Abstract
Although surgical resection is the only treatment of choice that can offer prolonged survival and a chance of cure in patients with colorectal liver metastases (CRLM), nearly 80% of patients are deemed to be unresectable at the time of diagnosis. Considerable efforts have been made to overcome this initial unresectability, including expanding the indication of surgery, the advent of conversion chemotherapy, and development and modification of specific surgical techniques, regulated under multidisciplinary approaches. In terms of specific surgical techniques, portal vein ligation/embolization can increase the volume of future liver remnant and thereby reduce the risk of hepatic insufficiency and death after major hepatectomy. For multiple bilobar CRLM that were traditionally considered unresectable even with preoperative chemotherapy and portal vein embolization, two‐stage hepatectomy was introduced and has been adopted worldwide with acceptable short‐ and long‐term outcomes. Recently, ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) was reported as a novel variant of two‐stage hepatectomy. Although issues regarding safety remain unresolved, rapid future liver remnant hypertrophy and subsequent shorter intervals between the two stages lead to a higher feasibility rate, reaching 98%. In addition, adding radiofrequency ablation and vascular resection and reconstruction techniques can allow expansion of the pool of patients with CRLM who are candidates for liver resection and thus a cure. In this review, we discuss specific techniques that may expand the criteria for resectability in patients with initially unresectable CRLM.
Keywords: ALPPS, colorectal liver metastases, conversion surgery, two‐stage hepatectomy
1. INTRODUCTION
The liver is the most common site of metastasis from colorectal cancer, due to portal venous drainage from colon to liver. Approximately half of patients with colorectal cancer develop liver metastases at some point during the course of their disease.1, 2, 3, 4 Surgical resection remains the only treatment of choice for curative strategy, with a 5‐year survival rate of up to 67% in selected patients.5, 6, 7, 8, 9, 10, 11 Nevertheless, only 20%‐30% of patients with colorectal liver metastases (CRLM) are initially considered to be eligible for surgery,6, 12, 13, 14 and liver metastases remain the predominant cause of death for colorectal cancer patients.15, 16 In patients with metastatic colorectal cancer treated with chemotherapy alone, survival beyond 5 years is unusual.8, 17, 18, 19, 20, 21
The development of surgical techniques, the increasing efficacy of modern chemotherapy with or without biological agents, and the emergence of multidisciplinary approaches have allowed patients with conventionally unresectable CRLM to undergo surgery. Given that surgical resection remains the only form of treatment that offers the possibility of prolonged survival, expanding the potentially resectable pool of patients is crucial.
In confronting unresectability, one of the most important issues is that the definition of unresectability differs among institutions. The definition of unresectability is also evolving with the development of surgical techniques and chemotherapy. Nowadays, unresectability should be considered based on both technical and oncological criteria.22, 23, 24 Historically, several tumor factors that represent massive tumor loads such as multinodular tumors, larger tumor size, bilobar distribution, and the presence of extrahepatic disease were used to classify liver metastases as unresectable, because these variables were prognostic factors for survival after hepatectomy for CRLM. However, recent advances in surgical techniques, in combination with modern effective chemotherapy, enable surgeons to overcome these issues, and these factors are no longer an absolute contraindication for surgery. According to European Society of Medical Oncology (ESMO) consensus guideline 2016, patients with CRLM can be categorized into groups on technological and oncological criteria (Table 1). However, because the decision of unresectability will change according to the transition of the time, a precise definition in response to the demands of the times should be required.
Table 1.
Contraindications to hepatic resection in patients with colorectal liver metastases (European Society of Medical Oncology consensus guideline 2016, Van Custem et al.)
| Category | |
|---|---|
| Technical (A) | |
| 1. Absolute | Impossibility of R0 resection with ≥30% liver remnant |
| Presence of unresectable extrahepatic disease | |
| 2. Relative | R0 resection possible only with complex procedure (portal vein embolization, two‐stage hepatectomy, hepatectomy combined with ablation) |
| R1 resection | |
| Oncological (B) | |
| 1 | Concomitant extrahepatic disease (unresectable) |
| 2 | Number of lesions ≥ 5 |
| 3 | Tumor progression |
Patients should be categorized as A1 or A2/B1, B2 or B3.
How can we increase the resectability of initially unresectable CRLM? We enumerate three material factors for improving resectability (Figure 1): (a) expanding the indication of surgery (eg, for older patients, larger tumor size, and R1 resection by necessity); (b) conversion chemotherapy (eg, considering biological agents and the duration of chemotherapy); and (c) specific surgical techniques (eg, portal vein embolization [PVE], two‐stage hepatectomy [TSH], and associating liver partition and portal vein ligation for staged hepatectomy [ALPPS]); which are regulated under multidisciplinary management (eg, involving surgeons, oncologists, and radiologists).
Figure 1.
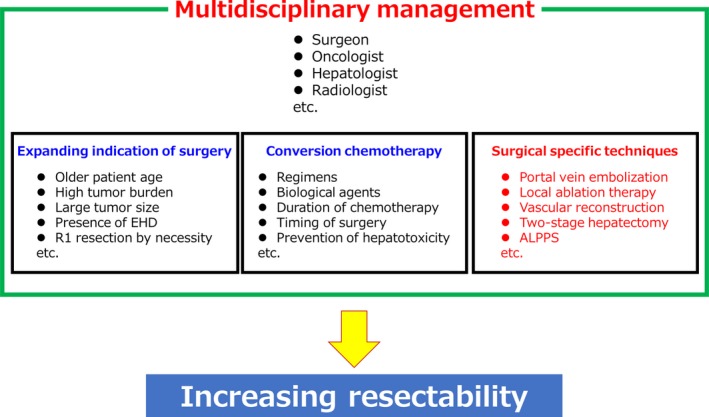
Three material factors for increasing resectability. EHD, extrahepatic disease; ALPPS, associating liver partition and portal vein ligation for staged hepatectomy
Among the three, this review discusses the specific surgical techniques that may expand the criteria for resectability in patients with initially unresectable CRLM.
