Supplemental Digital Content is Available in the Text.
Keywords: Temporomandibular disorder, Epidemiology, Segmental central sensitization
Abstract
Background:
Chronic facial pain often overlaps with pain experienced elsewhere in the body, although previous studies have focused on a few, selected pain conditions when assessing the degree of overlap.
Aim:
To quantify the degree of overlap between facial pain and pain reported at multiple locations throughout the body.
Methods:
Data were from a case–control study of US adults participating in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) project. They were interviewed to determine the presence of chronic facial pain (n = 424 cases) or its absence (n = 912 controls). A mailed questionnaire with a body drawing asked about pain at other locations. Odds ratios (ORs) and 95% confidence limits (95% CLs) quantified the degree of overlap between facial pain and pain at other locations. For replication, cross-sectional data were analyzed from the UK Biobank study (n = 459,604 participants) and the US National Health Interview Survey (n = 27,731 participants).
Results:
In univariate analysis, facial pain had greatest overlap with headache (OR = 14.2, 95% CL = 9.7–20.8) followed by neck pain (OR = 8.5, 95% CL = 6.5–11.0), whereas overlap decreased substantially (ORs of 4.4 or less) for pain at successively remote locations below the neck. The same anatomically based ranking of ORs persisted in multivariable analysis that adjusted for demographics and risk factors for facial pain. Findings were replicated in the UK Biobank study and the US National Health Interview Survey. The observed anatomical selectivity in the degree of overlap could be a consequence of neurosensory and/or affective processes that differentially amplify pain according to its location.
1. Introduction
Painful temporomandibular disorder (TMD) is the most frequent form of chronic orofacial pain, affecting an estimated 11.5 million US adults with annual incidence of 3.5%.23 As with several other types of chronic, musculoskeletal pain, the symptoms are not sufficiently explained by clinical findings such as injury, inflammation, or other proximate cause. Moreover, studies consistently report that TMD symptoms exhibit significant statistical overlap with other chronic pain conditions,25 suggesting the existence of common etiologic pathways.1 Most studies of overlap with orofacial pain have focused on selected pain conditions, classified according to clinical criteria (eg, headaches, cervical spine dysfunction, and fibromyalgia5), location of self-reported pain (eg, back, chest, stomach, and head2), or the number of comorbid pain conditions.11 Although there is a long tradition of depicting overlap between pain conditions qualitatively using Venn diagrams,28 we know of few studies that have quantified the degree of overlap between TMD and pain at multiple locations throughout the body.24
Overlap of pain symptoms can occur when there are common etiologic factors contributing to each of the overlapping pain conditions.1 One example is diabetes that contributes, etiologically, to neuropathy in the feet and retinopathy in the eye, thereby creating overlap, statistically, of diseases at opposite ends of the body. The etiologic factor most widely cited to account for overlap of pain conditions is central sensitization, defined as “amplification of neural signaling within the central nervous system (CNS) that elicits pain hypersensitivity.”27 The amplification means that otherwise innocuous sensations are perceived as painful (ie, allodynia) and that formerly mildly painful stimuli now evoke severe pain (ie, hyperalgesia). However, somatosensory afferent inputs into the CNS are segmentally organized, making it plausible that sensitization is not uniform throughout the neuraxis.17 Jensen10 coined the term “segmental central sensitization” to explain heightened thermal sensitivity and palpation tenderness specific to cranial sites during episodes of tension-type headache. Segmental sensitization is now recognized as an important component of many chronic pain conditions.3
Regardless of pain location, overlap creates serious problems for patients, adding to the suffering and disability caused by a single pain condition, and potentially complicating diagnosis and treatment for one or all of the overlapping conditions. This has broader implications for patients with multiple chronic illnesses who have poorer health outcomes and generate significantly greater health care costs than patients with a single illness.18 Thus, the aim of this epidemiological study was to quantify the degree of overlap between facial pain and pain reported elsewhere in the body.
