Abstract
Aim
Gastric cancer is the second leading cause of cancer death worldwide. Surgery is the mainstay treatment for gastric cancer. There are no prediction models that examine the severity of postoperative morbidity. Herein, we constructed prediction models that analyze the risk for postoperative morbidity based on severity.
Methods
Perioperative data were retrieved from the National Clinical Database in patients who underwent elective gastric cancer resection between 2011 and 2012 in Japan. Severity of postoperative complications was determined by Clavien‐Dindo classification. Patients were randomly divided into two groups, the development set and the validation set. Logistic regression analysis was used to build prediction models. Calibration powers of the models were assessed by a calibration plot in which linearity between the observed and predicted event rates in 10 risk bands was assessed by the Pearson R 2 statistic.
Results
We obtained 154 278 patients for the analysis. Prediction models were constructed for grade ≥2, grade ≥3, grade ≥4, and grade 5 in the development set (n = 77 423). Calibration plots of these models showed significant linearity in the validation set (n = 76 855): R 2 = 0.995 for grade ≥2, R 2 = 0.997 for grade ≥3, R 2 = 0.998 for grade ≥4, and R 2 = 0.997 for grade 5 (all: P < 0.001).
Conclusion
Prediction models for postoperative morbidity based on grade will provide a comprehensive risk of surgery. These models may be useful for informed consent and surgical decision‐making.
Keywords: gastric cancer, morbidity, National Clinical Database, risk model, surgery
1. INTRODUCTION
Gastric cancer is the fourth leading cause of cancer incidence and second leading cause of cancer death worldwide.1 Surgery is the only treatment that provides a chance for a cure against gastric cancer except for endoscopically resectable early tumors.2 Surgery for gastric cancer can be safely carried out in most cases. Thirty‐day postoperative mortality rates of gastric cancer resections were <1% according to a national database in Japan.3, 4 Nevertheless, elderly patients who require gastric cancer resections are increasing with an aging society in developed countries. Some of these patients have multiple comorbidity conditions, such as hypertension, diabetes mellitus, old myocardial infarction, and old brain infarction, and patients sometimes go back and forth between nursing homes and hospitals. These patients have limited reserve capacity and sometimes suffer from postoperative complications. Therefore, prediction of postoperative morbidity is still important.
There are several prediction models of postoperative morbidity for gastric cancer patients.5, 6, 7, 8, 9, 10 Nevertheless, patients may be uncertain about how much damage they will sustain because there are no prediction models based on severity. For example, patients can eat meals and function normally because of a wound infection. By contrast, patients are kept in bed with various tubes because of an abdominal abscess. To share information on postoperative morbidity, prediction of grade‐specific morbidity rates is needed for both patients and doctors.
The National Clinical Database (NCD) in Japan was developed in collaboration with the National Surgical Quality Improvement Program (NSQIP) in USA with a shared goal of creating a standardized surgery database for quality improvement. NCD and NSQIP have developed systems using standardized variable definitions to collect data on risk factors and outcomes after surgery.11 These databases collect prospective rather than retrospective data. Because patient registration for the board certification system by the Japan Surgical Society can be carried out only by the NCD system, the NCD now covers more than 97% of total surgical procedures in Japan.12 This study was undertaken to construct prediction models to estimate grade‐specific postoperative morbidity in gastric cancer resection using large NCD data.
2. METHODS
2.1. Study design
This study was prepared in response to a public call for research using NCD data by the Japanese Gastric Cancer Association (JGCA) in 2014. The study protocol was approved by the Council of JGCA on June 3, 2014. This was a retrospective analysis of data from NCD data.
2.2. Patients
Patients were selected who underwent partial gastrectomy, total gastrectomy, total gastrectomy with splenectomy, and total gastrectomy with distal pancreatectomy and splenectomy in combination with the main disease of gastric cancer between 2011 and 2012. Exclusion criteria were emergency operations, concomitant cancer, preoperative systemic inflammatory response syndrome (SIRS), preoperative sepsis, and recurrent disease because standard operations are usually avoided under these conditions.
