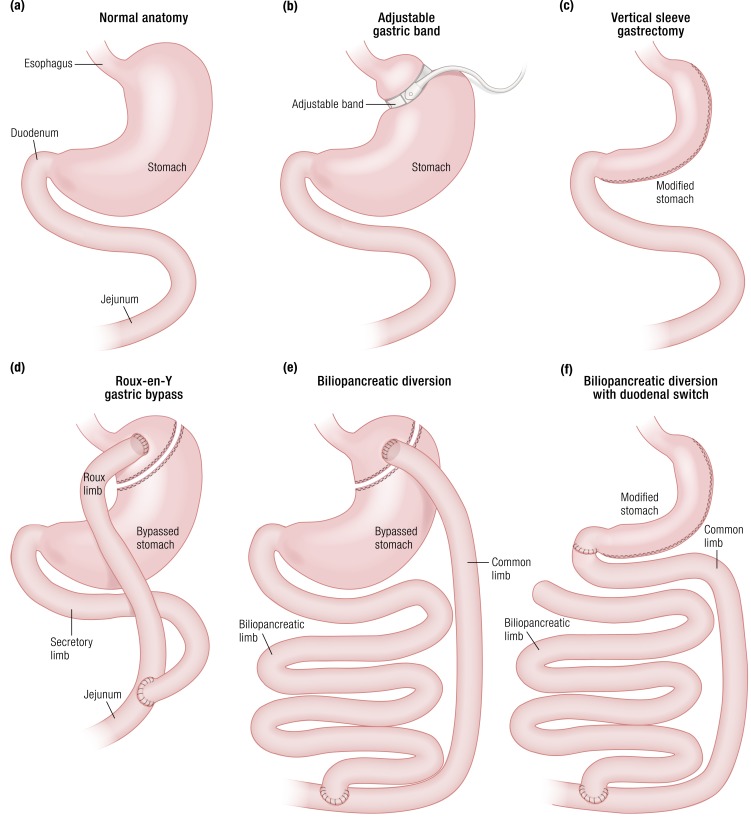
Schematic depictions of the major bariatric procedures currently in use are shown in Fig. 1 and described in detail elsewhere (61). The key anatomic features of the RYGB are the small gastric pouch emptying directly into the upper jejunum and the diversion of biliopancreatic secretions to the distal small intestine. With VSG a large percentage of the body of the stomach is removed, converting what is naturally a distensible muscular organ into a tight sleeve. Adjustable gastric banding is done by placement of an adjustable band on the proximal end of the stomach, restricting entry of food to a small pouch that empties slowly into the remainder of the stomach and eventually the small bowel. BPD, performed with or without a sleeve gastrectomy, shares some intestinal anatomy with RYGB but has significantly shorter alimentary and common limbs, and it has been demonstrated to limit caloric absorption (54). BPD is used only in a limited number of centers and comprises only a small percentage of bariatric procedures. All four of these procedures were initially thought to reduce food intake by physically restricting gastric volume, although this mechanism has been questioned in recent years (62–65).
Figure 1.
Gastric and foregut anatomy of RYGB, VSG, AGB, and BPD. (a) Normal upper GI anatomy. (b) RYGB surgically transforms the stomach into a small pouch, then bypasses most of the stomach and duodenum by attaching the distal jejunum directly to the stomach. (c) VSG surgically removes most of the stomach, turning the gastric pouch into a tight sleeve. (d) AGB applies an adjustable band to the proximal portion of the stomach to restrict food entry. (e) BPD surgically modifies the stomach in a manner similar to RYGB and connects the duodenum directly to the jejunum, bypassing most of the intestine. (f) BPD-DS includes surgical modification of the stomach much like a VSG coupled with the intestinal rerouting of a BPD.
The technical developments in surgery influenced a rise in the number of bariatric procedures performed in the United States to nearly half a million in 2013 (66), with RYGB and VSG being the two most common operations (>175,000 each in 2013); as of 2015, VSG had become the most common procedure performed in the United States (67). Refinements of RYGB and VSG have enhanced the speed and efficiency of surgery and reduced surgical morbidity dramatically (68). However, even with a greater number of procedures performed, and much more clinical investigation directed at their effects, many questions remain as to the physiologic basis of weight loss and other metabolic outcomes.
Effects of Modified GI Anatomy on Enteral Nutrient Flux
Gastric emptying
Current bariatric procedures all limit meal size. However, despite the anatomic dissimilarities between different surgical methods, the physiologic and clinical outcomes of surgeries that modify GI anatomy (e.g., RYGB, VSG, and BPD-DS) are largely comparable. One common feature of these three surgical approaches is that of accelerated gastric emptying (69); this has been confirmed in each of these procedures using current state-of-the-art scintigraphic methods. There is general consensus in the literature that the rate of gastric pouch emptying in RYGB patients is increased by ∼2.5-fold for liquids and ∼3-fold for solids relative to nonoperated controls (70–75). Similarly, VSG increases the gastric emptying of liquid and solid nutrients by 2.5-fold and 2-fold, respectively (76–86). In contrast, although AGB restricts entry of nutrients from the gastric pouch above the band to the body of the stomach, passage through the pylorus to the intestine is not modified (87). It is likely that gastric restriction is the primary, and perhaps sole, mechanism by which AGB changes body weight (61). Despite the long clinical application of conventional BPD with a horizontal distal gastrectomy, the effects of this procedure on gastric emptying have not been as well studied as the other procedures. Rapid emptying has been attributed to the wide gastroenterostomy (88), but this effect may be mitigated to some extent by significantly reduced intestinal motility (89).
“AGB improves glycemia in patients with diabetes in a manner that parallels weight loss.”
Nutrient absorption
Elevated gastric emptying rates after RYGB and VSG influence nutrient absorption. There is some variability in the absorption of different nutrient substrates, which has implications for islet regulation and insulin secretion after surgery.
Glucose
The antidiabetic effect of early bariatric surgeries was attributed to malabsorption of glucose (28); this is not true of most modern bariatric procedures. There is a distinctive pattern to glucose appearance and disposal following both RYGB and VSG, particularly early in the postoperative course. This entails a rapid, elevated peak in blood glucose followed by dramatic glucose clearance from the circulation (90–93). Although increased gastric emptying and alimentary motility can decrease exposure to the absorptive brush border of the intestine in some GI disorders (94), there appear to be specific adaptations of the Roux limb (the upper jejunum connected to the gastric pouch) after RYGB that causes increased glucose absorption into circulation (77). This may involve increased expression of the glucose transporters GLUT2 and SGLT-1, as well as other glucose-sensing machinery, within the portion of the intestine exposed to nutrients (95–98). For example, SGLT-1 expression in the ileum is positively correlated with peak glucose concentration in one study (95). Research addressing the regulation of intestinal glucose transport after VSG is lacking, although glucose appearance after ingestion mimics that of RYGB (99). However, rigorous studies comparing nutrient absorption in VSG and RYGB could be very informative. For one thing, better understanding of carbohydrate digestion and absorption would contribute to the understanding of glucose tolerance. For another, the role of upper intestinal bypass on micronutrient and mineral absorption, for example, iron and calcium, would provide valuable clinical information for patient care.
Absorption of other nutrients
A number of malabsorptive pathologies accompany bariatric surgery. Iron deficiency after surgery is commonplace, occurring in nearly 40% of patients receiving RYGB (96, 100, 101), which is noteworthy given the role of iron in regulating islet function (102, 103). Modest fat malabsorption has been reported after RYGB (104, 105), with a decrease in the coefficient of fat absorption from ∼90% to 70% per day (∼10 to 12 g or <100 kcal) along with reduction of fat intake (104, 105). In fact, this degree of malabsorption has been proposed to contribute modestly to weight loss. There is also a reduction in cholesterol absorption and synthesis in patients with RYGB (106, 107).
BPD is frankly malabsorptive, with the shorter common limb for mixing nutrients with digestive factors contributing to ∼50% reduced lipid absorption (54, 108). Interestingly, lower rates of cholesterol absorption after BPD are associated with increased rates of cholesterol synthesis (88). Absorptive capacity after BPD is reduced and seems to be fixed for lipid and energy substrates. Increased caloric consumption increases the degree of malabsorption, but not the amount of nutrient absorbed (109), with a 50-cm common limb maximal caloric absorption estimated to be ∼1250 kcal (88).
In contrast to fat absorption, protein digestion and subsequent amino acid absorption seem to be elevated after RYGB surgery (74). This finding is somewhat counterintuitive given the diversion of pancreatic proteases to the distal small intestine, but it was the clear outcome of an experiment with robust methodology and is consistent with other studies of protein absorption after meals (110). This effect of RYGB on protein absorption stands in contrast to the 30% reduction of the coefficient for protein absorption with BPD (108). It is notable that glutamine, a stimulus for glucagon-like peptide 1 (GLP-1) secretion and a putative α-cell proliferation factor (111), displays increased absorption after surgery (112). These particular modifications to nutrient absorption after RYGB raise the possibility that changes in amino acid flux could influence islet function after surgery and contribute to glucose homeostasis.
The fixed absorptive capacity of the gut for energy absorption after BPD presumably involves starch (108), so that carbohydrate is also malabsorbed after this procedure, similar to fat and protein. Alternatively, there was no evidence of glucose malabsorption during an OGTT in a small group of subjects studied 1 month after BPD (113); this question needs formal study with more subjects for clarity. There have been few studies of intestinal function following VSG, but these would be useful in understanding the physiologic responses to this common procedure.
Effects of Bariatric Surgery on Glucose Metabolism
Effects on chronic glucose control in diabetes
AGB improves glycemia in patients with diabetes in a manner that parallels weight loss (114). Conversely, RYGB, VSG, and BPD have dramatic effects to reduce fasting glycemia and improve prandial glucose control almost immediately after surgery (115, 116). Whereas initial reports of surgical improvement in patients with diabetes were from observational studies (115–117), more recent randomized clinical trials have compared RYGB, VSG, and AGB to conventional treatments for diabetes. These studies have been essential for increasing the acceptance of bariatric surgery more widely among health care providers as a useful and appropriate treatment of the disease.
One of the remarkable features of bariatric surgery that has driven its use in treating diabetes is the apparent disease resolution in some patients. A meta-analysis of studies done through 2003 noted that soon after a bariatric procedure the great majority of patients achieved normal glycemic parameters without continued use of medication (13). These types of observations raised the possibility that surgery could permanently eliminate dysglycemia and its sequelae. However, observational studies of large postsurgery cohorts reported somewhat lower rates of 40% to 60% for disease remission (118) when using more formal criteria, that is, nondiabetic values for fasting glucose and HbA1c without medication for 1 year (119). These rates of remission are comparable to longitudinal trials with VSG and RYGB. A number of investigators have developed models to predict which patients with diabetes are likely to have remission following surgery based on different variables that include age, weight, and duration/severity of diabetes (120–122). More recently, the focus on remission as the primary criteria for assessing surgical effects on diabetes has been questioned because there is substantial improvement in glycemic control, and often reduction or cessation of diabetes medication use, even in patients who do not achieve criteria for remission (123). Thus, the focus on an optimal outcome may actually understate a more general benefit of surgery on diabetes. Moreover, patients with RYGB and VSG have distinct glycemic patterns with higher peaks and lower nadirs that may have an impact on glycemic exposure of tissues. These distinct responses have raised questions as to the applicability of conventional measures of average glycemia and diabetes control to bariatric surgery patients (124–126).
As longer systematic follow-up of bariatric cohorts has become more common, it is apparent that some patients who initially remit have a later return of diabetic hyperglycemia. Data from observational studies (127), retrospective database analyses (128), and randomized clinical trials (129) document relapses of diabetes after surgically-induced remission with a broad range of estimates from 3% to 30% over 5 years. Thus, there is now general agreement that long-term remission does not occur in all patients. The broad question raised by these observations is whether diabetes relapse is due to the steady and inherent progression of T2DM that has been confounding medical management for years (130), to factors specific to individual patients, or to mechanisms specific to a given surgical procedure. To date there are no validated predictors of diabetes relapse following a period of surgically-induced remission.
Longitudinal, observational studies suggest that bariatric surgery reduces the incidence of vascular complications (131), findings consistent with a recent meta-analysis (132). These findings are supported by analysis of a large patient database that noted fewer microvascular complications among patients with diabetes who had remission following bariatric surgery, mostly RYGB (133). In this analysis the length of time in remission was proportional to the reduction in rates of retinopathy, nephropathy, and neuropathy. However, in a retrospective analysis such as this (133), it is not possible to attribute reduced complications to remission per se; the results could also be explained by reduction of cumulative hyperglycemic exposure, the model for microvascular disease derived from medical intervention trials such as the Diabetes Chronic Complications Trial and the United Kingdom Prospective Diabetes Study. The attention focused on diabetes remission and relapse has been valuable for framing the impact of bariatric surgery on glucose metabolism. However, the critical question that remains only partially addressed is how effects of surgery on glucose metabolism translate into reduction of microvascular and macrovascular outcomes, and ultimately mortality related to diabetes.
Clinical trials
The observed therapeutic potential of bariatric surgery on diabetes outcomes (115, 116) set the stage for a rigorous test of this effect. A major hurdle was creating research infrastructure and recruitment strategies for randomization of patients with obesity to either conventional or surgical interventions. However, during the past 10 years a number of clinical trials designed to compare the effects of bariatric surgery with standard medical management on diabetes control have been completed and reported. These have included tests of all four common surgical procedures.
Adjustable gastric band.
The first randomized clinical trial (RCT) of bariatric surgery directed specifically at treatment of diabetes used AGB as the surgical procedure (114). In this trial 60 subjects with obesity with T2DM were randomized to AGB or a medical/lifestyle strategy that emphasized caloric restriction and exercise and were followed for 2 years. During that period subjects given AGB had a mean 20% decrease in body weight compared with 1.4% in the lifestyle group. Rates of diabetes remission were significantly greater with surgery (73%) than with medical/lifestyle intervention (13%), and the amount of weight loss predicted nearly 50% of the variance in remission rate. Subsequently, two other RCTs have been reported that measured effects of AGB against nonsurgical management (134, 135). The first of these compared 22 subjects with AGB with 23 subjects following a lifestyle program for 1 year (134). Weight loss averaged 17% of starting weight in the AGB group, and 50% had partial or complete remission of diabetes (119). In contrast, subjects participating in the intensive lifestyle management program had 10% weight loss and none had diabetes remission. The final trial compared 18 subjects with diabetes with AGB to 22 subjects in a comprehensive weight management program for 1 year. In this trial the subjects treated with surgery lost 13% of starting body weight and the lifestyle management group lost ∼9%; diabetes remission was comparable in the two groups, 33% for surgery and 23% for lifestyle.
Although the RCTs of AGB vs nonsurgical management for diabetes are all of relatively small size and differ in patient characteristics, specifics of lifestyle intervention, and duration, they do suggest several conclusions. First, T2DM is amenable to remission with significant weight loss, and more weight loss leads to greater effects. Second, AGB causes more weight loss than intensive lifestyle management programs, even those that include expert counseling on diet, exercise, behavioral programs, and medication management. Thus, even the least efficacious weight loss surgery has superior efficacy to nonsurgical approaches for glycemic control in patients with diabetes. However, AGB does not appear to elicit major effects on glucose homeostasis beyond those explained by weight reduction.
Roux-en-Y gastric bypass.
The Surgical Treatments and Medication Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial is the landmark study of bariatric surgery and diabetes (8, 136). This trial randomized 150 patients with obesity with poorly controlled T2DM to RYGB, VSG, or intensive medical/lifestyle management. The study had high rates of completion, extended follow-up (129), and a number of novel and important findings that have shifted the view of bariatric surgery as a clinical intervention. In this trial the medically treated group lost >5 kg in the first year with a decrease of HbA1c from 8.9% to 7.5%, outcomes that would be considered above average for the standard management of T2DM. However, the RYGB and VSG groups lost 29 and 25 kg, respectively. Furthermore, ∼40% of both surgical groups met the primary outcome of an HbA1c ≤6%, significantly surpassing the 12% of medically treated patients achieving this target. These results demonstrated in dramatic fashion the powerful effects of surgery on diabetes control, with surgically treated subjects having reductions in HbA1c of ∼3%. After 5 years, 90% of the original cohort was retained in the study. The medically treated group maintained their mean 5-kg weight loss, but glucose control worsened over time, with the group mean HbA1c rising to 8.5%. The RYGB group had modest weight regain (∼6 kg) and an increase of the mean HbA1c from 6.4 at 1 year to 7.4 at 5 years; for patients given VSG, the changes were ∼7-kg weight regain and an increase in HbA1c from 6.6% to 7.4%. Nonetheless, nearly one fourth of the surgically treated patients continued to meet the primary endpoint of a nondiabetic HbA1c after 5 year. STAMPEDE demonstrated the large difference in effect size of surgery compared with top-rate medical management, and somewhat surprisingly the only slightly less powerful effect of VSG compared with RYGB. Moreover, the excellent rates of retention in the trial allowed the clear detection of glycemic worsening in all three study arms; these results are compatible with underlying progression of T2DM even in patients with a generally good response to surgery, although the average effects among the groups blur intersubject variability.
Other trials including RYGB have mostly confirmed the results of the STAMPEDE trial (10, 134, 137). For example, Ikramuddin et al. (10) reported that 6 years after RYGB there was a modest weight regain from the 1-year postoperative nadir of ∼25% of baseline weight, and an increase in HbA1c from ∼6.2% 1 year after surgery to 7%. However, the rate of diabetes progression was about twofold higher in a group of subjects randomized to medical treatment during the course of the trial. In sum, there is strong evidence for potent effects of RYGB to improve glycemic control well beyond what conventional clinical measures can achieve. Although this effect is generally proportional to the amount of weight lost, an observational study of glucose lowering that compared AGB and RYGB suggests that the degree of diabetes resolution per pound of weight lost is greater with bypass (138), a view that is widely shared although not yet definitively established. Despite the magnitude of the surgical effect on HbA1c and other measures of glycemic control, it is apparent that diabetes progresses in some surgical patients although it is not clear whether this is a general phenomenon (128).
Vertical sleeve gastrectomy.
