Supplemental digital content is available in the text.
Key Words: pancreatic cancer, pancreatic enzymes, pancreatic enzyme replacement therapy, pancreatic exocrine insufficiency, symptom alleviation, weight loss
Objectives
Pancreatic cancer (PC) and its treatments can result in pancreatic exocrine insufficiency that requires pancreatic enzyme replacement therapy (PERT). Appropriate PERT usage is during meals and snacks. The aim was to determine the frequency of appropriate use of PERT and its impact on symptom alleviation in PC through a patient-reported outcomes online platform.
Methods
Users in the Pancreatic Cancer Action Network's Patient Registry were prompted to answer a standalone questionnaire about their experience with PERT.
Results
Two hundred sixty-two users completed the PERT questionnaire (January 2016–January 2018). Patients who reported taking PERT with meals had higher alleviation of symptoms compared with those taking PERT prior to or after meals. Specifically, “feeling of indigestion,” “light-colored or orange stools,” and “visible food particles in stool” were significantly decreased. Patients taking PERT with meals reported weight gain and less weight loss.
Conclusions
Of the 89% of PC patients prescribed PERT, 65% were prescribed PERT appropriately with all meals and snacks. Overall compliance with PERT administration guidelines was low (50% [105/208]). Improvement in symptoms significantly correlated with appropriate use of PERT. Increase in PC patient and provider education about appropriate PERT usage and administration is warranted.
Pancreatic cancer (PC) is the third leading cause of cancer-related death and is projected to surpass colorectal cancer to become the second deadliest cancer by 2030.1,2 The overall 5-year survival rate is 8%,2 and while surgical resection remains the best therapeutic option, approximately only 20% of patients are eligible for attempt at surgical resection at diagnosis. Even with resection, postoperative recurrences are very common.3,4 Additionally, PC and its therapies are associated with many complications including pancreatic exocrine insufficiency (PEI), depression, weight loss, diabetes, pain, delayed gastric emptying, biliary obstruction, and hypercoagulability.5–9 These complications contribute to suboptimal quality of life for PC patients, which impacts their ability to tolerate treatment.
One specific complication of PC is PEI.9 Pancreatic enzyme insufficiency can result from multiple etiologies associated with PC including obstruction of the pancreatic duct in the head by the tumor itself, from surgical resection of the pancreas with resultant loss of significant parenchyma, denervation of the pancreas at time of surgery, and from alterations in intestinal anatomy postoperatively.8,9
Pancreatic enzyme insufficiency affects patients with both resectable and unresectable disease.9 Pancreatic enzyme insufficiency has a negative effect on survival and quality of life of PC patients. Pancreatic cancer treatment including radiation therapy and surgical resection is associated with PEI10,11 and a negative impact on quality of life.11 Additionally, low levels of fecal elastase, a marker for malabsorption, is correlated with poor survival in advanced PC patients.12
Pancreatic enzyme replacement therapy (PERT) as treatment for PEI has been shown to be beneficial for both resected PC patients and those with advanced disease.13–15 Two randomized controlled trials have demonstrated benefit of PERT for patients with chronic pancreatitis or pancreatic surgery and PEI; the benefits included weight gain, reduced stool frequency, increased fat absorption, improved stool consistency, and symptom improvement.16–18 While specific guidelines for PERT dosing and administration do not exist in PC, the Pancreatic Cancer Action Network (PanCAN) initiated Supportive Care Working Group recommended all patients with evidence or suspicion of PEI should be initiated on doses of 72,000 lipase units per meal and 36,000 lipase units per snack, and doses should be modified as needed based on clinical symptoms, steatorrhea present, and the fat content of the diet.19 Further, PERT is optimally taken at the start of the meal or at the start and throughout a meal if multiple pills are taken.20
The aim of this study was to determine the frequency of appropriate use of PERT administration in PC patients via PanCAN's Patient Registry, a patient-reported outcome (PRO) online platform, and to determine the impact of appropriate use of PERT on PEI symptom alleviation in PC patients. Pancreatic enzyme insufficiency can cause discomfort and weight loss, symptoms that can greatly affect patients' quality of life and their ability to tolerate treatment.