2. Methods
We first quantified overlap between facial pain and pain at other locations using data from a community-based case–control study that was part of the OPPERA project (Orofacial Pain: Prospective Evaluation and Risk Assessment). We then replicated the associations in 2 large population-based surveys that asked about pain at multiple anatomical locations. This article follows STROBE guidelines.26 The study was reviewed and approved by the UNC Office of Human Research Ethics (study 13-2232).
2.1. Primary data collection: the OPPERA case–control study of chronic temporomandibular disorder
The primary data collection was a case–control study of chronic TMD conducted in the second phase of the OPPERA project (NIDCR Study Protocol 12-052-E). Enrollment began in November 2013 and ended in May 2016. The target population was adults, aged 18 to 74 years, living in counties near 4 US study sites: Baltimore, MD, Buffalo, NY, Chapel Hill, NC, and Gainesville, FL. Other inclusion criteria were living or working in sampled counties; and fluency in written and spoken English. Exclusion criteria were any of 7 self-reported health conditions: kidney failure or dialysis; heart disease not controlled with medication; chronic respiratory disease not controlled with medication; hypertension not controlled with medication; epilepsy or medication to control grand mal seizures; diabetes not controlled with medication or diet; and psychiatric disorders or conditions that required hospitalization in the preceding 6 months.
A sampling frame of listed telephone numbers in counties near each study site was purchased and used for “cold-calling” recruitment that was managed by the Data Coordinating Center. Study sites also used flyers, advertisements, and email solicitations, asking potential study participants to phone the call center. Trained interviewers at the call center used a computer-assisted telephone interview system to explain the nature of the study, seek verbal consent for an interview, determine eligibility, assess demographic characteristics, and ask screening questions that were used to classify the presence or absence of facial pain (see Supplementary Material: Telephone Screening Interview, http://links.lww.com/PR9/A43).
2.2. Telephone interview to determine facial pain case-classification
Interviewers asked about pain in each of 6 craniofacial locations: face, jaw, ear, front of the ear, headaches in temples, and pain in your temples other than headaches (Supplementary Material: Telephone Screening Interview, Q9, available at http://links.lww.com/PR9/A43). A separate question asked about toothache and ear infection. Subjects were classified as chronic facial pain cases if they reported pain in one or more craniofacial locations that occurred for 5 days or more per month for at least 6 of the preceding 12 months. However, toothache or ear infection was exclusionary, consistent with similar exclusionary criteria applied for clinical classification of TMD.20 Controls reported no such facial pain in the preceding 6 months, were not wearing a night guard occlusal splint, and had never been diagnosed with TMD.
2.3. Self-completed questionnaire
Subjects selected as cases or controls during the telephone interview were mailed a consent form and a self-completed questionnaire (see Supplementary Material: Subject-Completed Questionnaire, available at http://links.lww.com/PR9/A43). The 3-page questionnaire included anterior and posterior views of a line drawing, depicting a body manikin labelled with 41 check boxes naming specific anatomical locations. A lead-in question asked “During the past 3 months, have you had any aches or pains anywhere in the body that have lasted for one day or longer?” Those responding affirmatively were asked to endorse one or more check boxes signifying the location of pain. Another question asked “In the past 30 days, how many headaches of any type have you had?” and the response was recorded in days.