The results from the STAMPEDE trial established VSG as significantly more effective for treating hyperglycemia than medical management alone (129). Moreover, subjects with VSG had only modestly less weight loss and rates of diabetes remission than those with RYGB. These findings are supported by the results of two trials comparing the efficacy of RYGB and VSG in subjects with obesity for 5 years (139, 140). One of these studies, SLEEVEPASS, a randomized trial comparing RYGB or VSG, included a substantial number (∼40%) of subjects with T2DM. Weight loss at 1 year was comparable between the surgeries, and diabetes resolved or improved in 84% of the subjects with VSG and 93% of the RYGB group (141). After 5 years, weight loss was generally maintained, and HbA1c reduction was similar with the two surgeries (∼6.6%) despite the RYBG group having ∼14% greater body weight loss (140). Similar results were reported in the SM-BOSS trial (139), with comparable, although slightly greater, weight loss in RYGB compared with VSG that was maintained for 5 years. At this last follow-up time point, >60% of the subjects had remission of diabetes with mean HbA1c of 6.2% in the VSG group and 5.9% in the RYGB subjects. Overall, VSG has effects on body weight and glycemic control that compare with RYGB; although not a universal finding, there is a trend in these comparisons for VSG to be slightly less effective.
“Overall, VSG has effects on body weight and glucose control that compare with RYGB…”
Biliopancreatic diversion.
Previous retrospective analyses (142, 143) and a systematic review (144) report greater amounts of weight loss and diabetes resolution in patients with BPD compared with those with RYGB, albeit with greater adverse surgical effects. BPD has been compared with medical treatment of diabetes outcomes in a small randomized controlled trial that compared effectiveness to medical management (11, 145). In this study, 60 patients with obesity with T2DM were randomized to medical/lifestyle treatment, BPD, or RYGB; the different operations were performed by separate surgical teams. At 2 years, 19 of the 20 subjects given BPD, 15 of 20 with RYGB, and none of the medically treated group reached the primary outcome of diabetes remission (fasting glucose <100 mg/dL and HbA1c <6.5% with use of no glucose-lowering medications). The medical/lifestyle-treated subjects lost ∼5% of starting body weight, whereas the surgical groups both lost ∼33%. The subjects with diabetes started this trial with HbA1c values of 8.6% and reached values of 8.3%, 6.3%, and 4.9% in the medical, RYGB, and BPD groups, respectively, at 2 years. When examined 5 years after surgery, 7 of the 19 RYGB patients and 12 of 19 BPD patients maintained diabetes remission, and the BPD group had lower mean HbA1c; the medically treated group had no diabetes remission. Notably there was not a clear correlation of diabetes remission with amount of weight loss. A second trial compared 60 patients randomized to RYGB or BPD-DS and followed for 5 years; only a small number of patients in this cohort (≤20% in each group) had diabetes before surgery (146). The BPD group had greater weight loss at the 5-year follow-up, and mean fasting glucose and HbA1c were lower than in the RYGB group. However, both groups had similar diabetes remission rates.
The results of these prospective studies with BPD are compatible with those of trials with RYGB and VSG in demonstrating the significant difference in diabetes improvement with surgery compared with medical management. Although there is general belief that the effects of BPD on weight loss and diabetes improvement are greater than other procedures, larger randomized comparisons are needed to validate the results of observational studies on potential differences in beneficial and adverse effects among procedures.
Mechanisms by which surgery affects glucose metabolism
The superior efficacy of bariatric surgeries to lower blood glucose compared with conventional medical or lifestyle interventions has spurred numerous studies to determine the mechanisms involved. What has been particularly compelling is the rapidity of this response. In a study of 31 patients with T2DM, blood glucose was reduced ∼2 mM 6 days after RYGB, coincident with an ∼60% decrease in fasting insulin (147). A more recent study including 18 women with diabetes undergoing RYGB reported an ∼20% decrease in fasting glucose within 3 days of surgery (148). These findings are in keeping with clinical impressions that many patients with diabetes who required medical management before undergoing RYGB do not need treatment of hyperglycemia in their postoperative hospital course (115, 116). Two points that have been debated as explanations for the rapid glucose lowering after surgery are: (i) the effect of caloric restriction per se, independent of anatomic changes to the gut to mediate this effect; and (ii) whether amelioration of insulin resistance is central to the response.
Role of caloric restriction
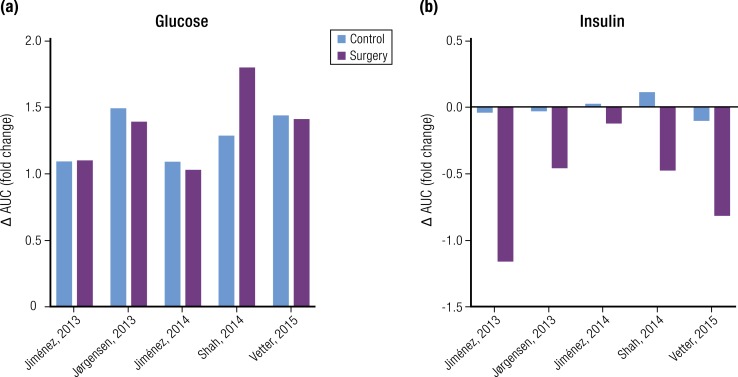
There is evidence that dietary caloric restriction for as little as 1 week can improve insulin action and insulin secretion (149, 150). In these studies, low calorie intake was associated with 2 to 3 kg of weight loss and significant reduction in fasting glucose. Thus, a number of studies have compared the effects of a very low-calorie diet (VLCD) to RYGB, during periods of 1 to 3 weeks, to determine the effects of decreased food intake on parameters of glucose metabolism. The study designs used in these experiments were either parallel assessments of diet- and surgery-treated subjects (151–155) or within-subjects comparisons of patients given VLCD before surgery and standard treatment after RYGB (156–159). Most of these studies were small, with ∼10 subjects per group, and the discrepant results may be due to their modest statistical power to distinguish differences between interventions. For example, studies using either parallel or within-subjects comparisons noted similar reductions in fasting glucose and insulin with either RYGB or VLCD and concluded that caloric restriction after surgery accounts for the rapid reduction of blood glucose in subjects with diabetes (152, 157, 160); a similar result was reported for subjects with diabetes studied 3 days after BPD (161). Alternatively, a study using both experimental approaches reported significant reductions in fasting glucose among subjects with diabetes with RYGB compared with those receiving a VLCD (156). A recent paper reported results from 20 nondiabetic subjects with obesity assessed before and 1 week after a 600 kcal/d diet, and again 3 months later, before and 1 week after RYGB (158). Following dietary restriction there was a 2-kg weight loss but no significant changes in fasting glucose and insulin, nor in fasting or insulin-stimulated glucose turnover. In 10 of these subjects who subsequently had RYGB there was a 5-kg weight loss, with weight-adjusted improvement in hepatic and peripheral insulin sensitivity. Although this study supports effects of RYGB on glucose metabolism independent of caloric intake, the small sample and normal glucose tolerance of the subjects limits extension to the salutary effects of surgery on diabetes.
Comparisons of dietary restriction with surgery on parameters of glucose metabolism are confounded by the known effects of surgical stress to cause insulin resistance (162–164). Thus, equivalent improvements in glucose regulation in unstressed subjects on VLCD, and those with recent RYGB, who have pain, inflammation, medication effects, and other factors impacting metabolism, must be considered carefully. This point is exemplified by the study of Lingvay et al. (157) in which patients treated sequentially with VLCD and RYGB had similar reductions in fasting glucose during 10 days, but with differing patterns. During the diet intervention, fasting glucose decreased steadily, whereas after surgery there was an increase in glycemia on the first postoperative day and relative hyperglycemia for the next 5 days before levels decreased below preoperative values. Indeed, it is not uncommon for endocrinologists to consult on patients whose diabetes has worsened after abdominal surgery even with caloric restriction.
One means of controlling for postoperative stress while investigating the impact of caloric restriction after surgery is comparison of gastric-restrictive procedures such as AGB to RYGB before significant weight loss; the assumption here is that AGB strictly reduces food intake, whereas the more substantial changes to GI anatomy with RYGB elicits effects independent of energy balance. Korner et al. (165) reported similar reductions in fasting insulin and glucose in groups of nondiabetic subjects with obesity with ABG and RYGB 2 weeks after surgery. Similarly, Kashyap et al. (166) observed similar glucose lowering with banding and bypass at 1 week postoperative but noted a greater decrease in fasting insulin in the bypass group and inferred a greater improvement of insulin sensitivity. More recently, Gastaldelli et al. (158) compared fasting measures and the response to a euglycemic–hyperinsulinemic clamp among nondiabetic subjects 1 week after AGB or RYGB. In this study subjects lost ∼5 kg of body weight with both procedures and had comparable, modest reductions in fasting glucose and insulin. Although endogenous glucose production was reduced and hepatic insulin sensitivity improved after both AGB and RYGB, only the subjects with bypass had improvements of peripheral insulin sensitivity, measured as either adipose tissue or skeletal muscle responses. The measure with the most convincing difference between the two surgery groups was insulin clearance, which was significantly increased after RYGB. This finding has been reported 1 week after RYGB by a second group (167), and the difference between band and bypass noted by a third (168). The results of the Gastaldelli et al. (158) study support differences in the physiology induced by RYGB and ABG, but similar to the other short-term comparisons of these procedures they have only marginal statistical power to identify definitive differences.
When taken together, studies comparing RYGB and either VLCD or ABG indicate that the rapid reduction in fasting glycemia, reflecting improved glucose regulation and the potential to stop antidiabetic medications (115, 116), is accounted for in great part by reduced caloric intake. However, there is evidence that RYGB has additional effects beyond acute energy balance on insulin clearance and insulin sensitivity. Determining the nature and impact of these changes will require a directed and amply powered study.
Effects of bariatric surgery on insulin sensitivity
The improvement of insulin sensitivity in the early postoperative course has been studied in a series of small but rigorous studies, focused mostly on patients with RYGB. These studies have measured hepatic glucose production (HGP) using isotope dilution methods and used euglycemic–hyperinsulinemic glucose clamps to determine insulin sensitivity; at present, these are the most accurate and precise techniques available for studies of small and medium size, for example, 20 to 50 subjects. One week following RYGB, Bojsen-Møller et al. (169) noted reduced fasting HGP, a trend toward greater suppression of HGP by insulin, and no change in peripheral insulin sensitivity. Similar results were published by Gastaldelli et al. (158) in 10 nondiabetic subjects 1 week after RYGB, including a significant reduction of fasting HGP, a threefold suppression of HGP with insulin that did not reach statistical significance, and no change in peripheral insulin sensitivity. Two studies involving small groups of subjects 2 weeks after RYGB (170, 171) reported comparable findings: significant reductions in fasting insulin and glucose, reduced basal HGP, and no effect on hepatic or peripheral insulin sensitivity. By 1 month after surgery HGP is more robustly suppressed during an insulin clamp in both subjects with diabetes and nondiabetic subjects compared with their preoperative state (172). Some groups also report a small improvement of peripheral insulin sensitivity at this time (173, 174), whereas others see no change (172, 175). Taken together, these data, obtained with the currently accepted best analytic methods, do not provide a definitive explanation for immediate/early changes in glucose metabolism after surgery. The most consistent and significant observation is a large decrease in basal insulin concentrations. There seems to be a tendency for HGP to be reduced in the early period following surgery, yet it is unclear whether this is a function of insulin sensitivity or some other regulatory input. Peripheral insulin sensitivity seems to lag behind hepatic changes, with improved glucose disposal starting to become apparent only 4 weeks postoperatively.
“There is evidence that RYGB has additional effects beyond acute energy balance on insulin clearance and insulin sensitivity.”
Multiple studies have reported that insulin resistance as determined by HOMA modeling of fasting insulin and glucose concentrations improves following RYGB (176–178), VSG (179–181), and AGB (182). HOMA modeling to derive an index of insulin sensitivity (HOMA-S) (183) has been the most common assessment of changes in glucose metabolism used in studies of bariatric surgery because of its simplicity. HOMA-S requires only fasting values of insulin and glucose, and it is thought to reflect primarily hepatic insulin sensitivity, although in recent iterations the model also accounts for peripheral insulin action (183). This approach has been used to support the view that the acute effects of surgery on glycemia are due to rapid improvement of insulin resistance in the liver. Importantly, however, note that the application of HOMA in surgical subjects has been challenged in studies that also use hyperinsulinemic glucose clamps to measure insulin action (163, 175, 184). Although HOMA-S measures generally correlate with estimates of insulin sensitivity derived from clamp studies, there are differences in precision, a problem that is magnified in studies with small sample sizes (183). Moreover, because insulin secretion is pulsatile, proper application of HOMA modeling should use three fasting blood samples taken at 5-minute intervals to determine plasma insulin and glucose concentrations (183). This protocol recommendation has not been followed, or not been noted, in many studies of bariatric surgical subjects, and use of single measures of insulin for HOMA computations reduces the accuracy of the measure, another problem that can affect studies with relatively small sample sizes.
A major potential confounder of HOMA-derived insulin sensitivity measures in studies of RYGB subjects is the rapid and significant enhancement of hepatic insulin clearance that occurs in the first postoperative week (150). An early increase in hepatic insulin clearance has been noted for RYGB, VSG, and BPD in both subjects with diabetes and nondiabetic patients (167, 185, 186). This causes fasting insulin levels to decrease by 25% to 50% (158, 169, 174, 187) and significantly affects estimates of insulin sensitivity from models that use plasma insulin as a divisor; this includes the HOMA-S, QUICKI, and Matsuda models, and even indices using tracer-derived measures of HGP (158, 169). It is not clear how these early changes in hepatic insulin clearance after surgery are related to insulin sensitivity. Although it has been proposed that hepatic insulin clearance is roughly equivalent to hepatic insulin sensitivity (174), this has not been proven experimentally. In fact, whereas changes in hepatic insulin sensitivity in the week after RYGB are equivocal when measured by direct methods (i.e., euglycemic insulin clamps with isotopic dilution), estimates based on HOMA almost uniformly demonstrate large effects. Recent work in an animal model suggests that hepatic insulin clearance is related to peripheral, but not hepatic, insulin sensitivity, although this relationship has not been extended to humans (188). What is needed are direct, independent measures of hepatic insulin clearance and action to determine whether changes in these parameters after surgery are related or coincidental. Until a relationship between hepatic insulin clearance and insulin sensitivity in postoperative humans is established, the assumption that HOMA is a reliable reflection of hepatic insulin sensitivity should be made with caution (184).
It is now very clearly established that in both subjects with diabetes and nondiabetic subjects who have substantial (e.g., >15%) weight loss following surgery, hepatic and peripheral insulin sensitivity improves significantly (152, 169, 180, 189, 190). Although the rate of weight loss is faster, and generally greater in absolute terms, for RYGB, BPD, and VSG than for ABG (189, 191–194), resolution of insulin resistance is predictable past a general threshold of reduced body weight. This is evident in longitudinal studies assessing the temporal pattern of insulin sensitivity after surgery. For example, when insulin action has been measured using glucose clamps in RYGB subjects studied in the first month after their operation, results have been disparate (158, 169, 174, 184). However, by 3 months there is generally a large reduction in insulin resistance that persists for a year or more (99, 169, 170, 195, 196); similar longitudinal results have been described for BPD (197). Improved insulin sensitivity after weight loss is detectable in both skeletal muscle and adipose tissue and is associated with changes in molecular mediators of insulin action (198, 199). Once successful weight loss becomes stabilized, insulin sensitivity is proportional to body weight when measured using glucose clamps (99, 185, 189, 200). However, when estimated using a formula based on fasting insulin (e.g., HOMA), the relationship of insulin sensitivity to body weight is lost (197, 198), possibly because increased insulin clearance after RYGB is not related to BMI.
There are two studies that have reported the course of insulin sensitivity following BPD using glucose clamps. In a study by Guidone et al. (201), 10 subjects with obesity with T2DM had resolution of hyperglycemia 1 week after BPD that was associated with a doubling of insulin sensitivity; glucose tracers were not used to differentiate hepatic and peripheral insulin action. Insulin sensitivity did not change further at 4 weeks, and there was a proportional decrease in fasting insulin secretion. Astiarraga et al. (202) reported compatible findings, with a significant improvement of insulin sensitivity 2 months following BPD in patients with T2DM. In this study, fasting and insulin suppression of HGP were lower at 2 months than before surgery. This is a limited set of evidence on which to make firm conclusions, but these findings of more rapid improvement of insulin sensitivity with BPD are in keeping with the notion that this procedure has a greater impact on metabolic physiology than do other surgeries.
It is notable that several studies have reported that subjects with diabetes and nondiabetic subjects receiving bariatric surgery are actually more insulin sensitive than are weight-matched subjects without surgery (170, 198). This observation has not been pursued with more comprehensive studies, for example, of body composition and tissue-specific insulin action, but it raises the possibility that surgery has an impact on insulin action that is out of proportion to the effects on weight loss. Finally and importantly, note that weight loss and improved insulin sensitivity do not predict diabetes remission across all patient populations (203, 204); in fact, improved glucose control has been described to be largely independent of improved insulin sensitivity (91, 205).
Effects of surgery on postprandial glycemia
Although postprandial glycemic profiles in subjects with AGB are similar to controls (87, 206), both RYGB and VSG have marked effects on postprandial glucose excursions. The more rapid delivery of carbohydrate to the absorptive surface of the intestine (207) leads to a sharp upward deflection of blood glucose that is higher than peak levels in subjects without GI surgery (189, 206). Despite the elevated peak glucose levels after either an OGTT or mixed meal tolerance test (MMTT) there is an improvement in glucose tolerance following RYGB and VSG as reflected in a reduction in the area under the curve for glucose during the meal (176, 208–210). This prandial profile demonstrates both the accelerated meal glucose appearance in VSG and RYGB (185, 209), but also the superior clearance of glucose conferred by surgery. In fact, it is common for RYGB patients, and also for those with VSG, to have glucose nadirs during test meals that are significantly lower than their fasting levels (185). This exaggerated pattern of glucose dynamics reflects both the impact of altered GI anatomy as well as adaptive changes that allow the homeostatic challenge of increased nutrient flux to be managed.