2.3. Data collection
Thirty‐eight preoperative conditions including comorbidities, past history, and functional status were collected with 17 preoperative laboratory data. Sixteen intraoperative data were also collected including type of surgery, American Society of Anesthesiologists Physical Status (ASA‐PS), operative duration, blood loss, intraoperative problems, and information of metastatic organs.11 The outcome of this study was postoperative morbidity according to the Clavien‐Dindo classification.13 Postoperative complications were defined as adverse events that occurred within 30 days after operation. Absence or presence of 47 complications was recorded with Clavien‐Dindo grades. Other complications were also recorded with their name and Clavien‐Dindo grade.
2.4. Statistical analysis
Patients were divided into two groups, a development set and a validation set, by a random sampling method. Univariate analysis of each predictor was conducted using chi‐squared tests with Yates correction when appropriate. Using the significant variables by univariate analysis, a stepwise increase logistic regression analysis was carried out to construct a prediction model for graded postoperative morbidity. When entering the variables into the multivariate analyses, we excluded the variables with ambiguous definitions, such as “transport by ambulance.” We also excluded the variables with an incidence less than 1%, such as “ventilator dependent.” We further excluded the variables with odds ratios that were close to 1 even though they were significant. When the variables were closely related to each other, such as hemoglobin levels and hematocrit, we excluded the variable with the smaller odds ratio.
Discriminative and calibration power of the model was carried out using area under receiver‐operating characteristics curve (AUC) and a calibration plot, respectively. In the calibration plot, patients were divided into 10 risk bands according to the predicted event rates. Each risk band was set to have an equal number of patients. Linearity between the observed and predicted event rates was assessed by Pearson's R 2 statistic.
3. RESULTS
We obtained 154 278 patient datasets for analysis and divided them into two groups, a development set and a validation set. Demographic data are shown in Table 1. Both groups were well balanced in preoperative factors, intraoperative factors, and postoperative morbidity.
Table 1.
Demographic data of patients who underwent elective gastric cancer resection between 2011 and 2012 in Japan
| Development set (n = 77 423) | Validation set (n = 76 855) | |
|---|---|---|
| Preoperative factors | ||
| Age, y, mean (SD) | 69.3 (11.3) | 69.2 (11.3) |
| Male | 53 529 (69.1%) | 53 056 (69.0%) |
| Diabetes mellitus | 12 659 (16.4%) | 12 366 (16.1%) |
| COPD | 3032 (3.9%) | 3067 (4.0%) |
| Cerebrovascular disease | 2958 (3.8%) | 2890 (3.8%) |
| Previous PCI | 2002 (2.6%) | 1959 (2.5%) |
| Previous cardiac surgery | 906 (1.2%) | 900 (1.2%) |
| Previous PVD surgery | 407 (0.5%) | 443 (0.6%) |
| Bleeding disorder | 2709 (3.5%) | 2676 (3.5%) |
| Weight loss ≥10% | 4445 (5.7%) | 4474 (5.8%) |
| Respiratory distress, any | 1598 (2.1%) | 1519 (2.0%) |
| ADL, any assistance | 3295 (4.3%) | 3303 (4.3%) |
| Ascites | 1065 (1.4%) | 1096 (1.4%) |
| BMI, median (IQR) | 22.0 (19.8‐24.2) | 22.0 (19.8‐24.2) |
| ASA‐PS ≥3 | 7577 (9.8%) | 7366 (9.6%) |
| Disseminated disease | 1348 (1.7%) | 1276 (1.7%) |
| Laboratory data | ||
| WBC >11 000/μL | 1493 (1.9%) | 1482 (1.9%) |
| Platelet <80 000/μL | 384 (0.5%) | 363 (0.5%) |
| Albumin <4.0 g/dL | 27 811 (35.9%) | 27 310 (35.5%) |
| Na <135 mmol/L | 2357 (3.0%) | 2236 (2.9%) |
| Creatinine >1.2 mg/dL | 5356 (6.9%) | 5135 (6.7%) |
| AST >35 IU/mL | 6009 (7.8%) | 5950 (7.7%) |
| ALP >600 IU/mL | 467 (0.6%) | 481 (0.6%) |
| CRP >1.0 mg/dL | 6573 (8.5%) | 6428 (8.4%) |
| Prothrombin time‐INR >1.25 | 1866 (2.4%) | 1928 (2.5%) |
| Intraoperative factors | ||
| Blood loss, g, median (IQR) | 190 (70‐400) | 194 (70‐405) |
| Operation time, min, median (IQR) | 250 (196‐315) | 251 (196‐315) |
| Type of gastrectomy | ||
| Partial gastrectomy without LN dissection | 4577 (5.9%) | 4643 (6.0%) |
| Partial gastrectomy | 46 411 (59.9%) | 45 913 (59.7%) |
| Total gastrectomy | 23 832 (30.8%) | 23 735 (30.9) |
| Proximal gastrectomy | 647 (0.8%) | 673 (0.9%) |
| Total gastrectomy with splenectomy | 2277 (2.9%) | 2207 (2.9%) |
| Total gastrectomy with PS | 327 (0.4%) | 358 (0.5%) |
| Postoperative morbidity | ||
| Grade 2 | 12 806 (16.5%) | 12 828 (16.7%) |
| Grade 3 | 6006 (7.8%) | 6046 (7.9%) |
| Grade 4 | 1604 (2.1%) | 1552 (2.1%) |
| Grade 5 | 1069 (1.4%) | 1031 (1.3%) |
ADL, activities of daily living; ALP, alkaline phosphatase; ASA‐PS, American Society of Anesthesiologists Physical Status; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; INR, international normalized ratio; IQR, interquartile range; LN, lymph node; PCI, percutaneous coronary intervention; PS, pancreatosplenectomy; PVD, peripheral vascular disease; WBC, white blood cell count.