Other questions asking about the presence or absence of 6 jaw symptoms in the preceding 30 days (eg, stiffness and cramping) were used to create a count of nonspecific jaw symptoms. Likewise, the presence or absence of 8 common health conditions (eg, arthritis and acid reflux) was used to create a count of comorbid conditions. Extent of distress from nonpainful somatic symptoms in the preceding 7 days was assessed using a 7-question subscale (eg, faintness, hot or cold spells) from the somatization scale of the SCL90-R questionnaire.6,15 Questions about cigarette smoking was also included in the questionnaire because smoking is a risk factor for TMD.19
2.4. Replication data sets: the UK Biobank study and the US National Health Interview Survey
Corroborating evidence was sought using publicly available data from 2 studies. The UK Biobank study enrolled volunteers aged 40 to 69 years who were patients registered with the UK National Health Service living within an approximate 25-mile radius of 22 assessment centers in England, Scotland, and Wales. Of the 9.2 million people invited, 503,325 enrolled between 2007 and 201012 and 459,604 participants answered questions about pain. The first was a screening question that asked “In the past month, have you experienced any of the following that interfered with your usual activities?” Response options were one or more of: Headache; Facial pain; Neck or shoulder pain; Back pain; Stomach or abdominal pain; Hip pain; Knee pain; or Pain all over the body. For each affirmative response, a second question asked “Have you had [type of pain] for more than 3 months?,” with response options of yes or no. For the purpose of comparison with the OPPERA study, this analysis was limited to 6 types of pain (stomach and widespread pain were excluded). The UK Biobank variable codes for those 6 types of pain were 4067 (facial pain), 3799 (headache), 3404 (neck/shoulder pain), 3571 (back pain), 3414 (hip pain), and 3773 (knee pain). For each type of pain, cases were subjects who responded positively to both questions (eg, facial pain that interfered with usual activities AND facial pain that had lasted for more than 3 months), whereas controls were subjects who responded negatively to the first question (ie, did not report facial pain that interfered with usual activities). Subjects were excluded from the analysis if they reported any specific type of pain that had lasted for less than 3 months.
The second replication data set was from the US National Health Interview Survey (NHIS) conducted annually by the National Center for Health Statistics. It selects a nationally representative sample of the civilian, noninstitutionalized population of the United States. Sampled subjects complete face-to-face interviews conducted by trained interviewers from the US Census Bureau. To match the age range studied in OPPERA, this analysis of data from the 2009 survey was restricted to the 27,731 participants aged 18 to 74 years who answered 4 questions about pain experienced in the preceding 3 months: “Facial ache or pain in the jaw muscles or the joint in front of the ear,” “Severe headache or migraine,” “Neck pain,” and “Lower back pain,” each reported as “Yes” or “No.” (“Don't know” responses were coded as missing). Other questions asked about joint pain experienced during the preceding 12 months, and those responding affirmatively were asked to list one or more affected joints from a list of 16 joints below the neck (shoulders, elbows, fingers, hips, wrist, knee, ankle, and toes, each listed bilaterally).
2.5. Statistical analysis
By definition, the concept of overlap begins with an “index” condition,7 which, in this OPPERA study, was the case–control classification of chronic facial pain determined during the telephone screening interview. Other potential overlapping pain conditions were derived from responses to the self-completed questionnaire, which were used to create a binary indicator for the presence or absence of headache (zero vs one or more days of headache) and for the presence or absence of pain at each of 6 body locations, aggregated from the 35 noncranial checkbox locations depicted in the manikin: neck, shoulders, trunk, hips, arms, and legs. To quantify overlap between facial pain and each of the other pain locations, odds ratios (ORs) and their 95% confidence limits (95% CLs) were computed using contingency tables.
Recognizing that the degree of overlap could be confounded by risk factors for facial pain that are likewise associated with other pain conditions, we used multivariable logistic regression models to estimate unconfounded associations. Three groups of potential risk factors were used in the multivariable models: demographics, health-related behavior, and biopsychosocial risk profile. Demographic variables were age (in years, modeled as a continuous variable), sex (2 categories: male and female), self-reported race (4 categories: White, African American, Asian, and other/mixed), and self-reported ethnicity (2 categories: Hispanic and non-Hispanic). The single health-related behavior variable was history of cigarette smoking (3 categories: current, former, or never).
Among the many relevant biopsychosocial risk factors for facial pain,8 the available risk factor variables were limited to responses from one self-completed questionnaire, which were used to assign subjects to a “pain symptom cluster,” hence serving as a maker of a subject's overall biopsychosocial risk profile. The clustering method was developed and validated in a previous OPPERA study using hundreds of pain-related phenotypic characteristics.4 For this analysis, cluster membership was classified with a statistical prediction model based on 3 predictor variables used in both the current study and the previous OPPERA study: the nonpain somatization subscale of the SCL90-R, the count of nonspecific jaw symptoms, and the count of comorbid conditions. The first step in developing the prediction model used data from n = 2,118 subjects in the previous OPPERA study.4 The data set was randomly partitioned into a training set and a test set (with equal numbers of participants in both partitions). The training data set was used to fit a nearest centroid model to predict membership in the global symptoms cluster based on the 3 summary variables. After validating the model, it was applied to the data collected in this study to assign participants to either the global symptoms cluster or the combined adaptive- and pain-sensitive clusters.