BPD performed with a distal gastrectomy does not cause the rapid rise in prandial glycemia typical of RYGB and VSG (113, 202), but formal measures of gastric emptying or meal glucose appearance were not performed in these studies. Of note, patients with BPD-DS studied several days after surgery had glycemic excursions following a mixed meal that were completely blunted, and much smaller than the excursion seen in a matched group of patients with sleeve gastrectomy despite similar gastropyloric reconstruction with the two operations (211). Although not formally measured, this result suggests impaired glucose absorption after BPD-DS. Despite, or perhaps because of, impaired absorption of glucose in the transposed ileum, GLP-1 levels in these early postsurgery subjects were substantially elevated. This effect of BPD on glucose absorption appears to wane over time, as prandial glycemia levels in patients with BPD-DS match those of patients with SG when studied at 3 months and 1 year postoperatively (212). These findings suggest the interesting possibility that there is adaptation in the alimentary limb of patients with BPD over time that enhances carbohydrate digestion and uptake (211).
Effects of bariatric surgery on insulin secretion
Although procedures such as VSG, RYGB, and BPD have a number of reported effects on islet function, there remains some uncertainty as to what the proximate actions of surgery on insulin secretion are, and whether all procedures have similar effects. However, what is clear is that postsurgical patients with T2DM show more immediate and definite improvements of insulin secretion as measured by a number of methodological approaches than do those without antecedent diabetes (173, 213, 214). This difference in response raises the possibility that bariatric procedures induce specific responses to rectify abnormalities in β-cell function, a tractable but as yet unsubstantiated hypothesis. Regardless, the current state of literature in this area suggests that postsurgical effects on insulin secretion are more readily understood by considering the responses of subjects with diabetes and nondiabetic subjects separately.
“…GLP-1 does not seem to account for the greater glucose tolerance among patients with diabetes after surgery.”
Nondiabetic subjects.
Insulin secretion predictably mirrors the glucose excursion during either an OGTT or MMTT following RYGB, VSG, and BPD. Studies of subjects with VSG and RYGB are consistent with meal stimulation engaging a variety of factors that drive hyperinsulinemia, starting with steeper glycemic excursions (90, 206, 215–217), and including greater stimulation from enteral factors such as incretins (218). Therefore, the early β-cell response to meals is predictably increased following either RYGB or VSG, with an excursion that peaks and returns to baseline more rapidly than the profile of subjects without surgery. In contrast, the pattern of prandial insulin secretion in persons with BPD, with or without the DS, is not as dynamic as described in those with VSG or RYGB (113, 202, 212, 219). These findings support the importance of ambient glycemia, independent of other effects of surgery, in shaping the β-cell response to meals, and they indicate that differences of 2 to 4 mM in peak blood glucose concentrations have a major impact on the magnitude of secretion in nondiabetic persons (91, 189, 211). One challenge this has posed to assessing insulin secretion using meal stimuli is that the rapid dynamics of meal absorption, glycemia, and insulin responses among surgical patients adds a temporal factor to comparisons and can confound simple summaries of secretion (e.g., area under the curve).
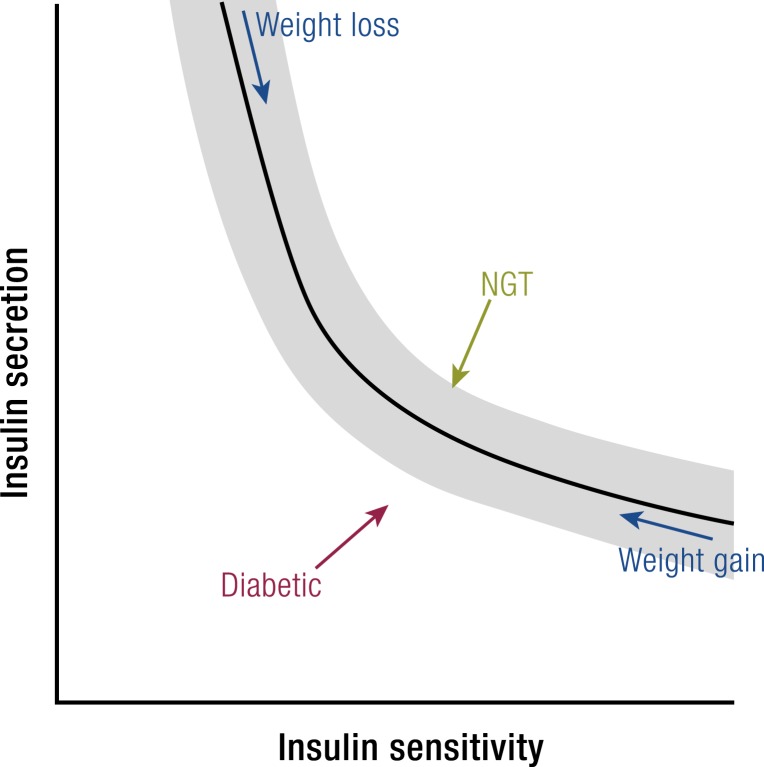
Perhaps the most important consideration when evaluating the effects of bariatric surgery on insulin secretion is the impact of insulin sensitivity. Among nonsurgical patients there is an inverse relationship between measures of insulin secretion and insulin sensitivity that is generally interpreted as β-cell compensations to provide appropriate amounts of insulin to maintain glucose homeostasis (Fig. 2) (220). In both healthy subjects and subjects with diabetes this relationship has been described by a rectangular hyperbola such that the product of insulin secretion and insulin sensitivity are a constant, termed the disposition index (DI) (221). Application of the DI after bariatric surgery is exemplified by the study of Bradley et al. (189), which examined nondiabetic subjects before and after 20% weight loss with either AGB and RYGB. Insulin sensitivity measured by euglycemic glucose clamps increased 50% to 60%, and total insulin secretion rate (ISR) during a mixed meal decreased ∼20%, with both surgeries. Thus, the DI was increased by nearly 75% in both groups and was taken as evidence of enhanced β-cell function that would not have been identified were plasma insulin or C-peptide levels considered in isolation. Similar relationships have been described before and after VSG (91) and BPD (113) and emphasize the importance of interpreting insulin secretion in the context of insulin sensitivity. Importantly, however, note that the validation of the DI as a hyperbolic function of insulin secretion and sensitivity has only been done in nonsurgical subjects (221–223). It is plausible that surgery alters this relationship, and formal testing of the interaction of secretion and sensitivity after RYGB, VSG, or BPD would be an important addition to the knowledge base in this area.
Figure 2.
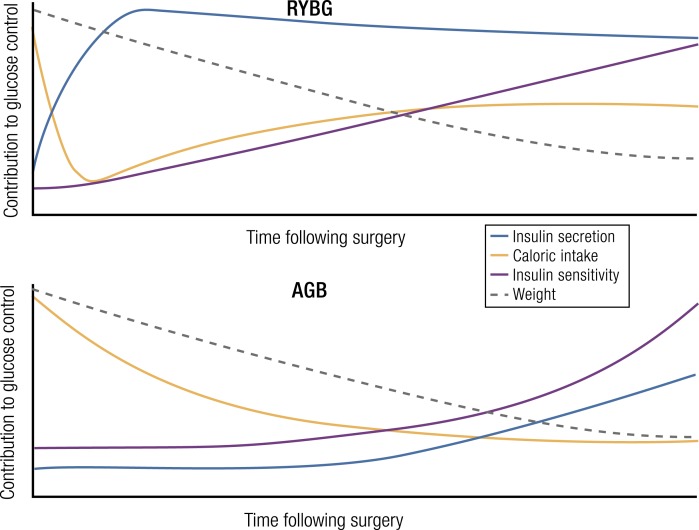
Model of changes in β-cell function following RYGB. An inverse relationship between insulin secretion and insulin sensitivity exists in surgical and nonsurgical patients. This empirically derived relationship is generally interpreted as β-cell function adapting to meet the demands of increasing insulin sensitivity (with weight loss) or increasing insulin resistance (with weight gain). Nondiabetic persons have decreased insulin secretion after RYGB, as β-cell function is blunted to account for greater insulin action. In contrast, subjects with diabetes have improvements in both insulin secretion and insulin sensitivity. See (213, 214).
A number of studies have used IV glucose as a stimuli to assess insulin secretion following RYGB, VSG, or BPD (173, 196, 214, 224–226). This approach removes the effects of rapid enteral nutrient flux on β-cell function and allows simpler comparisons between subjects before and after surgery, or with nonoperated controls. Additionally, the frequently sampled IV glucose tolerance test protocol can be used with the minimal model of glucose kinetics to estimate insulin secretion and sensitivity from the same data set (196, 226), an approach that minimizes day-to-day variability and adds greater precision to the connection between insulin secretion and action. These studies demonstrate minimal change (173), or even a reduction (196, 226), in the acute insulin response to glucose over time as postsurgical patients lose weight and reduce insulin resistance. However, when insulin secretion is corrected for insulin sensitivity as the DI, most studies report that β-cell responsiveness to glucose stimulation is improved in glucose-tolerant subjects with obesity after surgery (173, 196, 226). Similar to studies using oral challenges to glucose tolerance, the body of work using IV glucose tolerance tests supports improved glucose homeostasis following bariatric surgery as a function of both insulin secretion and insulin sensitivity. The question raised by studies examining insulin secretion in response to IV stimuli is how improved β-cell function is mediated, as improved responsiveness occurs in the absence of inputs from the gut and central nervous system and thus is independent of postsurgical anatomy. The data available at present are compatible with surgery causing adaptations intrinsic to the islet, a conclusion supported by recent findings in a preclinical model (93).
There is recently published evidence that some inherent capabilities for insulin secretion may be attenuated after surgery. Salehi et al. (227, 228) reported that subjects with RYGB for several years have reduced β-cell sensitivity to glucose and incretins. In these experiments, subjects were given graded infusions of glucose, glucose-dependent insulinotropic polypeptide (GIP), or GLP-1, and ISR compared with a group of weight-matched subjects without surgery. The RYGB subjects had almost uniformly lower insulin secretion in response to comparable glycemic or incretin stimulation that was not accounted for by differences in insulin sensitivity derived from a glucose clamp. These findings suggest that, at later time points following surgery, β-cell sensitivity is dampened to factors such as glucose and GLP-1 that circulate at high concentrations after meals, plausibly to prevent hypersecretion and hypoglycemia. Although these findings require confirmation, they do raise the possibility that β-cell function adapts over time after surgery to optimize or maintain glucose homeostasis.
Finally, assessment of insulin secretion in patients after VSG, RYGB, and BPD must account for the increase in hepatic insulin clearance that occurs soon after surgery (167, 185, 186). Using changes in plasma insulin alone can be misleading and mute the magnitude of insulin secretion (167). Accurate assessments of β-cell function require measurement of C-peptide, and it has become common to use deconvolution models to derive ISRs (113, 229), which provides a more precise estimation of β-cell function and allows accurate calculation of insulin clearance. However, deconvolution of C-peptide kinetics relies on estimates derived from nondiabetic subjects (230), a parameter that has not been generated for people with bariatric surgery. Although there is no reason to expect that surgery would alter C-peptide kinetics, this has not been directly tested.
Patients with diabetes.
Although the effect of bariatric surgery on insulin secretion can be a subtle or contingent finding in nondiabetic subjects, the effect is more obvious in persons with preoperative diabetes. A number of studies have reported restoration, if not normalization, of first phase insulin release (FPIR) to IV glucose in subjects with T2DM studied 1 to 4 weeks after RYGB or BPD (173, 214, 224, 225). This finding is notable for its consistency across a range of cohorts, the rapidity of the response (often before significant weight loss or change in insulin sensitivity), and because loss of FPIR is one of the hallmark β-cell lesions associated with diabetes (231). The association of rapidly corrected fasting hyperglycemia and enhanced FPIR supports a model in which β-cell adaptation contributes to diabetes resolution after procedures such as RYGB, VSG, and BPD.
Diabetic patients also have improved insulin responses to enteral challenges following RYGB, VSG, and BPD. Measures of insulin secretion in response to mixed nutrient meals (185, 213, 225, 232) or oral glucose (173, 180, 225, 233) are increased in the first month following common bariatric procedures; a single report comparing RYGB and AGB suggests that gastric restriction alone does not have this prompt action to increase insulin release (166). Improved insulin secretion in response to carbohydrate ingestion is maintained in subjects with diabetes even after substantial weight loss and reduced insulin resistance (169, 215, 232). This pattern of concurrent improvement of insulin secretion and insulin sensitivity differs from that of nondiabetic subjects after surgery in whom these parameters have an inverse relationship (196, 226), and it raises the possibility that bariatric surgical procedures have distinct actions on diabetic and nondiabetic β-cells (Fig. 2).
One exception to the pattern of increased insulin secretion and sensitivity after surgery was reported by Grenier-Larouche et al. (197) in a cohort of patients with mild T2DM (A1c ∼6.6%) who were followed for 12 months following BPD. These subjects had ISRs measured with graded IV infusions of glucose and related to insulin sensitivity measured with hyperinsulinemic clamps. There was no difference in ISR or DI 3 days after surgery compared with before surgery. However, by 3 months the subjects had mild, nonsignificant decreases in ISR with significant improvement in insulin sensitivity leading to a higher DI; this pattern was maintained at 12 months. Similar findings were reported in a group of subjects studied during 3 years after RYGB with oral and graded IV glucose tolerance tests (234). This group of 16 subjects with diabetes, who all had remission through the study, had measured ISR responses to oral glucose that were comparable with nondiabetic controls starting 1 month after surgery and extending for 3 years. However, there was much less improvement in IV glucose-stimulated insulin secretion. The findings suggest that although enhanced incretin action can normalize prandial insulin responses in subjects who were formerly diabetic, more subtle stimuli, such as graded glucose infusions, may be a more sensitive measure of the capacity inherent in β-cells.
The importance of β-cell function in the response of patients with diabetes to bariatric surgery is also reflected in the prediction of disease remission. Most prediction models incorporate some index of diabetes severity such as fasting or stimulated C-peptide. These measures have been shown repeatedly to be independent predictors of diabetes outcomes from surgery (235–238). Although C-peptide is included in some broader prediction models (239), that compromised β-cell function before bariatric surgery reflects the resolution of diabetes afterward speaks to the importance of improved insulin secretion to mediate surgical effects.
Hyperinsulinemic hypoglycemia syndrome.
As more patients have had bariatric procedures during the past two to three decades, clinicians have started to recognize a syndrome of postprandial hypoglycemia, mostly in patients several years following RYGB. Originally attributed to the dumping syndrome that has long been observed in patients with gastric surgery (240), several case series described a more severe condition, often involving neuroglycopenic symptoms (241). Since these initial descriptions it has become clear that a subset of patients with RYGB develop recurrent hypoglycemia, with glucose levels <3 mM occurring 1 to 3 hours after meals. These hypoglycemic periods are characterized by hyperadrenergic and neuroglycopenic symptoms (242–244).
The prevalence of hyperinsulinemic hypoglycemia syndrome is not well established, in great part because of a lack of consensus on diagnostic criteria (245). Rates have been estimated at <1% based on hospitalization for hypoglycemia (246) or self-reporting (247), 13% based on a longitudinal cohort study (248), up to 30% based on an OGTT or MMTT (249, 250), and as high as 75%, mostly asymptomatic, based on continuous glucose monitoring (250, 251). Affected patients have hypoglycemia after some, but not all, meals (252), which may be accounted for by differences in amounts of carbohydrate ingested (253). Clinical hypoglycemia has been described rarely in patients with VSG (254), and almost never after AGB. A single case report of hypoglycemia after BPD-DS was associated with advanced liver disease (255).
Studies of subjects with the postsurgery hypoglycemia syndrome have consistently demonstrated relative meal-induced hyperinsulinemia compared with matched subjects with RYGB without a history of symptomatic low glucose (244, 254, 256, 257). This difference becomes magnified when plasma insulin is adjusted for prevailing glycemia (243). There is also a tendency for subjects with the most dramatic symptoms to have both higher rates of insulin secretion and lower rates of insulin clearance (186). The high rates of meal-induced insulin secretion in subjects with the hypoglycemia syndrome were initially attributed to increased GLP-1 secretion and action (241, 243), but subsequent studies including greater numbers of subjects did not demonstrate significant differences in plasma GLP-1 (229). Moreover, the insulinotropic activity of meal-induced GLP-1 was not increased in RYGB subjects with hypoglycemia compared with a group of matched, asymptomatic RYGB individuals (229). However, blockade of the GLP-1 receptor (GLP-1R) with the peptide exendin-(9–39) almost completely mitigates meal-induced hypoglycemia in symptomatic RYGB subjects (217, 258). These studies implicate increased sensitivity to GLP-1 as a mechanism for the syndrome of hyperinsulinemic hypoglycemia in a subset of gastic bypass subjects, and they suggest a therapeutic strategy for their treatment.
Some post-RYGB patients have had recurrent hypoglycemia to such severity that partial or subtotal pancreatectomy was performed to blunt hypersecretion of insulin (242, 259, 260). Microscopic examination of surgical specimens from these patients were described as showing features of nesidioblastosis, with hyperplasia of islet β-cells and increased nuclear size, suggesting changes at the tissue level that could account for hypersecretion of insulin (242, 260–262). The possibility that islet cell growth after RYGB underlies clinical hypoglycemia is supported by the typical delay of 1 to 2 years before the onset of symptoms and by a handful of cases that describe recurrence of symptoms following partial but not total pancreatectomy [e.g., (259)]. However, postsurgical β-cell hypertrophy has been questioned by other investigators who could not confirm the histologic picture of nesidioblastosis in a reexamination of some of these pancreatectomy samples (263). Furthermore, a general concern about the pathological investigation of the post-RYGB hypoglycemia syndrome is a lack of pancreas samples from asymptomatic subjects with surgery. Moveover, two lines of evidence suggest that prandial hyperinsulinemia is not due to an increase in β-cell mass. First, the syndrome has been corrected by reoperation, either to increase gastric restriction (260) or reverse the RYGB (264). Second, subjects with hypoglycemia after RYGB have comparable insulin secretion in response to IV glucose as for control subjects without surgery (265), suggesting that they do not have generalized β-cell hyperfunction. Thus, although at present several hypotheses speak to the causative mechanisms for hyperinsulinemic hypoglycemia after RYGB, none has been definitively established.