2. SPECIFIC TECHNIQUES TO INCREASE RESECTABILITY
2.1. PVE
PVE was first described by Makuuchi et al25 in the 1980s. This procedure induces atrophy of the embolized liver lobe with compensatory hypertrophy of the non‐embolized contralateral liver lobe, and can thereby reduce the risk of hepatic insufficiency after major hepatectomy in patients with an insufficient future liver remnant (FLR).26, 27, 28 Several studies have reported that PVE has no adverse effect on survival in patients with CRLM who have undergone major hepatectomy.28, 29, 30, 31, 32
PVE approaches include transileocolic and transhepatic techniques. The transileocolic approach requires a mini‐laparotomy. Although a previous meta‐analysis demonstrated that the major complication rates of transileocolic and transhepatic PVE were comparable,27 the transhepatic approach has become standard due to the recent development of radiological intervention techniques. Transhepatic PVE is performed percutaneously, by an ipsilateral or contralateral approach. The advantage of the ipsilateral approach is that it does not require puncture of the FLR; that is, it can reduce the risk of injury of vessels in the FLR. Given that the vessels of the FLR should be carefully preserved for the subsequent planned surgery, the transhepatic ipsilateral approach is recommended if possible, despite its relative complexity.
One of the concerns about preoperative PVE is that stimulation of liver hypertrophy can also accelerate tumor growth in the embolized and non‐embolized liver lobe.32, 33, 34, 35, 36 Portal flow reduction in the embolized liver leads to an increase of arterial blood flow and, subsequently, growth of tumors including micrometastases could be induced, because liver tumors are mostly supplied by arterial blood. Although tumors in the embolized liver lobe will be removed by surgery, those in the non‐embolized liver lobe could be also stimulated, leading to a cause of unresectability or a risk of early tumor recurrence. One possible way to reduce the risk of unresectability would be chemotherapy after PVE, although, to our knowledge, there are no studies providing evidence of the efficacy of chemotherapy between PVE and hepatectomy. Meanwhile, preoperative PVE should probably be indicated for unilobar disease, and in the case of multiple bilobar diseases, which requires hypertrophy of the FLR, two‐stage surgery may be indicated.
2.2. Local ablation therapy in combination with hepatectomy
Local ablation therapy including radiofrequency ablation (RFA) and microwave ablation (MWA) is a thermal ablation technique that is designed to produce localized tumor destruction by heating the tumor and the surrounding liver tissue. Nowadays RFA is adopted worldwide as a safe, effective, well tolerated, and less invasive technique for small liver tumors. However, the therapeutic role of RFA is yet to be decided for CRLM. A randomized CLOCC study (RFA + chemotherapy vs chemotherapy alone) reported that RFA + chemotherapy offered better long‐term survival than chemotherapy alone in patients with unresectable CRLM (8‐year overall survival [OS] rate: 35.9% vs 8.9%, respectively).37 We previously reported that RFA in combination with hepatectomy achieved outcomes comparable to hepatectomy alone in a propensity score‐matched setting.38 Another study reported that RFA in combination with hepatectomy gave poorer survival than hepatectomy alone; however, in the setting of tumor number ≥ 4, long‐term survival rates were similar between the two groups.39 These results suggest that in selected patients and selected tumors, adding RFA to hepatectomy may be justified in the treatment of multiple CRLM.
In essence, we should not support the unconsidered use of RFA in the treatment of CRLM. Surgeons should always strive for resection with a clear margin. However, most patients with CRLM are not candidates for hepatectomy at the time of diagnosis. In these circumstances, adding RFA to hepatectomy would expand the pool of patients who can receive radical treatment.23 With this view, RFA should be strictly indicated for appropriate patients and appropriate tumors. We consider appropriate indications to be as follows: (a) unresectable or deeply located small tumors (≤2 cm) within the FLR, which require extended parenchymal resection; and (b) tumors that have responded to preoperative chemotherapy. Tumors that demonstrate no response to chemotherapy have potentially more malignant behavior and a higher local recurrence rate, and RFA should therefore be contraindicated for such tumors.40
In addition to its lower invasiveness, the benefit of adding RFA to hepatectomy is that it allows the uninvolved functional liver parenchyma to be preserved. Several recent studies have reported the usefulness of parenchyma‐preserving hepatectomy for CRLM, and Mise and colleagues found that parenchyma‐preserving hepatectomy improved survival in the event of intrahepatic recurrence, possibly due to an increase in the likelihood that the patients would be able to undergo salvage repeat hepatectomy.41 In our previous study, salvage hepatectomy for intrahepatic recurrence was more frequently performed in patients who underwent RFA in combination with hepatectomy than in those who underwent hepatectomy alone.38 Endeavoring to preserve liver parenchyma should lead to an increase in the number of patients who can undergo salvage hepatectomy for recurrence and in subsequent prolonged survival.
During the past several years MWA has gained acceptance alternative to RFA. Theoretical benefits of MWA over RFA include larger ablation zone, shorter duration, and no heat‐sink effect.42 There are some studies reporting the outcomes of MWA for colorectal liver metastases as a safe and effective modality for use in the treatment of CRLM patients.43, 44, 45, 46, 47, 48, 49 Correa‐Gallego et al. reported that ablation‐site recurrence of CRLM was lower with MWA compared with RFA (6% vs 20%) in the matched cohort analysis including 254 tumors from 134 patients. For RFA, it is said that there is relationship between higher rate of ablation‐site recurrence and proximity of the tumor to large venous structure because of a heat‐sink effect.50 In contrast, MWA may be less affected by the heat‐sink effect, leading to better local control rate. However, van Tilborg et al47 reported that MWA has a higher complication rate than RFA for peribiliary CRLM, maybe due to its more aggressive heat production. It is important to use these thermal ablation techniques after understanding each characteristic enough.