For descriptive purposes, heat maps were created to graphically depict the percentage of subjects in the OPPERA case–control study who reported pain in each bodily location. The heat maps were generated separately according to facial pain case-classification and the dichotomized pain symptom clusters. To quantify overlap between facial pain and each other pain locations, univariate ORs and their 95% CLs were computed using contingency tables. Multivariable binary logistic regression was then used to estimate adjusted ORs that accounted for demographics, cigarette smoking, and pain symptom cluster.
The UK Biobank data were analyzed using binary logistic regression models, with each model estimating the univariate association between facial pain and one other pain condition: headache, neck/shoulder pain, back pain, hip pain, or knee pain. Each model additionally controlled for age (in years), sex (2 categories: male and female), and 5 racial eigenvectors derived from principal component analysis of ancestry-informative genetic markers. The NHIS data were analyzed with SAS survey estimation procedures that used survey design variables and sampling weights to generate estimates that were generalizable to the US adult population. Odds ratios and 95% CLs were estimated using binary logistic regression models to quantify the association between facial pain and each of 4 other pains: headache, neck pain, back pain, and 2 or more below-neck joints. The models adjusted for age (in years, modeled as a continuous variable), sex (2 categories: male and female), self-reported race (4 categories: White, African American, Asian, and other/mixed), and self-reported ethnicity (2 categories: Hispanic and non-Hispanic). There was no additional multivariable modeling of data from the UK Biobank and NHIS studies because they did not collect the same set of covariates used in multivariable analysis of the OPPERA study. Instead, judgments about replication were based on comparisons across the 3 studies of the univariate ORs.
3. Results
3.1. Primary data collection: the OPPERA case–control study of chronic temporomandibular disorder
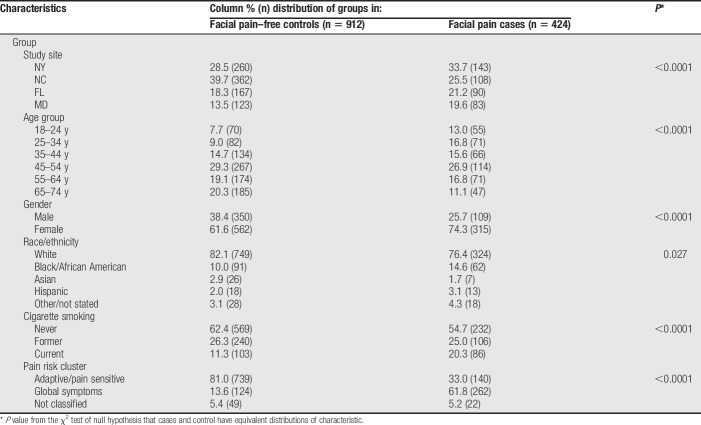
During the 30-month recruitment period, 783,739 attempts were made to call 166,062 phone numbers in the OPPERA case–control study. Many yielded no response after 6 attempts and others were excluded, yielding 2,430 subjects who completed the screening interview and were eligible for the case–control study: 725 subjects were facial pain cases and 1,705 were controls (Appendix Fig. 1, http://links.lww.com/PR9/A43). Fifty-five percent of them (n = 1,336 subjects; 424 cases and 912 controls) completed mailed questionnaires and hence were included in this analysis. The percentage of eligible subjects who completed mailed questionnaires varied according to study site, age, race/ethnicity, and screening case-classification, although not according to sex (Appendix Table 1, http://links.lww.com/PR9/A43). Compared to the reference standard for cluster classification in our previous study,4 the predictive accuracy of the model used to classify pain symptom cluster was found to be very good: sensitivity was 69.5%, specificity was 89.7%, and percent agreement was 86.1%.