Glucagon secretion following bariatric surgery
A surprising but now well-established finding is that patients with RYGB, BPD, or VSG, as well as rodents with VSG (93), have significant increases in circulating glucagon following meal ingestion (93, 266–268). The profile of prandial glucagon in surgical patients follows closely the temporal pattern of GLP-1 secretion (217, 244), leading to conjecture that enteroendocrine L-cells produce and secrete glucagon after RYGB. However, a recent report suggested that elevated plasma glucagon in surgical patients may be an assay artifact due to increased concentrations of cross-reacting proglucagon peptides (269). It is noteworthy that plasma glucagon levels in subjects with RYGB more than double after a mixed nutrient meal, but they do not increase further with postprandial hypoglycemia (217). These findings suggest substantial changes in α-cell function in these people, possibly leading to defective counterregulation. α-Cell function following surgery has not been well characterized, but the more extreme glucose excursions in surgical patients after meals may be accounted for in some measure by defective glucagon secretion or action.
GLP-1 and the incretin effect following bariatric surgery
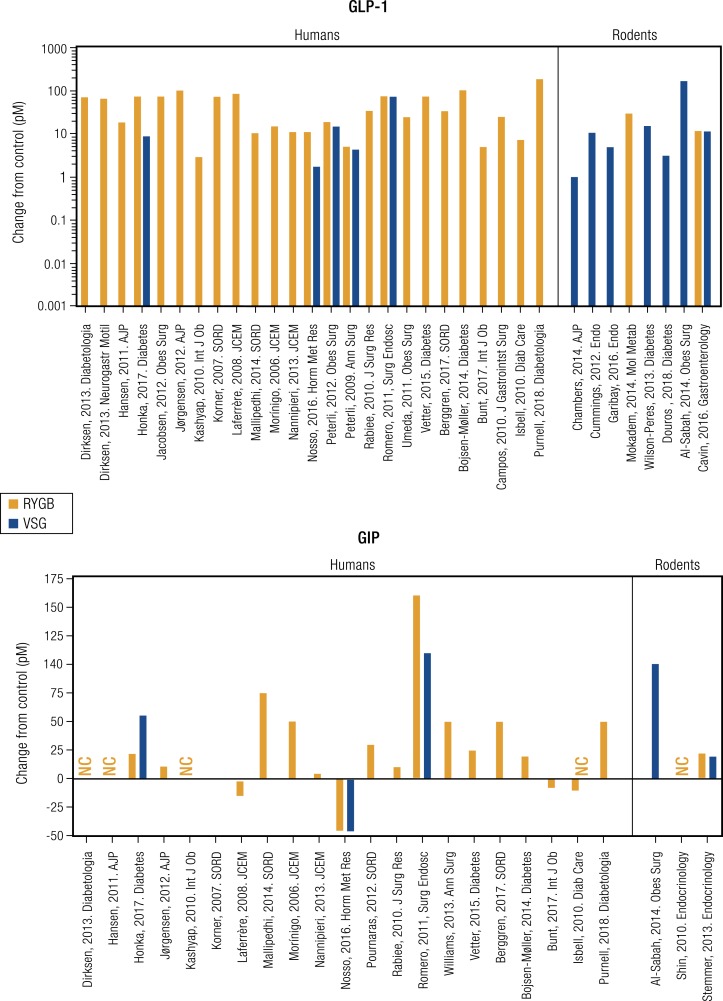
The rapid GI transit that is one of the proximal results of RYGB and VSG has dramatic effects on GI hormone secretion (Fig. 3). A hallmark of both procedures is the huge increases in GLP-1 release after meals (Fig. 3). Prandial GLP-1 levels rise ∼2-fold in humans with intact GI tracts, but after surgeries that speed gastric emptying the peptide increases to 10-fold and more above basal concentrations (267, 270–272). The increase in circulating GLP-1 after surgery is likely a function of more rapid passage of nutrients into the intestine because GLP-1 release has previously been demonstrated to be more sensitive to the rate, rather than the amount, of nutrient entry into the intestine (273–275). Exemplifying this point, AGB, which does not enhance nutrient delivery to the absorptive surface of the gut, has minimal effect on GLP-1 secretion compared with controls without surgery (276). The case with BPD is more complex. These patients do not have the dramatic changes in prandial glucose that mark VSG and RYGB, but they do experience elevated meal-induced GLP-1 concentrations (161, 202, 219) presumably due to increased rates of enteral nutrient flow through the small bowel.
Figure 3.
Incretin secretion after RYGB or VSG. GLP-1 (top) and GIP (bottom) secretion following RYGB (red bars) and VSG (blue bars). Data are presented as change relative to control subjects in studies of humans and rodents. References for each study are cited below.
GLP-1 is produced primarily by enteroendocrine L-cells that are distributed in a graded fashion throughout the intestine, with fewer cells in the upper gut, and the highest concentration in the ileum and colon. However, this distribution is modified following bariatric surgery. Following an RYGB a dense population of L-cells begins to appear in the Roux limb of the small intestine (277), the portion of the midjejunum anastomosed to the gastric pouch. Conversely, after VSG, L-cells are generated in greater density in the upper jejunum (277). Thus, it appears that there is a rearrangement of enteroendocrine cells within the GI tract that differs depending on the surgical procedure performed, but these modified distributions are consistent with increased GLP-1 secretion.
The incretin effect is potentiated following RYGB (278, 279), an effect that has been attributed in great part to GLP-1. In a comparison of nondiabetic RYBG and weight-matched control subjects without surgery, insulin secretion to IV glucose stimulation was similar between groups. However, insulin release in response to ingested glucose was substantially greater in the RYGB group, even when circulating glucose was matched by a glucose clamp (229). In this experimental setting, GLP-1R blockade caused a twofold to threefold greater reduction of insulin secretion in postsurgical subjects compared with a control group with an intact GI tract and significantly lower plasma GLP-1 concentrations. These findings demonstrate that higher prandial GLP-1 secretion following RYGB is associated with an enhanced GLP-1 effect on β-cell function.
The high levels of meal-induced GLP-1 and augmented GLP-1–stimulated insulin secretion among RYGB subjects raises the question of whether these effects can account for the dramatic improvement in glucose regulation in patients with diabetes undergoing surgery. In fact, this does not appear to be the case. Meal-induced GLP-1 did not vary among subjects who had remission of preexisting T2DM and those who remained diabetic following either RYGB (280) or VSG (281). Moreover, five studies have examined the impact of GLP-1 on postsurgical glucose control during meal tolerance tests with and without GLP-1R blockade (281–285). In these studies treatment with the GLP-1R antagonist exendin-(9–39) impaired glucose tolerance in subjects with RYGB (282–285) or VSG (281), but not to a greater degree than before surgery, or in comparison with nonoperated control subjects [Fig. 4(a)]. The lack of a disproportionate exendin-9 effect on glucose homeostasis after surgery indicates that GLP-1 signaling is not the primary mediator of improved diabetes (286). Although GLP-1 does not seem to account for the greater glucose tolerance among patients with diabetes after surgery, it does contribute significantly to the increased insulin secretion seen after RYGB or VSG [Fig. 4(b)]. In every study where postsurgical subjects with diabetes were given exendin-9, prandial insulin was disproportionately reduced (281–285). Similar findings have been reported in nondiabetic subjects with RYBG (229). This discrepant set of responses to GLP-1R blockade in postbariatric surgery patients, that is, a significant diminution of insulin secretion but no effect on acute glucose regulation, has not been explained. However, the reproducibility of the finding is clear. It is plausible that glucose disposition after surgery is not a linear function of circulating insulin, or that there are compensatory mechanisms that maintain homeostasis independent of β-cell secretion.
Figure 4.
Effects of GLP-1R blockade with exendin-(9–39) in subjects with diabetes with and without RYGB or VSG. (a) The effect of Ex-9 on glycemic responses to meal tests is shown for five separate studies. There is little difference in the magnitude of glucose intolerance induced by exendin-9 in subjects with or without surgery. (b) The effect of exendin-9 on insulin responses to meals. Insulin secretion after GLP-1R blockade was less in subjects with surgery than in controls. The surgical patients in Jiménez 2014 had VSG; the surgical groups in the other studies had RYBG.
Secretion of GIP and other GI peptides
It is noteworthy that the secretion of GIP, the other major incretin, after bariatric surgery is still a matter of debate. Postprandial GIP levels have been reported as increased, decreased, or unchanged in RYGB subjects (180, 218, 278, 287). Most of the K-cells that produce and release GIP are located in the duodenum and upper jejunum, regions that are bypassed with RYGB. However, in subjects receiving supplemental feeding through gastrostomy tubes after surgery, GIP responses were comparable whether a test meal was administered into the remnant stomach and bypassed the upper gut or was taken orally into the Roux limb (288). These findings suggest adequate distribution of K-cells throughout the small intestine to maintain GIP release, and, in fact, these cells are increased in the postgastroenterostomy mucosa 10 to 12 months after gastric bypass (289, 290). Although subjects with RYGB maintain a degree of GIP release that is only modestly higher or lower than normal, these changes in no way approach the enormous increase in GLP-1 that is a hallmark of this procedure.
There is much less information about GIP release after VSG. Because GIP release has been described as a linear function of enteral glucose delivery (273), and because subjects with VSG have increased rates of nutrient entry into the upper GI tract, a logical inference would be that they would have higher prandial levels of this incretin. However, the limited information that is available does not support this hypothesis, and GIP secretion is unchanged compared with preoperative or nonoperated subjects in most postsurgical subjects (180, 209) (Fig. 3). The prandial secretion of GIP is reduced after BPD (201, 219).
In addition to uncertainty about the relative amounts of GIP secretion following bariatric surgery, the role of GIP receptors on α-cells and β-cells to modulate islet function in postoperative patients remains almost wholly unexplored. The lack of information regarding GIP action after surgery is an important gap in the knowledge base that could explain some of the changes in insulin secretion among patients with T2DM. Although the insulinotropic actions of GLP-1 are moderately attenuated in persons with diabetes (291), the β-cell response to GIP in this group is almost entirely absent (292–296). Insulin treatment to improve HbA1c can restore β-cell sensitivity to GIP (297, 298), as well as the incretin effect (299), in subjects with T2DM. This specific and correctable defect in incretin action among patients with diabetes and the particular effectiveness of bariatric procedures to restore insulin secretion in this group raise the possibility that at least some of the effects of surgery are mediated by GIP. This hypothesis is testable using similar approaches to those used to define the role of GLP-1 in bariatric surgery.
A number of other insulinotropic gut peptides are also known to be differentially regulated following surgery. Gastrin secretion decreases after RYGB and increases with VSG in humans (185, 187, 300, 301). Secretion of peptide YY and cholecystokinin is increased after VSG and RYGB (300, 302, 303). Postprandial pancreatic polypeptide and somatostatin did not change following RYGB (187), whereas other studies show that fasting pancreatic polypeptide is decreased following both RYGB and VSG (185). Rats with BPD have been reported to have increased cholecystokinin and ghrelin cell content in the ileum; however, there are no data regarding the secretion of these peptides in humans or rodents after this surgery (304). The role these factors play in normal physiology is not established in any depth, and their impact after bariatric surgery is unknown.
Other humoral factors influencing islet function
A number of islet-regulating hormonal factors and metabolites change in the context of either an OGTT or MMTT following bariatric surgery. None of these has been characterized as thoroughly as GLP-1, but they are worth mentioning to provide a thorough overview of how modified gut anatomy may affect islet function. Surgery generally decreases free fatty acids and cholesterol (228, 305, 306); it is curious that RYGB-induced, postprandial hypoglycemia correlates with high triglycerides (307). BPD decreases adipocyte lipolysis within 3 days and improves the sensitivity of adipocytes to insulin (197); RYGB also has a rapid effect to enhance adipocyte insulin sensitivity (158).
Protein digestion and absorption increase after RYGB in humans (74). Amino acids, including the metabolically important branch-chain amino acids (BCAAs), display rapid rates of appearance and clearance similar to glucose (308). BCAAs are reduced in nondiabetic subjects after weight loss from RYGB and AGB (309, 310), but not in subjects with weight loss from dietary restriction (310). Increased plasma concentrations of BCAAs have been associated with insulin resistance (311), and this relationship was observed in subjects with RYGB and AGB (309).
Plasma bile acids increase twofold in subjects who have had RYGB, but they do not change following AGB (241, 312). Although proposed to account for some of the weight loss–independent effects of RYGB on metabolism (313), there was no association of bile acid concentrations with insulin secretion or insulin sensitivity among nondiabetic subjects who had lost 20% of body weight with RYGB (312).
Insights From Preclinical Studies of Bariatric Surgery
Surgical effects on body weight and food intake in preclinical models
A number of preclinical, mostly rodent, models of bariatric surgery have been developed. The systemic physiology of these rodent models is largely consistent with surgery in human subjects. These studies, and particularly the application of surgery in mouse genetic models, has allowed testing of more refined mechanistic hypotheses of bariatric surgery. However, there are several features of preclinical models that need to be considered when extending the results to humans (272, 314, 315). Rodent studies generally implement high-fat feeding regimens for 2 to 4 months in genetically identical animals under controlled conditions to achieve obesity and relative glucose intolerance (316, 317). This represents a relatively acute, homogeneous metabolic challenge, whereas humans are subject to decades of lifestyle, genetic, and environmental factors that contribute to the development of obesity and diabetes (318). Rodents given either bariatric or sham surgery lose up to 20% of their body weight during a matter of weeks before returning to a trajectory of weight gain parallel to, but never returning to, that of control animals fed ad libitum (177, 271, 314). This is an accelerated version of the dynamics of weight loss in humans who have maximal weight loss during 6 to 12 months before reaching a plateau or slow weight regain (8). The rapid weight loss observed in rodent studies improves glucose control in both treatment and sham-operated control groups in the early postoperative time period. A key benefit available for experiments with preclinical models is the ability to pair-feed a sham-operated control group, allowing matched caloric intake and generally comparable body weights to animals receiving surgery. This allows a more direct test of effects of surgery that are independent of energy balance, a key question raised in human research of bariatric surgery.
Food consumption is initially reduced after bariatric surgery in rodents (319). However, mice subjected to VSG subsequently increase their caloric intake to similar levels as controls within 2 weeks of surgery, even though their preference shifts toward less energy-dense foods (319). Lean body mass tends to increase, whereas fat mass is reduced, after surgery (319, 320). The mechanisms underlying this effect are unknown but raise the possibility that these models could be used to identify novel therapeutic targets. Rats given either RYBG or VSG, and mice with VSG, have lower fasting blood glucose compared with sham-operated controls (302, 321, 322). Fasting blood glucose is often lowered in a weight-independent manner in rodents when comparisons are made to weight-matched and/or pair-fed animals (302, 321). Alternatively, rats given an AGB have no change in fasting blood glucose (323). This suggests a weight-independent enhancement of insulin sensitivity and/or reduction in HGP after procedures that have been more effective for diabetes resolution in humans. There is a need for further research regarding changes to nutrient handling in critical tissues such as the liver after bariatric surgery, as well as how these changes are integrated at the level of the islet to modify basal glucose levels.
Surgical effects on glucose control and insulin secretion in preclinical models
Similar to nearly all available human data, glucose tolerance is changed in response to either a glucose or mixed-nutrient gavage in rodents after surgery, frequently with a more rapid initial excursion but an overall reduction in glycemic exposure (i.e., glucose area under the curve) (93, 177, 210, 324–326). This glycemic response is accompanied by elevated plasma insulin (177, 210, 324, 325) and higher plasma concentrations of postprandial GLP-1 (93, 272, 326, 327); the latter has been associated with increased rates of enteral nutrient flux after surgery in rodents (328, 329). Additionally, postgavage GIP appears to be elevated after VSG in rats and mice, suggesting hyperstimulation of duodenal K-cells after this procedure (93, 330), whereas GIP secretion after RYGB is mixed, with some studies reporting elevated levels (330) and others no change (303). Notably, many studies demonstrate improved glucose control during either IP or IV glucose tolerance tests, which bypass the surgically modified gut and do not affect secretion of incretins (93, 326, 331). These findings are in keeping with those in humans with diabetes and suggest that surgery causes changes in the β-cell responsiveness to glucose that are independent of acute stimulation by gut factors. Indeed, a recent study by Douros et al. (93) demonstrates that pancreatic islets isolated from mice with a VSG undergo intrinsic changes within a week of surgery that sensitize the insulin response to glucose and uniquely modify the transcriptomic profile compared with calorically restricted, weight-matched controls. It is unclear how the changes in GI anatomy are communicated to the islet to evoke these changes.
Mechanistic studies in mice with candidate gene deletions
A number of studies have been reported where genetic mouse lines, particularly those with single gene knockouts, were used to test specific mechanisms underlying the metabolic benefit of bariatric surgery. This candidate gene approach is highlighted by several studies utilizing GLP-1R knockout models, a logical choice given the consistently high circulating levels after surgery and potent actions for glycemic reduction. In two studies with VSG (320, 326) and one with RYGB (177), deletion of the GLP-1R did not attenuate the benefit of surgery on weight loss or glucose tolerance, suggesting that other mechanisms compensate, or substitute, for the absence of GLP-1 signaling. A single study of VSG in mice with a β-cell deletion of the GLP-1R did show a modest reduction of the surgical effect on glucose tolerance (332).
Beyond the studies of GLP-1R knockout lines, it has been shown that ghrelin (331), the GLP-2 receptor (333), acyl CoA:monoacylglycerol acyltransferase-2 (MGAT2) (334), Magel2 (a Prader–Willi syndrome model) (335), gustducin (177), apo AIV (329), serotonin (336), and lipocalin-type prostaglandin D2 (L-PGDS) (335) are dispensable for the glycemic benefits of VSG. The effect of the bile acid–binding receptor TGR5 is not required for glucose lowering after RYGB (337), but it may contribute to the glucose lowering response after VSG (338). Conversely, leptin appears to be required for improving glycemic control after RYGB in mice (339), as ob/ob animals given surgery failed to improve glucose tolerance, insulin sensitivity, and hyperinsulinemia despite weight loss (339). Perhaps the best example of a single factor with an effect to mediate the actions of VSG is the farnesoid-X receptor (FXR, a nuclear bile acid receptor) (316). Mice with a global deletion of this gene failed to lose weight or improve glucose tolerance after surgery. However, this model has some limitations in that the knockout mice were leaner than wild-type controls and had better glucose tolerance prior to surgery.