2.3. Vascular resection and reconstruction
Major vascular invasion is one of the common reasons for unresectability in patients with CRLM. When the tumor is invading either the portal vein bifurcation, all three hepatic veins, or the inferior vena cava (IVC), vascular resection and reconstruction would be required. Removal of a tumor with involvement of these major vessels has been thought to be technically demanding. However, recently, considerable advances in vascular surgery and liver transplantation have made these procedures possible.
For portal vein resection and reconstruction, primary end‐to‐end anastomosis is preferred over the use of grafts if possible.51 If primary anastomosis is impossible, several potential autologous conduits are available, including the left renal vein, superficial femoral vein, hepatic vein (from the resected liver), jugular veins, or saphenous vein modified to a spiral graft.
Hepatic vein/IVC resection and reconstruction, with liver resection, is now accepted for most liver tumors including CRLM. Reconstructions of IVC include primary suture under a side‐biting clamp, patch reconstruction, or complete replacement of the vena cava with a synthetic or biological graft.51 For reconstruction of the hepatic vein, patch reconstruction, autogenous vein graft (eg, external iliac vein, left renal vein, hepatic vein [from resected liver], jugular veins, or customized saphenous vein), or artificial graft have been used.52, 53, 54, 55, 56
Because many patients with CRLM have received chemotherapy, some of them suffer from chemotherapy‐induced liver injury, and for such patients the FLR volume may be insufficient due to their impaired liver function. In such a situation, even if the tumor is solitary, or if not all three hepatic veins are involved, hepatic vein reconstruction can sometimes be required. Several recent studies reported the usefulness of vascular resection and reconstruction with hepatectomy for CRLM, with acceptable short‐ and long‐term outcomes.53, 54, 55 A larger‐scale study will be required to confirm its benefit, in terms of safety, acceptability, and impact on long‐term outcomes.
2.4. Two‐stage hepatectomy
From 1992, a Paul Brousse team introduced the concept of TSH, based on two sequential procedures to remove multiple bilateral tumors that were impossible to remove by a single hepatectomy and then using the liver regeneration obtained after the first procedure; the work was published in 2000.57 During the next decade, TSH was developed in combination with portal vein ligation (PVL)/PVE and effective chemotherapy, achieving a 5‐year survival rate of 32%‐64%. The indication of TSH for CRLM is only bilateral, multinodular diseases which could not be resected by a single hepatectomy, even in combination with other specific techniques such as PVE, local ablation therapy, or vascular reconstruction.58
TSH is usually classified into four types as follows (Figure 2). (A) Right‐first approach: the more invaded hemiliver (usually the right lobe) is resected at the first stage, leading to hypertrophy of the contralateral liver lobe. PVL/PVE is not required. At the second stage, tumor cleaning of the FLR is performed, usually by non‐anatomical partial resection. (B) Left‐first approach with PVL/PVE: the less invaded liver lobe (FLR, usually the left lobe) is cleaned of its metastases in combination with intraoperative PVL/PVE at the first stage. At the second stage, the tumor‐bearing liver lobe (deportalized liver lobe) is anatomically removed. (C) Left‐first approach followed by PVE: percutaneous PVE is performed between the first and second stages. (D) ALPPS: a novel form of conventional TSH. The less invaded liver lobe is cleaned of its metastases in combination with intraoperative PVL/PVE and in situ splitting of the hemiliver at the first stage. At the second stage, usually 7‐14 days later, the tumor‐bearing liver lobe is removed. The details are described in the next section. From the viewpoint that liver hypertrophy after PVL/PVE may accelerate tumor growth,32, 33, 34, 35, 36 the left‐first approach has now become a mainstream strategy of TSH.
Figure 2.
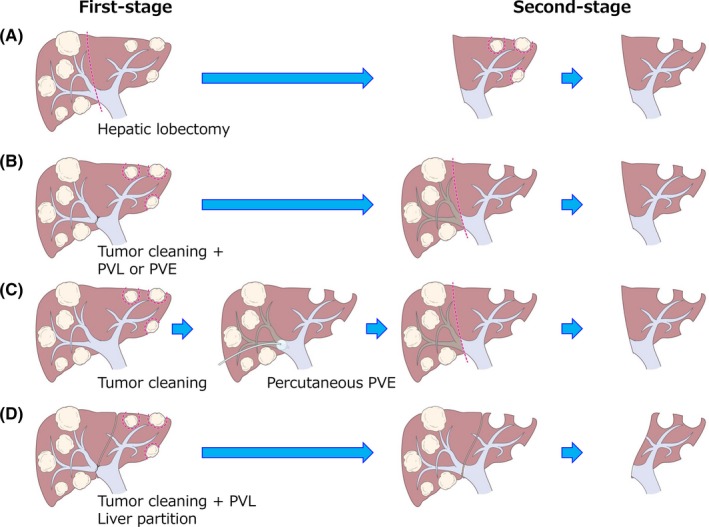
Scheme of staged hepatectomy for colorectal liver metastases. (A) Right‐first approach: most of the invaded hemiliver (usually the right lobe) is resected at the first stage, leading to hypertrophy of the contralateral liver lobe. At the second stage, tumor cleaning of the future liver remnant (FLR) is performed, usually by non‐anatomical partial resection. (B) Left‐first approach with portal vein ligation/embolization (PVL/PVE): The less invaded liver lobe (FLR, usually the left lobe) is cleaned of its metastases in combination with intraoperative PVL/PVE at the first stage. At the second stage, the tumor‐bearing liver lobe (deportalized liver lobe) is anatomically removed. (C) Left‐first approach followed by PVE: percutaneous PVE is performed between the first and second stages. (D) ALPPS: the less invaded liver lobe is cleaned of its metastases in combination with intraoperative PVL/PVE and in situ splitting of the hemiliver at the first stage. At the second stage, usually 7‐14 days later, the tumor‐bearing liver lobe is removed
The main drawback of the TSH strategy is the failure to complete both sequential procedures; that is, dropout from the second‐stage hepatectomy. A systematic review demonstrated that the failure rate of TSH ranged 0%‐36% (median, 23%), the main reason for failure being disease progression after first‐stage surgery.59 We previously reported a failure rate of 35.2%, and main reason was disease progression after first‐stage surgery (88.6%).60 In this study, four independent predictive factors for the failure of TSH were identified, namely tumor progression while on first‐line chemotherapy, number of chemotherapy cycles >12, tumor size >40 mm, and carcinoembryonic antigen at hepatectomy >30 ng/mL. A predictive model for failure of TSH was created based on the logistic model. To complete both sequential procedures in the TSH strategy is crucial for prolonged survival in patients who are planned for TSH, and to achieve that, preventing disease progression after first‐stage surgery is the most important issue.
