This article reviews current methods and challenges and provides recommendations for future design and conduct of clinical trials of interventions to treat acute pain.
Keywords: Clinical trial, Acute pain, Analgesic trial
Abstract
Introduction:
The clinical setting of acute pain has provided some of the first approaches for the development of analgesic clinical trial methods.
Objectives:
This article reviews current methods and challenges and provides recommendations for future design and conduct of clinical trials of interventions to treat acute pain.
Conclusion:
Growing knowledge about important diverse patient factors as well as varying pain responses to different acute pain conditions and surgical procedures has highlighted several emerging needs for acute pain trials. These include development of early-phase trial designs that minimize variability and thereby enhance assay sensitivity, minimization of bias through blinding and randomization to treatment allocation, and measurement of clinically relevant outcomes such as movement-evoked pain. However, further improvements are needed, in particular for the development of trial methods that focus on treating complex patients at high risk of severe acute pain.
1. Introduction
Analgesic trials for acute pain are typically investigated in the context of traumatic injury or surgical procedures. Because of the relatively high, and consistent, frequency of surgical procedures all over the world, development and refinement of clinical trial methodologies has been most prominent in the setting of acute postoperative pain. Other conditions such as migraine and dysmenorrhea, serious illnesses such as pancreatitis or herpes zoster, acute neuropathic pain associated with chemotherapies, and acute exacerbations (“flares”) of chronic conditions such as osteoarthritis, sickle cell disease, and low back pain, although often associated with brief self-resolving periods of intense pain are less typically used to evaluate analgesics for acute pain.29 Distinctive features of acute pain include generally short duration (eg, days to weeks) and often predictable onset (eg, postsurgical and posttraumatic pain). Acute pain, particularly when related to trauma or surgery, is also often associated with psychological and physiological stress responses,63 hemodynamic changes and other fluid shifts, and exposure to multiple medications and nondrug interventions. The purpose of this article is to review current methods and challenges, and to consider the needs for improvement of future design and conduct of clinical trials of interventions to treat acute pain. Although the majority of current knowledge in this area comes from the setting of pharmacological interventions to treat postsurgical pain, a great many principles and considerations may be applied to other nonsurgical acute pain conditions and, also may be applied to some degree to nonpharmacological interventions.
2. Historical perspective on acute pain trial design
Before the initial development of clinical trial designs for the evaluation of pain treatment interventions, much research was first done to better understand the nature of subjective responses to analgesic interventions in the setting of acute pain.7 However, earlier investigations on the effects of analgesic interventions were often limited to open-label observational studies,69 which were susceptible to various sources of bias. The study of the efficacy of new analgesic drugs using randomized, placebo-controlled clinical trials in patients experiencing acute postoperative pain started with Beecher at Harvard in the 1940s and 50s,6,8,9 in whose group a number of other investigators were trained.5 Many of the earliest designs for placebo-controlled, single-dose, parallel group and cross-over trials were developed and advocated by these pioneering investigators. Their earliest publications documented the human dose–response and time action characteristics of prototypical analgesics in patients with acute pain.5,60,75,108 A primary focus of the earliest studies was the characterization of the efficacy and limitations of opioid drugs, which were so commonly used in hospital wards at that time. These study designs have also been applied to evaluate nondrug interventions such as preoperative patient education as to the nature, duration and anticipated pain quality and intensity during the perioperative interval.35
From the outset, there was interest in, and support for, these methods by the pharmaceutical industry and the U.S. Food and Drug Administration (FDA) as finally there seemed to be reproducible methods to assess the efficacy of new analgesic drugs. These early investigators stressed the need for placebo and active controls to judge the sensitivity of individual studies.74 Critical learnings from these early trials included understanding of the pharmacological principles underlying opioid analgesics (eg, relative potency, oral/parenteral conversion ratios, and additive effects of combination drug products), recognition of the risks of short-term administration of opioid analgesics, and an enduring appreciation of the importance of placebo effects in analgesic trials. Most trials were performed in hospital wards by trained research nurse observers and soon, a more standardized process for analgesic drug development seemed feasible. This progress spurred the development of the FDA's Guideline for the Clinical Evaluation of Analgesic Drugs.111
Several factors unfortunately constrained innovation in the study of new analgesics. First, the contingent of trained clinician–investigators was relatively small and concentrated in a few teaching hospitals. Second, clinical development teams within the pharmaceutical industry were generally reluctant to explore new study designs and models outside established hospital-based postoperative pain models. “Pivotal” postoperative pain studies consisted almost entirely of single-dose analgesic assays. Long-term safety studies for analgesic drugs focused on their 6-month use in disorders such as osteoarthritis. Longer-term efficacy studies were not performed.
In 1975, a new outpatient pain model—the Dental Impaction Pain Model (DIPM)—was introduced,28 which seemed to simplify and accelerate analgesic drug development in several ways. Patients eligible for molar extraction studies were relatively plentiful and generally healthy, the studies could be conducted in outpatients or inpatients, and the perioperative and surgical interventions became standardized within the protocols. Hence, highly reproducible study results could be obtained in relatively short periods. Recruitment methods (newspaper, radio, television, and even billboard advertising approved by IRBs), which had been disdained by academic medical centers, now became tools for rapid study enrollment. Studies that previously were conducted in 12 to 18 months could now be conducted in 3 to 6 months. Now, enrollment of a relatively homogeneous population of younger, healthy patients yielded studies with excellent assay sensitivity (ie, the ability of a trial to detect a meaningful treatment effect compared with a placebo and active comparators), high reproducibility, and greatly accelerated time lines. Because of these factors, this pain model became the model of choice for analgesic drug development in the 1980s and 1990s when many new nonsteroidal anti-inflammatory drugs and later, cyclooxygenase-2 (“COX-2”) inhibitors were being developed.
In the late 1990s and early 2000s, Desjardins et al. developed the Post-Bunionectomy Pain Model (PBPM) that in large part was modelled on the lessons learned from the now refined DIPM.2,31,100 The PBPM offered plentiful patient recruitment, standardized postoperative care in specialized clinical research centers, and standardized surgical, perioperative, and anesthesia protocols. The PBPM's popularity among drug and device developers surged because it was recognized that these patients experienced a more prolonged course of acute pain than did patients after dental impaction surgery. It now became possible to retain patients in research settings for 2 to 5 days to better understand their pain course over time. Although there remain questions about optimal repeat dosing schedules, possible interactions between rescue drug and investigational drug, and assay sensitivity over time, this relatively new acute pain model has proven to be useful in drug development.
Even with these methodological innovations, limitations remain. Because clinical drug development emphasizes speed of enrollment and reproducibility, contemporary drug development plans typically limit the number of postoperative studies to 2 or 3 types of acute postoperative or posttraumatic injury pain. There remains a reluctance to explore new models or new study designs until FDA regulators agree to accept them. Only after FDA regulators indicated they would no longer accept DIPM studies as confirmatory trials did sponsors and contract research organizations invest in the development of new models. Other orthopedic surgical models (total knee arthroplasty and hip arthroplasty) were included by sponsors in their drug development plans, despite being seen as relatively challenging and less predictable. The focus for much industry-funded, acute pain research remains the development of drugs under well-controlled, standardized conditions—not on their study in today's hospital setting with its growing focus on sicker, medically compromised patients, cared for using methods such as multimodal analgesia that may promote earlier hospital discharge. These factors each contribute to the complexity of hospital-based studies and unpredictability of their outcomes, ultimately leading to a greater chance of failed studies and slow enrollment.
Fortunately, as most industry-supported scientists focused their efforts on individual drug development, a few groups59 continued to investigate the efficacy of new analgesics in specific postoperative populations of patients to develop clinical guidance for surgeons and anesthesiologists. In many acute pain conditions, however, there remain unanswered questions that plague surgical patients. Those questions will be highlighted below.
3. Trial design features
3.1. Patient stratification
Although acute pain occurs in many nonsurgical clinical situations, the vast majority of acute pain research has been conducted in the setting of treating acute pain after surgery. Therefore, most of the considerations discussed in the area of patient selection are related to postoperative pain. However, several of these trial design considerations also apply to other acute pain conditions.