Table 2 shows the parameters and coefficients of the grade‐specific models for postoperative morbidity that were generated in the development set. The predicted event rate (p) for each outcome was calculated as follows: ln[p/(1 − p)] = β0 + ∑βi X i, where β0 is a constant, βi is a coefficient, and X i is a variable.
Table 2.
Coefficients of logistic regression models for postoperative morbidity
| Variables | Grade ≥2 | Grade ≥3 | Grade ≥4 | Grade 5 |
|---|---|---|---|---|
| Age, y | — | — | 0.244 | 0.291 |
| Distal gastrectomy without LN dissection | — | — | — | 0.231 |
| With colectomy | 0.568 | 0.541 | — | — |
| Total or proximal gastrectomy | 0.413 | 0.463 | 0.580 | 0.566 |
| With splenectomy | 0.523 | 0.413 | — | — |
| Male | — | 0.460 | 0.330 | 0.199 |
| Respiratory distress, any | 0.420 | — | 0.474 | 0.670 |
| Weight loss ≥10% | 0.271 | 0.286 | 0.431 | 0.534 |
| Previous PCI | 0.314 | 0.256 | 0.377 | 0.305 |
| Previous cardiac surgery | 0.253 | — | — | — |
| Previous PVD surgery | 0.314 | — | — | — |
| ADL, any assistance | 0.515 | 0.612 | 0.715 | 0.821 |
| Cerebrovascular disease | 0.403 | 0.292 | 0.509 | 0.412 |
| Ascites, any | 0.491 | 0.454 | 0.830 | 1.040 |
| COPD | 0.294 | 0.365 | 0.214 | — |
| PVD | — | 0.541 | — | — |
| Disseminated disease | 0.266 | 0.515 | 0.922 | 1.092 |
| Bleeding disorder | 0.561 | — | 0.766 | 0.691 |
| ASA‐PS ≥4 | 0.819 | 0.535 | 0.723 | — |
| ASA‐PS ≥3 | 0.360 | 0.336 | 0.410 | 0.423 |
| BMI >25 | — | — | 0.203 | — |
| BMI >26 | 0.287 | — | — | — |
| WBC >11 000/μL | 0.261 | — | 0.670 | 0.691 |
| Platelets <80 000/μL | 0.596 | 0.782 | 0.803 | 0.879 |
| Albumin <4.0 g/dL | 0.294 | 0.276 | — | — |
| Albumin <3.8 g/dL | — | — | 0.446 | 0.624 |
| Na <138 mmol/L | — | — | 0.379 | 0.463 |
| Na <135 mmol/L | 0.298 | — | — | — |
| Creatinine >2.0 mg/dL | — | — | 0.500 | — |
| Creatinine >1.2 mg/dL | — | 0.282 | 0.518 | — |
| AST >35 IU/mL | — | — | 0.290 | 0.341 |
| ALP >600 IU/mL | — | — | 0.781 | 0.868 |
| CRP >1.0 mg/dL | — | — | 0.276 | 0.322 |
| Prothrombin time‐INR >1.25 | — | — | 0.245 | 0.364 |
| Constant | −2.441 | −3.337 | −6.094 | −6.880 |
ADL, activities of daily living; ALP, alkaline phosphatase; ASA‐PS, American Society of Anesthesiologists Physical Status classification; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; INR, international normalized ratio; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; WBC, white blood cell count.