TSH is now an established strategy for patients with multiple bilobar CRLM, in terms of short‐ and long‐term outcomes. According to our recent review,58 postoperative complication rates after the first and second stages are 0%‐37% and 11%‐60%, and mortality rates are 0%‐4% and 0%‐6%, respectively. Regarding long‐term outcome, the 5‐year OS rate after completion of TSH ranged 32%‐64%, with a median survival time of 22‐44 months. In this review, we present analyses comparing the survival between TSH and standard one‐stage hepatectomy performed at Paul Brousse Hospital. Among the patients who underwent liver‐curative surgery (R0 or R1 resection), survival for patients who completed TSH (n = 93) was comparable with that of those who underwent one‐stage hepatectomy (n = 940) (5‐year OS: 41.3% vs 48.0%, median 44.3 vs 56.6 months, P = .40).58 These results suggest that if both of the sequential TSH procedures are completed, a long‐term outcome that is comparable with standard one‐stage hepatectomy can be expected.
Because of their more extensive tumor load, patients who undergo TSH experience an extremely high recurrence rate: the 3‐year disease‐free survival rate after TSH was reported as 6%‐27% (median, 20 months).61 In our recent report, 1‐, 3‐, and 5‐year disease‐free survival rates were 28.7%, 12.3%, and 10.5%, respectively, in 93 patients who completed TSH.62 Among the 81 patients who were able to undergo potentially curative surgery, disease recurrence was observed in 62 (76.5%) during the study period. After TSH completion, repeat surgery for recurrence was performed in 38 patients (51.4%), and their survival was significantly better than those who could not undergo repeat surgery (5‐year survival rate: 45.8% vs 26.3%, P = .0041). These findings suggest that repeat surgery for recurrence may be crucial for ensuring long‐term survival, even after very invasive surgery such as TSH.
2.5. ALPPS
ALPPS is a novel form of TSH. The first report of 25 cases who underwent the ALPPS procedure in five German centers was published in 2012.63 In this paper, rapid growth of the FLR with a median hypertrophy rate of 74% after 9 days and a complete resection rate of 100% were reported. Despite initial concerns about high mortality and morbidity rates, this innovative concept has been adopted by many specialized centers around the world.
The main criticism of ALPPS was its high morbidity and mortality rates. Indeed, the first paper reported morbidity and mortality rates of 68% and 12%, respectively.63 An international ALPPS registry was subsequently initiated to collect information from multiple centers worldwide from 2012, and in the first report from this registry (202 patients, including 141 patients with CRLM), the 90‐day mortality rate was 8% and the major complication rate (≥ Clavien‐Dindo IIIa) was 36% in CRLM patients.64 After that, with accumulating experience, considerable efforts have been made to improve safety, such as development of technical modifications, stricter patient selection, and a deeper understanding of the procedure. Recent data from the ALPPS registry (486 CRLM patients) revealed that the 90‐day mortality rate was 7% and the major complication rate (≥ Clavien‐Dindo IIIa) was 39%, with a completion rate of both stages of 98%.65
Since ALPPS was introduced, many surgeons have reported various modifications of this procedure to improve postoperative short‐ and long‐term outcomes, including anterior‐approach ALPPS (using anterior approach with or without the liver hanging technique),66, 67 hybrid ALPPS (anterior approach + PVE after the first stage),68 partial ALPPS (partial liver partition),69 mini‐ALPPS (partial liver partition to a depth not exceeding 3‐5 cm + intraoperative PVE)70, ALTPS (tourniquet instead of liver partition),71 RALLP (radiofrequency ablation instead of liver partition),72 ALPTIPS (partial liver partition until the middle hepatic vein + intraoperative transileocecal PVE),73 and modified ALPPS with preservation of portal pedicles (preserving portal pedicles at the transection plane) (Table 2).74 In addition, ALPPS modifications using minimally invasive approaches, such as laparoscopic75 or robotic ALPPS76 have been described. However, the reports of these modifications consist of small case series, and larger‐scale or prospective controlled studies will be needed to establish the real roles of these modifications.
Table 2.
Several modifications of ALPPS procedure
| Procedure | Technical features | Liver transection | Benefits | Scheme | Ref |
|---|---|---|---|---|---|
| Anterior‐approach ALPPS |
|
|
|
|
66, 67 |
| Hybrid ALPPS |
|
|
|
|
68 |
| Partial ALPPS |
|
|
|
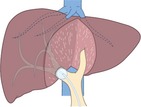
|
69 |
| Mini‐ALPPS |
|
|
|
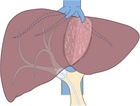
|
70 |
| ALTPS |
|
|
|
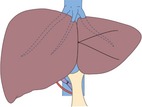
|
71 |
| RALLPS |
|
|
|
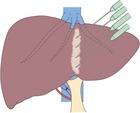
|
72 |
| ALPTIPS |
|
|
|
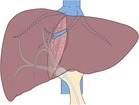
|
73 |
| Modified ALPPS with preservation of portal pedicles |
|
|
|
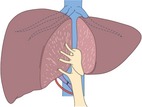
|
74 |
ALPPS, Associating liver partition and portal vein ligation for staged hepatectomy; ALTPS, associating liver tourniquet and portal vein occlusion for staged hepatectomy; RALPP, radiofrequency‐assisted liver partition with portal vein ligation; ALPTPS, associating liver partial partition and transileocecal portal vein embolization for staged hepatectomy; PVE, portal vein embolization; IVC, inferior vena cava; MHV, middle hepatic vein.