Despite much research and several international guidelines, overall treatment of postoperative pain remains suboptimal.27 This problem echoes a tension now evident throughout much of medical and surgical translational research. Evidence-based guidelines usually translate aggregated, population-based observations into clinical recommendations. However, individual patients–particularly those whose postoperative pain is difficult to control in practice–may be outliers often excluded from relevant clinical trials by virtue of age, previous treatment, or other patient-related factors. Although existing guidelines for trial design have provided a sound basis for improvements in research methods, the broader question remains as to whether future strategies should be focused primarily on specific patient groups vs specific surgical procedures. The importance of the latter is emphasized by the huge interindividual variations in pain intensity trajectories and opioid consumption after the same operation in patients receiving the same analgesic treatment.11 Also, the aggregation of clinical trial results must now take into consideration the markedly different analgesic efficacy of the same analgesic in relation to the nature, location, and severity of the surgical trauma.50 Consequently, we propose that future analgesic trials in acute postoperative pain should be more clearly differentiated regarding different patient-related and surgery-related factors (see Table 1 for recommendations). This strategy of disaggregation may clarify whether some low-pain patient responders only require relatively simple, standardized multimodal opioid-sparing analgesia, whereas high-pain or high-risk patients should have preoperative plans in place to provide additional multimodal or specific high-pain intensity analgesic treatment. This strategy also addresses several key principles and challenges of current population health science, eg, that we can predict health in populations with much more certainty than we can predict health in individuals.70
Table 1.
Recommendations for patient stratification in acute pain randomized controlled trials.
Recent qualitative systematic reviews have suggested that type of surgery, age, and psychological distress (eg, depression, anxiety, and pain catastrophizing) are significant predictors for postoperative pain and analgesic consumption, whereas sex is less (or not) important.32,57 However, despite including 48 eligible studies in 1 review, the overall predictive value of these predictors was less than 54% calling for better prediction methods in future studies. In addition, the studies included did not stratify by other important factors, for example, different types of analgesic management or surgical procedures, both of which may influence pain intensity and other pain-related outcomes.
The hypothesis that the large interindividual variation in pain response to a given operation could be assessed by the pain response to a preoperative nociceptive stimulus was reviewed recently in relation to predictive, ie, preoperative, experimental pain testing. Although there was some predictive value, it was less than 50%, based on relatively few studies and lack of statistical power in relation to specific type of surgery or analgesic treatment.96,114
Although the type of surgical injury is important for prediction of acute postoperative pain,57 an often-overlooked factor is whether or not the operation was performed for preexisting pain (ie, knee replacement for painful arthritis vs thoracotomy for painless lung cancer). It is well established that high-intensity preoperative pain increases the risk of acute postoperative pain.57,114 Therefore, preoperative pain intensity should be considered as a stratification factor in future analgesic trials, and then on a procedure-specific basis.59
Because preoperative psychological distress (anxiety, depression, pain catastrophizing, etc.) is also a well-established predictor of acute postoperative pain,57 targeted trials are needed in these high-risk subjects compared with low-risk ones.14 Unfortunately, only a few trials are available with such an approach79 calling for more studies to allow recommendations for design of future trials. Thus, although this particular trial found only a limited analgesic effect during the first postoperative week when added to the multimodal opioid-sparing strategy, the approach of supplementing analgesic regimens with mood-enhancing or anxiolytic agents calls for future studies after this enriched trial design.14 Also, improved preoperative characterization of the endogenous pain modulation system as a predictor of postoperative pain response should be considered.4,51,95,120 Finally, stratification for other population-based vulnerabilities may be considered in future trials that enroll very old or very young subjects and those with cognitive dysfunction.
Many surgical patients with preoperative pain at the site of surgery or other sites present for operation with a history of ongoing use of opioids or other analgesics. In this context, it is well established that patients receiving preoperative opioid treatment are more difficult to manage because of their higher postoperative pain intensity and behavioral responses as especially demonstrated in orthopedic procedures.1,38,47,94 Here, too, these observations again call for specific analgesic studies in such high-pain responders that apply nonopioid agents such as ketamine.10,78,113
In addition to patients receiving preoperative opioids, there is a need for studies in patient groups receiving nonopioid analgesics and adjuvant agents preoperatively, again on a procedure-specific basis.
In summary, several studies during the past decade have focused on the identification of subsets of high-pain responders to surgery to allow improved future analgesic trial design. Potentially, the results of such trials could demonstrate that certain low-pain responders can effectively be managed by simple, standard nonopioid multimodal analgesic regimens. By contrast, those high-risk or high-pain intensity responders require focused attention to define the relative efficacy of more complex multimodal opioid-sparing therapy vs efficacious interventional analgesic techniques including regional anesthesia, which might avoid opioids entirely.
3.2. Study treatment and comparators
Trial design features should be closely aligned with the purpose of the trial (Table 2 for recommendations). For example, an early proof of concept trial of a new molecular entity might use a dose range, including the maximally tolerated dose, as guided by phase 1 data.17,29,46 Alternatively, a comparative effectiveness trial might use a more thoroughly characterized drug dose that has been deemed efficacious, safe, and well tolerated in previous trials.23 Control comparators to be included alongside the study treatment of interest may include placebo, a different active comparator, and/or a lower dose of the study treatment.29 Evaluation of 2 or more different doses of a study medication can provide clinically useful information. For example, the observation of an ascending dose–response for analgesic efficacy suggests an effect specific to the pharmacological actions of the study drug and, further, provides a rationale for using the higher dose as long as the safety profile is acceptable. In a trial comparing either 2 or more doses of 1 treatment or 2 different treatments, downside sensitivity is demonstrated if the lower dose (or inferior treatment) is shown to be superior to placebo, whereas upside sensitivity is demonstrated if the higher dose (or superior treatment) is shown to be superior to the lower dose (or inferior treatment).29 In settings where delaying analgesic treatment will not result in long-term complications and in study designs that allow participant withdrawal from the trial and/or participant access to a nonstudy drug rescue analgesic treatment, current consensus favors using a placebo comparator—ie, an inactive intervention otherwise identical to the study intervention.29 If, however, the purpose of the trial is to evaluate the comparative effectiveness of 2 or more previously proven analgesic treatments, then inclusion of a placebo arm may not be necessary although downside assay sensitivity may not be demonstrable. In such cases, a noninferiority study design might be preferable.
Table 2.
Recommendations for selecting treatment comparators in acute pain randomized controlled trials.
Including a previously proven active treatment comparator and a placebo in a trial of a new analgesic is one approach to demonstrating assay sensitivity.29,83 For example, if an investigational agent demonstrates no superiority over placebo and the active comparator does demonstrate superiority, then the trial has demonstrated assay sensitivity (ie, trial methods are sensitive to differences in treatment effect) and lack of effect of the investigational agent. Also, growing evidence of the potential benefits of combination analgesic therapy and its increased use for acute pain41,115 has led to a growing number of factorial trial designs that compare, eg, a 2-drug combination with each of its single-agent components.45,82,87,92 In multidose studies involving an active comparator, it is important to consider the optimal timing of outcome assessments with respect to the pharmacokinetics and pharmacodynamics of the study treatment and, in particular, to avoid or otherwise account for active comparators with time courses that differ from those of the study treatment.
3.3. Internal validity, generalizability, and assay sensitivity
As with all other types of trials, randomized controlled trials of acute pain treatments must minimize sources of bias to achieve a high degree of internal validity (Table 3). This is facilitated by randomizing study patients to the different treatment arms in the trial and also by blinding participants and research staff to treatment allocation, outcome assessment, and data analysis.53,58 Another important consideration of internal validity is assay sensitivity, ie, the ability of a trial to detect a meaningful treatment effect compared with a placebo and/or with active comparators.29 On the other hand, maximizing assay sensitivity, for example, by applying restrictive criteria to enroll a homogeneous population of uncomplicated patients can conflict with the external validity or generalizability, and possibly limit relevance of randomized controlled trial results to “real world” practice.
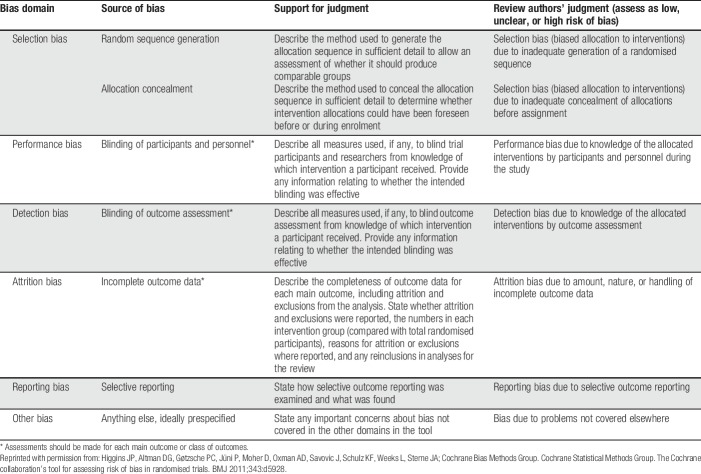
Table 3.
Cochrane collaboration's tool for assessing risk of bias.