Overall, studies with rodent models have provided some valuable insights into the physiology of bariatric surgery and should continue to augment clinical research in this area. Ongoing technical refinements and more widespread availability and expertise will enhance the applications and breadth of questions that can be tested in animal models. The published literature to date indicates that improved glucose homeostasis after surgery is unlikely to be mediated by single gene products, whether they be receptors, their ligands, or other regulatory factors. Rather, the view from preclinical work suggests more complex mechanisms that involve the interplay of a number of mediators and systems. Preclinical models are amenable to unbiased discovery methods such as gene transcription analysis, metabolomics, and proteomics that are useful for interrogating complex systems, and it is easy to foresee these sorts of approaches leading to important advances in the future.
Conceptualizing the Potential Mechanisms for Surgery to Improve Glucose Control
Connecting anatomic modifications of surgery to islet function
Work during the last century has demonstrated that surgery to the GI tract has multiple, potent actions on metabolic physiology. However, only recently have these procedures been sufficiently standardized to allow for a comparison of effects between surgical approaches. Even with widespread clinical use of RYGB and VSG, and to a lesser degree AGB and BPD, there have been few studies designed to compare and contrast the effects of various surgically revised GI anatomies. The bulk of evidence suggests that AGB works mainly through decreasing food intake, with few of the changes seen with the other procedures such as GI motility, absorption, and hormone secretion. Indeed, AGB has been applied in comparisons with RYGB in a manner similar to dietary and lifestyle measures under the assumption that it has few effects beyond reduction of caloric consumption (189, 340).
It is also clear that BPD causes significantly more malabsorption than do the other bariatric procedures, and in fact it may cause effects that are independent of a diet based on the limitations imposed on enteral nutrient uptake (108). However, there are several other features of the BPD that also distinguish this surgery from the others. First, the larger gastric remnant (300 mL compared with ∼30 mL for RYGB and ∼100 mL for VSG and BPD-DS) and distal gastrectomy (341) may actually delay rather than accelerate passage of nutrients through the gut (89), although this is not true of BPD-DS (69). Second, the resolution of insulin resistance seems to be faster for BPD than for the other procedures, with unequivocal improvement after 1 week (201). Third, the improvement of insulin secretion after surgery does not seem to be as great for BPD as for VSG and RYGB (180, 201), although this may be partly due to adaptation for a greater degree of insulin sensitivity. Interestingly, the DI undergoes greater enhancement after BPD-DS than after VSG in the only study comparing the two (211). Notably, responses that are consistently enhanced by RYGB such as insulin clearance and the incretin effect are only minimally affected by BPD (113). Finally, although the GLP-1 response is enhanced approximately twofold after BPD (161, 197, 211), it does not appear to be as substantial as in patients with VSG and RYGB, whereas GIP seems to be significantly reduced, an observation unique to this surgery (201, 219). These examples cannot be considered definitive in the absence of more direct comparisons in well-defined surgical groups. However, the differences support distinct mechanisms of action for specific surgical manipulation and support comparative studies with robust physiologic testing.
It is notable that VSG and RYGB, two procedures that have major differences in postsurgical anatomy, have the most comparable acute and chronic effects on glucose metabolism and weight loss. Although the results of one study suggest that enhanced insulin secretion in the early postoperative phase may be greater with VSG (99), there is insufficient evidence to draw more distinctions between the two procedures. What is notable is that VSG and RYGB share the effect of greatly accelerated passage of ingested nutrients into the intestine, with the rapid absorption of glucose, amino acids, and other small molecules challenging homeostasis with each meal. The need to adapt to this challenge may provide the impetus for physiologic changes that improve glucose metabolism (65). Research to determine whether these two procedures have distinct or shared mechanisms of action on glucose tolerance would provide a major advance.
More direct and refined comparisons of the physiologic changes following different bariatric procedures, interpreted in the context of surgical anatomy, are likely to provide insight into the mechanisms by which VSG, RYGB, and BPD act. Inclusion of measures of GI motility and substrate absorption are important for this sort of investigation because meal appearance of substrates is important for assessing systemic fluxes and may in fact play a role in initiating metabolic mechanisms. This line of investigation would also benefit from validation of simple metrics such as HOMA modeling and the DI among the various procedures, as well as from a rigorous test of the relationship between insulin clearance and insulin sensitivity.
Temporally dynamic model
There are longitudinal data available to suggest that processes involved in glucose regulation change and adapt over time after surgery. There is also evidence for adaptation of intestinal histology and function (70, 224). Although the temporal profile of adaptations cannot be mapped with great detail, a general description is possible (Fig. 5). Initial reductions in glycemia and insulin concentrations seem to be driven in great part by decreased caloric intake. At least with VSG and RYGB, there appears to be an enhancement of β-cell function in the first postoperative month, mostly as a result of increased enteral stimulation of insulin secretion. This heightened β-cell function is concomitant with small, gradual suppression of HGP, possibly driven by increased hepatic insulin action. These early phenomena could contribute to what has been termed weight loss (<15%)–independent effects of surgery, although they occur in a setting of negative energy balance. As the postoperative time frame progresses and patients begin to lose substantial amounts of weight, there are detectable changes in global insulin sensitivity, and insulin secretion is dampened to accommodate this, even when the DI is ultimately relatively increased. The likelihood that the response to surgery evolves over time is an important consideration for the design of experiments, where the timing of cross-sectional comparisons or longitudinal measures might be expected to impact outcomes.
Figure 5.
Schematic of a hypothetical model of adaptations of insulin secretion and insulin sensitivity after RYGB and AGB. Against a background of decreased caloric intake and decreasing body weight, parameters of glucose metabolism have varying courses with RYGB and AGB. As body weight decreases, insulin sensitivity improves after body surgeries. In nondiabetic subjects, insulin secretion is fairly static after AGB, but with the significant increase of insulin action the DI improves slightly in nondiabetic subjects. Following RYGB enhanced incretin stimulation leads to greater insulin responses; in subjects with diabetes, FPIR is restored and secretion after meals is also improved. Over time, as weight loss progresses, there is a decrease in the absolute amount of insulin released among nondiabetic subjects, although the DI improves. The dampening of the β-cell response after RYGB compensates for greater insulin sensitivity, mitigating the risk of hypoglycemia. See (165, 168, 185, 213).
Summary
The last 20 years have seen a sharp rise in the clinical application of bariatric surgery. There has been growth in the number of surgeons specializing in the treatment of obesity and diabetes, a refinement and standardization of procedures, and an increase in the acceptance and utilization of surgery across the spectrum of health care providers and payers. A parallel rise has also occurred in the depth and quality of research focused on bariatric surgery, with much of the work addressing its effects on diabetes. Data from carefully done observational studies have now been bolstered by results from randomized clinical trials to provide a clearer picture of safety, efficacy, and outcomes. Valuable subdomains of bariatric surgery research have also grown and developed, including applications of epidemiology, quantitative modeling, and health economics.
There has also been progress in understanding the physiology underlying the mechanisms whereby surgery affects glucose metabolism, processes fundamental to the treatment of diabetes and the focus of this review. The large effects of surgery to quickly reduce diabetic hyperglycemia are one of the most dramatic shifts of metabolism in clinical medicine. Although decreased caloric consumption and negative energy balance play an important role in the factors leading to diabetes resolution, there is solid evidence that surgery also changes parameters such as insulin secretion and regulation of HGP, which can reduce glycemia. Understanding how surgery to the GI tract co-opts normal physiology to amplify glucose clearance is an area of clinical investigation that is still at an early stage. However, the potential for research in this area seems very promising because the dramatic effects of surgery suggest underlying mechanisms that are central to metabolic physiology and could serve as therapeutic targets. The state of current knowledge in this area is sufficient to support the design and application of larger, more powerful studies directed at specific mechanisms affected by surgery. This is arguably the next frontier in the application of surgery to treat diabetes.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants F32DK115031 (J.D.D.), R01DK097550 (J.T.), and R01DK101991 (D.A.D.).
Glossary
Abbreviations:
- AGB
adjustable gastric band
- BCAA
branched-chain amino acid
- BMI
body mass index
- BPD
biliopancreatic diversion
- BPD-DS
BPD with duodenal switch
- DI
disposition index
- FPIR
first phase insulin release
- GI
gastrointestinal
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide 1
- GLP-1R
GLP-1 receptor
- HbA1c
glycated hemoglobin
- HGP
hepatic glucose production
- HOMA
homeostasis model assessment
- HOMA-S
HOMA of insulin sensitivity
- ISR
insulin secretion rate
- MMTT
mixed meal tolerance test
- OGTT
oral glucose tolerance test
- RCT
randomized controlled trial
- RYGB
Roux-en-Y gastric bypass
- STAMPEDE
Surgical Treatments and Medication Potentially Eradicate Diabetes Efficiently
- T2DM
type 2 diabetes mellitus
- VLCD
very low-calorie diet
- VSG
vertical sleeve gastrectomy
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References and Notes.
References and Notes
- 1. Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323–2330. [DOI] [PubMed] [Google Scholar]
- 2. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. [DOI] [PubMed] [Google Scholar]
- 3. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and –United States, 1988–2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Data and statistics: US national, state, and county diabetes data. www.cdc.gov/diabetes/data/. Accessed 21 January 2019.
- 6. Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. Pharmacoeconomics. 2015;33(7):707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGMM, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27(1):2–21. [DOI] [PubMed] [Google Scholar]
- 10. Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang LM, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309(21):2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. [DOI] [PubMed] [Google Scholar]
- 12. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447–1463. [DOI] [PubMed] [Google Scholar]
- 13. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. [DOI] [PubMed] [Google Scholar]
- 14. Faria GR. A brief history of bariatric surgery. Porto Biomed J. 2017;2(3):90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moshiri M, Osman S, Robinson TJ, Khandelwal S, Bhargava P, Rohrmann CA. Evolution of bariatric surgery: a historical perspective. AJR Am J Roentgenol. 2013;201(1):W40–W48. [DOI] [PubMed] [Google Scholar]
- 16. Hopkins KD, Lehmann ED. Successful medical treatment of obesity in 10th century Spain. Lancet. 1995;346(8972):452. [DOI] [PubMed] [Google Scholar]
- 17. Ramada Faria GF, Nunes Santos JM, Simonson DC. Quality of life after gastric sleeve and gastric bypass for morbid obesity. Porto Biomed J. 2017;2(2):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pach R, Orzel-Nowak A, Scully T. Ludwik Rydygier—contributor to modern surgery. Gastric Cancer. 2008;11(4):187–191. [DOI] [PubMed] [Google Scholar]
- 19. Fallis LS, Szilagyi DE. Observations on some metabolic changes after total pancreatoduodenectomy. Ann Surg. 1948;128(4):639–667. [PubMed] [Google Scholar]
- 20. Barnes CG. Hypoglycaemia following partial gastrectomy; report of three cases. Lancet. 1947;2(6476):536–539. [DOI] [PubMed] [Google Scholar]
- 21. Clausen EG, Jake RJ. Subtotal gastric resection; an appraisal of a means of treatment of benign peptic ulceration of the stomach and duodenum. Calif Med. 1959;90(6):407–410. [PMC free article] [PubMed] [Google Scholar]
- 22. Komlos J, Brabec M. The trend of BMI values of US adults by centiles, birth cohorts 1882–1986. NBER Working Paper No. 16252. Available at: www.nber.org/papers/w16252. Accessed December 2011.
- 23. Cuff T. The body mass index values of mid-nineteenth-century West Point cadets. Hist Methods. 1993;26(4):171–182. [Google Scholar]
- 24. Carson SA. Racial differences in body mass indices of men imprisoned in 19th century Texas. Econ Hum Biol. 2009;7(1):121–127. [DOI] [PubMed] [Google Scholar]
- 25. Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. 1954;140(3):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faxon HH, Schoch WG Jr. Gastrojejunocolic fistula; review of nine cases, with use of vagus resection as part of the operative procedure in one. N Engl J Med. 1949;240(3):81–87. [DOI] [PubMed] [Google Scholar]
- 27. Sherman CD Jr, May AG, Nye W, Waterhouse C. Clinical and metabolic studies following bowel by-passing for obesity. Ann N Y Acad Sci. 1965;131(1):614–622. [DOI] [PubMed] [Google Scholar]
- 28. Shibata HR, MacKenzie JR, Long RC. Metabolic effects of controlled jejunocolic bypass. Arch Surg. 1967;95(3):413–428. [DOI] [PubMed] [Google Scholar]
- 29. Melissinos K, Katsaros I. Pathogenesis of the disturbance in the blood-sugar regulation after gastrectomy; a research study. Am J Dig Dis. 1954;21(10):288–292. [DOI] [PubMed] [Google Scholar]
- 30. Holdsworth CD. The gut and oral glucose tolerance. Gut. 1969;10(6):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman MN, Sancetta AJ, Magovern GJ. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet. 1955;100(2):201–204. [PubMed] [Google Scholar]
- 32. Holdsworth CD, Dawson AM. The absorption of monosaccharides in man. Clin Sci. 1964;27:371–379. [PubMed] [Google Scholar]
- 33. Cameron AJ, Ellis JP, McGill JI, Le Quesne LP. Insulin response to carbohydrate ingestion after gastric surgery with special reference to hypoglycaemia. Gut. 1969;10(10):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hastings-James R. Spontaneous hypoglycaemia. Lancet. 1949;1(6559):814–817. [PubMed] [Google Scholar]
- 35. MacDonald JA, Welsh WK. The immediate results of operations for duodenal ulcer: a comparative study of the morbidity and mortality of vagotomy and pyloroplasty versus subtotal gastrectomy. Can Med Assoc J. 1965;92:652–657. [PMC free article] [PubMed] [Google Scholar]
- 36. Stevens GA, Kipen CS, Ross WL. Comparative concurrent results of vagotomy procedures, conventional gastric resection and hemigastrectomy with subtotal vagotomy. Calif Med. 1950;73(3):229–231. [PMC free article] [PubMed] [Google Scholar]
- 37. Burstein R, Epstein Y, Charuzi I, Suessholz A, Karnieli E, Shapiro Y. Glucose utilization in morbidly obese subjects before and after weight loss by gastric bypass operation. Int J Obes Relat Metab Disord. 1995;19(8):558–561. [PubMed] [Google Scholar]
- 38. DeFronzo RA. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988;37(6):667–687. [DOI] [PubMed] [Google Scholar]
- 39. Porte D., Jr Banting lecture 1990. β-Cells in type II diabetes mellitus. Diabetes. 1991;40(2):166–180. [DOI] [PubMed] [Google Scholar]
- 40. Pories WJ, Caro JF, Flickinger EG, Meelheim HD, Swanson MS. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville gastric bypass. Ann Surg. 1987;206(3):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lauritsen KB, Christensen KC, Stokholm KH. Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol. 1980;15(4):489–495. [DOI] [PubMed] [Google Scholar]
- 42. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. [DOI] [PubMed] [Google Scholar]
- 43. Starkloff GB, Donovan JF, Ramach KR, Wolfe BM. Metabolic intestinal surgery. Its complications and management. Arch Surg. 1975;110(5):652–657. [DOI] [PubMed] [Google Scholar]
- 44. Kish GF, Parker FW, Joseph WL. Intestinal bypass in morbid obesity: long-term metabolic sequelae. Am Surg. 1975;41(12):786–792. [PubMed] [Google Scholar]
- 45. Buckwalter JA. Clinical trial of jejunoileal and gastric bypass for the treatment of morbid obesity: four-year progress report. Am Surg. 1980;46(7):377–381. [PubMed] [Google Scholar]
- 46. Gazet JC, Pilkington TR, Kalucy RS, Crisp AH, Day S. Treatment of gross obesity by jejunal bypass. BMJ. 1974;4(5940):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buchwald H, Varco RL. Ileal bypass in patients with hypercholesterolemia and atherosclerosis. Preliminary report on therapeutic potential. JAMA. 1966;196(7):627–630. [PubMed] [Google Scholar]
- 48. Michael P, Hocking MD, Margaret C, Duerson RN, O’Leary JP, Woodward ER. Jejunoileal bypass for morbid obesity—late follow-up in 100 cases. N Engl J Med. 1983;308(17):995–999. [DOI] [PubMed] [Google Scholar]
- 49. Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47(6):1345–1351. [DOI] [PubMed] [Google Scholar]
- 50. Mason EE. Vertical banded gastroplasty for obesity. Arch Surg. 1982;117(5):701–706. [DOI] [PubMed] [Google Scholar]