Compared to conventional TSH, ALPPS has the following advantages: (a) rapid and greater increase of the FLR, (b) shortening of the interval between the two stages, thereby minimizing the dropout risk, (c) maximization of the rate of R0 resections to approaching 100%, and (d) salvage from PVE or PVL failure.77 However, the oncological outcomes of ALPPS, relative to TSH, have not been determined. Oldhafer and colleagues reported the risk of early tumor recurrence with disease progression after ALPPS for CRLM.78 We previously published a study comparing ALPPS and conventional TSH for CRLM in an early phase of introduction of ALPPS, demonstrating that the OS rate of ALPPS was significantly worse than that of TSH, even in intention‐to‐treat analysis.79 However, two other studies reported no difference in OS between ALPPS and TSH.80, 81 Recently, multicenter randomized controlled trial (LINGO Trial) including 97 patients with CRLM and FLR < 30% (ALPPS, n = 48; TSH, n = 49) reported that resection rate was significantly higher in the ALPPS arm than in the TSH arm (92% vs 57%, P < .0001), and there was no difference in terms of complication and 90‐day mortality rates.82 Moris et al83 conducted a meta‐analysis comparing the operative results and oncological outcomes between ALPPS and conventional TSH in patient with unresectable CRLM. This meta‐analysis included 657 patients (ALPPS, n = 186; TSH, n = 471), and the kinetic growth rate was significantly faster with the ALPPS vs TSH, although there was no difference in final postoperative FLR. TSH had lower overall and major morbidity compared to ALPPS, and OS was comparable following ALPPS vs TSH. In spite of the initial concerns regarding higher morbidity and mortality rates, considerable efforts have made this technically demanding procedure much safer compared to when the ALPPS procedure was introduced.84 However, there are many issues left unsolved in ALPPS, such as still high morbidity and mortality rates, early tumor recurrence, possible stimulation of tumor proliferation due to unprecedent liver hypertrophy, and unconfirmed criteria of patient selection. The important thing is that ALPPS and TSH could be complementary in the treatment of multiple bilateral CRLM, and establishment of patient selection criteria will be warranted in further studies.
3. OTHER POSSIBLE METHODS FOR IMPROVING PATIENTS’ OUTCOMES
3.1. Minimally invasive liver surgery
Laparoscopic liver resection is nowadays adopted worldwide for the treatment of liver tumors including CRLM. Numerous studies have reported the benefits of laparoscopic liver resection compared with the standard open liver resection, such as reduced intraoperative bleeding, a lower morbidity rate, cost‐effectiveness, and shorter in‐hospital stay.85, 86, 87, 88, 89, 90 In addition, laparoscopic liver resection has been reported to provide comparable long‐term oncological outcomes with open liver resection.88, 89, 91 Another possible benefit is that less invasiveness of laparoscopic liver resection may lead to rapid initiation of postoperative chemotherapy.92, 93, 94 In light of this reduced invasiveness without compromising short‐ and long‐term outcomes, laparoscopic liver resection will probably continue to expand in the treatment of CRLM.
Robotic liver surgery is another possible minimally invasive treatment for CRLM. Robotic liver surgery has the potential to overcome some of the limits of laparoscopic surgery, such as limited degrees of motion of the instruments, unstable camera platforms, and two‐dimensional vision.95 However, in the consensus opinion during the second international laparoscopic liver forum held in 2014, there was insufficient data to comment on the applicability of robotic liver resection.96 Further data accumulation and prospective large‐scale studies will be required.
3.2. Liver transplantation
The indication for liver transplantation for CRLM has been restrictive over time because of high recurrence rates and poor outcomes. Given the systemic nature of metastatic disease and need for immunosuppressive therapy after surgery, liver transplantation for CRLM has been controversial. However, recent advances in surgical techniques, advent of more effective systemic chemotherapy, development of imaging modality, and improvements of perioperative management including novel immune‐modulating agents have led to the re‐evaluation of liver transplantation as a treatment option for unresectable CRLM. A recent systematic review reported that estimated cumulative survival rate after liver transplantation was 85.2% (at 1 year), 48% (at 3 years), and 34.6% (at 5 years), respectively, and disease‐free survival rate at 1 year was 38.9%.97 Due to the small sample size and lack of studies comparing liver transplantation with current standard of care, it is difficult to evaluate its applicability as a treatment of choice in patients with unresectable CRLM. Now some clinical trials are ongoing and will provide high‐quality evidence regarding the role of liver transplantation in the management of CRLM.
4. CONCLUSIONS
For patients with CRLM, surgical resection is the only treatment of choice for prolonged survival and a chance of cure. Given this, how to increase resectability is one of the most crucial outstanding issues. Because we currently have various surgical options to improve resectability, we should use all available techniques to explore the possibilities of surgical resection, working in a multidisciplinary manner with specialists in hepatobiliary, colorectal, and thoracic surgery, and across hepatology, oncology, and radiology.
DISCLOSURE
Funding: There was no funding for the present study.
Conflict of interest: The authors declare they have no conflict of interest.