3.4. Trial designs
Postinjury/postsurgical pain is commonly short lived and generally diminishes to mild or zero intensity within days to weeks depending on the site and magnitude of the injury.12,119 Therefore, a parallel group design is commonly used, in which all participants are randomized, in a double-blind fashion, to receive only one of the treatments under evaluation.29 In this design, not all participants are exposed to the study treatment and the obvious ethical aspects of pain undertreatment in placebo-treated participants must be considered. Given the brief duration of pain associated with acute pain settings such as surgery or trauma, cross-over trial designs are seldom used. Particularly, in early-phase exploratory clinical trials, there may be a role for adaptive trial designs, ie, involving a predefined plan to modify 1 or more aspects of the study design (eg, drug dose and sample size) based on interim data analyses at predefined time points throughout the trial.29
3.4.1. Preemptive analgesia trial design
Over 25 years ago, the hypothesis that interventions to suppress mechanisms of postinjury neuronal sensitization could lead to more effective reductions in postoperative pain, led to the concept of “preemptive analgesia,” ie, the administration of antinociceptive treatments before starting surgery.112,116 For several years later, many clinical trials were conducted using a “preemptive trial design” involving the randomization of surgical patients to 2 groups, 1 receiving the investigational analgesic treatment before surgery and the other receiving it just after surgery.116 Overall, an overwhelmingly positive impact of preemptive analgesia was not consistently seen72 and it has been suggested that an important reason for this is that mechanisms of neuronal sensitization related to postinjury/postsurgical inflammation are at least as important to the pathogenesis of acute pain as is the initial nociceptive barrage related to the injury/surgery itself.61
3.5. Efficacy outcome measures
This section addresses efficacy measures and identifies 4 key trends relevant to acute pain treatment (see Table 4 for recommendations). First, after decades of relative indifference to studying acute pain among academic researchers, who often viewed its assessment and treatment as mechanical and formulaic, it is seen more and more as a complex multidimensional entity having much in common with chronic pain.67,109 Second, there is increasing understanding that distress—particularly as related to social isolation and stigmatization—is as integral to the acute pain experience as is nociception, and is important to patients with acute pain. Third, we have begun to enter an era of shared decision making between patient and clinician, with expectations of personalized, precision medicine. Finally, related to the third trend and as emphasized above, clinicians' ability to provide evidence-based clinical interventions has evolved beyond population-based recommendations50 towards identifying prospectively those subpopulations most likely to benefit from particular interventions after specific procedures.11,57
Table 4.
Recommendations for selecting efficacy outcome measures in acute pain randomized controlled trials.
Tissue injury and the resultant experience of acute pain evoke multidimensional responses,18,26 not all of which reach consciousness. Paralleling the history of medicine in general, at one time or another some subset of these intertwined responses has received greatest attention. For clinical trials in acute pain, at different times, attention has centered on personal experience as reflected in pain intensity, pain relief, or satisfaction with care13; local or systemic measures of stress or catabolism; generic measures of the quality of postoperative recovery; procedure-specific functional outcomes such as ability to ambulate or breathe deeply45,117,118; psychosocial outcomes such as return to work- and family-related roles vs progression to chronic pain and disability; and more recently, population-based outcomes with society-wide implications such as the proportion of patients maintained long term on opioid analgesics initiated postoperatively.
Clinically relevant efficacy outcomes in analgesic trials assess pain intensity and the temporal pattern of onset and offset of pain relief.99 A second group of outcomes in analgesic trials assess physical function, either directly related to the surgical procedure (eg, pulmonary function after thoracotomy) or more generic (eg, ability to ambulate without assistance). A third group of outcomes are behavioral, such as anxiety or perceived isolation. These 3 groups of outcomes taken together determine health-related quality of life (HRQOL) during postoperative recovery and, when HRQOL is impaired, permit tracking of disability.106 Disappointingly, early analyses of biochemical measures of systemic stress22,62 or surgical site inflammatory mediators proved to be poor surrogates for clinical outcomes,16 although more recent studies have shown inflammatory/immunological responses to surgery to be relevant for postoperative recovery, including analgesia.42 Comparing differences in stress hormone secretion and systemic inflammatory and immunological responses across various analgesic regimens may help to understand which regimen is of most clinical benefit.
Early in the 20th century, Heisenberg said of experiments, “what we observe is not nature itself, but nature exposed to our method of questioning.” Each of the above 3 groups of outcomes illustrates this dictum, particularly in multidose acute pain trials where the care setting, patient heterogeneity and expectations, pharmacokinetics and pharmacodynamics, patient–staff interactions, the success of the operation, and the dynamic nature of the healing process generate variations in outcomes.
3.5.1. The primary outcome: pain intensity
Measures of pain intensity will always be integral to acute pain efficacy trials and, for the foreseeable future, will continue to be the primary outcome in acute pain trials.20 It is increasingly common to see the primary outcome of pain intensity assessed during relevant activity such as sitting or standing, and deep breathing or coughing, after abdominal surgery (Fig. 1),45 or joint flexion at rest, or during walking after total knee arthroplasty. The importance of the measurement of movement-related pain after surgery is highlighted by observations that movement-related pain is substantially more intense than pain at rest104 and seems to be more closely associated with pain-related functional impairment.36,46 In addition to the obvious generalizability of such a strategy to the target population, such an outcome may offer greater sensitivity to change. Pain intensity measures are currently preferred over pain relief measures because the latter require recall of the initial pain, and the cognitive ability to assess the degree to which current pain intensity differs from starting pain intensity.20 For a multiple-dose trial, “starting” may have been hours or days earlier than “current.” Despite this concern, in a large number of acute pain trials which have included both pain intensity and pain relief, the pain relief measures seem to show greater assay sensitivity.101 In virtually all trials which include both measures, changes in pain relief mirror those seen in pain intensity. Even when a clear concept such as “pain now” is assessed, some patients will misunderstand or incorrectly score their pain on a numeric rating scale or visual analogue scale, thereby diluting trial power. Pretreatment patient training can reduce error-based variance and increase trial power.
Figure 1.
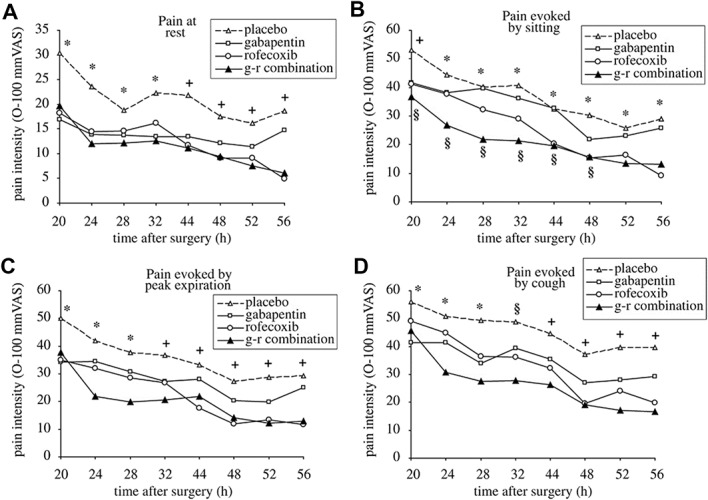
Measurement of acute pain at rest, and immediately following functionally relevant movements in analgesic clinical trials. Spontaneous and movement-evoked pain during postoperative days 1 and 2. (A) Pain at rest: Analysis revealed a significant treatment by time interaction (P<0.001). *Placebo different from gabapentin (P=0.009–0.02), rofecoxib (P=0.01–0.032) and combination (P=0.005–0.013). +Placebo different only from rofecoxib (P=0.006–0.008) and combination (P=0.003–0.006). (B) Pain evoked by sitting: Analysis revealed a significant treatment by time interaction (P<0.001). +Placebo different only from combination (P<0.001), *Placebo different only from rofecoxib (P=0.002–0.044) and combination (P<0.001 throughout). §Combination different from gabapentin (P=0.04–0.047). (C) Pain evoked by peak expiration: Analysis revealed a significant treatment by time interaction (P<0.001). *Placebo different from gabapentin (P=0.028–0.046), rofecoxib (P=0.008–0.019) and combination (P<0.001). +Placebo different only from rofecoxib (P=0.002–0.005) and combination (P<0.001 throughout). (D) Pain evoked by cough: Analysis revealed a significant treatment by time interaction (P<0.001). *Placebo different only from gabapentin (P=0.04–0.045) and combination (P=0.001–0.003). §Placebo different only from combination (P<0.001). +Placebo different only from rofecoxib (P=0.014–0.022) and combination (P<0.001). Reprinted with permission from: Gilron I, Orr E, Tu D, O'Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib, and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. PAIN 2005;113:191–200. VAS, visual analogue scale.