MATERIALS AND METHODS
A retrospective analysis of PROs related to PERT was completed of patients who participated in PanCAN's Patient Registry. Launched in January 2016, the Patient Registry was created to look for patterns in treatment options, adverse effects, and diagnostics. The Patient Registry was established as a research tool and has been approved by the Western Institutional Review Board. Users were recruited from PanCAN's Patient Central call-in department, as well as through marketing efforts. Patients, as well as caregivers on behalf of a patient, were encouraged to create an account. Registry enrollment was emphasized to patients enrolling in PanCAN's Know Your Tumor precision medicine service. The registry includes patients in all 50 states, as well as 28 countries outside the United States. Participants were included in this analysis if they created a Patient Registry account, consented to allowing PanCAN to view and analyze their data, are based in the United States, and completed the Pancreatic Enzymes (PE) survey or the pancreatic enzyme-specific questions in the Pancreatic Cancer Experience Basics (Basics) survey.
There are currently 23 different individual surveys a user can complete in the registry. Survey topics include diagnosis, health assessment, treatment(s) received and associated adverse effects, test results, symptom management, family history, and medical history. Users are required to first take the Basics survey before they are able to take any additional surveys. The Basics survey includes 25 to 30 general questions about symptoms, diagnosis, treatment, current disease status, PanCAN services used, and why users participate in the registry. The Basics survey includes patient characteristics (age, disease stage, etc) and lays the foundation for other surveys. Patients who joined the registry and completed the Basics survey were given the option to answer a standalone PE survey.
The PE survey was introduced at the same time as the Registry launch (January 2016); at that time, 25 PEI-related and experience with PERT–related questions were asked (Fig. 1). In the PE survey, users are first asked if they have discussed pancreatic enzymes with their health care professional (HCP). If they have not discussed them with their HCP, they are then asked if they are aware of enzymes. If aware, they are asked why they have not discussed enzymes with their HCP. If unaware, they are prompted to learn more about enzymes by contacting PanCAN's call center for information. In all cases, users are given the opportunity to provide additional information or context through an open-field text box at the end of the PE survey.
FIGURE 1.
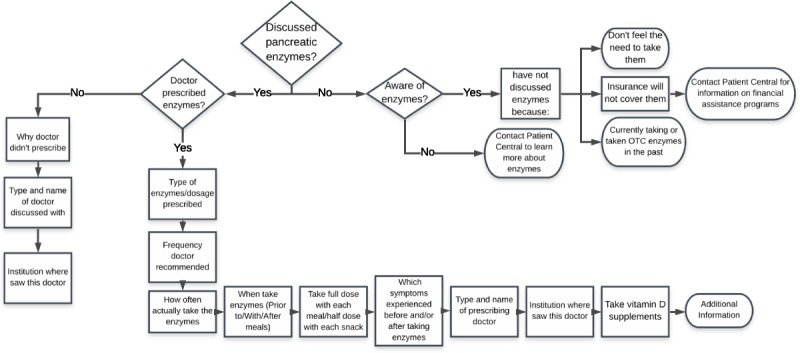
Questions from the PE survey.
In May 2016, 5 supplementary questions were added to the PE survey to capture additional symptoms and adverse effects (Fig. 2).
FIGURE 2.
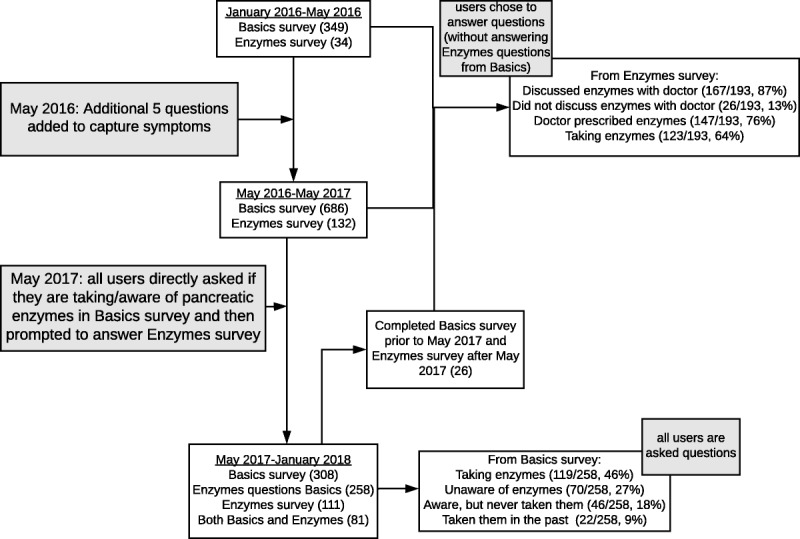
Flow diagram of data collection process.