Imai K, Adam R, Baba H. How to increase the resectability of initially unresectable colorectal liver metastases: A surgical perspective. Ann Gastroenterol Surg. 2019;3:476–486. 10.1002/ags3.12276
REFERENCES
- 1. Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg. 1999;86(2):158–69. [DOI] [PubMed] [Google Scholar]
- 2. Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23(9):2038–48. [DOI] [PubMed] [Google Scholar]
- 3. Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population‐based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93(4):465–74. [DOI] [PubMed] [Google Scholar]
- 4. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–22, discussion 722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–25, discussion 825–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94(7):982–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97(7):1110–8. [DOI] [PubMed] [Google Scholar]
- 9. Shah SA, Bromberg R, Coates A, Rempel E, Simunovic M, Gallinger S. Survival after liver resection for metastatic colorectal carcinoma in a large population. J Am Coll Surg. 2007;205(5):676–83. [DOI] [PubMed] [Google Scholar]
- 10. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long‐term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–35. [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10‐year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–80. [DOI] [PubMed] [Google Scholar]
- 12. Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long‐term survival. Ann Surg. 2004;240(4):644–57, discussion 657–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pwint TP, Midgley R, Kerr DJ. Regional hepatic chemotherapies in the treatment of colorectal cancer metastases to the liver. Semin Oncol. 2010;37(2):149–59. [DOI] [PubMed] [Google Scholar]
- 14. Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, et al. Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–21. [DOI] [PubMed] [Google Scholar]
- 15. Adam R. Developing strategies for liver metastases from colorectal cancer. Semin Oncol. 2007;34(2 Suppl 1):S7–11. [DOI] [PubMed] [Google Scholar]
- 16. Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol. 2014;21(2):501–6. [DOI] [PubMed] [Google Scholar]
- 17. Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Liver resection for colorectal cancer metastases. Curr Oncol. 2013;20(3):e255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masi G, Cupini S, Marcucci L, Cerri E, Loupakis F, Allegrini G, et al. Treatment with 5‐fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol. 2006;13(1):58–65. [DOI] [PubMed] [Google Scholar]
- 19. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. [DOI] [PubMed] [Google Scholar]
- 21. Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80. [DOI] [PubMed] [Google Scholar]
- 22. Cauchy F, Faivre S, Belghiti J. Surgical results after downstaging of initially marginal or non‐resectable liver metastases. Dig Dis. 2012;30(Suppl 2):143–9. [DOI] [PubMed] [Google Scholar]
- 23. Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17(10):1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422. [DOI] [PubMed] [Google Scholar]
- 25. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107(5):521–7. [PubMed] [Google Scholar]
- 26. Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–59. [DOI] [PubMed] [Google Scholar]
- 27. Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta‐analysis. Ann Surg. 2008;247(1):49–57. [DOI] [PubMed] [Google Scholar]
- 28. Giglio MC, Giakoustidis A, Draz A, Jawad ZA, Pai M, Habib NA, et al. Oncological outcomes of major liver resection following portal vein embolization: a systematic review and meta‐analysis. Ann Surg Oncol. 2016;23(11):3709–17. [DOI] [PubMed] [Google Scholar]
- 29. Huiskens J, Olthof PB, van der Stok EP, Bais T, van Lienden KP, Moelker A, et al. Does portal vein embolization prior to liver resection influence the oncological outcomes – a propensity score matched comparison. Eur J Surg Oncol. 2018;44(1):108–14. [DOI] [PubMed] [Google Scholar]
- 30. Adam R, Frilling A, Elias D, Laurent C, Ramos E, Capussotti L, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97(3):366–76. [DOI] [PubMed] [Google Scholar]
- 31. Ardito F, Vellone M, Barbaro B, Grande G, Clemente G, Giovannini I, et al. Right and extended‐right hepatectomies for unilobar colorectal metastases: impact of portal vein embolization on long‐term outcome and liver recurrence. Surgery. 2013;153(6):801–10. [DOI] [PubMed] [Google Scholar]
- 32. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34(2):267–72. [DOI] [PubMed] [Google Scholar]
- 33. Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, Van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256(5):812–7, discussion 817–818. [DOI] [PubMed] [Google Scholar]
- 34. Elias D, De Baere T, Roche A, Mducreux Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86(6):784–8. [DOI] [PubMed] [Google Scholar]
- 35. Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100(4):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simoneau E, Hassanain M, Shaheen M, Aljiffry M, Molla N, Chaudhury P, et al. Portal vein embolization and its effect on tumour progression for colorectal cancer liver metastases. Br J Surg. 2015;102(10):1240–9. [DOI] [PubMed] [Google Scholar]
- 37. Ruers T PC, van Coevorden F, Pierie JP, Rinkes IB, Ledermann J. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): long‐term survival results of a randomized phase II study of the EORTC‐NCRI CCSG‐ALM Intergroup 40004 (CLOCC). ASCO Annual Meeting 2015. J Clin Oncol. 2015;33(Suppl):Abstract 3501. [Google Scholar]
- 38. Imai K, Allard MA, Castro Benitez C, Vibert E, Sa Cunha A, Cherqui D, et al. Long‐term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104(5):570–9. [DOI] [PubMed] [Google Scholar]
- 39. Masuda T, Margonis GA, Andreatos N, Wang J, Warner S, Mirza MB, et al. Combined hepatic resection and radio‐frequency ablation for patients with colorectal cancer liver metastasis: a viable option for patients with a large number of tumors. Anticancer Res. 2018;38(11):6353–60. [DOI] [PubMed] [Google Scholar]