3.5.2. Secondary outcomes
Secondary outcomes are expected to be favorably impacted if pain intensity is diminished meaningfully by the experimental intervention. Examples might include functional physiological measures that reflect muscular effort such as vital capacity or timed, forced expiratory volume after upper abdominal or thoracic surgery. Sleep quantity and quality are other important physiological measures. Failure to demonstrate benefit on secondary measures suggests that even if superiority in the primary outcome is evident, it may have limited clinical significance; it is also possible, of course, that the trial did not have adequate statistical power for the secondary measures. Recovery of, or surpassing, preoperative health status and autonomy depends not only on recovery of physiological function but also harnessing an improving physiology to hasten social reintegration. The latter process is multidimensional and captured by generic or condition- and procedure-specific HRQOL measures (see 3.5.3 below). As we continue to refine the taxonomy of acute pain to align it with that used for chronic pain,67 we will be challenged to translate advances in descriptive ontogeny into novel outcome measures suitable for application in acute pain trials.
Opioid use and opioid sparing, used for decades as secondary outcomes in acute pain trials, have recently attracted special attention because of the possible link between providing prescriptions for opioid analgesics at the time of discharge, and the misuse, abuse, and diversion of such medications with profound societal harm. Researchers are developing novel abuse deterrent formulations of opioids, improved formulations of existing nonopioids (eg, controlled-release local anesthetics), and new molecular entities that minimize opioid side effects or avoid opioids entirely. Whether their use perioperatively will reduce opioid-induced adverse events and accelerate discharge remains to be evaluated across a spectrum of agents and procedures.17 Nevertheless, future perioperative analgesic trials that assess opioid-sparing effects should include a detailed assessment of opioid-related side effects such as the “opioid-related symptom distress scale” (see below).3 Statistically significant decreases in opioid dose requirements per se are not sufficient to argue for superiority of test drug vs placebo or other drug (or nondrug intervention); the key question is whether such decreases are of a magnitude sufficient to reduce opioid-related adverse effects to a clinically meaningful degree.
3.5.3. Tertiary outcomes
The outcome of interest just cited, namely, opioid use or misuse long after discharge postoperatively, is a population-based outcome that would not ordinarily be assessed in the confines of a registration trial designed to capture data on safety and efficacy. Yet, as the use of electronic health records becomes routine clinical practice, acquisition of unprecedentedly large amounts of long-term, population-based data permits analyses of what might be termed tertiary outcomes. These could include the continuing use of opioids for pain control long after a typical acute pain trial would have ended, or a reduced incidence of chronic postsurgical pain with 1 clinical management algorithm vs another. Unfortunately, the assumption that even to ask inpatients about their experience with pain control will increase the likelihood that they will be offered prescriptions for opioids on discharge,71 has recently in the United States led to calls to drop such questions entirely from routine quality improvement surveys. If that occurs, a potentially valuable source of routine data capture will be lost, leaving a “big data” gap and challenging clinicians and researchers to resume collection of pain-related data on addressing quality of inpatient care. Additional socially meaningful group statistics include resumption of employment and leaving the disability system, or racial and ethnic differences in short- or long-term outcomes. Although complex and technically difficult to estimate the effects of specific acute, time-limited interventions on chronic outcomes, such analyses are important76 and posttrauma studies97 are likely to increase in the near term.
3.5.4. Qualitative and narrative-based outcomes
Many believe that unidimensional verbal, numerical, or visual analogue scales simply cannot capture the complex experience of pain. Responding to this challenge, a “clinically aligned patient assessment tool” has been developed that begins with a guided conversation between nurse and patient about several aspects of the pain being experienced by the patient, after which the nurse steps away from the patient and completes a multidimensional categorical pain instrument.33 Assessment instruments that include both qualitative social conversation and quantitative measures suitable for conventional statistical analysis merit further evaluation. In this context, it is worth recalling that the McGill-Melzack Pain Questionnaire was derived from the descriptors volunteered by patients during their recounting of personal pain narratives.84
The tension between clinical trial methods in many cases designed to reduce the effect of outliers on estimates of treatment efficacy, and the growing importance of personalized, precision medicine is a major challenge for biomedicine beyond just pain research. Current approaches to reducing patient heterogeneity by pretesting so as to exclude placebo responders, or to enrich enrollment with likely treatment responders embody efforts to increase the sensitivity of analgesic trials while identifying target populations likely to benefit. If successful, such strategies will be important for the clinical development of new chemical identities whose marketing for use in clinical practice depends on proving efficacy in commercially meaningful numbers of patients.
Durations of postsurgical hospital stays have been shortened or even eliminated (ie, by conversion from inpatient to outpatient procedures) after the adoption of minimally invasive surgical techniques, prolonged delivery of postoperative regional anesthesia, and other advances in the standard of care. Thus, postoperative stays during which patient responses to single- or multiple-dose analgesic therapies may be scrutinized are becoming less frequent. This reality may be dealt with by planning to extend the duration of acute, in-hospital observation but doing so adds expense to the trial (the sponsor would be expected to cover the extra per diem costs). Furthermore, the opportunity to see whether the investigational treatment may shorten the time to discharge during routine care would be lost. Alternatively, such investigations could transition from the inpatient to the outpatient setting as is often performed with chronic pain trials. At the same time, there is ongoing interest in optimizing perioperative management so as to benefit long-term outcomes by reducing persistent pain or accelerating rehabilitation. Investigators' desire to identify short-term efficacy outcomes that can predict long-term outcomes has led to the use of early postoperative “pain trajectories” for this purpose.25,121 An unmet need is to supplement early measures such as pain intensity with other indicators of physical function or psychological resilience, to better predict the long-term benefits of postoperative analgesic interventions delivered within ever-briefer inpatient stays.
Evolving surgical methods and routine, consensus acute analgesic practice impact as well on the selection or de novo development of efficacy assessments most suitable to determine optimal analgesic regimens for specific operations in particular subgroups (eg, children vs older persons). For example, the routine application of multimodal regimens such as prolonged duration local anesthetics may reduce opioid requirements in a “standard therapy” control group to sufficiently low levels as to negate the value of opioid sparing as a postoperative outcome measure. Furthermore, a functional outcome measure appropriate for application in patients undergoing knee replacement using conventional general anesthesia and systemic opioids might simply consist of a measurement of maximal knee flexion. Yet, if regional anesthesia were combined with an anti-inflammatory agent as the baseline regimen, so many patients may have substantial knee flexion that this outcome may not be sensitive to the addition of an experimental analgesic. In the latter patients, distance walked during timed ambulation is better suited to assess analgesic efficacy. A similar point may be made for other topics related to acute pain control, eg, the application of programs to enhance recovery after surgery.39,66 One may consider the assessment of postoperative recovery as a special case of HRQOL assessment. Health-related quality of life assessment instruments may be generic, such as the SF-36, or condition-specific, such as Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT). The generic postoperative quality of recovery scales developed by Myles et al. has been validated and widely applied (including a shortened 15 question version of the 40-question instrument).49,90,105 Efforts are now under way to adapt the Patient-Reported Outcomes Measurement Information System (PROMIS), a computerized dynamic testing instrument developed to assess outcomes of chronic pain treatments, to the acute postoperative setting. Finally, future pain trials may include the question “Why is the patient remaining in the hospital today?”55 when addressing the effect of pain management on length of stay, using well-defined discharge criteria.
Challenges in the development of efficacy outcomes related to behavioral factors are presently among the most intriguing to those developing future acute pain trial designs and interpreting their results. A decade ago, in a monograph issued to “display the evolution of ideas and information for these 30 years” (since the founding of the International Association for the Study of Pain), none of the 35 chapters addressed acute pain.85 Instead, the contents were divided roughly equally between laboratory research and the assessment and treatment of chronic noncancer pain. Yet, in the past 2 decades, research and practice on acute pain have increasingly explored social and behavioral dimensions such that the core outcome domains of interest in acute and chronic pain trials may now be converging.40,67 For example, the initial 2003 IMMPACT recommendations for “core domains for clinical trials of chronic pain treatment efficacy and effectiveness”110—pain, physical functioning, emotional functioning, participant ratings of global improvement, symptoms and adverse events, and participant disposition (including adherence to the treatment regimen and reasons for premature withdrawal from the trial)—now routinely are reported in acute pain trials.