- 51. Flickinger EG, Pories WJ, Meelheim HD, Sinar DR, Blose IL, Thomas FT. The Greenville gastric bypass. Progress report at 3 years. Ann Surg. 1984;199(5):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waters GS, Pories WJ, Swanson MS, Meelheim HD, Flickinger EG, May HJ. Long-term studies of mental health after the Greenville gastric bypass operation for morbid obesity. Am J Surg. 1991;161(1):154–157. [DOI] [PubMed] [Google Scholar]
- 53. Franco JV, Ruiz PA, Palermo M, Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21(9):1458–1468. [DOI] [PubMed] [Google Scholar]
- 54. Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66(9):618–620. [DOI] [PubMed] [Google Scholar]
- 55. Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: 1. An experimental study in dogs. Br J Surg. 1979;66(9):613–617. [DOI] [PubMed] [Google Scholar]
- 56. Scopinaro N, Gianetta E, Pandolfo N, Anfossi A, Berretti B, Bachi V. Bilio-pancreatic bypass. Proposal and preliminary experimental study of a new type of operation for the functional surgical treatment of obesity [in Italian]. Minerva Chir. 1976;31(10):560–566. [PubMed] [Google Scholar]
- 57. Lagacé M, Marceau P, Marceau S, Hould FS, Potvin M, Bourque RA, Biron S. Biliopancreatic diversion with a new type of gastrectomy: some previous conclusions revisited. Obes Surg. 1995;5(4):411–418. [DOI] [PubMed] [Google Scholar]
- 58. Kuzmak LI, Rickert RR. Pathologic changes in the stomach at the site of silicone gastric banding. Obes Surg. 1991;1(1):63–68. [DOI] [PubMed] [Google Scholar]
- 59. Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4(4):353–357. [DOI] [PubMed] [Google Scholar]
- 60. Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg. 2003;13(4):649–654. [DOI] [PubMed] [Google Scholar]
- 61. Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132(6):2253–2271. [DOI] [PubMed] [Google Scholar]
- 62. Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20(4):447–453. [DOI] [PubMed] [Google Scholar]
- 63. Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–1521. [DOI] [PubMed] [Google Scholar]
- 64. Bernstine H, Tzioni-Yehoshua R, Groshar D, Beglaibter N, Shikora S, Rosenthal RJ, Rubin M. Gastric emptying is not affected by sleeve gastrectomy—scintigraphic evaluation of gastric emptying after sleeve gastrectomy without removal of the gastric antrum. Obes Surg. 2009;19(3):293–298. [DOI] [PubMed] [Google Scholar]
- 65. Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 2015;21(3):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–1832. [DOI] [PubMed] [Google Scholar]
- 67. English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–263. [DOI] [PubMed] [Google Scholar]
- 68. Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47(5):531–535. [DOI] [PubMed] [Google Scholar]
- 69. Hedberg J, Hedenström H, Karlsson FA, Edén-Engström B, Sundbom M. Gastric emptying and postprandial PYY response after biliopancreatic diversion with duodenal switch. Obes Surg. 2011;21(5):609–615. [DOI] [PubMed] [Google Scholar]
- 70. Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–2009. [DOI] [PubMed] [Google Scholar]
- 71. Riccioppo D, Santo MA, Rocha M, Buchpiguel CA, Diniz MA, Pajecki D, de Cleva R, Kawamoto F. Small-volume, fast-emptying gastric pouch leads to better long-term weight loss and food tolerance after Roux-en-Y gastric bypass. Obes Surg. 2018;28(3):693–701. [DOI] [PubMed] [Google Scholar]
- 72. Wang G, Agenor K, Pizot J, Kotler DP, Harel Y, Van Der Schueren BJ, Quercia I, McGinty J, Laferrère B. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP) [published correction appears in Obes Surg. 2013;23(7):1016]. Obes Surg. 2012;22(8):1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akkary E, Sidani S, Boonsiri J, Yu S, Dziura J, Duffy AJ, Bell RL. The paradox of the pouch: prompt emptying predicts improved weight loss after laparoscopic Roux-Y gastric bypass. Surg Endosc. 2009;23(4):790–794. [DOI] [PubMed] [Google Scholar]
- 74. Bojsen-Møller KN, Jacobsen SH, Dirksen C, Jørgensen NB, Reitelseder S, Jensen JE, Kristiansen VB, Holst JJ, van Hall G, Madsbad S. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am J Clin Nutr. 2015;102(3):600–607. [DOI] [PubMed] [Google Scholar]
- 75. Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, Naver LS, Worm D, Holst JJ, Madsbad S, Hansen DL, Madsen JL. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346-e255. [DOI] [PubMed] [Google Scholar]
- 76. Shah S, Shah P, Todkar J, Gagner M, Sonar S, Solav S. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6(2):152–157. [DOI] [PubMed] [Google Scholar]
- 77. Baumann T, Kuesters S, Grueneberger J, Marjanovic G, Zimmermann L, Schaefer AO, Hopt UT, Langer M, Karcz WK. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg. 2011;21(1):95–101. [DOI] [PubMed] [Google Scholar]
- 78. Mans E, Serra-Prat M, Palomera E, Suñol X, Clavé P. Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am J Clin Nutr. 2015;102(3):540–547. [DOI] [PubMed] [Google Scholar]
- 79. Vives M, Molina A, Danús M, Rebenaque E, Blanco S, París M, Sánchez A, Sabench F, Del Castillo D. Analysis of gastric physiology after laparoscopic sleeve gastrectomy (LSG) with or without antral preservation in relation to metabolic response: a randomised study. Obes Surg. 2017;27(11):2836–2844. [DOI] [PubMed] [Google Scholar]
- 80. Vigneshwaran B, Wahal A, Aggarwal S, Priyadarshini P, Bhattacharjee H, Khadgawat R, Yadav R. Impact of sleeve gastrectomy on type 2 diabetes mellitus, gastric emptying time, glucagon-like peptide 1 (GLP-1), ghrelin and leptin in non-morbidly obese subjects with BMI 30–35.0 kg/m2: a prospective study. Obes Surg. 2016;26(12):2817–2823. [DOI] [PubMed] [Google Scholar]
- 81. Mion F, Tolone S, Garros A, Savarino E, Pelascini E, Robert M, Poncet G, Valette PJ, Marjoux S, Docimo L, Roman S. High-resolution impedance manometry after sleeve gastrectomy: increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26(10):2449–2456. [DOI] [PubMed] [Google Scholar]
- 82. Burgerhart JS, van Rutte PW, Edelbroek MA, Wyndaele DN, Smulders JF, van de Meeberg PC, Siersema PD, Smout AJ. Association between postprandial symptoms and gastric emptying after sleeve gastrectomy. Obes Surg. 2015;25(2):209–214. [DOI] [PubMed] [Google Scholar]
- 83. Garay M, Balagué C, Rodríguez-Otero C, Gonzalo B, Domenech A, Pernas JC, Gich IJ, Miñambres I, Fernández-Ananín S, Targarona EM. Influence of antrum size on gastric emptying and weight-loss outcomes after laparoscopic sleeve gastrectomy (preliminary analysis of a randomized trial). Surg Endosc. 2018;32(6):2739–2745. [DOI] [PubMed] [Google Scholar]
- 84. Parikh M, Eisner J, Hindman N, Balthazar E, Saunders JK. Tests of correlation between immediate postoperative gastroduodenal transit times and weight loss after laparoscopic sleeve gastrectomy. Surg Endosc. 2012;26(12):3548–3551. [DOI] [PubMed] [Google Scholar]
- 85. Sista F, Abruzzese V, Clementi M, Carandina S, Cecilia M, Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg Obes Relat Dis. 2017;13(1):7–14. [DOI] [PubMed] [Google Scholar]
- 86. Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, Karkavitsas N. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258(6):976–982. [DOI] [PubMed] [Google Scholar]
- 87. de Jong JR, van Ramshorst B, Gooszen HG, Smout AJ, Tiel-Van Buul MM. Weight loss after laparoscopic adjustable gastric banding is not caused by altered gastric emptying. Obes Surg. 2009;19(3):287–292. [DOI] [PubMed] [Google Scholar]
- 88. Scopinaro N. Biliopancreatic diversion: mechanisms of action and long-term results. Obes Surg. 2006;16(6):683–689. [DOI] [PubMed] [Google Scholar]
- 89. Bonalumi U, Moresco L, Gianetta E, Civalleri D, De Cata T, Brignole E, Scopinaro N. Intestinal transit time in bilio-pancreatic bypass [in Italian]. Minerva Chir. 1980;35(13–14):993–996. [PubMed] [Google Scholar]
- 90. Jacobsen SH, Bojsen-Møller KN, Dirksen C, Jørgensen NB, Clausen TR, Wulff BS, Kristiansen VB, Worm D, Hansen DL, Holst JJ, van Hall G, Madsbad S. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254. [DOI] [PubMed] [Google Scholar]
- 91. Bradley D, Magkos F, Eagon JC, Varela JE, Gastaldelli A, Okunade AL, Patterson BW, Klein S. Matched weight loss induced by sleeve gastrectomy or gastric bypass similarly improves metabolic function in obese subjects. Obesity (Silver Spring). 2014;22(9):2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dirksen C, Eiken A, Bojsen-Møller KN, Svane MS, Martinussen C, Jørgensen NB, Holst JJ, Madsbad S. No islet cell hyperfunction, but altered gut-islet regulation and postprandial hypoglycemia in glucose-tolerant patients 3 years after gastric bypass surgery. Obes Surg. 2016;26(9):2263–2267. [DOI] [PubMed] [Google Scholar]
- 93. Douros JD, Niu J, Sdao S, Gregg T, Fisher-Wellman K, Bharadwaj M, Molina A, Arumugam R, Martin M, Petretto E, Merrins MJ, Herman MA, Tong J, Campbell J, D’Alessio D. Sleeve gastrectomy rapidly enhances islet function independently of body weight. JCI Insight. 2019;4(6):126688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Read NW. Diarrhée motrice. Clin Gastroenterol. 1986;15(3):657–686. [PubMed] [Google Scholar]
- 95. Nguyen NQ, Debreceni TL, Bambrick JE, Chia B, Deane AM, Wittert G, Rayner CK, Horowitz M, Young RL. Upregulation of intestinal glucose transporters after Roux-en-Y gastric bypass to prevent carbohydrate malabsorption. Obesity (Silver Spring). 2014;22(10):2164–2171. [DOI] [PubMed] [Google Scholar]
- 96. Blume CA, Boni CC, Casagrande DS, Rizzolli J, Padoin AV, Mottin CC. Nutritional profile of patients before and after Roux-en-Y gastric bypass: 3-year follow-up. Obes Surg. 2012;22(11):1676–1685. [DOI] [PubMed] [Google Scholar]
- 97. Baud G, Raverdy V, Bonner C, Daoudi M, Caiazzo R, Pattou F. Sodium glucose transport modulation in type 2 diabetes and gastric bypass surgery. Surg Obes Relat Dis. 2016;12(6):1206–1212. [DOI] [PubMed] [Google Scholar]
- 98. Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, Pigny P, Klein A, Kerr-Conte J, Gmyr V, Caiazzo R, Pattou F. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab. 2016;23(3):547–553. [DOI] [PubMed] [Google Scholar]
- 99. Nannipieri M, Mari A, Anselmino M, Baldi S, Barsotti E, Guarino D, Camastra S, Bellini R, Berta RD, Ferrannini E. The role of β-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(9):E1372–E1379. [DOI] [PubMed] [Google Scholar]
- 100. Sahebzamani FM, Berarducci A, Murr MM. Malabsorption anemia and iron supplement induced constipation in post-Roux-en-Y gastric bypass (RYGB) patients. J Am Assoc Nurse Pract. 2013;25(12):634–640. [DOI] [PubMed] [Google Scholar]
- 101. DeFilipp Z, Lister J, Gagné D, Shadduck RK, Prendergast L, Kennedy M. Intravenous iron replacement for persistent iron deficiency anemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(1):129–132. [DOI] [PubMed] [Google Scholar]
- 102. Tuomainen TP, Nyyssönen K, Salonen R, Tervahauta A, Korpela H, Lakka T, Kaplan GA, Salonen JT. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care. 1997;20(3):426–428. [DOI] [PubMed] [Google Scholar]
- 103. McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49(7):1661–1669. [DOI] [PubMed] [Google Scholar]
- 104. Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, Porter JL, Asplin J, Kuhn JA, Fordtran JS. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92(4):704–713. [DOI] [PubMed] [Google Scholar]
- 105. Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, Bergstralh EJ, Li X. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149(5):654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Benetti A, Del Puppo M, Crosignani A, Veronelli A, Masci E, Frigè F, Micheletto G, Panizzo V, Pontiroli AE. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes Care. 2013;36(6):1443–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Pihlajamäki J, Grönlund S, Simonen M, Käkelä P, Moilanen L, Pääkkönen M, Pirinen E, Kolehmainen M, Kärjä V, Kainulainen S, Uusitupa M, Alhava E, Miettinen TA, Gylling H. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism. 2010;59(6):866–872. [DOI] [PubMed] [Google Scholar]
- 108. Scopinaro N. Laparoscopic BPD. Obes Surg. 2000;10(6):524. [DOI] [PubMed] [Google Scholar]
- 109. Scopinaro N, Marinari GM, Pretolesi F, Papadia F, Murelli F, Marini P, Adami GF. Energy and nitrogen absorption after biliopancreatic diversion. Obes Surg. 2000;10(5):436–441. [DOI] [PubMed] [Google Scholar]
- 110. Khoo CM, Chen J, Pamuklar Z, Torquati A. Effects of Roux-en-Y gastric bypass or diabetes support and education on insulin sensitivity and insulin secretion in morbidly obese patients with type 2 diabetes. Ann Surg. 2014;259(3):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dean ED, Li M, Prasad N, Wisniewski SN, Von Deylen A, Spaeth J, Maddison L, Botros A, Sedgeman LR, Bozadjieva N, Ilkayeva O, Coldren A, Poffenberger G, Shostak A, Semich MC, Aamodt KI, Phillips N, Yan H, Bernal-Mizrachi E, Corbin JD, Vickers KC, Levy SE, Dai C, Newgard C, Gu W, Stein R, Chen W, Powers AC. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 2017;25(6):1362–1373.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wolff BS, Meirelles K, Meng Q, Pan M, Cooney RN. Roux-en-Y gastric bypass alters small intestine glutamine transport in the obese Zucker rat. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G594–G601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Salinari S, Mingrone G, Bertuzzi A, Previti E, Capristo E, Rubino F. Downregulation of insulin sensitivity after oral glucose administration: evidence for the anti-incretin effect. Diabetes. 2017;66(11):2756–2763. [DOI] [PubMed] [Google Scholar]
- 114. Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–323. [DOI] [PubMed] [Google Scholar]
- 115. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7(5):477–484. [DOI] [PubMed] [Google Scholar]
- 118. Blackstone R, Bunt JC, Cortés MC, Sugerman HJ. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis. 2012;8(5):548–555. [DOI] [PubMed] [Google Scholar]
- 119. Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL II, Robertson RP, Rubino F, Kahn R, Kirkman MS. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lee WJ, Chong K, Chen SC, Zachariah J, Ser KH, Lee YC, Chen JC. Preoperative prediction of type 2 diabetes remission after gastric bypass surgery: a comparison of DiaRem scores and ABCD scores. Obes Surg. 2016;26(10):2418–2424. [DOI] [PubMed] [Google Scholar]
- 121. Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, Ibele A, Seiler J, Irving BA, Celaya MP, Blackstone R, Gerhard GS, Argyropoulos G. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ahuja A, Tantia O, Chaudhuri T, Khanna S, Seetharamaiah S, Majumdar K, Goyal G. Predicting remission of diabetes post metabolic surgery: a comparison of ABCD, diarem, and DRS scores. Obes Surg. 2018;28(7):2025–2031. [DOI] [PubMed] [Google Scholar]
- 123. Ramos-Levi AM, Sanchez-Pernaute A, Cabrerizo L, Matia P, Barabash A, Hernandez C, Calle-Pascual AL, Torres AJ, Rubio MA. Remission of type 2 diabetes mellitus should not be the foremost goal after bariatric surgery. Obes Surg. 2013;23(12):2020–2025. [DOI] [PubMed] [Google Scholar]
- 124. Jiménez A, Ceriello A, Casamitjana R, Flores L, Viaplana-Masclans J, Vidal J. Remission of type 2 diabetes after Roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profile. Ann Surg. 2015;261(2):316–322. [DOI] [PubMed] [Google Scholar]
- 125. Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol. 2013;169(6):885–889. [DOI] [PubMed] [Google Scholar]
- 126. Nosso G, Lupoli R, Saldalamacchia G, Griffo E, Cotugno M, Costabile G, Riccardi G, Capaldo B. Diabetes remission after bariatric surgery is characterized by high glycemic variability and high oxidative stress. Nutr Metab Cardiovasc Dis. 2017;27(11):949–955. [DOI] [PubMed] [Google Scholar]
- 127. Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, O’Connor PJ, Theis MK, Campos GM, McCulloch D, Selby J. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23(1):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 131. Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–2304. [DOI] [PubMed] [Google Scholar]
- 132. Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27(10):2724–2732. [DOI] [PubMed] [Google Scholar]
- 133. Coleman KJ, Haneuse S, Johnson E, Bogart A, Fisher D, O’Connor PJ, Sherwood NE, Sidney S, Sidney S, Theis MK, Anau J, Schroeder EB, O’Brien R, Arterburn D. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39(8):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, Toledo FG, Jakicic JM. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ding SA, Simonson DC, Wewalka M, Halperin F, Foster K, Goebel-Fabbri A, Hamdy O, Clancy K, Lautz D, Vernon A, Goldfine AB. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab. 2015;100(7):2546–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2014;336(17):1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, Hamdy O, Abrahamson M, Clancy K, Foster K, Lautz D, Vernon A, Goldfine AB. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Purnell JQ, Selzer F, Wahed AS, Pender J, Pories W, Pomp A, Dakin G, Mitchell J, Garcia L, Staten MA, McCloskey C, Cummings DE, Flum DR, Courcoulas A, Wolfe BM. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the longitudinal assessment of bariatric surgery study. Diabetes Care. 2016;39(7):1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, Schultes B, Beglinger C, Drewe J, Schiesser M, Nett P, Bueter M. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Helmiö M, Victorzon M, Ovaska J, Leivonen M, Juuti A, Peromaa-Haavisto P, Nuutila P, Vahlberg T, Salminen P. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg. 2014;103(3):175–181. [DOI] [PubMed] [Google Scholar]
- 142. Dorman RB, Abraham AA, Al-Refaie WB, Parsons HM, Ikramuddin S, Habermann EB. Bariatric surgery outcomes in the elderly: an ACS NSQIP study. J Gastrointest Surg. 2012;16(1):35–44. [DOI] [PubMed] [Google Scholar]
- 143. Topart P, Becouarn G, Sallé A, Ritz P. Biliopancreatic diversion requires multiple vitamin and micronutrient adjustments within 2 years of surgery. Surg Obes Relat Dis. 2014;10(5):936–941. [DOI] [PubMed] [Google Scholar]
- 144. Hedberg J, Sundström J, Sundbom M. Duodenal switch versus Roux-en-Y gastric bypass for morbid obesity: systematic review and meta-analysis of weight results, diabetes resolution and early complications in single-centre comparisons. Obes Rev. 2014;15(7):555–563. [DOI] [PubMed] [Google Scholar]
- 145. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–973. [DOI] [PubMed] [Google Scholar]