Relative to controls, chronic facial pain cases had a younger age distribution, greater percentages of cases were females and non-whites, and a greater percentage of cases were smokers. Cases were much more likely than controls to be in the global symptoms cluster and, conversely, less likely to be in the adaptive/pain-sensitive cluster (all P value <0.05; Table 1).
Table 1.
Distribution of demographic characteristics and covariates: the OPPERA case–control study of facial pain.
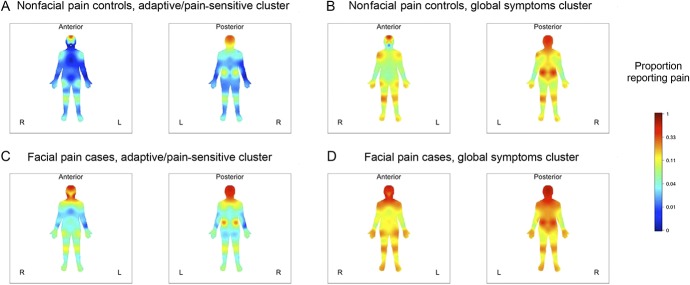
Pain was endorsed on the body manikin most frequently in the head and least frequently in the forearms, a pattern that was consistent for each cluster within each case–control group (Fig. 1). For a given anatomical location, there was an expected pattern of variation in pain frequency according to facial pain classification and symptom cluster: for example, controls with nonglobal symptoms had the lowest frequency, whereas cases with global symptoms had the greatest frequency. The other 2 groups had intermediate pain frequency, although the second- and third-ranking varied according to pain location. That is, facial pain cases with nonglobal symptoms were more likely to endorse headache than controls with global symptoms, whereas for virtually all anatomical locations below the neck, the order was reversed: facial pain cases with nonglobal symptoms were less likely to endorse pain than controls with global symptoms. Neck pain was reported with similar frequency in the 2 groups: 36.8% and 40.9%, respectively (Appendix Table 2, http://links.lww.com/PR9/A43).
Figure 1.
Anatomical distribution of pain in 4 study groups: OPPERA case–control study of facial pain. Color coding signifies location and proportion of subjects reporting ≥1 headache during the preceding 30 days or reporting pain at any of 35 noncraniofacial locations that had lasted ≥1 day during the preceding 3 months. Subjects are stratified according to the presence or absence of facial pain (classified as ≥5 days per month for ≥6 of the preceding 12 months) and type of pain symptom cluster: global symptoms or nonglobal symptoms. (A) Nonfacial pain controls, nonglobal symptoms (n = 739); (B) nonfacial pain controls, global symptoms (n = 124); (C) facial pain cases, nonglobal symptoms (n = 140); and (D) facial pain cases, global symptoms (n = 262).
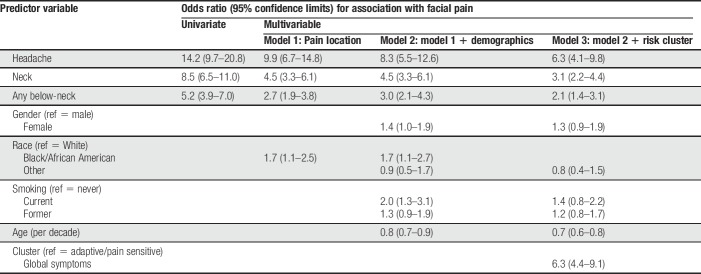
In univariate analysis, facial pain was most strongly associated with headache (OR = 14.2, 95% CL = 9.7–20.8) followed by neck pain (OR = 8.5, 95% CL = 6.5–11.0; Table 2). Pain at other locations was also significantly associated with facial pain, although ORs were 4.6 or less. The same anatomical ranking of ORs was seen in multivariable analysis that aggregated the data to 3 types of pain: headache, neck pain, and any below-neck pain (Table 3, Model 1). The corresponding ORs for association with facial pain were again ranked anatomically in models that additionally adjusted for demographics and smoking (Table 3, Model 2) and also for pain symptom cluster (Table 3, Model 3). In each instance, ORs were attenuated somewhat after adjustment for other variables, although the OR for headache was approximately twice that of neck pain and 3 times that of below-neck pain.
Table 2.