The Beecher group's seminal 1954 paper characterizing placebo responders in analgesic trials in acute pain explicitly identified patients' high regard for the hospital staff, as well as presurgical social connectedness as gauged by regular church attendance and interest in church affairs, as predictors of placebo responsivity.74 To borrow Beecher's phrase, there is a need to develop “data stat[ing] what is known to all thoughtful clinical observers”: patients' purposefulness and resilience, sense of connectedness and alliance with the clinicians caring for them, and motivation to recover from postoperative or other acute pain so as to rejoin and support their family and community, are all important determinants of efficacy outcomes. As noted above in this guide, current systematic reviews of factors to predict high-pain intensity scores and analgesic consumption after surgery are able to account for only about half of the observed variance. Thus, both as predictive variables as well as outcomes per se, factors related to social connectedness with health care providers during routine care or analgesic trials, with family, and with society in general, may account for much of the still-uncharacterized half of the variation in pain intensity and analgesic consumption after surgery.
Although not a trial in the conventional sense, the capture of effectiveness data during routine clinical care is now possible with unprecedented speed and breadth and is increasingly informing postmarketing appraisals of pharmacological effectiveness and safety. Observations and analysis of “big data” permit evaluations of benefits and risks of pain treatment in the naturalistic fashion that Lasagna envisioned in 1974.73,121 In addition to demographic data, pain scores, and physiological parameters, patient satisfaction with care can now be captured and quickly available for review.52 Early studies of acute pain-related quality improvement programs48 found a surprisingly weak correlation between pain intensity and patient satisfaction. Instead, patient satisfaction was influenced by whether staff members communicated the importance of pain control, and whether they projected concern for achieving good pain control. When such findings initially emerged, they were viewed as if patients were reporting satisfaction in a naive and misunderstood fashion because nociception and pain intensity were viewed as paramount to the pain experience. However, given the distress, dysphoria, and in particular, sense of social isolation that we now realize we are hardwired to experience during acute and chronic pain, we would have performed well listening to these patients. Now, we understand that their ratings of satisfaction were based on a more global construct within which both nociception and distress are included. Going forward, we must partner with patients to guide us because we seek to address still-unmet challenges in the design, measurement, and interpretation of efficacy outcomes in analgesic trials.19,21
3.6. Safety assessment and reporting
Assessment and reporting of adverse effects/events (AEs) in acute pain trials is critical to evaluate the safety and tolerability of study treatments.37,54 In addition to AEs resulting in unpleasant symptoms experienced by the patient, other important safety assessments—particularly relevant to novel experimental drugs—may require more specific objective assessments such as vital signs, laboratory tests, and electrocardiograms as guided by early-phase trial results. Despite reports nearly 2 decades ago suggesting the need to improve safety reporting in acute pain trials,34 recent evidence suggests that progress in this area has been too slow.54,102,103 In a recent systematic review of acute pain trials of gabapentin and pregabalin, nearly 10% of trials provided no information about AE assessment/reporting.54 Such deficiencies may be, in part, related to limitations in investigator performance and journal editorial policies. However, more effective knowledge translation about importance of, and methods for, AE assessment/reporting is clearly needed.37
Standards concerning safety assessment and reporting in clinical trials come from the 2004 extension to the CONSORT statement.56 One early, fundamental recommendation is to indicate in the title, abstract, and introduction of the trial report that data about treatment harms were collected. Regarding methods of AE assessment, periodic open-ended questions may be useful to detect previously unrecognized AEs in early-phase trials. It is recognized that open-ended questioning about AEs (eg, “Are you experiencing any other symptoms?”) may result in underreporting of AEs, whereas targeted questioning about specific AEs (eg, “Are you having any nausea?”) may lead to overreporting. However, in phase 3/4 trials of treatments with recognized AEs, it may be appropriate to ask specific questions about defined AEs at pharmacokinetically or pharmacodynamically relevant time points (ie, at peak effect of study treatment).
One example of a treatment-specific AE assessment tool is the opioid-related symptom distress scale (SDS) developed by Apfelbaum et al.3 This tool assesses 12 opioid side effects–including nausea, vomiting, constipation, and drowsiness–with respect to frequency, severity, and bothersomeness.3 This SDS has also been used to quantify reductions in opioid-related side effect burden by opioid-sparing analgesics.93 One example of the value of careful safety assessment is the trial of the COX-2 inhibitors parecoxib and valdecoxib for pain after coronary artery surgery.91 In this trial, the primary endpoint was the combined incidence of predefined AEs.91 Of 1,671 patients, AE rates were significantly higher in the active treatment groups (7.4%) vs the placebo group (4.0%), suggesting the inferior safety of parecoxib and valdecoxib in this setting,91 but not necessarily in other surgical settings.98
Thus, when designing an acute pain trial, careful attention should be given to using validated AE assessment methods that are sensitive to severity of anticipated AEs and appropriately timed so as to facilitate attribution to the study treatment. Together with such AE assessment and treatment strategies, acute pain trials should also include a prospective statistical analysis plan specific to treatment harms as statistical considerations for these outcomes may be unique and different from those for efficacy outcomes.56
We contend that AE reporting is as important as reporting of efficacy outcomes and should include a description of treatment-specific participant trial withdrawals.56 To estimate absolute risk of harm per treatment group, numbers of each AE should be reported with the appropriate denominator, ie, the number of patients exposed per arm. Subgroup analyses for harms should be described. Finally, the trial report should include a discussion of the perceived balance of benefits and harms in the context of the trial's limitations.
3.7. Trial execution
Execution of an acute pain trial starts with finalizing the trial protocol—including a predefined statistical analysis plan, obtaining operational funding (a critical prerequisite for trial execution), ethics approval, and registration in an external trial registry.30 Other issues include considering the establishment of a Data and Safety Monitoring Committee with predefined trial stopping guidelines for adverse safety outcomes, early efficacy signals, and/or futility for lack of evidence of efficacy. Some challenges may be anticipated with multicenter designs—commonly used for acute pain trials—depending on regional, national, or international regulations regarding use of multiple local research ethics approvals vs approval by a single “umbrella” research ethics board.44,68 Furthermore, some have posited that the most significant challenge to assay sensitivity in large multicenter trials is the variability in perioperative care and intraoperative anesthesia.65,101
Unique to acute pain trials is the setting in which they are conducted, eg, perioperative setting for postsurgical pain studies89 and emergency settings for posttraumatic pain studies. Common challenges to participant recruitment in acute care settings include other competing activities (eg, presurgical preparation, urgent diagnostic studies, family interactions, etc.), last minute scheduling changes, and patient anxiety or stress that may affect the informed consent process.77 Also, transient mobilization of endogenous analgesic processes in response to physiological stress may potentially reduce the apparent analgesic effect of active medication compared with placebo.15 For studies involving elective surgery, engaging trial candidates as far as possible in advance of the procedure may mitigate these challenges.
3.8. Trial analysis and interpretation
Essential aspects of statistical analysis of pain trials are discussed in detail elsewhere. However, a number of issues related to trial analysis of acute pain trials are worthy of brief discussion here. In earlier postsurgical single-dose analgesic trials where only patients developing moderate to severe pain were randomized to a relatively short-acting treatment (or placebo), common analytical methods107 involved the estimation of area under the time–analgesic effect curve using either measures of pain intensity (SPID4, summed pain intensity difference over 4 hours) or of pain relief (TOTPAR4, total pain relief over 4 hours)—typically over a period of 4 to 6 hours. More recently, studies of longer acting agents have used an 8-hour or a 12-hour measure such as TOTPAR12 or SPID12,81 and multidose studies have used even longer measures such as SPID24 and a patient-reported global measure of response to therapy.23 Use of these longer-duration measures in multidose studies may be problematic in the setting of missing data (eg, because of trial patient dropouts) and/or use of rescue medication during the relevant period.
Although statistical analyses in most earlier acute pain trials involved comparing treatment group means for SPID and TOTPAR measures, it has been recognized that the distribution of analgesic responses within treatment groups may be nonnormal such that few individual treatment responses are similar to the mean.88 Therefore, a more clinically relevant measure that could be used as a dichotomous outcome, namely 50% pain reduction, has been used in several recent acute pain trials and could be considered.86 However, some controversy remains over this because using a dichotomous outcome could result in diminished statistical power.
3.9. Remaining challenges
Consideration of clinical and research needs over the past few decades has led to the recognition of at least 3 divergent goals of acute pain clinical trials: (1) to provide valid and efficient methods with high assay sensitivity that can identify new molecular entities with first evidence of efficacy in the treatment of human clinical pain (eg, dental impaction model or bunionectomy model for early phase 2 regulatory trials), (2) to optimize designs for standard phase 3 trials conducted to obtain regulatory approval of new treatments, and (3) to provide designs for longer-term (eg, days to weeks) comparative effectiveness trials that identify optimal acute pain treatment strategies for patient subpopulations with the greatest clinical need (eg, major surgical procedures, preexisting chronic pain/opioid use, and predictors of high acute pain severity).