- 146. Risstad H, Søvik TT, Engström M, Aasheim ET, Fagerland MW, Olsén MF, Kristinsson JA, le Roux CW, Bøhmer T, Birkeland KI, Mala T, Olbers T. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015;150(4):352–361. [DOI] [PubMed] [Google Scholar]
- 147. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–481. [DOI] [PubMed] [Google Scholar]
- 148. Shankar SS, Mixson LA, Chakravarthy M, Chisholm R, Acton AJ, Jones R, Mattar SG, Miller DL, Petry L, Beals CR, Stoch SA, Kelley DE, Considine RV. Metabolic improvements following Roux-en-Y surgery assessed by solid meal test in subjects with short duration type 2 diabetes. BMC Obes. 2017;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77(5):1287–1293. [DOI] [PubMed] [Google Scholar]
- 150. Malandrucco I, Pasqualetti P, Giordani I, Manfellotto D, De Marco F, Alegiani F, Sidoti AM, Picconi F, Di Flaviani A, Frajese G, Bonadonna RC, Frontoni S. Very-low-calorie diet: a quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am J Clin Nutr. 2012;95(3):609–613. [DOI] [PubMed] [Google Scholar]
- 151. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pournaras DJ, Nygren J, Hagström-Toft E, Arner P, le Roux CW, Thorell A. Improved glucose metabolism after gastric bypass: evolution of the paradigm. Surg Obes Relat Dis. 2016;12(8):1457–1465. [DOI] [PubMed] [Google Scholar]
- 155. Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, Taylor R. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders [published correction appears in Diabetes Care. 2018;41(6):1321]. Diabetes Care. 2016;39(5):808–815. [DOI] [PubMed] [Google Scholar]
- 156. Foo J, Krebs J, Hayes MT, Bell D, Macartney-Coxson D, Croft T, Stubbs RS. Studies in insulin resistance following very low calorie diet and/or gastric bypass surgery. Obes Surg. 2011;21(12):1914–1920. [DOI] [PubMed] [Google Scholar]
- 157. Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? [published correction appears in Diabetes Care. 2013;36(9):2872] Diabetes Care. 2013;36(9):2741–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gastaldelli A, Iaconelli A, Gaggini M, Magnone MC, Veneziani A, Rubino F, Mingrone G. Short-term effects of laparoscopic adjustable gastric banding versus Roux-en-Y Gastric bypass. Diabetes Care. 2016;39(11):1925–1931. [DOI] [PubMed] [Google Scholar]
- 159. Berggren J, Lindqvist A, Hedenbro J, Groop L, Wierup N. Roux-en-Y gastric bypass versus calorie restriction: support for surgery per se as the direct contributor to altered responses of insulin and incretins to a mixed meal. Surg Obes Relat Dis. 2017;13(2):234–242. [DOI] [PubMed] [Google Scholar]
- 160. Pop LM, Mari A, Zhao TJ, Mitchell L, Burgess S, Li X, Adams-Huet B, Lingvay I. Roux-en-Y gastric bypass compared with equivalent diet restriction: mechanistic insights into diabetes remission. Diabetes Obes Metab. 2018;20(7):1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Plourde CE, Grenier-Larouche T, Caron-Dorval D, Biron S, Marceau S, Lebel S, Biertho L, Tchernof A, Richard D, Carpentier AC. Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity (Silver Spring). 2014;22(8):1838–1846. [DOI] [PubMed] [Google Scholar]
- 162. Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol. 1999;277(4):E733–E741. [DOI] [PubMed] [Google Scholar]
- 163. Ljunggren S, Nyström T, Hahn RG. Accuracy and precision of commonly used methods for quantifying surgery-induced insulin resistance: prospective observational study. Eur J Anaesthesiol. 2014;31(2):110–116. [DOI] [PubMed] [Google Scholar]
- 164. Baban B, Thorell A, Nygren J, Bratt A, Ljungqvist O. Determination of insulin resistance in surgery: the choice of method is crucial. Clin Nutr. 2015;34(1):123–128. [DOI] [PubMed] [Google Scholar]
- 165. Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on β-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2010;34(3):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Hansen DL, Worm D, Naver L, Kristiansen VB, Holst JJ, Madsbad S. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab. 2013;98(6):E1066–E1071. [DOI] [PubMed] [Google Scholar]
- 168. Bunt JC, Blackstone R, Thearle MS, Vinales KL, Votruba S, Krakoff J. Changes in glycemia, insulin and gut hormone responses to a slowly ingested solid low-carbohydrate mixed meal after laparoscopic gastric bypass or band surgery. Int J Obes. 2017;41(5):706–713. [DOI] [PubMed] [Google Scholar]
- 169. Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63(5):1725–1737. [DOI] [PubMed] [Google Scholar]
- 170. Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, Baldi S, Nannipieri M, Rebelos E, Anselmino M, Muscelli E, Ferrannini E. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54(8):2093–2102. [DOI] [PubMed] [Google Scholar]
- 171. de Weijer BA, Aarts E, Janssen IM, Berends FJ, van de Laar A, Kaasjager K, Ackermans MT, Fliers E, Serlie MJ. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity (Silver Spring). 2013;21(6):1143–1147. [DOI] [PubMed] [Google Scholar]
- 172. Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K, Feurer ID, Tamboli RA. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35(1):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Salinari S, Bertuzzi A, Guidone C, Previti E, Rubino F, Mingrone G. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg. 2013;257(3):462–468. [DOI] [PubMed] [Google Scholar]
- 174. Severino A, Castagneto-Gissey L, Raffaelli M, Gastaldelli A, Capristo E, Iaconelli A, Guidone C, Callari C, Bellantone R, Mingrone G. Early effect of Roux-en-Y gastric bypass on insulin sensitivity and signaling. Surg Obes Relat Dis. 2016;12(1):42–47. [DOI] [PubMed] [Google Scholar]
- 175. Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, Chaim EA, Geloneze B. Acute effect of Roux-en-Y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(8):3871–3875. [DOI] [PubMed] [Google Scholar]
- 176. Lin E, Davis SS, Srinivasan J, Sweeney JF, Ziegler TR, Phillips L, Gletsu-Miller N. Dual mechanism for type-2 diabetes resolution after Roux-en-Y gastric bypass. Am Surg. 2009;75(6):498–502. [PMC free article] [PubMed] [Google Scholar]
- 177. Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2013;3(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vaurs C, Brun JF, Bérard E, Chalret du Rieu M, Hanaire H, Ritz P. β-Cell pancreatic dysfunction plays a role in hyperglycemic peaks observed after gastric bypass surgery of obese patients. Surg Obes Relat Dis. 2016;12(4):795–802. [DOI] [PubMed] [Google Scholar]
- 179. Liu T, Zhong MW, Liu Y, Sun D, Wei M, Huang X, Cheng YG, Wu QZ, Wu D, Zhang XQ, Wang KX, Hu SY, Liu SZ. Diabetes recurrence after metabolic surgeries correlates with re-impaired insulin sensitivity rather than beta-cell function. World J Gastroenterol. 2017;23(19):3468–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mallipedhi A, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):860–869. [DOI] [PubMed] [Google Scholar]
- 181. Roslin MS, Dudiy Y, Weiskopf J, Damani T, Shah P. Comparison between RYGB, DS, and VSG effect on glucose homeostasis. Obes Surg. 2012;22(8):1281–1286. [DOI] [PubMed] [Google Scholar]
- 182. Dixon JB, Dixon AF, O’Brien PE. Improvements in insulin sensitivity and β-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med. 2003;20(2):127–134. [DOI] [PubMed] [Google Scholar]
- 183. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 184. Bojsen-Møller KN, Dirksen C, Svane MS, Jørgensen NB, Holst JJ, Richter EA, Madsbad S. Variable reliability of surrogate measures of insulin sensitivity after Roux-en-Y gastric bypass. Am J Physiol Regul Integr Comp Physiol. 2017;312(5):R797–R805. [DOI] [PubMed] [Google Scholar]
- 185. Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98(11):4391–4399. [DOI] [PubMed] [Google Scholar]
- 186. Junqueira Vasques AC, Pareja JC, de Oliveira MS, Satake Novaes F, Miranda de Oliveira Lima M, Chaim EA, Piccinini F, Dalla Man C, Cobelli C, Geloneze B. β-Cell function improvements in grade I/II obese subjects with type 2 diabetes 1 month after biliopancreatic diversion: results from modeling analyses of oral glucose tolerance tests and hyperglycemic clamp studies. Diabetes Care. 2013;36(12):4117–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, Rehfeld JF, Wulff BS, Clausen TR, Hansen DL, Holst JJ, Madsbad S. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–1096. [DOI] [PubMed] [Google Scholar]
- 188. Asare-Bediako I, Paszkiewicz RL, Kim SP, Woolcott OO, Kolka CM, Burch MA, Kabir M, Bergman RN. Variability of directly measured first-pass hepatic insulin extraction and its association with insulin sensitivity and plasma insulin. Diabetes. 2018;67(8):1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Papamargaritis D, Tzovaras G, Sioka E, Zachari E, Koukoulis G, Zacharoulis D. Comparison of glucose homeostasis parameters between patients with high and low risk of diabetes at 6 weeks and 6 months after sleeve gastrectomy. Surg Obes Relat Dis. 2017;13(6):1016–1024. [DOI] [PubMed] [Google Scholar]
- 191. Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–1456. [DOI] [PubMed] [Google Scholar]
- 192. Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252(6):966–971. [DOI] [PubMed] [Google Scholar]
- 193. Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, Krause KR, Schram JL, Kole KL, Finks JF, Birkmeyer JD, Share D, Birkmeyer NJ; Michigan Bariatric Surgery Collaborative. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg. 2013;257(5):791–797. [DOI] [PubMed] [Google Scholar]
- 195. Casella G, Soricelli E, Castagneto-Gissey L, Redler A, Basso N, Mingrone G. Changes in insulin sensitivity and secretion after sleeve gastrectomy. Br J Surg. 2016;103(3):242–248. [DOI] [PubMed] [Google Scholar]
- 196. Purnell JQ, Johnson GS, Wahed AS, Dalla Man C, Piccinini F, Cobelli C, Prigeon RL, Goodpaster BH, Kelley DE, Staten MA, Foster-Schubert KE, Cummings DE, Flum DR, Courcoulas AP, Havel PJ, Wolfe BM. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61(5):1142–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Grenier-Larouche T, Carreau AM, Geloën A, Frisch F, Biertho L, Marceau S, Lebel S, Hould FS, Richard D, Tchernof A, Carpentier AC. Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes. 2017;66(11):2743–2755. [DOI] [PubMed] [Google Scholar]
- 198. Bikman BT, Zheng D, Pories WJ, Chapman W, Pender JR, Bowden RC, Reed MA, Cortright RN, Tapscott EB, Houmard JA, Tanner CJ, Lee J, Dohm GL. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93(12):4656–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Albers PH, Bojsen-Møller KN, Dirksen C, Serup AK, Kristensen DE, Frystyk J, Clausen TR, Kiens B, Richter EA, Madsbad S, Wojtaszewski JF. Enhanced insulin signaling in human skeletal muscle and adipose tissue following gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2015;309(5):R510–R524. [DOI] [PubMed] [Google Scholar]
- 200. Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrannini E. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med. 2005;118(1):51–57. [DOI] [PubMed] [Google Scholar]
- 201. Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025–2031. [DOI] [PubMed] [Google Scholar]
- 202. Astiarraga B, Gastaldelli A, Muscelli E, Baldi S, Camastra S, Mari A, Papadia F, Camerini G, Adami G, Scopinaro N, Ferrannini E. Biliopancreatic diversion in nonobese patients with type 2 diabetes: impact and mechanisms. J Clin Endocrinol Metab. 2013;98(7):2765–2773. [DOI] [PubMed] [Google Scholar]
- 203. Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24(5):1005–1010. [DOI] [PubMed] [Google Scholar]
- 204. Aminian A, Jamal M, Augustin T, Corcelles R, Kirwan JP, Schauer PR, Brethauer SA. Failed surgical weight loss does not necessarily mean failed metabolic effects. Diabetes Technol Ther. 2015;17(10):682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chen CY, Lee WJ, Asakawa A, Fujitsuka N, Chong K, Chen SC, Lee SD, Inui A. Insulin secretion and interleukin-1β dependent mechanisms in human diabetes remission after metabolic surgery. Curr Med Chem. 2013;20(18):2374–2388. [DOI] [PubMed] [Google Scholar]
- 206. Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring). 2008;16(2):298–305. [DOI] [PubMed] [Google Scholar]
- 207. Billeter AT, Fischer L, Wekerle AL, Senft J, Müller-Stich B. Malabsorption as a therapeutic approach in bariatric surgery. Viszeralmedizin. 2014;30(3):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Inabnet WB, Milone L, Harris P, Durak E, Freeby MJ, Ahmed L, Sebastian M, Lifante JC, Bessler M, Korner J. The utility of [11C] dihydrotetrabenazine positron emission tomography scanning in assessing beta-cell performance after sleeve gastrectomy and duodenal-jejunal bypass. Surgery. 2010;147(2):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48(5):312–317. [DOI] [PubMed] [Google Scholar]
- 210. Zhu Z, Yang X, Wang K, Wang Z, Zhao Y, Yu M. The effects of sleeve gastrectomy on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Eur Surg. 2014;46(5):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Michaud A, Grenier-Larouche T, Caron-Dorval D, Marceau S, Biertho L, Simard S, Richard D, Tchernof A, Carpentier AC. Biliopancreatic diversion with duodenal switch leads to better postprandial glucose level and beta cell function than sleeve gastrectomy in individuals with type 2 diabetes very early after surgery. Metabolism. 2017;74:10–21. [DOI] [PubMed] [Google Scholar]
- 212. Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9(5):667–677. [DOI] [PubMed] [Google Scholar]
- 213. Jørgensen NB, Jacobsen SH, Dirksen C, Bojsen-Møller KN, Naver L, Hvolris L, Clausen TR, Wulff BS, Worm D, Lindqvist Hansen D, Madsbad S, Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–E131. [DOI] [PubMed] [Google Scholar]
- 214. Lin E, Liang Z, Frediani J, Davis SS Jr, Sweeney JF, Ziegler TR, Phillips LS, Gletsu-Miller N. Improvement in β-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2010;299(5):E706–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, Nannipieri M, Ciociaro D, Anselmino M, Mari A, Ferrannini E. Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes. 2013;62(11):3709–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Salehi M, D’Alessio DA. Effects of glucagon like peptide-1 to mediate glycemic effects of weight loss surgery. Rev Endocr Metab Disord. 2014;15(3):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Previti E, Salinari S, Bertuzzi A, Capristo E, Bornstein S, Mingrone G. Glycemic control after metabolic surgery: a Granger causality and graph analysis. Am J Physiol Endocrinol Metab. 2017;313(5):E622–E630. [DOI] [PubMed] [Google Scholar]
- 220. Kahn SE. Clinical review 135: the importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(9):4047–4058. [DOI] [PubMed] [Google Scholar]
- 221. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. [DOI] [PubMed] [Google Scholar]
- 222. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Berkowitz K, Marroquin A, Goico J, Ochoa C, Azen SP. Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes. 2000;49(5):782–788. [DOI] [PubMed] [Google Scholar]
- 223. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels [published correction appears in Diabetes Care. 2009;32(7):1355]. Diabetes Care. 2009;32(2):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Reed MA, Pories WJ, Chapman W, Pender J, Bowden R, Barakat H, Gavin TP, Green T, Tapscott E, Zheng D, Shankley N, Yieh L, Polidori D, Piccoli SP, Brenner-Gati L, Dohm GL. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(8):2525–2531. [DOI] [PubMed] [Google Scholar]
- 225. Martinussen C, Bojsen-Møller KN, Dirksen C, Jacobsen SH, Jørgensen NB, Kristiansen VB, Holst JJ, Madsbad S. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab. 2015;308(6):E535–E544. [DOI] [PubMed] [Google Scholar]
- 226. Inge TH, Prigeon RL, Elder DA, Jenkins TM, Cohen RM, Xanthakos SA, Benoit SC, Dolan LM, Daniels SR, D’Alessio DA. Insulin sensitivity and β-cell function improve after gastric bypass in severely obese adolescents. J Pediatr. 2015;167(5):1042–1048.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to glucose is impaired after gastric bypass surgery. Diabetes Obes Metab. 2018;20(4):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Salehi M, Gastaldelli A, D’Alessio DA. Beta-cell sensitivity to insulinotropic gut hormones is reduced after gastric bypass surgery. Gut. 2019;gutjnl-2018-317760. [DOI] [PubMed] [Google Scholar]
- 229. Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1–stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 231. Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17(3):164–174. [DOI] [PubMed] [Google Scholar]
- 232. Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. The exaggerated glucagon-like peptide-1 response is important for the improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013:62(9):3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32(3):375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, McGinty J, Laferrère B. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63(4):1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Mallipedhi A, Min T, Prior SL, MacIver C, Luzio SD, Dunseath G, Bracken RM, Islam S, Barry JD, Caplin S, Stephens JW. Association between the preoperative fasting and postprandial C-peptide AUC with resolution of type 2 diabetes 6 months following bariatric surgery. Metabolism. 2015;64(11):1556–1563. [DOI] [PubMed] [Google Scholar]
- 236. Souteiro P, Belo S, Neves JS, Magalhães D, Silva RB, Oliveira SC, Costa MM, Saavedra A, Oliveira J, Cunha F, Lau E, Esteves C, Freitas P, Varela A, Queirós J, Carvalho D. Preoperative beta cell function is predictive of diabetes remission after bariatric surgery. Obes Surg. 2017;27(2):288–294. [DOI] [PubMed] [Google Scholar]
- 237. Aarts EO, Janssen J, Janssen IM, Berends FJ, Telting D, de Boer H. Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes Surg. 2013;23(7):867–873. [DOI] [PubMed] [Google Scholar]
- 238. Zhao L, Li W, Su Z, Liu Y, Zhu L, Zhu S. Preoperative fasting C-peptide predicts type 2 diabetes mellitus remission in low-BMI Chinese patients after Roux-en-Y gastric bypass. J Gastrointest Surg. 2018;22(10):1672–1678. [DOI] [PubMed] [Google Scholar]
- 239. Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147(5):664–669. [DOI] [PubMed] [Google Scholar]
- 240. Vecht J, Masclee AA, Lamers CB. The dumping syndrome. Current insights into pathophysiology, diagnosis and treatment. Scand J Gastroenterol Suppl. 1997;223:21–27. [PubMed] [Google Scholar]
- 241. Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236–2240. [DOI] [PubMed] [Google Scholar]
- 242. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249–254. [DOI] [PubMed] [Google Scholar]