(Predicted event rate (p) for each outcome was calculated as follows: ln [p/(1 − p)] = β0 + ∑βi X i, where β0 is a constant, βi is a coefficient, and X i is a variable).
"–" means no coefficients.
Figure 1 shows the AUC of models in the validation set. When dependent variables included lower grades of complications, the AUC became lower. The AUC (95% CI) were 0.656 (0.650‐0.661) for grade ≥2, 0.668 (0.661‐0.675) for grade ≥3, 0.794 (0.783‐0.806) for grade ≥4, and 0.839 (0.828‐0.851) for grade 5.
Figure 1.
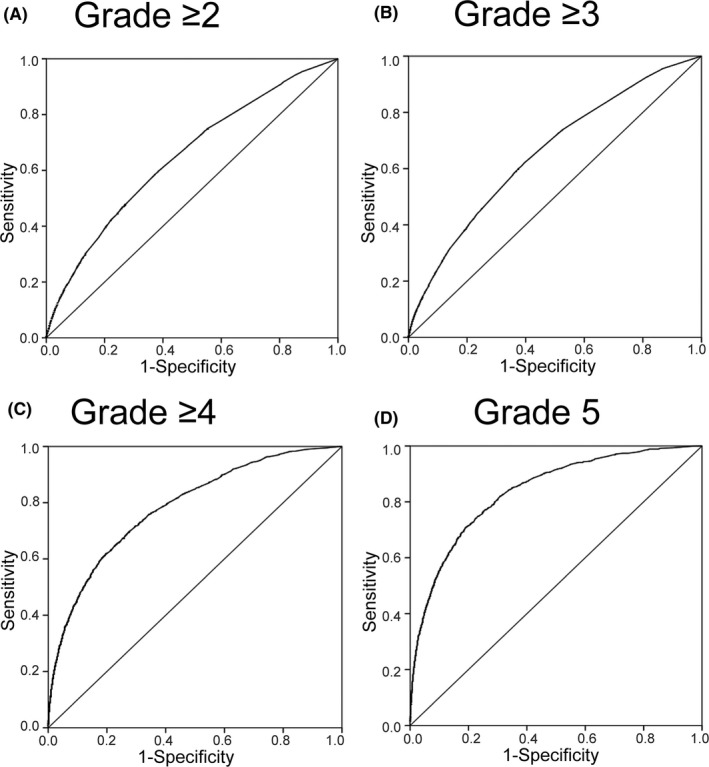
Discriminative power of prediction models for postoperative morbidity in gastric cancer resection. Receiver operating characteristic curve analysis for each prediction model was carried out in a validation set (n = 76 855)
Figure 2 shows calibration plots of the models in the validation set. All the models fit well from the lower to the higher risk groups. These models showed a significant linearity between the observed and predicted event rates in 10 risk bands: R 2 = 0.995 for grade ≥2, R 2 = 0.997 for grade ≥3, R 2 = 0.998 for grade ≥4, and R 2 = 0.997 for grade 5 (all: P < 0.001). Tables 3 and 4 show the observed event rates in each risk band determined by the prediction models for grade ≥2 and grade ≥3, respectively.
Figure 2.