Although estimates of analgesic efficacy derived from acute pain trials applying methods with high assay sensitivity (eg, the dental impaction model) do not always generalize to all clinical pain conditions, such methods have demonstrated their merits and will likely continue to be useful in drug development. Perhaps, the greater challenge will be to further cultivate valid methods to generate evidence in support of best care for patients at highest risk of developing moderate to severe acute pain. Doing so will require well-powered, likely multicenter, trials that focus on a specific surgical procedure or injury and, further, on a specific subpopulation (eg, chronic pain patients experiencing acute pain) with careful consideration given to multiple outcomes of interest and possibly more sophisticated analytical methods. Also, more attention needs to be paid to AEs, especially in trials including multimodal analgesia, where little information is available on potential undesirable interactions of different analgesics. Although persistent postsurgical/postinjury pain is not the topic of this review, studying the transition from acute to chronic pain logically begins in the setting of acute pain. Therefore, it is worth noting here the need to further improve trial designs to evaluate interventions for the prevention of the transition from acute to chronic pain.24,43,64,80
4. Conclusion
Over the past 50 years, clinical trial methods for the evaluation of treatments for acute pain have dramatically evolved and improved. These include development of early-phase trial designs that minimize variability and thereby enhance assay sensitivity, minimization of bias through blinding and randomization to treatment allocation, and measurement of clinically relevant outcomes such as movement-evoked pain. However, further improvements are needed, including: (1) refinement of trial designs that address specific factors (eg, patient-related and injury-/surgery-specific) relevant to acute pain either through a focus on a specific subpopulation or through patient stratification; (2) development and implementation of new patient-centered outcome measures most relevant to the acute pain condition and/or treatment intervention of interest; (3) more robust development of trial designs for acute pain conditions other than postsurgical pain; (4) greater attention to trial quality, including more comprehensive assessment and reporting of safety outcomes; and (5) development of trial methods that focus on treating complex patients at high risk of severe acute pain.
Disclosures
The authors have no conflict of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Aasvang EK, Lunn TH, Hansen TB, Kristensen PW, Solgaard S, Kehlet H. Chronic pre-operative opioid use and acute pain after fast-track total knee arthroplasty. Acta Anaesthesiol Scand 2016;60:529–36. [DOI] [PubMed] [Google Scholar]
- [2].Apfelbaum JL, Desjardins PJ, Brown MT, Verburg KM. Multiple day efficacy of parecoxib sodium treatment in postoperative bunionectomy pain. Clin J Pain 2008;24:784–92. [DOI] [PubMed] [Google Scholar]
- [3].Apfelbaum JL, Gan TJ, Zhao S, Hanna DB, Chen C. Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg 2004;99:699–709. [DOI] [PubMed] [Google Scholar]
- [4].Baert IA, Lluch E, Mulder T, Nijs J, Noten S, Meeus M. Does pre-surgical central modulation of pain influence outcome after total knee replacement? A systematic review. Osteoarthritis Cartilage 2016;24:213–23. [DOI] [PubMed] [Google Scholar]
- [5].Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. J Pharmacol Exp Ther 1978;207:92–100. [PubMed] [Google Scholar]
- [6].Beecher HK. The powerful placebo. J Am Med Assoc 1955;159:1602–6. [DOI] [PubMed] [Google Scholar]
- [7].Beecher HK. The measurement of pain; prototype for the quantitative study of subjective responses. Pharmacol Rev 1957;9:59–209. [PubMed] [Google Scholar]
- [8].Beecher HK: Measurement of subjective responses: quantitative effects of drugs. New York: Oxford University, 1959. [Google Scholar]
- [9].Beecher HK, Keats AS, Mosteller F, Lasagna L. The effectiveness of oral analgesics (morphine, codeine, acetylsalicylic acid) and the problem of placebo “reactors” and “non-reactors”. J Pharmacol Exp Ther 1953;109:393–400. [PubMed] [Google Scholar]
- [10].Bhatia A. Ketamine as an adjunct to patient-controlled analgesia: why, for whom, and how much? Can J Anaesth 2016;63:262–4. [DOI] [PubMed] [Google Scholar]
- [11].Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. PAIN 2001;90:261–9. [DOI] [PubMed] [Google Scholar]
- [12].Boscariol R, Gilron I, Orr E. Chronobiological characteristics of postoperative pain: diurnal variation of both static and dynamic pain and effects of analgesic therapy. Can J Anaesth 2007;54:696–704. [DOI] [PubMed] [Google Scholar]
- [13].Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth 2008;101:17–24. [DOI] [PubMed] [Google Scholar]
- [14].Brummett CM, Clauw DJ. Flipping the paradigm: from surgery-specific to patient-driven perioperative analgesic algorithms. Anesthesiology 2015;122:731–3. [DOI] [PubMed] [Google Scholar]
- [15].Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol 2009;88:184. [DOI] [PubMed] [Google Scholar]
- [16].Carr DB. Caveats in the evaluation of stress hormone responses in analgesic trials. In: Max MB, Portenoy RK, Laska EM, editors. The design of analgesic clinical trials (advances in pain research and therapy). Vol. 18 New York: Raven Press, 1991. p. 599–605. [Google Scholar]
- [17].Carr DB, Cohen RI. “Are perioperative opioids obsolete?” Proceedings of an IASP Acute Pain Special Interest Group Satellite Symposium. September 25, 2016 Yokohama, Japan. Pain Rep 2017;2 e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carr DB, Goudas LC. Acute pain. Lancet 1999;353:2051–8. [DOI] [PubMed] [Google Scholar]
- [19].Casarett D, Karlawish J, Sankar P, Hiirschman K, Asch DA. Designing pain research from the patient's perspective: what trial endpoints are important to patients with chronic pain? Pain Med 2001;2:309–16. [DOI] [PubMed] [Google Scholar]
- [20].Center for Drug Evaluation and Research (CDER), US Food and Drug Administration (FDA). Guidance for industry. Analgesic indications: developing drug and biological products (draft). Silver Spring, 2014. [Google Scholar]
- [21].Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? PAIN 2003;105:151–7. [DOI] [PubMed] [Google Scholar]
- [22].Cepeda MS, Carr DB. The stress response and regional anesthesia. In: Brown DL, editor. Regional anesthesia and analgesia. Philadelphia: Saunders, 1996. p. 108–23. [Google Scholar]
- [23].Chang DJ, Desjardins PJ, Bird SR, Black P, Chen E, Petruschke RA, Geba GP. Comparison of rofecoxib and a multidose oxycodone/acetaminophen regimen for the treatment of acute pain following oral surgery: a randomized controlled trial. Curr Med Res Opin 2004;20:939–49. [DOI] [PubMed] [Google Scholar]
- [24].Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chapman CR, Donaldson GW, Davis JJ, Bradshaw DH. Improving individual measurement of postoperative pain: the pain trajectory. J Pain 2011;12:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine and immune interactions. J Pain 2008;9:122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131–57. [DOI] [PubMed] [Google Scholar]
- [28].Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther 1976;20:241–50. [DOI] [PubMed] [Google Scholar]
- [29].Cooper SA, Desjardins PJ, Turk DC, Dworkin RH, Katz NP, Kehlet H, Ballantyne JC, Burke LB, Carragee E, Cowan P, Croll S, Dionne RA, Farrar JT, Gilron I, Gordon DB, Iyengar S, Jay GW, Kalso EA, Kerns RD, McDermott MP, Raja SN, Rappaport BA, Rauschkolb C, Royal MA, Segerdahl M, Stauffer JW, Todd KH, Vanhove GF, Wallace MS, West C, White RE, Wu C. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. PAIN 2016;157:288–301. [DOI] [PubMed] [Google Scholar]
- [30].De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJ, Schroeder TV, Sox HC, Van Der Weyden MB; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 2004;351:1250–1. [DOI] [PubMed] [Google Scholar]
- [31].Desjardins PJ, Shu VS, Recker DP, Verburg KM, Woolf CJ. A single pre-operative oral dose of valdecoxib, a new cyclooxygenase-2 specific inhibitor, relieves post-oral surgery or bunionectomy pain. Anesthesiol 2002;97:565–73. [DOI] [PubMed] [Google Scholar]
- [32].Dorow M, Löbner M, Stein J, Konnopka A, Meisel HJ, Günther L, Meixensberger J, Stengler K, König HH, Riedel-Heller SG. Risk factors for postoperative pain intensity in patients undergoing lumbar disc surgery: a systematic review. PLoS One 2017;12:e0170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Drew DJ, Topham D. Implementation of the CAPA (clinically aligned pain assessment) tool: pain is more than just a number. Presentation at American Society of Pain Management Nursing. Available at: http://www.aspmn.org/Documents/2014%20Conference%20Documents/Friday/Drew%20Topham%20CAPA.pdf. Accessed March 18, 2018.