All users who enrolled in the registry and completed the required Basics survey were prompted to answer the PE survey, either through email engagement or by having the PE survey added to the user's dashboard. Users were encouraged to complete the survey via an email blast in April 2016 and December 2017. In May 2017, 2 additional questions about pancreatic enzymes were added to the Basics survey so that all users were directly asked if they were experiencing symptoms related to PEI. If a user indicated having experienced 1 or more of the related symptoms, they were asked if they are taking and/or are aware of pancreatic enzymes. They were then asked if they would be willing to answer the full PE survey. If they answered affirmatively, the PE survey was added to the list of surveys to be taken.
In the PE survey, if users indicated they had discussed enzymes with and were prescribed enzymes by their doctor, they were prompted to answer questions about the type and dosage of enzymes prescribed, the recommended frequency, and how often and when users actually took (prior to, with, or after meals) the enzymes (Fig. 1). Additionally, users were prompted to report if they experienced any of the 10 PEI-related symptoms either before and/or after taking the enzymes (Fig. 1). The prescribing doctor and institution where they saw this doctor were captured.
In February 2018, 2 questions were removed, 1 changed, and 6 questions were added in order to better capture dose taken and severity of symptoms, as well as the date started taking the enzymes and date ended, if applicable (Supplemental Figure 1, http://links.lww.com/MPA/A725).
RESULTS
Collection of Data
From January 2016 to May 2016, 34 users completed the PE survey, and 132 users completed it from May 2016 (since the addition of the 5 questions) to May 2017 (Fig. 2). Starting in May 2017, all users were directly asked if they were taking enzymes or if they are aware of enzymes in the Basics survey (all users are prompted to answer the Basics survey prior to any other survey) and then prompted to complete the PE survey if they wish to do so. This new method was implemented to eliminate any possible answer bias as all users are required to complete the Basics survey, but the PE survey is completed only by those who choose to complete it. Since the questions specific to enzyme use were added to the Basics survey (from May 2017 to January 2018), 308 users answered the Basics survey, with 258 users completing the enzymes specific questions, 105 completing the PE survey, and 81 users completing both the Basics survey and the PE survey. Because the registry is an ongoing platform and users are encouraged to log in periodically to complete additional surveys, 26 users completed the Basics survey prior to May 2017 (prior to addition of enzymes specific questions) and completed the PE survey after May 2017. These 26 users did not answer the enzyme-specific questions in the Basics survey. Unlike all other surveys, the Basics survey can be completed only once by each user.
Enzyme Specific Questions From Basics Survey (May 2017–January 2018)
Of the 258 users who answered the enzymes specific questions (added May 2017) from the Basics survey, 119 (46%) reported taking enzymes, 70 (27%) reported being unaware of enzymes, 46 (18%) were aware of enzymes but never took them, and 22 (9%) had taken them in the past but were not taking them at the time they answered the question (Fig. 2).
Enzymes Survey (January 2016–January 2018)
Patient Characteristics
From January 2016 to January 2018, the PE survey was completed 277 times by 262 patients. Of the 262 patients, 110 (42%) were female, 117 (45%) were male, and 35 (13%) did not report their sex. The median age at PC diagnosis was 61 years (range, 21–86 years). There were 14 users who either did not report a date of diagnosis or a date of birth that were excluded from the median age of diagnosis calculation. Two hundred sixty-one users reported the histologic type of PC they were diagnosed with, with adenocarcinoma being the most common (202 patients [77%]), followed by pancreatic neuroendocrine tumors (19 patients [7%]) and other types of PC (11 patients [4%]), and 29 patients (11%) either skipped the question or did not know the specific histologic type of PC (Table 1).
TABLE 1.