Univariate associations with facial pain: the OPPERA case–control study of facial pain (n = 1,336 subjects).
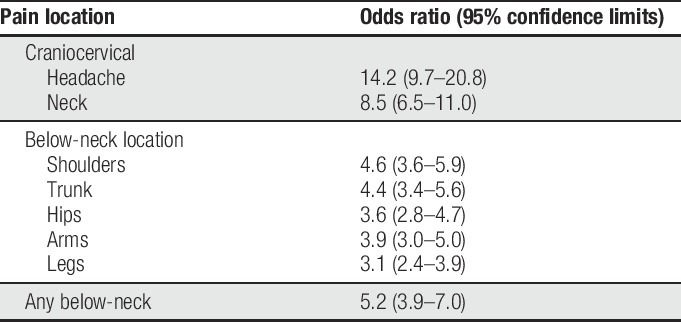
Table 3.
Unadjusted and adjusted associations with facial pain: the OPPERA case–control study of facial pain (n = 1,265 subjects).
3.2. Replication data sets: the UK Biobank study and the US National Health Interview Survey
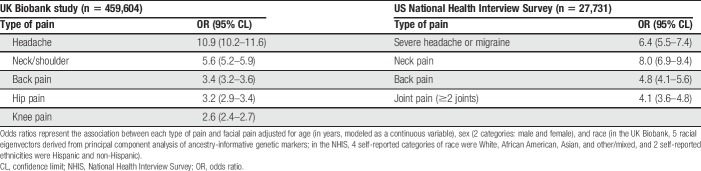
In the UK Biobank study (n = 459,604 participants), 0.9% of subjects reported chronic facial pain (95% CL = 0.8–0.9), whereas the percentage reporting other types of chronic pain varied from 9% for headache to 18% for back pain. The univariate association with facial pain was greatest for headache (OR = 10.9, 95% CL = 10.2–11.6) and smallest for knee pain (OR = 2.6, 95% CL = 2.4–2.7), with intermediate values for neck/shoulder, back, and hip pain (Table 4).
Table 4.
Associations with facial pain in 2 replication cohorts of UK and US adults.
In the NHIS study (n = 27,731 participants), the population-weighted estimate of prevalence of facial pain among US adults was 5.2% (95% CL = 4.9–5.5) and prevalence of other pain conditions varied from 16% for neck pain to 28% for back pain. Univariate associations with facial pain were more pronounced for neck pain (OR = 8.0, 95% CL = 6.9–9.4; Table 4) and headache (OR = 6.4, 95% CL = 5.5–7.4) than for back pain (OR = 4.8, 95% CL = 4.1–5.6) or joint pain (OR = 4.1, 95% CL = 3.6–4.8).
4. Discussion
In this community-based sample of adults in the OPPERA project, there was, as expected, considerable overlap between facial pain and pain elsewhere in the body. The noteworthy finding was that the degree of overlap was much greater for craniocervical pain (ie, headache or neck pain) than for pain in anatomical locations below the neck. Odds ratios signifying the magnitude of overlap between craniocervical pain and facial pain were about twice the value observed for below-neck pain and facial pain, a pattern that was independent of pain symptom cluster and other potential confounding characteristics. A similarly distinctive anatomical pattern of univariate associations was seen in 2 large replication cohorts, suggesting that this phenomenon of anatomical selectivity in the degree of overlap with facial pain was generalizable to diverse population samples.
Overlap of orofacial pain and headache may appear unsurprising, given the trigeminal nerve's innervation of the head and face. However, the underlying mechanism cited most frequently to account for overlap of the pain symptoms studied here is central sensitization.27 In this instance, segmental sensitization provides a more nuanced explanation that might account for anatomical variation in the degree of overlap observed here. Early evidence of segmental sensitization came from a study of patients with tension-type headache who were assessed for palpation tenderness in muscles and tendons of the head and neck and thermal pain sensitivity in the head and hand.10 Compared to periods without headache, both palpation tenderness and cranial thermal pain sensitivity increased during episodes of headache, but thermal pain sensitivity in the hand did not change. The authors concluded that headache contributes selectively to sensitization in the pericranial region, not remotely, a pattern that is consistent with segmental sensitization. Likely, neurologic mechanisms underlying segmental sensitization were suggested in a rodent study demonstrating that nociceptive neurons in the first cervical dorsal horn receive an extensive afferent input from multiple tissues in the craniocervical region, but little input from afferents caudal to C2 innervation.13 The authors concluded that afferent convergence in first cervical dorsal horn nociceptive neurons was a plausible explanation for pain referral in clinical conditions such as TMDs, whiplash, and headache.