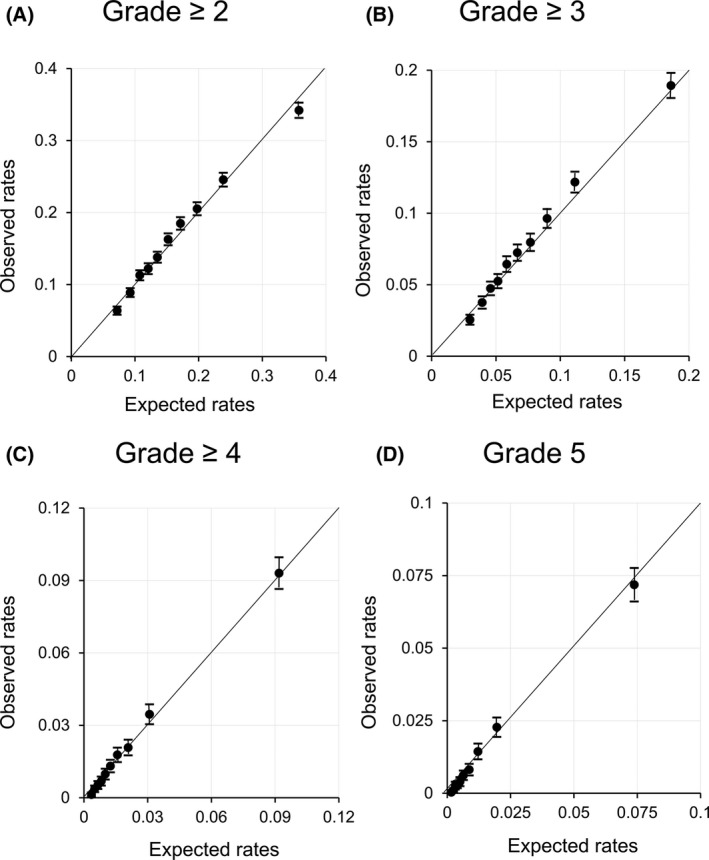
Calibration plot of prediction models for postoperative morbidity in gastric cancer resection. Observed event rates (95% CI) were plotted with predicted event rates in 10 risk bands for each prediction model in the validation set (n = 76 855)
Table 3.
Risk of postoperative morbidity of patients classified as grade ≥2
| Risk band | Predicted event rates | Observed event rates (95% CI) |
|---|---|---|
| 1 | <8.5% | 6.4% (5.8% to 7.0%) |
| 2 | 8.5% to <10.0% | 8.9% (8.3% to 9.5%) |
| 3 | 10.0% to <11.4% | 11.3% (10.6% to 12.0%) |
| 4 | 11.4% to <12.7% | 12.2% (11.5% to 13.0%) |
| 5 | 12.7% to <14.3% | 13.8% (13.0% to 14.6%) |
| 6 | 14.3% to <16.2% | 16.3% (15.5% to 17.1%) |
| 7 | 16.2% to <18.3% | 18.5% (17.6% to 19.4%) |
| 8 | 18.3% to <21.5% | 20.5% (19.6% to 21.4%) |
| 9 | 21.5% to <27.0% | 24.6% (23.6% to 25.5%) |
| 10 | ≥27.0% | 34.2% (33.2% to 35.3%) |
Table 4.
Risk of postoperative morbidity of patients classified as grade ≥3
| Risk band | Predicted event rates | Observed event rates (95% CI) |
|---|---|---|
| 1 | <3.5% | 2.6% (2.2% to 2.9%) |
| 2 | 3.5% to <4.3% | 3.8% (3.3% to 4.2%) |
| 3 | 4.3% to <4.8% | 4.8% (4.3% to 5.2%) |
| 4 | 4.8% to <6.2% | 5.3% (4.8% to 5.8%) |
| 5 | 6.2% to <7.1% | 6.4% (5.9% to 7.0%) |
| 6 | 7.1% to <8.2% | 7.3% (6.7% to 7.8%) |
| 7 | 8.2% to <9.8% | 8.0% (7.4% to 8.6%) |
| 8 | 9.8% to <12.8% | 9.6% (9.0% to 10.3%) |
| 9 | 12.8% to <25.2% | 12.2% (11.5% to 12.9%) |
| 10 | ≥25.2% | 18.9% (18.1% to 19.8%) |
4. DISCUSSION
We constructed and validated prediction models for postoperative morbidity following gastric cancer resections according to severity using large‐scale national data. Several prediction models have been reported for individual postoperative complications. Kikuchi et al14 developed prediction models for surgical site infections, anastomotic leakage, pancreatic fistula, pneumonia, prolonged pneumonia, and renal failure following total gastrectomy. Tu et al15 developed a nomogram to predict anastomotic leakage after gastrectomy for gastric cancer. However, there are no prediction models to account for all postoperative complications following gastric cancer resections. To the best of our knowledge, this is the first report regarding a grade‐stratified morbidity prediction system in this area. Postoperative complications may decrease long‐term survival in various cancer surgeries.16 Prediction of postoperative morbidity is becoming more important when considering early and long‐term prognosis. This system will help clinicians and patients with surgical decision‐making.