- 40. Mao R, Zhao JJ, Zhao H, Zhang YF, Bi XY, Li ZY, et al. Non‐response to preoperative chemotherapy is a contraindication to hepatectomy plus radiofrequency ablation in patients with colorectal liver metastases. Oncotarget. 2017;8(43):75151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal‐sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263(1):146–52. [DOI] [PubMed] [Google Scholar]
- 42. Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis. 2011;13(9):e252–65. [DOI] [PubMed] [Google Scholar]
- 43. Song P, Sheng L, Sun Y, An Y, Guo Y, Zhang Y. The clinical utility and outcomes of microwave ablation for colorectal cancer liver metastases. Oncotarget. 2017;8(31):51792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ierardi AM, Floridi C, Fontana F, Chini C, Giorlando F, Piacentino F, et al. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med. 2013;118(6):949–61. [DOI] [PubMed] [Google Scholar]
- 45. Leung U, Kuk D, D'angelica MI, Kingham TP, Allen PJ, DeMatteo RP, et al. Long‐term outcomes following microwave ablation for liver malignancies. Br J Surg. 2015;102(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–75 e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Tilborg AA, Scheffer HJ, de Jong MC, Vroomen LG, Nielsen K, van Kuijk C, et al. MWA versus RFA for perivascular and peribiliary CRLM: a retrospective patient‐ and lesion‐based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39(10):1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meijerink MR, Puijk RS, van Tilborg AA, Henningsen KH, Fernandez LG, Neyt M, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta‐analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Correa‐Gallego C, Fong Y, Gonen M, D'Angelica MI, Allen PJ, DeMatteo RP, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol. 2014;21(13):4278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta‐analysis and review of contributing factors. Ann Surg. 2005;242(2):158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berumen J, Hemming A. Vascular reconstruction in hepatic malignancy. Surg Clin North Am. 2016;96(2):283–98. [DOI] [PubMed] [Google Scholar]
- 52. Hemming AW, Reed AI, Langham MR, Fujita S, van der Werf WJ, Howard RJ. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002;235(6):850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aoki T, Sugawara Y, Imamura H, Seyama Y, Minagawa M, Hasegawa K, et al. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer. J Am Coll Surg. 2004;198(3):366–72. [DOI] [PubMed] [Google Scholar]
- 54. Saiura A, Yamamoto J, Sakamoto Y, Koga R, Seki M, Kishi Y. Safety and efficacy of hepatic vein reconstruction for colorectal liver metastases. Am J Surg. 2011;202(4):449–54. [DOI] [PubMed] [Google Scholar]
- 55. Ko S, Kirihataya Y, Matsusaka M, Mukogawa T, Ishikawa H, Watanabe A. Parenchyma‐sparing hepatectomy with vascular reconstruction techniques for resection of colorectal liver metastases with major vascular invasion. Ann Surg Oncol. 2016;23(Suppl 4):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miyazaki M, Ito H, Kimura F, Shimizu H, Togawa A, Ohtsuka M, et al. Hepatic vein reconstruction using autologous vein graft for resection of advanced hepatobiliary malignancy. Hepatogastroenterology. 2004;51(60):1581–5. [PubMed] [Google Scholar]
- 57. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two‐stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232(6):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Imai K, Adam R. Two‐stage liver surgery In: de Santibañes E, Ardiles V, Alvarez F, Cano Busnelli V, de Santibañes M, editors. Extreme hepatic surgery and other strategies: increasing resectability in colorectal liver metastases. Springer International Publishing, 2017; p. 203–15. 10.1007/978-3-319-13896-1 [DOI] [Google Scholar]
- 59. Chua TC, Liauw W, Chu F, Morris DL. Summary outcomes of two‐stage resection for advanced colorectal liver metastases. J Surg Oncol. 2013;107(2):211–6. [DOI] [PubMed] [Google Scholar]
- 60. Imai K, Benitez CC, Allard MA, Vibert E, Cunha AS, Cherqui D, et al. Failure to achieve a 2‐stage hepatectomy for colorectal liver metastases: How to prevent it? Ann Surg. 2015;262(5):772–8, discussion 778–779. [DOI] [PubMed] [Google Scholar]
- 61. Lam VW, Laurence JM, Johnston E, Hollands MJ, Pleass HC, Richardson AJ. A systematic review of two‐stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford). 2013;15(7):483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Imai K, Benitez CC, Allard MA, Vibert E, Cunha AS, Cherqui D, et al. Impact of surgical treatment for recurrence after 2‐stage hepatectomy for colorectal liver metastases, on patient outcome. Ann Surg. 2019;269(2):322–30. [DOI] [PubMed] [Google Scholar]
- 63. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2‐staged extended right hepatic resection in small‐for‐size settings. Ann Surg. 2012;255(3):405–14. [DOI] [PubMed] [Google Scholar]
- 64. Schadde E, Ardiles V, Robles‐Campos R, Malago M, Machado M, Hernandez‐Alejandro R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260(5):829–36, discussion 836–8. [DOI] [PubMed] [Google Scholar]
- 65. Huiskens J, Schadde E, Lang H, Malago M, Petrowsky H, de Santibañes E, et al. Avoiding postoperative mortality after ALPPS‐development of a tumor‐specific risk score for colorectal liver metastases. HPB (Oxford). 2019. [Epub ahead of print]. 10.1016/j.hpb.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 66. Chan AC, Pang R, Poon RT. Simplifying the ALPPS procedure by the anterior approach. Ann Surg. 2014;260(2):e3. [DOI] [PubMed] [Google Scholar]
- 67. Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg. 2016;263(2):e14–6. [DOI] [PubMed] [Google Scholar]
- 68. Li J, Kantas A, Ittrich H, Koops A, Achilles EG, Fischer L, et al. Avoid “All‐Touch” by hybrid ALPPS to achieve oncological efficacy. Ann Surg. 2016;263(1):e6–7. [DOI] [PubMed] [Google Scholar]