Patient Characteristics
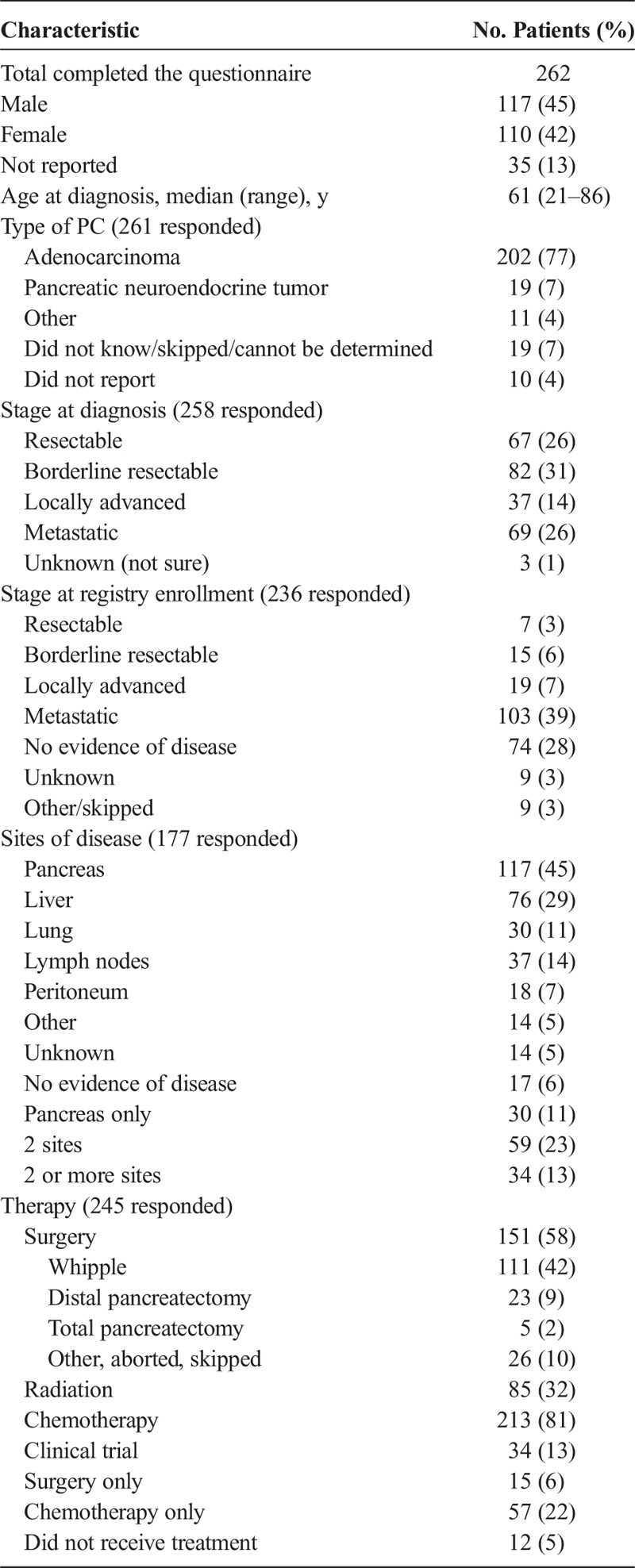
Two hundred fifty-eight users reported the stage of PC at diagnosis. Sixty-seven (26%) reported having resectable disease, 82 (31%) had borderline resectable disease, 37 (14%) had locally advanced disease, 69 (26%) had metastatic disease, and 3 (1%) were either unsure or staging was unknown. Two hundred thirty-six users provided their stage of disease at Registry enrollment, of which 7 (3%) indicated having resectable disease, 15 (6%) borderline resectable, 19 (7%) locally advanced, 103 (39%) metastatic, 74 (28%) reported no evidence of disease, and 18 (6%) reported not sure or skipped. Of the 74 users who reported no evidence of disease at Registry enrollment, 29 (39%) reported their stage at first diagnosis as resectable, 31 (42%) borderline resectable, 12 (16%) locally advanced, 1 (<1%) metastatic, and 1 (<1%) not sure.
Overall, 177 reported on their site of disease; 117 (66%) reported cancer in their pancreas, 76 (43%) in the liver, 30 (17%) in the lungs, 37 (21%) in lymph nodes, 18 (10%) in peritoneum, and 14 (8%) other sites; and 17 (10%) reported no evidence of disease and 14 (8%) unknown. Twenty-nine reported cancer in their pancreas only, 59 (50%) reported cancer in 2 sites, and 34 (29%) reported cancer in 2 or more sites.
Two hundred forty-five reported on the type of therapy they received, 151 (58%) had surgery (15 reported having surgery only), 85 (32%) had radiation, 213 (81%) had chemotherapy (57 reported having chemotherapy only), 34 (13%) enrolled in a clinical trial, and 12 (5%) reported that they did not receive treatment.