Postoperative complications following major gastrointestinal surgeries are an important outcome for patients. Complications decrease quality of life, prolong hospital stay, and increase medical costs. Postoperative complications include surgical complications that relate to surgery, and non‐surgical complications that are not related to surgery. Surgical complications, such as anastomotic leakage, may result from technical problems in addition to patient factors such as malnourishment and diabetes.14, 17 Non‐surgical complications, such as pneumonia, may depend on the patient's physiological conditions.14, 18 For both surgical and non‐surgical complications, the balance between patient reserve capacity and the degree of surgical stress may play a key role in the genesis of postoperative complications.19 Therefore, we postulate that postoperative morbidity by severity can be predicted using physiological and tumor‐related variables. For surgical factors, such as blood loss and operational time, these factors are dependent on tumor status and patient characteristics. For example, advanced carcinoma in the upper portion with significant lymph node enlargement requires total gastrectomy with extended lymph node dissection, which results in a longer operational time and a larger blood loss. If the patient has a higher body mass index, operational time and blood loss will be further increased. Therefore, these surgical factors are thought to be intermediate variables in the multivariate analysis, which may lead to overadjustment bias.20 The current models were constructed by analyzing the variables of physiological variables, metastatic status, and type of surgery. In preliminary analyses, we also constructed prediction models by adding surgical factors. Nevertheless, the predictive power of models with surgical factors was similar to models without surgical factors. Therefore, we used models without surgical factors.
The model's discriminative power became lower when lower grade complications were included as dependent variables. This phenomenon may result from the difficulty of predicting diverse complications. For example, the etiology of surgical site infection and postoperative pneumonia may be different. A previous study on total gastrectomy showed that predicting surgical complications is more difficult than predicting non‐surgical complications.17 For complications of grade ≥4, impaired patient reserve capacity may be important, and our model can predict these complications more precisely. The discriminative power indicates how well the model distinguishes events and non‐events on an individual patient basis. Because the AUC of the model for grade ≥2 or grade ≥3 was lower, these predicted event rates should not be used for patients that need an operation. However, when we classified a group of patients using the system as shown in Tables 3 and 4, we can assume the risk of these patients. In contrast, the models for grade ≥4 and grade 5 showed good discriminative and calibration power. Therefore, we can directly use the predicted event rates for these patients. Using these models, physicians and patients can be informed of operation risks more specifically.
When using these models, the quality of care of hospitals should be considered because a volume outcome relationship has been reported for gastric cancer surgeries.21, 22, 23, 24 The present models provide an average risk in Japan. Before using the models, each hospital should calculate postoperative morbidity rates according to the Clavien‐Dindo grading and compare them with NCD data. If the rates are far from NCD data, the predicted event rates should be adjusted in parallel to the observed rates.
A limitation of the present study was that the present validation set was not truly an external subset. We must analyze the predictive power in a truly external set such as future data. A validation study outside Japan will also add generalizability. Furthermore, the present models include many independent variables, which may burden the working time for data input. However, these variables are already incorporated into the NSQIP/NCD system and will not increase the workload at participating hospitals. The authors will upload a computer file which can compute risk of postoperative morbidity using the current models.
In conclusion, we constructed prediction models for grade‐based postoperative morbidity using large national data. These models show a clear portrait of postoperative risk in which clinicians and patients can share the same information.
DISCLOSURE
Funding: This work was supported by a grant from the Japanese Gastric Cancer Association.
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Ethical Statement: This study used only anonymized data from the NCD system. Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan defines no ethical obligation on such a study.
Haga Y, Miyata H, Tsuburaya A, et al. Development and validation of grade‐based prediction models for postoperative morbidity in gastric cancer resection using a Japanese web‐based nationwide registry. Ann Gastroenterol Surg. 2019;3:544–551. 10.1002/ags3.12269
REFERENCES
- 1. Global Burden of Disease Cancer Collaboration . The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Japan Gastric Cancer Society [internet]: Guidelines for Diagnosis and Treatment of Carcinoma of the Stomach April 2004 edition. [cited 2 May 2019]. Available from: http://www.jgca.jp/pdf/Guidelines2004_eng.pdf.