- 69. Petrowsky H, Gyori G, de Oliveira M, Lesurtel M, Clavien PA. Is partial‐ALPPS safer than ALPPS? A single‐center experience Ann Surg. 2015;261(4):e90–2. [DOI] [PubMed] [Google Scholar]
- 70. de Santibanes E, Alvarez FA, Ardiles V, Pekolj J, de Santibanes M. Inverting the ALPPS paradigm by minimizing first stage impact: the Mini‐ALPPS technique. Langenbecks Arch Surg. 2016;401(4):557–63. [DOI] [PubMed] [Google Scholar]
- 71. Robles R, Parrilla P, López‐Conesa A, Brusadin R, De La Peña J, Fuster M, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg. 2014;101(9):1129–34, discussion 1134. [DOI] [PubMed] [Google Scholar]
- 72. Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, et al. Radio‐frequency‐assisted liver partition with portal vein ligation (RALPP) for liver regeneration. Ann Surg. 2015;261(2):e45–6. [DOI] [PubMed] [Google Scholar]
- 73. Sakamoto Y, Inagaki F, Omichi K, Ohkura N, Hasegawa K, Kokudo N. Associating liver partial partition and transileocecal portal vein embolization for staged hepatectomy. Ann Surg. 2016;264(6):e21–2. [DOI] [PubMed] [Google Scholar]
- 74. Tanaka K, Kikuchi Y, Kawaguchi D, Murakami T, Hiroshima Y, Matsuo K. Modified ALPPS procedures avoiding division of portal pedicles. Ann Surg. 2017;265(2):e14–20. [DOI] [PubMed] [Google Scholar]
- 75. Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg. 2012;256(3):e13; author reply e16–19. [DOI] [PubMed] [Google Scholar]
- 76. Vicente E, Quijano Y, Ielpo B, Fabra I. First ALPPS procedure using a total robotic approach. Surg Oncol. 2016;25(4):457. [DOI] [PubMed] [Google Scholar]
- 77. Torzilli G, Adam R, Viganò L, Imai K, Goransky J, Fontana A, et al. Surgery of colorectal liver metastases: pushing the limits. Liver Cancer. 2016;6(1):80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oldhafer KJ, Donati M, Jenner RM, Stang A, Stavrou GA. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg. 2014;38(6):1504–9. [DOI] [PubMed] [Google Scholar]
- 79. Adam R, Imai K, Castro Benitez C, Allard MA, Vibert E, Sa Cunha A, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two‐stage hepatectomy for colorectal liver metastases. Br J Surg. 2016;103(11):1521–9. [DOI] [PubMed] [Google Scholar]
- 80. Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, et al. Strategies to increase the resectability of patients with colorectal liver metastases: a multi‐center case‐match analysis of ALPPS and conventional two‐stage hepatectomy. Ann Surg Oncol. 2015;22(6):1933–42. [DOI] [PubMed] [Google Scholar]
- 81. Kambakamba P, Linecker M, Alvarez FA, Samaras P, Reiner CS, Raptis DA, et al. Short chemotherapy‐free interval improves oncological outcome in patients undergoing two‐stage hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2016;23(12):3915–23. [DOI] [PubMed] [Google Scholar]
- 82. Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, et al. ALPPS improves resectability compared with conventional two‐stage hepatectomy in patients with advanced colorectal liver metastasis: results from a scandinavian multicenter randomized controlled trial (LIGRO trial). Ann Surg. 2018;267(5):833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moris D, Ronnekleiv‐Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two‐stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta‐analysis. World J Surg. 2018;42(3):806–15. [DOI] [PubMed] [Google Scholar]
- 84. Linecker M, Björnsson B, Stavrou GA, Oldhafer KJ, Lurje G, Neumann U, et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann Surg. 2017;266(5):779–86. [DOI] [PubMed] [Google Scholar]
- 85. Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250(5):849–55. [DOI] [PubMed] [Google Scholar]
- 86. Vanounou T, Steel JL, Nguyen KT, Tsung A, Marsh JW, Geller DA, et al. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol. 2010;17(4):998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi‐institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250(5):842–8. [DOI] [PubMed] [Google Scholar]
- 88. Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery. 2015;157(2):211–22. [DOI] [PubMed] [Google Scholar]
- 89. Ciria R, Ocaña S, Gomez‐Luque I, Cipriani F, Halls M, Fretland ÅA, et al. A systematic review and meta‐analysis comparing the short‐ and long‐term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc. 2019. [Epub ahead of print]. 10.1007/s00464-019-06774-2 [DOI] [PubMed] [Google Scholar]
- 90. Fretland ÅA, Dagenborg VJ, Bjørnelv GM, Kazaryan AM, Kristiansen R, Fagerland MW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO‐COMET randomized controlled trial. Ann Surg. 2018;267(2):199–207. [DOI] [PubMed] [Google Scholar]
- 91. Parks KR, Kuo YH, Davis JM, O'Brien B, Hagopian EJ. Laparoscopic versus open liver resection: a meta‐analysis of long‐term outcome. HPB (Oxford). 2014;16(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mbah N, Agle SC, Philips P, Egger ME, Scoggins CR, McMasters KM, et al. Laparoscopic hepatectomy significantly shortens the time to postoperative chemotherapy in patients undergoing major hepatectomies. Am J Surg. 2017;213(6):1060–4. [DOI] [PubMed] [Google Scholar]
- 93. Tohme S, Goswami J, Han K, Chidi AP, Geller DA, Reddy S, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015;19(12):2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kawai T, Goumard C, Jeune F, Savier E, Vaillant JC, Scatton O. Laparoscopic liver resection for colorectal liver metastasis patients allows patients to start adjuvant chemotherapy without delay: a propensity score analysis. Surg Endosc. 2018;32(7):3273–81. [DOI] [PubMed] [Google Scholar]
- 95. Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez‐Ciccarelli LF, Kim J, Benedetti E. Robotic liver surgery: technical aspects and review of the literature. Hepatobiliary Surg Nutr. 2016;5(4):311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261(4):619–29. [DOI] [PubMed] [Google Scholar]
- 97. Moris D, Tsilimigras DI, Chakedis J, Beal EW, Felekouras E, Vernadakis S, et al. Liver transplantation for unresectable colorectal liver metastases: a systematic review. J Surg Oncol. 2017;116(3):288–97. [DOI] [PubMed] [Google Scholar]