PE Replacement Therapy Prescription and Usage
Two hundred thirty-five (85%) of 277 users who completed the survey reported speaking to their HCP about PERT. Two hundred eight (89%) of 235 were prescribed PERT (of which 89% were prescribed Creon) (Fig. 3). The type of HCP that prescribed PERT was specified by 201 users: 45% reported that PERT was prescribed by a medical oncologist, 25% by a surgeon, 17% by a gastroenterologist, and 15% by another type of HCP. Two hundred five users provided an answer to how PERT was prescribed by their HCP.
FIGURE 3.
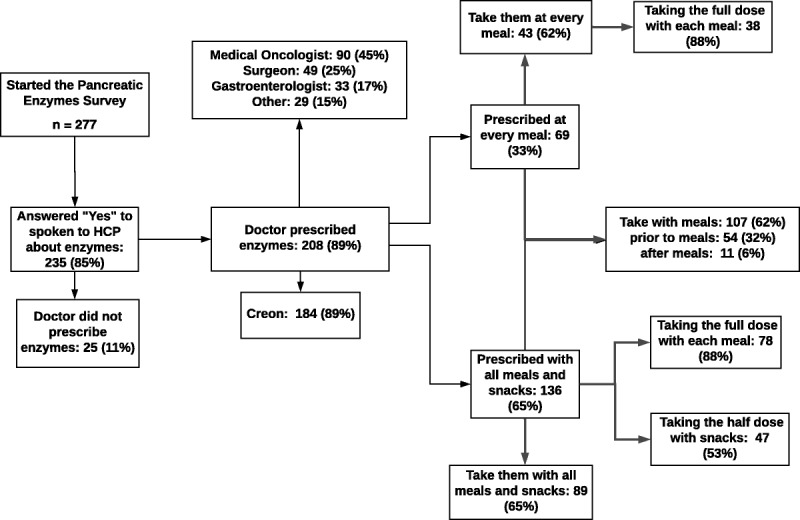
Results of questionnaire from PE survey (January 2016–January 2018).
One hundred thirty-six (65%) of 205 were prescribed PERT appropriately (with all meals and snacks), 89 (66%) of whom were compliant and took PERT with all meals and snacks. Of the 89 users who were compliant and took PERT with all meals and snacks, 88% took their full dose with each meal, and 53% reported taking the half dose with snacks. Sixty-nine users reported that their HCP prescribed PERT only at every meal, and of these, 43 (62%) reported taking them at every meal. Of the 43 who reported taking them at every meal, 38 reported taking the full dose with meals.
Additionally, 172 users provided information on how PERT was taken: 62% reported taking PERT with meals, 32% reported taking PERT prior to meals, 6% reported taking PERT after meals, and 9% reported taking PERT with meals and prior to/after meals.
Alleviation of Symptoms With PERT
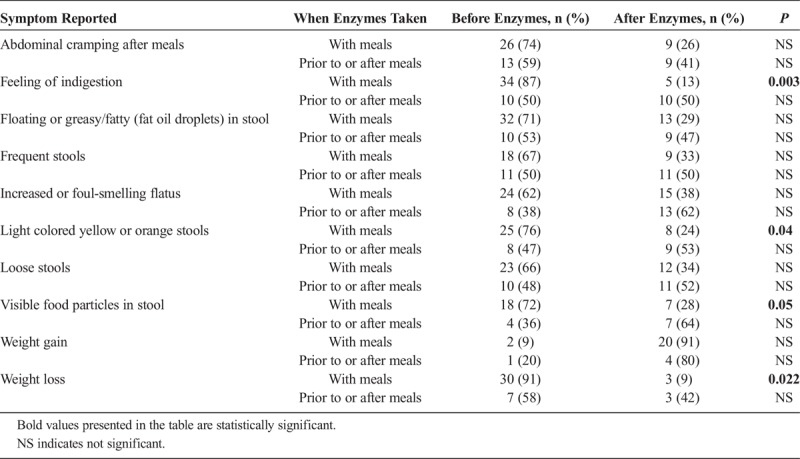
Users were asked about symptoms they experienced before and after taking PERT. Patients who took PERT with meals reported higher alleviation of symptoms (Table 2). Feeling of indigestion, light-colored yellow or orange stool, and visible food particles in stool were significantly decreased (P = 0.003, P = 0.04, and P = 0.05, respectively) when taking PERT with meals compared with those who took PERT prior to or after meals. Patients also reported less weight loss when taking PERT with meals compared with those who took PERT prior to or after meals (P = 0.022). Additionally, only 1 (14%) of 7 patients who took enzymes prior to or after meals reported weight loss before taking PERT and weight gain after taking PERT, whereas 13 patients (43%) who took PERT with meals reported losing weight before taking PERT and reported weight gain after taking PERT.