- [34].Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. J Pain Symptom Manage 1999;18:427–37. [DOI] [PubMed] [Google Scholar]
- [35].Egbert LD, Battit GE, Welch CE, Bartlett MK. Reduction of postoperative pain by encouragement and instruction of patients. A study of doctor-patient rapport. N Engl J Med 1964;270:825–7. [DOI] [PubMed] [Google Scholar]
- [36].Erb J, Orr E, Mercer CD, Gilron I. Interactions between pulmonary performance and movement-evoked pain in the immediate postsurgical period: implications for perioperative research and treatment. Reg Anesth Pain Med 2008;33:312–9. [DOI] [PubMed] [Google Scholar]
- [37].Fabritius ML, Mathiesen O, Wetterslev J, Dahl JB. Post-operative analgesia: focus has been on benefit—are we forgetting the harm? Acta Anaesthesiol Scand 2016;60:839–41. [DOI] [PubMed] [Google Scholar]
- [38].Farrell C, McConaghy P. Perioperative management of patients taking treatment for chronic pain. BMJ 2012;345:e4148. [DOI] [PubMed] [Google Scholar]
- [39].Feldman LS, Lee L, Fiore J., Jr What outcomes are important in the assessment of enhanced recovery after surgery (ERAS) pathways? Can J Anesth 2015;62:120–30. [DOI] [PubMed] [Google Scholar]
- [40].Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fletcher D, Fermanian C, Mardaye A, Aegerter P; Pain and Regional Anesthesia Committee of the French Anesthesia and Intensive Care Society (SFAR). A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. PAIN 2008;137:441–51. [DOI] [PubMed] [Google Scholar]
- [42].Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 2014;6:255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, Markman JD, Oaklander AL, Polydefkis MJ, Raja SN, Robinson JP, Woolf CJ, Ziegler D, Ashburn MA, Burke LB, Cowan P, George SZ, Goli V, Graff OX, Iyengar S, Jay GW, Katz J, Kehlet H, Kitt RA, Kopecky EA, Malamut R, McDermott MP, Palmer P, Rappaport BA, Rauschkolb C, Steigerwald I, Tobias J, Walco GA. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. PAIN 2015;156:1184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gillan MG, Ross S, Gilbert FJ, Grant AM, O'Dwyer PJ. Recruitment to multicentre trials: the impact of external influences. Health Bull (Edinb) 2000;58:229–34. [PubMed] [Google Scholar]
- [45].Gilron I, Orr E, Tu D, O'Neill JP, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. PAIN 2005;113:191–200. [DOI] [PubMed] [Google Scholar]
- [46].Gilron I, Max MB, Lee G, Booher SL, Sang CN, Chappell AS, Dionne RA. Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain. Clin Pharmacol Ther 2000;68:320. [DOI] [PubMed] [Google Scholar]
- [47].Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, Brummett CM. Trends and predictors of opioid use after total knee and total hip arthroplasty. PAIN 2016;157:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gordon DB, Dahl JL, Miaskowski C, McCarberg B, Todd KH, Paice JA, Lipman AG, Bookbinder M, Sanders SH, Turk DC, Carr DB. American Pain Society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med 2005;165:1574–80. [DOI] [PubMed] [Google Scholar]
- [49].Gornall BF, Myles PS, Smith CL, Burke JA, Leslie K, Pereira MJ, Bosto JE, Kluiwers KB, Nilsson UG, Tanaka Y, Forbes A. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth 2013;111:161–9. [DOI] [PubMed] [Google Scholar]
- [50].Gray A, Kehlet H, Bonnet F, Rawal N. Predicting postoperative analgesia outcomes: NNT league tables or procedure-specific evidence? Br J Anaesth 2005;94:710–14. [DOI] [PubMed] [Google Scholar]
- [51].Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M, Drewes AM. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: a prospective observational cohort study. PLoS One 2014;9:e90185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Haythornthwaite JA, Fauerbach JA. Assessment of acute pain, pain relief, and patient satisfaction. In: Turk DC, Melzack R, editors. Handbook of pain assessment, 2nd ed New York: Guilford Press, 2001. p. 417–30. [Google Scholar]
- [53].Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hoffer D, Smith SM, Parlow J, Allard R, Gilron I. Adverse event assessment and reporting in trials of newer treatments for post-operative pain. Acta Anaesthesiol Scand 2016;60:842–51. [DOI] [PubMed] [Google Scholar]
- [55].Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop 2011;82:679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, Moher D; CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781–8. [DOI] [PubMed] [Google Scholar]
- [57].Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009;111:657–77. [DOI] [PubMed] [Google Scholar]
- [58].Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- [59].Joshi GP, Kehlet H. Procedure-specific pain management: the road to improve postsurgical pain management? Anesthesiology 2013;118:780–82. [DOI] [PubMed] [Google Scholar]
- [60].Kantor TG, Sunshine A, Laska E, Meisner M, Hopper M. Oral analgesic studies: pentazocine hydrochloride, codeine, aspirin and placebo and their influence on response to placebo. Clin Pharmacol Ther 1966;7:447–54. [DOI] [PubMed] [Google Scholar]
- [61].Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesth Analg 2011;113:1242–53. [DOI] [PubMed] [Google Scholar]
- [62].Kehlet H. Modification of responses to surgery by neural blockade. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. 3rd ed Philadelphia: Lippincott-Raven, 1998. p. 129–75. [Google Scholar]
- [63].Kehlet H. Labat lecture 2005: surgical stress and postoperative outcome-from here to where? Reg Anesth Pain Med 2006a;31:47–52. [DOI] [PubMed] [Google Scholar]
- [64].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [65].Kehlet H, Joshi GP. Systematic reviews and meta-analyses of randomized controlled trials on perioperative outcomes: an urgent need for critical reappraisal. Anesth Analg 2015;121:1104–7. [DOI] [PubMed] [Google Scholar]
- [66].Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98. [DOI] [PubMed] [Google Scholar]
- [67].Kent ML, Tighe PJ, Belfer I, Brennan TJ, Bruehl S, Brummett CM, Buckenmaier CC, Buvanendran A, Cohen RI, Desjardins P, Edwards D, Fillingim R, Gewandter J, Gordon DB, Hurley RW, Kehlet H, Loeser JD, Mackey S, McLean SA, Polomano R, Rahman S, Raja S, Rowbotham M, Suresh S, Schachtel B, Schreiber K, Schumacher M, Stacey B, Stanos S, Todd K, Turk DC, Weisman SJ, Wu C, Carr DB, Dworkin RH, Terman G: The ACTTION-APS-AAPM Pain Taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. Pain Med 2017;18:947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kenyon GM, Mendelow AD, Gregson BA, Rowan E. Obtaining regulatory approval for multicentre randomised controlled trials: experiences in the STICH II trial. Br J Neurosurg 2011;25:352–6. [DOI] [PubMed] [Google Scholar]
- [69].Keutmann E, Foldes FF. The analgesic effect of dromoran hydrobromide (3-hydroxy-N-methyl morphinan hydrobromide) in postoperative pain. N Engl J Med 1951;244:286–8. [DOI] [PubMed] [Google Scholar]
- [70].Keyes KM, Galea S. Population health science. New York: Oxford University Press, 2016. [Google Scholar]
- [71].Kharasch ED, Brunt LM. Perioperative opioids and public health. Anesthesiology 2016;124:960–5. [DOI] [PubMed] [Google Scholar]
- [72].Kissin I. Preemptive analgesia: why its effect is not always obvious. Anesthesiology 1996;84:1015–19. [DOI] [PubMed] [Google Scholar]
- [73].Lasagna L. A plea for the “naturalistic” study of medicines. Eur J Clin Pharmacol 1974;7:153–4. [DOI] [PubMed] [Google Scholar]
- [74].Lasagna L, Mosteller F, von Felsinger JM, Beecher HK. A study of the placebo response. Am J Med 1954;16:770–9. [DOI] [PubMed] [Google Scholar]
- [75].Laska EM, Sunshine A, Wanderling JA, Meisner MJ. Quantitative differences in aspirin analgesia in three models of clinical pain. J Clin Pharmacol 1982;22:531–42. [DOI] [PubMed] [Google Scholar]
- [76].Lavand'homme PM, Grosu I, France MN, Thienpont E. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: an observational study. Clin Orthop Relat Res 2014;472:1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lawton J, Snowdon C, Morrow S, Norman JE, Denison FC, Hallowell N. Recruiting and consenting into a peripartum trial in an emergency setting: a qualitative study of the experiences and views of women and healthcare professionals. Trials 2016;17:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010;113:639–46. [DOI] [PubMed] [Google Scholar]