- 243. Goldfine AB, Mun EC, Devine E, Bernier R, Baz-Hecht M, Jones DB, Schneider BE, Holst JJ, Patti ME. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. [DOI] [PubMed] [Google Scholar]
- 244. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. [DOI] [PubMed] [Google Scholar]
- 247. Sarwar H, Chapman WH III, Pender JR, Ivanescu A, Drake AJ III, Pories WJ, Dar MS. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014;24(7):1120–1124. [DOI] [PubMed] [Google Scholar]
- 248. Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98(7):E1208–E1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Capristo E, Panunzi S, De Gaetano A, Spuntarelli V, Bellantone R, Giustacchini P, Birkenfeld AL, Amiel S, Bornstein SR, Raffaelli M, Mingrone G. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metab. 2018;103(6):2136–2146. [DOI] [PubMed] [Google Scholar]
- 250. Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-en-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564–569. [DOI] [PubMed] [Google Scholar]
- 251. Panosian J, Ding SA, Wewalka M, Simonson DC, Goebel-Fabbri A, Foster K, Halperin F, Vernon A, Goldfine AB. Physical Activity in Obese Type 2 Diabetes After Gastric Bypass or Medical Management. Am J Med. 2017;130(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Tharakan G, Behary P, Wewer Albrechtsen NJ, Chahal H, Kenkre J, Miras AD, Ahmed AR, Holst JJ, Bloom SR, Tan T. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. Eur J Endocrinol. 2017;177(6):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, Buchwald H, Ikramuddin S. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4(4):492–499. [DOI] [PubMed] [Google Scholar]
- 254. Nannipieri M, Belligoli A, Guarino D, Busetto L, Moriconi D, Fabris R, Mari A, Baldi S, Anselmino M, Foletto M, Vettor R, Ferrannini E. Risk factors for spontaneously self-reported postprandial hypoglycemia after bariatric surgery. J Clin Endocrinol Metab. 2016;101(10):3600–3607. [DOI] [PubMed] [Google Scholar]
- 255. Aasheim ET, Björkman S, Søvik TT, Engström M, Hanvold SE, Mala T, Olbers T, Bøhmer T. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90(1):15–22. [DOI] [PubMed] [Google Scholar]
- 256. Papamargaritis D, Zacharoulis D, Sioka E, Zachari E, Bargiota A, Koukoulis G, Tzovaras G. Differences in anthropometric and metabolic parameters between subjects with hypoglycaemia and subjects with euglycaemia after an oral glucose tolerance test six months after laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(11):2747–2755. [DOI] [PubMed] [Google Scholar]
- 257. Vaurs C, Brun JF, Bertrand M, Burcelin R, du Rieu MC, Anduze Y, Hanaire H, Ritz P. Post-prandial hypoglycemia results from a non-glucose-dependent inappropriate insulin secretion in Roux-en-Y gastric bypassed patients. Metabolism. 2016;65(3):18–26. [DOI] [PubMed] [Google Scholar]
- 258. Craig CM, Liu LF, Deacon CF, Holst JJ, McLaughlin TL. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Clancy TE, Moore FD Jr, Zinner MJ. Post-gastric bypass hyperinsulinism with nesidioblastosis: subtotal or total pancreatectomy may be needed to prevent recurrent hypoglycemia. J Gastrointest Surg. 2006;10(8):1116–1119. [DOI] [PubMed] [Google Scholar]
- 260. Z'graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, Komminoth P, Horber F. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18(8):981–988. [DOI] [PubMed] [Google Scholar]
- 261. Rumilla KM, Erickson LA, Service FJ, Vella A, Thompson GB, Grant CS, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol. 2009;22(2):239–245. [DOI] [PubMed] [Google Scholar]
- 262. Vella A, Service FJ. Incretin hypersecretion in post-gastric bypass hypoglycemia—primary problem or red herring? J Clin Endocrinol Metab. 2007;92(12):4563–4565. [DOI] [PubMed] [Google Scholar]
- 263. Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased β-cell turnover. Diabetes Care. 2006;29(7):1554–1559. [DOI] [PubMed] [Google Scholar]
- 264. Davis DB, Khoraki J, Ziemelis M, Sirinvaravong S, Han JY, Campos GM. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: confirming a non-pancreatic etiology. Mol Metab. 2018;9:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Kim SH, Liu TC, Abbasi F, Lamendola C, Morton JM, Reaven GM, McLaughlin TL. Plasma glucose and insulin regulation is abnormal following gastric bypass surgery with or without neuroglycopenia. Obes Surg. 2009;19(11):1550–1556. [DOI] [PubMed] [Google Scholar]
- 266. Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, Morínigo R, Lacy AM. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94(3):884–891. [DOI] [PubMed] [Google Scholar]
- 267. Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS, Hansen DL, Worm D, Holst JJ, Madsbad S. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia. 2013;56(12):2679–2687. [DOI] [PubMed] [Google Scholar]
- 268. Eickhoff H, Louro T, Matafome P, Seiça R, Castro e Sousa F. Glucagon secretion after metabolic surgery in diabetic rodents. J Endocrinol. 2014;223(3):255–265. [DOI] [PubMed] [Google Scholar]
- 269. Roberts GP, Kay RG, Howard J, Hardwick RH, Reimann F, Gribble FM. Gastrectomy with Roux-en-Y reconstruction as a lean model of bariatric surgery. Surg Obes Relat Dis. 2018;14(5):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Basso N, Soricelli E, Castagneto-Gissey L, Casella G, Albanese D, Fava F, Donati C, Tuohy K, Angelini G, La Neve F, Severino A, Kamvissi-Lorenz V, Birkenfeld AL, Bornstein S, Manco M, Mingrone G. Insulin resistance, microbiota, and fat distribution changes by a new model of vertical sleeve gastrectomy in obese rats. Diabetes. 2016;65(10):2990–3001. [DOI] [PubMed] [Google Scholar]
- 271. Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde S, Sorrell JE, Toure M, Berger J, D’Alessio DA, Sandoval DA, Seeley RJ, Woods SC. Similar effects of Roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav. 2011;105(1):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97(1):92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. O’Donovan DG, Doran S, Feinle-Bisset C, Jones KL, Meyer JH, Wishart JM, Morris HA, Horowitz M. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89(7):3431–3435. [DOI] [PubMed] [Google Scholar]
- 275. Pilichiewicz AN, Little TJ, Brennan IM, Meyer JH, Wishart JM, Otto B, Horowitz M, Feinle-Bisset C. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R668–R677. [DOI] [PubMed] [Google Scholar]
- 276. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse JP, Bado A, Le Gall M. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2016;150(2):454–464.e9. [DOI] [PubMed] [Google Scholar]
- 278. Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Fellici AC, Lambert G, Lima MM, Pareja JC, Rodovalho S, Chaim EA, Geloneze B. Surgical treatment of type 2 diabetes in subjects with mild obesity: mechanisms underlying metabolic improvements. Obes Surg. 2015;25(1):36–44. [DOI] [PubMed] [Google Scholar]
- 280. Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg. 2013;257(5):894–899. [DOI] [PubMed] [Google Scholar]
- 281. Jiménez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014;63(10):3372–3377. [DOI] [PubMed] [Google Scholar]
- 282. Jiménez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36(7):2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Shah M, Law JH, Micheletto F, Sathananthan M, Dalla Man C, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63(2):483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Vetter ML, Wadden TA, Teff KL, Khan ZF, Carvajal R, Ritter S, Moore RH, Chittams JL, Iagnocco A, Murayama K, Korus G, Williams NN, Rickels MR. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64(2):434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Vidal J, de Hollanda A, Jiménez A. GLP-1 is not the key mediator of the health benefits of metabolic surgery. Surg Obes Relat Dis. 2016;12(6):1225–1229. [DOI] [PubMed] [Google Scholar]
- 287. Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–2239. [DOI] [PubMed] [Google Scholar]
- 288. Pournaras DJ, Aasheim ET, Bueter M, Ahmed AR, Welbourn R, Olbers T, le Roux CW. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis. 2012;8(4):371–374. [DOI] [PubMed] [Google Scholar]
- 289. Nergård BJ, Lindqvist A, Gislason HG, Groop L, Ekelund M, Wierup N, Hedenbro JL. Mucosal glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide cell numbers in the super-obese human foregut after gastric bypass. Surg Obes Relat Dis. 2015;11(6):1237–1246. [DOI] [PubMed] [Google Scholar]
- 290. Rhee NA, Wahlgren CD, Pedersen J, Mortensen B, Langholz E, Wandall EP, Friis SU, Vilmann P, Paulsen SJ, Kristiansen VB, Jelsing J, Dalbøge LS, Poulsen SS, Holst JJ, Vilsbøll T, Knop FK. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58(10):2254–2258. [DOI] [PubMed] [Google Scholar]
- 291. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380– 386. [DOI] [PubMed] [Google Scholar]
- 292. Meier JJ, Hücking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes. 2001;50(11):2497–2504. [DOI] [PubMed] [Google Scholar]
- 293. Nauck MA, Heimesaat MM, Ørskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91(1):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Nauck MA, El-Ouaghlidi A, Gabrys B, Hücking K, Holst JJ, Deacon CF, Gallwitz B, Schmidt WE, Meier JJ. Secretion of incretin hormones (GIP and GLP-1) and incretin effect after oral glucose in first-degree relatives of patients with type 2 diabetes. Regul Pept. 2004;122(3):209–217. [DOI] [PubMed] [Google Scholar]
- 295. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(1):10–18. [DOI] [PubMed] [Google Scholar]
- 296. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. [DOI] [PubMed] [Google Scholar]
- 297. Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. [DOI] [PubMed] [Google Scholar]
- 298. Højberg PV, Vilsbøll T, Zander M, Knop FK, Krarup T, Vølund A, Holst JJ, Madsbad S. Four weeks of near-normalization of blood glucose has no effect on postprandial GLP-1 and GIP secretion, but augments pancreatic B-cell responsiveness to a meal in patients with type 2 diabetes. Diabet Med. 2008;25(11):1268–1275. [DOI] [PubMed] [Google Scholar]
- 299. An Z, Prigeon RL, D’Alessio DA. Improved glycemic control enhances the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2013;98(12):4702–4708. [DOI] [PubMed] [Google Scholar]
- 300. Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Grong E, Græslie H, Munkvold B, Arbo IB, Kulseng BE, Waldum HL, Mårvik R. Gastrin secretion after bariatric surgery-response to a protein-rich mixed meal following Roux-en-Y gastric bypass and sleeve gastrectomy: a pilot study in normoglycemic women. Obes Surg. 2016;26(7):1448–1456. [DOI] [PubMed] [Google Scholar]
- 302. Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, Chouinard ML, Havel PJ. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153(8):3620–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151(4):1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Mendieta-Zerón H, Larrad-Jiménez Á, Burrell MA, Rodríguez MM, Da Boit K, Frühbeck G, Diéguez C. Biliopancreatic diversion induces villi elongation and cholecystokinin and ghrelin increase. Diabetes Metab Syndr. 2011;5(2):66–70. [DOI] [PubMed] [Google Scholar]
- 305. Masuda T, Ohta M, Hirashita T, Kawano Y, Eguchi H, Yada K, Iwashita Y, Kitano S. A comparative study of gastric banding and sleeve gastrectomy in an obese diabetic rat model. Obes Surg. 2011;21(11):1774–1780. [DOI] [PubMed] [Google Scholar]
- 306. Thomas F, Smith GC, Lu J, Babor R, Booth M, Beban G, Chase JG, Murphy R. Differential acute impacts of sleeve gastrectomy, Roux-en-Y gastric bypass surgery and matched caloric restriction diet on insulin secretion, insulin effectiveness and non-esterified fatty acid levels among patients with type 2 diabetes. Obes Surg. 2016;26(8):1924–1931. [DOI] [PubMed] [Google Scholar]
- 307. Belligoli A, Sanna M, Serra R, Fabris R, Pra’ CD, Conci S, Fioretto P, Prevedello L, Foletto M, Vettor R, Busetto L. Incidence and predictors of hypoglycemia 1 year after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(12):3179–3186. [DOI] [PubMed] [Google Scholar]
- 308. Khoo CM, Muehlbauer MJ, Stevens RD, Pamuklar Z, Chen J, Newgard CB, Torquati A. Postprandial metabolite profiles reveal differential nutrient handling after bariatric surgery compared with matched caloric restriction. Ann Surg. 2014;259(4):687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, Newgard CB, Klein S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013;62(8):2757–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah SH, Svetkey LP, Newgard CB. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Laferrère B. Gut feelings about diabetes. Endocrinol Nutr. 2012;59(4):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Carmody JS, Muñoz R, Yin H, Kaplan LM. Peripheral, but not central, GLP-1 receptor signaling is required for improvement in glucose tolerance after Roux-en-Y gastric bypass in mice. Am J Physiol Endocrinol Metab. 2016;310(10):E855–E861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Lindqvist A, Spégel P, Ekelund M, Garcia Vaz E, Pierzynowski S, Gomez MF, Mulder H, Hedenbro J, Groop L, Wierup N. Gastric bypass improves β-cell function and increases β-cell mass in a porcine model. Diabetes. 2014;63(5):1665–1671. [DOI] [PubMed] [Google Scholar]
- 316. Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D’Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303(8):E1076–E1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. [DOI] [PubMed] [Google Scholar]
- 319. Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes. 2013;37(2):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Ye M, Huang R, Min Z, Zhang P, Wang T, Yu B. Comparison of the effect by which gastric plication and sleeve gastrectomy procedures alter metabolic and physical parameters in an obese type 2 diabetes rodent model. Surg Obes Relat Dis. 2017;13(11):1819–1828. [DOI] [PubMed] [Google Scholar]
- 322. Xu X, Wang J, Li L, Wang C, Li W, Zhang Q, Yang L. The role of obestatin in Roux-en-Y gastric bypass surgery in the obese, type 2 diabetes Zucker rat. Diabetes Res Clin Pract. 2016;119:57–64. [DOI] [PubMed] [Google Scholar]
- 323. Habegger KM, Kirchner H, Yi CX, Heppner KM, Sweeney D, Ottaway N, Holland J, Amburgy S, Raver C, Krishna R, Müller TD, Perez-Tilve D, Pfluger PT, Obici S, DiMarchi RD, D’Alessio DA, Seeley RJ, Tschöp MH. GLP-1R agonism enhances adjustable gastric banding in diet-induced obese rats. Diabetes. 2013;62(9):3261–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, Ley RE, Chouinard ML, Cummings BP. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Seyfried F, Miras AD, Rotzinger L, Nordbeck A, Corteville C, Li JV, Schlegel N, Hankir M, Fenske W, Otto C, Jurowich C. Gastric bypass-related effects on glucose control, β cell function and morphology in the obese Zucker rat. Obes Surg. 2016;26(6):1228–1236. [DOI] [PubMed] [Google Scholar]
- 326. Douros JD, Lewis AG, Smith EP, Niu J, Capozzi M, Wittmann A, Campbell J, Tong J, Wagner C, Mahbod P, Seeley R, D’Alessio DA. Enhanced glucose control following vertical sleeve gastrectomy does not require a β-cell glucagon-like peptide 1 receptor. Diabetes. 2018;67(8):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Lindqvist A, Ekelund M, Garcia-Vaz E, Ståhlman M, Pierzynowski S, Gomez MF, Rehfeld JF, Groop L, Hedenbro J, Wierup N, Spégel P. The impact of Roux-en-Y gastric bypass surgery on normal metabolism in a porcine model. PLoS One. 2017;12(3):e0173137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D’Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Pressler JW, Haller A, Sorrell J, Wang F, Seeley RJ, Tso P, Sandoval DA. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64(2):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Stemmer K, Bielohuby M, Grayson BE, Begg DP, Chambers AP, Neff C, Woods SC, Erben RG, Tschöp MH, Bidlingmaier M, Clemens TL, Seeley RJ. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology. 2013;154(6):2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144(1):50–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Garibay D, McGavigan AK, Lee SA, Ficorilli JV, Cox AL, Michael MD, Sloop KW, Cummings BP. β-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology. 2016;157(9):3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Patel A, Yusta B, Matthews D, Charron MJ, Seeley RJ, Drucker DJ. GLP-2 receptor signaling controls circulating bile acid levels but not glucose homeostasis in Gcgr−/− mice and is dispensable for the metabolic benefits ensuing after vertical sleeve gastrectomy. Mol Metab. 2018;16:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Mul JD, Begg DP, Haller AM, Pressler JW, Sorrell J, Woods SC, Farese RV Jr, Seeley RJ, Sandoval DA. MGAT2 deficiency and vertical sleeve gastrectomy have independent metabolic effects in the mouse. Am J Physiol Endocrinol Metab. 2014;307(11):E1065–E1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Arble DM, Pressler JW, Sorrell J, Wevrick R, Sandoval DA. Sleeve gastrectomy leads to weight loss in the Magel2 knockout mouse. Surg Obes Relat Dis. 2016;12(10):1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Carmody JS, Ahmad NN, Machineni S, Lajoie S, Kaplan LM. Weight loss after RYGB is independent of and complementary to serotonin 2C receptor signaling in male mice. Endocrinology. 2015;156(9):3183–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Hao Z, Leigh Townsend R, Mumphrey MB, Gettys TW, Yu S, Münzberg H, Morrison CD, Berthoud HR. Roux-en-Y gastric bypass surgery-induced weight loss and metabolic improvements are similar in TGR5-deficient and wildtype mice. Obes Surg. 2018;28(10):3227–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ding L, Sousa KM, Jin L, Dong B, Kim BW, Ramirez R, Xiao Z, Gu Y, Yang Q, Wang J, Yu D, Pigazzi A, Schones D, Yang L, Moore D, Wang Z, Huang W. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64(3):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Mokadem M, Zechner JF, Uchida A, Aguirre V. Leptin is required for glucose homeostasis after Roux-en-Y gastric bypass in mice. PLoS One. 2015;10(10):e0139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, McMahon DJ, Korner J. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring). 2011;19(11):2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Ceriani V, Lodi T, Porta A, Roncaglia O, Osio C, Faleschini E, Bignami P, Coladonato M, Elnabil-Mortada A, Belloni A, Baldoli D, Gaffuri P. Roux-en-Y reconstruction at greater curvature in biliopancreatic diversion: effects on early postoperative functional recovery. Obes Surg. 2011;21(8):1188–1193. [DOI] [PubMed] [Google Scholar]