By definition, pain is an unpleasant sensation, and it is also possible that the affective component of pain is influenced by anatomical location of the symptoms. For example, one study used a rodent model to show how craniofacial pain activates an “affective pain circuit” that amplified pain aversiveness.17 However, other cells and biological systems in the CNS also regulate pain, including Schwann cells and satellite cells in dorsal root ganglia and microglia and astrocytes in the spinal cord.21 Although those pathways probably influence pain symptoms reported here, it is not likely that they could fully account for anatomical variation in the degree of overlap.
Although spinal/trigeminal segmental sensitization seems a plausible explanation for these findings, this study did not conduct any quantitative sensory testing that would be needed help to confirm the explanation. It is also conceivable that prolonged nociceptive input from a particular anatomical location could promote cerebral reorganization, such that increased somatosensory or attentional resources are allocated to that region of the body. Indeed, cortical reorganization has been documented in multiple chronic pain conditions, particularly in somatosensory and motor regions.9,14,22
Another noteworthy finding in the OPPERA study was the independent contribution of pain symptom cluster classification to the distribution of pain symptoms. As indicated by the pain location heat maps, subjects in the global symptoms cluster reported pain more widely than subjects who were not in that cluster (ie, either “adaptive” or “pain sensitive”). The difference was likewise observed within facial pain cases, indicating that the condition of “facial pain” itself reflects a heterogeneous set of underlying and coexisting pain disorders. As expected, facial pain cases in the global symptoms cluster were more likely to have pain outside the orofacial region than facial pain cases who were not in that cluster. However, the effects were independent: the global symptoms cluster did not account for the distinctive anatomical ranking of ORs between each pain location and facial pain seen in the multivariable model.
One methodological limitation of this study was the extent of nonparticipation by people invited to participate in the OPPERA Protocol 12-052-E. While every effort was made to obtain a sample representative of the populations living near each study site, the low response rate means that the participants studied are effectively a convenience sample of people in those communities who were willing to complete study procedures. In principle, the UK Biobank sample is intended to represent the 95% of the United Kingdom that is registered with the National Health Service, although that study, like OPPERA, enrolled only a small fraction of people invited to participate. By contrast, however, the NHIS uses a rigorous sampling method to select a nationally representative sample, and participation rates have exceeded 85% in recent decades. Replication of the OPPERA findings in those cohorts suggests that biases due to uncertain representativeness were not a serious problem for this study of pain overlap. Another limitation arises from reliance upon self-reported history and symptoms of pain. For example, our classification of facial pain excluded toothache, which is sometimes the label used by people who actually have TMD. When examiners diagnose TMD using established criteria,20 they likewise exclude toothache, although the clinical evaluation permits greater accuracy making that exclusion than was possible in this study.
Aside from casting light on anatomical selectivity in overlap of bodily pain and facial pain, the presence and nature of pain comorbidities may have implications for TMD therapy. For example, a randomized controlled trial that evaluated efficacy of oral splints in treating TMD myalgia found significant improvement over controls for TMD cases having only regionally restricted myalgia, whereas TMD patients with more widespread pain did not benefit from the oral splints.16 Another practical implication of this study was the value of comorbid conditions assessed using simple methods that are practical to use in clinical care or clinical studies. Specifically, the brief set of questions about somatic symptoms, a checklist of health conditions, and nonspecific jaw symptoms proved adequate to classify subjects into pain symptom clusters that appear to be a good proxy for central sensitization. Likewise, the body manikin proved to be a simple method to capture widespread pain symptoms.
Disclosures
The authors have no conflict of interest to declare.