- [79].Lunn TH, Frokjaer VG, Hansen TB, Kristensen PW, Lind T, Kehlet H. Analgesic effect of perioperative escitalopram in high pain catastrophizing patients after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Anesthesiology 2015;122:884–94. [DOI] [PubMed] [Google Scholar]
- [80].Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [81].Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin Ther 2002;24:1549–60. [DOI] [PubMed] [Google Scholar]
- [82].Mathiesen O, Wetterslev J, Kontinen VK, Pommergaard HC, Nikolajsen L, Rosenberg J, Hansen MS, Hamunen K, Kjer JJ, Dahl JB; Scandinavian Postoperative Pain Alliance (ScaPAlli). Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand 2014;58:1182–98. [DOI] [PubMed] [Google Scholar]
- [83].Max MB, Laska EM. Single-dose analgesic comparisons. In: Max M, Portenoy R, Laska EM, editors. Advances in pain research and therapy. Vol. 18 Seattle: IASP Press, 1991. p. 55–96. [Google Scholar]
- [84].Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. PAIN 1975;1:277–99. [DOI] [PubMed] [Google Scholar]
- [85].Merskey H, Loeser JD, Dubner R, editors. The paths of pain: 1975–2005. Seattle: IASP Press, 2005. [Google Scholar]
- [86].Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev 2015;9:CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moore RA, Derry CJ, Derry S, Straube S, McQuay HJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. Eur J Pain 2012;16:585–91. [DOI] [PubMed] [Google Scholar]
- [88].Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. PAIN 1996;66:229–37. [DOI] [PubMed] [Google Scholar]
- [89].Myles PS, Fletcher HE, Cairo S, Madder H, McRae R, Cooper J, Devonshire D, Hunt JO, Richardson J, Machlin H, Morgan EB, Moloney J, Downey G. Randomized trial of informed consent and recruitment for clinical trials in the immediate preoperative period. Anesthesiology 1999;91:969–78. [DOI] [PubMed] [Google Scholar]
- [90].Myles P, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000;84:11–15. [DOI] [PubMed] [Google Scholar]
- [91].Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005;352:1081–91. [DOI] [PubMed] [Google Scholar]
- [92].Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg 2010;110:1170–9. [DOI] [PubMed] [Google Scholar]
- [93].Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, Chung F. Use of low-dose pregabalin in patients undergoing laparoscopic cholecystectomy. Br J Anaesth 2010;105:155–61. [DOI] [PubMed] [Google Scholar]
- [94].Pivec R, Issa K, Naziri Q, Kapadia BH, Bonutti PM, Mont MA. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop 2014;38:1159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ruscheweyh R, Viehoff A, Tio J, Pogatzki-Zahn EM. Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre-existing chronic pain. PAIN 2017;158:1030–8. [DOI] [PubMed] [Google Scholar]
- [96].Sangesland A, Støren C, Vaegter HB. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand J Pain 2017;15: 44–52. [DOI] [PubMed] [Google Scholar]
- [97].Schley M, Topfner S, Wiech K, Schaller HE, Konrad CJ, Schmelz M, Birbaumer N. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. Eur J Pain 2007;11:299–308. [DOI] [PubMed] [Google Scholar]
- [98].Schug SA, Joshi GP, Camu F, Pan S, Cheung R. Cardiovascular safety of the cyclooxygenase-2 selective inhibitors parecoxib and valdecoxib in the postoperative setting: an analysis of integrated data. Anesth Analg 2009;108:299–307. [DOI] [PubMed] [Google Scholar]
- [99].Schug SA, Palmer GM, Scott DA, Halliwell R, Trinka J; APM: SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: scientific evidence. 4th ed Melbourne: ANZCA & FPM, 2015. p. 49–50. [Google Scholar]
- [100].Singla NK, Desjardins PJ, Chang PD. A comparison of the clinical and experimental characteristics of four acute surgical pain models: dental extraction, bunionectomy, joint replacement and soft tissue surgery. PAIN 2014;155:441–56. [DOI] [PubMed] [Google Scholar]
- [101].Singla N, Hunsinger M, Chang PD, McDermott MP, Chowdhry AK, Desjardins PJ, Turk DC, Dworkin RH. Assay sensitivity of pain intensity versus pain relief in acute pain clinical trials: ACTTION systematic review and meta-analysis. J Pain 2015;16:683–91. [DOI] [PubMed] [Google Scholar]
- [102].Smith SM, Chang RD, Pereira A, Shah N, Gilron I, Katz NP, Lin AH, McDermott MP, Rappaport BA, Rowbotham MC, Sampaio C, Turk DC, Dworkin RH. Adherence to CONSORT harms-reporting recommendations in publications of recent analgesic clinical trials: an ACTTION systematic review. PAIN 2012;153:2415–21. [DOI] [PubMed] [Google Scholar]
- [103].Smith SM, Wang AT, Katz NP, McDermott MP, Burke LB, Coplan P, Gilron I, Hertz SH, Lin AH, Rappaport BA, Rowbotham MC, Sampaio C, Sweeney M, Turk DC, Dworkin RH. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: ACTTION systematic review and recommendations. PAIN 2013;154:997–1008. [DOI] [PubMed] [Google Scholar]
- [104].Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. PAIN 2011;152:1734–9. [DOI] [PubMed] [Google Scholar]
- [105].Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 2015;118:1332–40. [DOI] [PubMed] [Google Scholar]
- [106].Strassels SA, Carr DB. Assessment of acute pain and condition–specific health-related quality of life. In: Wittink HM, Carr DB. editors. Pain management: evidence, outcomes and quality of life. St. Louis: Elsevier, 2008. p. 171–5. [Google Scholar]
- [107].Sunshine A, Marrero I, Olson NZ, Laska EM, McCormick N. Oral analgesic efficacy of suprofen compared to aspirin, aspirin plus codeine, and placebo in patients with postoperative dental pain. Pharmacology 1983;27(suppl 1):31–40. [DOI] [PubMed] [Google Scholar]
- [108].Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery and chronic cancer pain. J Clin Pharmacol 1988;28:47S–54S. [DOI] [PubMed] [Google Scholar]
- [109].Tighe P, Buckenmaier CC, Boezaart AP, Carr DB, Clark L, Herring A, Jacobs W, Kent M, Mackey S, Mariano ER, Polomano RC, Reisfield GM. Acute pain medicine in the United States: a status report. Pain Med 2015;16:1806–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. PAIN 2003;106:337–45. [DOI] [PubMed] [Google Scholar]
- [111].US Food and Drug Administration Guideline for the Clinical Evaluation of Analgesic Drugs. Docket number 91D-0425 dockets management branch (HFA-305). Rockville, 1992. [Google Scholar]
- [112].Wall PD. The prevention of postoperative pain. PAIN 1988;33:289–90. [DOI] [PubMed] [Google Scholar]
- [113].Wang L, Johnston B, Kaushal A, Cheng D, Zhu F, Martin J. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth 2016;63:311–25. [DOI] [PubMed] [Google Scholar]
- [114].Werner MU, Mjobo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology 2010;112:1494–502. [DOI] [PubMed] [Google Scholar]
- [115].White PF, Kehlet H, Neal JM, Schricker T, Carr DB, Carli F; Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg 2007;104:1380–96. [DOI] [PubMed] [Google Scholar]
- [116].Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the Establishment of central sensitization. Anesth Analg 1993;77:362–79. [DOI] [PubMed] [Google Scholar]
- [117].Wu CL, Hurley RW. Postoperative pain management and patient outcome. In: Shorten G, Carr DB, Harmon D, Puig MM, Browne J, editors. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders Elsevier, 2006. p. 71–83. [Google Scholar]
- [118].Wu CL, Liu SS. Neural blockade: impact on outcome. In: Cousins MJ, Bridenbaugh PO, Carr DB, Horlocker TT, editors. Cousins & Bridenbaugh's neural blockade in clinical anesthesia and management of pain. 4th ed Philadelphia: Lippincott Williams & Wilkins, 2009. p. 144–58. [Google Scholar]
- [119].Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011;377:2215–25. [DOI] [PubMed] [Google Scholar]
- [120].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- [121].Zaslansky R, Rothaug J, Chapman CR, Bäckström R, Brill S, Fletcher D, Fodor L, Gordon DB, Komann M, Konrad C, Leykin Y, Pogatski-Zahn E, Puig MM, Rawal N, Ullrich K, Volk T, Meissner W. PAIN OUT: the making of an international acute pain registry. Eur J Pain 2015;19:490–502. [DOI] [PubMed] [Google Scholar]