TABLE 2.
Reported Symptoms After and Before Taking Enzymes
DISCUSSION
In this PRO study, we asked PC patients about their awareness, knowledge, and use of PERT.
Two hundred eight of 235 patients who discussed PERT with their HCP were prescribed PERT. Of the 75% (208 of 277 total study patients) of PC patients who were prescribed PERT, 65% were prescribed PERT appropriately with all meals and snacks. Of the 65% who were prescribed PERT appropriately, 65% reported compliance taking PERT with all meals and snacks. Overall, only 43% of all patients prescribed PERT took PERT with meals and snacks. However, if we take into account the way PERT was taken during meals, the efficiency rate is even lower; only 62% of patients reported taking PERT with meals. These patients who took PERT with meals reported significant reductions in PEI-related symptoms and significantly less weight loss. Patients who reported PEI-related symptoms both before and after PERT had significantly less feelings of indigestion, less visible food particles in stool, and less weight loss. Our findings are in accord with prior studies of patients with chronic pancreatitis or pancreatic resections and resultant PEI showing overall symptomatic improvement, with specific improvements in stool consistency and frequency and weight gain.16–18,21 Further, our results are again strengthened by the results of a recent study of patients with chronic pancreatitis and PEI receiving PERT that showed improvements in stool frequency and consistency with PERT, which correlated with improvements in fecal coefficient of fat absorption and mean stool fat.22
Other studies investigating PERT use in advanced or unresectable PC patients showed improvement in fat absorption and prevention of weight loss in patients who took PERT compared with those who did not, again consistent with our findings.13 In a nonrandomized retrospective study, Dominguez-Muñoz et al15 showed an increase in median survival of unresectable patients receiving PERT compared with standard palliative care. A more recent randomized study showed an increase in overall survival in patients randomized to taking PERT but did not reach statistical significance due to a low enrollment rate, with only 18 patients randomized prior to closure of the study.23 A prospective cohort study of unresectable PC patients receiving chemotherapy showed improved changes in body mass index and increased overall survival of patients who received PERT retrospectively compared with prior patients receiving chemotherapy as historical controls.24 These clinical trials investigating PERT used various doses, ranging from 40,000 to 72,000 units of lipase for meals and 20,000 to 36,000 units of lipase per snack.
Perhaps some of the causes of this notable gap in care may be due to the lack of established, clear guidelines for PERT administration. Dosing guidelines vary without standardization, and PC is currently not considered an “on-label” indication in and of itself for PERT by the Food and Drug Administration. Pancreatic enzyme replacement therapy may be dosed based on symptoms, fat content of diet, body weight, extent of steatorrhea, or usually as per meal/snack guidelines. In general, PERT administration guidelines in relation to food intake in PC patients also do not exist. However, the optimal way of taking PERT is at the start of the meal, and if taking multiple pills, they should be taken at the start of the meal and at intervals throughout the meal.20 A national increase in patient and provider education on the appropriate use and administration of PERT is therefore warranted.
Best supportive care recommendations for PC-associated weight loss were established by a PanCAN-initiated supportive care working group.19 The working group identified symptoms, diagnosis, and causes of PC-associated weight loss. The group recommends categorizing PC weight loss into 3 causes: anorexia, malabsorption, and cachexia. For each category, specific assessment and intervention are recommended. Pancreatic enzyme replacement therapy was emphasized as an intervention for patients presenting with malabsorption, with the authors suggesting doses of 72,000 lipase units per meal and 36,000 lipase units per snack. Patients are to be continually monitored at these doses, and if their weight is stable or if they gain weight, it is recommended to continue with the current dose. However, if patients continue to lose weight and/or have persistent symptoms, modification of dose was suggested.
In addition to the pancreatic enzyme–specific (PE) survey, all users completed an initial basic survey about their symptoms, diagnosis, and treatment. After 16 months of collecting data from the PE survey, we found a slight sample bias to the PE survey as most patients who completed the PE survey had spoken to their HCP about enzymes (85%), and of these, 89% were prescribed PERT. To capture information about awareness of PERT in a more representative population of PC patients, we added PERT-specific questions to the Basics survey, a survey that all users were required to complete prior to completing any other survey. Using this new method, we found that only 46% of patients were taking PERT (compared with 75% of users who specifically answered the PE survey), and 27% were unaware of PERT. This new method also confirms that users who are taking PERT are more likely to complete the standalone survey on PERT. This dual way of asking users questions about PERT (in the Basics survey and PE survey) allows us to understand both the overall PC populations' use of PERT, and using information from the PE survey specifically, we are able to explore the details of PERT use of those who have been prescribed and taking or have taken PERT.