- 3. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14(4):301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M, Kimura O. Evaluation of modified Estimation of Physiologic Ability and Surgical Stress in gastric carcinoma surgery. Gastric Cancer. 2012;15(1):7–14. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe M, Miyata H, Gotoh M, Baba H, Kimura W, Tomita N, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet‐based database. Ann Surg. 2014;260(6):1034–9. [DOI] [PubMed] [Google Scholar]
- 7. Kurita N, Miyata H, Gotoh M, Shimada M, Imura S, Kimura W, et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese Patients collected using a Nationwide Web‐based Data Entry System. Ann Surg. 2015;262(2):295–303. [DOI] [PubMed] [Google Scholar]
- 8. Hong S, Wang S, Xu G, Liu J. Evaluation of the POSSUM, p‐POSSUM, o‐POSSUM, and APACHE II scoring systems in predicting postoperative mortality and morbidity in gastric cancer patients. Asian J Surg. 2017;40(2):89–94. [DOI] [PubMed] [Google Scholar]
- 9. Kitano Y, Iwatsuki M, Kurashige J, Kuroda D, Kosumi K, Baba Y, et al. Estimation of Physiologic Ability and Surgical Stress (E‐PASS) versus modified E‐PASS for prediction of postoperative complications in elderly patients who undergo gastrectomy for gastric cancer. Int J Clin Oncol. 2017;22(1):80–7. [DOI] [PubMed] [Google Scholar]
- 10. Fang Y, Wu C, Gu X, Li Z, Xiang J, Chen Z. Perioperative mortality and morbidity prediction using POSSUM, P‐POSSUM and APACHE II in Chinese gastric cancer patients: surgical method is a key independent factor affecting prognosis. Int J Clin Oncol. 2014;19(1):74–80. [DOI] [PubMed] [Google Scholar]
- 11. Anazawa T, Miyata H, Gotoh M. Cancer registries in Japan: National Clinical Database and site‐specific cancer registries. Int J Clin Oncol. 2015;20(1):5–10. [DOI] [PubMed] [Google Scholar]
- 12. Iwanaka T. Past, present, and future of national clinical database. Kyobu Geka. 2017;70(1):35–40. [PubMed] [Google Scholar]
- 13. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 14. Kikuchi H, Miyata H, Konno H, Kamiya K, Tomotaki A, Gotoh M, et al. Development and external validation of preoperative risk models for operative morbidities after total gastrectomy using a Japanese web‐based nationwide registry. Gastric Cancer. 2017;20(6):987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tu RH, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, et al. Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur J Surg Oncol. 2017;43(2):485–92. [DOI] [PubMed] [Google Scholar]
- 16. Shimada H, Fukagawa T, Haga Y, Oba K. Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg. 2017;1(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee KG, Lee HJ, Yang JY, Oh SY, Bard S, Suh YS, et al. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien‐Dindo system. J Gastrointest Surg. 2014;18(7):1269–77. [DOI] [PubMed] [Google Scholar]
- 18. Kiuchi J, Komatsu S, Ichikawa D, Kosuga T, Okamoto K, Konishi H, et al. Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int J Clin Oncol. 2016;21(5):920–6. [DOI] [PubMed] [Google Scholar]
- 19. Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E‐PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29(3):219–25. [DOI] [PubMed] [Google Scholar]
- 20. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bachmann MO, Alderson D, Edwards D, Wotton S, Bedford C, Peters TJ, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89(7):914–22. [DOI] [PubMed] [Google Scholar]
- 22. Smith DL, Elting LS, Learn PA, Raut CP, Mansfield PF. Factors influencing the volume‐outcome relationship in gastrectomies: a population‐based study. Ann Surg Oncol. 2007;14(6):1846–52. [DOI] [PubMed] [Google Scholar]
- 23. Pasquer A, Renaud F, Hec F, Gandon A, Vanderbeken M, Drubay V, et al. Is centralization needed for esophageal and gastric cancer patients with low operative risk? A Nationwide Study. Ann Surg. 2016;264(5):823–30. [DOI] [PubMed] [Google Scholar]
- 24. Iwatsuki M, Yamamoto H, Miyata H, Kakeji Y, Yoshida K, Konno H, et al. Effect of hospital and surgeon volume on postoperative outcomes after distal gastrectomy for gastric cancer based on data from 145,523 Japanese patients collected from a nationwide web‐based data entry system. Gastric Cancer. 2019;22(1):190–201. [DOI] [PubMed] [Google Scholar]