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research (Award Number U01DE017018). Parts of this study were conducted under UK Biobank application number 20802.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A43.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001;134:868–81. [DOI] [PubMed] [Google Scholar]
- [2].Al-Harthy M, Michelotti A, List T, Ohrbach R. Influence of culture on pain comorbidity in women with and without temporomandibular disorder-pain. J Oral Rehabil 2017;44:415–25. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Mohr Drewes A. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. [DOI] [PubMed] [Google Scholar]
- [4].Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. PAIN 2016;157:1266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Costa YM, Conti PC, de Faria FA, Bonjardim LR. Temporomandibular disorders and painful comorbidities: clinical association and underlying mechanisms. Oral Surg Oral Med Oral Pathol Oral Radiol 2017;123:288–97. [DOI] [PubMed] [Google Scholar]
- [6].Derogatis L. The SCL-90-R: administration, scoring and procedures manual. Minneapolis: National Computer Systems, Inc, 1994. [Google Scholar]
- [7].Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis 1970;23:455–68. [DOI] [PubMed] [Google Scholar]
- [8].Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, Ohrbach R, Maixner W. Summary of findings from the OPPERA baseline case-control study: implications and future directions. J Pain 2011;12(11 suppl):T102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fujimaki Y, Ehara M, Kimura E, Shimada M, Aoki Y. Diethylcarbamazine, antifilarial drug, inhibits microtubule polymerization and disrupts preformed microtubules. Biochem Pharmacol 1990;39:851–6. [DOI] [PubMed] [Google Scholar]
- [10].Jensen R. Mechanisms of spontaneous tension-type headaches: an analysis of tenderness, pain thresholds and EMG. PAIN 1996;64:251–6. [DOI] [PubMed] [Google Scholar]
- [11].John MT, Miglioretti DL, LeResche L, Von Korff M, Critchlow CW. Widespread pain as a risk factor for dysfunctional temporomandibular disorder pain. PAIN 2003;102:257–63. [DOI] [PubMed] [Google Scholar]
- [12].Macfarlane TV, Beasley M, Macfarlane GJ. Self-reported facial pain in UK Biobank study: prevalence and associated factors. J Oral Maxillofac Res 2014;5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci 2007;26:142–54. [DOI] [PubMed] [Google Scholar]
- [14].Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair 2012;26:646–52. [DOI] [PubMed] [Google Scholar]
- [15].Ohrbach R, Turner JA, Sherman JJ, Mancl LA, Truelove EL, Schiffman EL, Dworkin SF. The research diagnostic criteria for temporomandibular disorders. IV: evaluation of psychometric properties of the axis II measures. J Orofac Pain 2010;24:48–62. [PMC free article] [PubMed] [Google Scholar]
- [16].Raphael KG, Marbach JJ. Widespread pain and the effectiveness of oral splints in myofascial face pain. J Am Dent Assoc 2001;132:305–16. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez E, Sakurai K, Xu J, Chen Y, Toda K, Zhao S, Han BX, Ryu D, Yin H, Liedtke W, Wang F. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat Neurosci 2017;20:1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sambamoorthi U, Tan X, Deb A. Multiple chronic conditions and healthcare costs among adults. Expert Rev Pharmacoecon Outcomes Res 2015;15:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. The J pain 2013;14(12 suppl):T51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF; International Rdc/Tmd Consortium Network IafDR, Orofacial Pain Special Interest Group IAftSoP. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–8. [DOI] [PubMed] [Google Scholar]
- [22].Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol 2011;24:515–23. [DOI] [PubMed] [Google Scholar]
- [23].Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T20–32 e21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Slade GD, Sanders AE, Bair E, Brownstein N, Dampier D, Knott C, Fillingim R, Maixner WO, Smith S, Greenspan J, Dubner R, Ohrbach R. Preclinical episodes of orofacial pain symptoms and their association with health care behaviors in the OPPERA prospective cohort study. PAIN 2013;154:750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009;7:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [27].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152(3 suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yunus MB. Primary fibromyalgia syndrome: current concepts. Compr Ther 1984;10:21–8. [PubMed] [Google Scholar]