Overall, only 46% of users reported taking PERT (from the Basics survey); however, the actual number of users who are not taking PERT may be as high as 54%, based on the number of those who reported experiencing 1 or more PEI-related symptoms and not previously or currently taking PERT in the Basics survey. This again highlights the need to bring awareness to both HCPs who care for PC patients and PC patients themselves about PERT and its importance.
A multidisciplinary approach to treating PEI should be considered. Almost half (45%) of users reported that PERT was prescribed by their medical oncologist, which closely correlates with the number of patients who reported metastatic disease (39%) at registry enrollment. One should note, however, that users reported their experiences with PERT at any time during their disease and the stage of disease when taking PERT was not captured. Thus, it is unclear whether patients who had surgery and then had a recurrence took PERT postoperatively or at recurrence. Of the 151 users who reported having surgery, 78% reported the type of HCP that prescribed PERT, and 39% reported that their surgeon prescribed PERT, 38% reported the medical oncologist prescribed PERT, and 16% by their gastroenterologist. Currently, there is not a standardized process as to what type of HCP prescribes PERT and what step of PC diagnosis or therapy PERT is prescribed or even discussed with patients. In an ideal system, patients would receive information about PEI-related symptoms and PERT when they are first diagnosed and at periodic follow-up visits. However, in the United States, PC patients may be diagnosed by a gastroenterologist and then referred to a surgeon/medical oncologist, depending on the stage of disease. It would be most effective if patients are informed about PERT at both steps of diagnosis and during treatment to avoid patients “falling through the cracks.”
Our study does have certain limitations inherent to the nature of a survey-based PRO study. This includes lack of correlation with patient medical records and potential recall bias as this was a survey-based study. Other issues and biases to consider are that those who contact the call center are likely to be more motivated, and those who join our registry are likely more technologically savvy. Additionally, users are more likely to have undergone a pancreatic resection. Lastly, in certain subsections of the survey, there were more limited responses, which therefore may be underpowered to perhaps show additional differences in patients who were appropriately taking PERT in comparison to those who were not appropriately prescribed or taking PERT.
In conclusion, we report on PERT use among PC patients in a geographically wide population in the United States. We found a general lack of awareness about PERT, inappropriate administration and use by both HCPs and patients, inconsistent timing of PERT prescription, and prescription of PERT by many different specialties of HCPs during the course of disease. It is imperative to bring awareness to these notable gaps in care and to the importance of appropriate PERT use as it results in improvements in PEI-related symptoms and associated weight loss in PC patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, caregivers, and their families who participated in this study. The authors also thank PanCAN's Patient Central team for their assistance in recruitment.
Footnotes
Pancreatic Cancer Action Network has received support unrelated to this work from companies who have or may have an interest in pancreatic enzyme replacement therapy, including Abbott Laboratories, AbbVie, Allergan, Aptalis Pharma, and Janssen Pharmaceuticals.
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Barugola G, Falconi M, Bettini R, et al. The determinant factors of recurrence following resection for ductal pancreatic cancer. JOP. 2007;8(suppl 1):132–140. [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366; discussion 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMagno EP. Pancreatic cancer: clinical presentation, pitfalls and early clues. Ann Oncol. 1999;10(suppl 4):140–142. [PubMed] [Google Scholar]
- 6.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485–492. [PubMed] [Google Scholar]
- 7.Passik SD, Breitbart WS. Depression in patients with pancreatic carcinoma. Diagnostic and treatment issues. Cancer. 1996;78(suppl 3):615–626. [DOI] [PubMed] [Google Scholar]
- 8.Othman MO, Harb D, Barkin JA. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018;72:e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel MJ, Asbun H, Stauffer J, et al. Pancreatic exocrine insufficiency in pancreatic cancer: a review of the literature. Dig Liver Dis. 2015;47:1013–1020. [DOI] [PubMed] [Google Scholar]
- 10.Barkin JS, Kalser MH, Thomsen S, et al. Defect in assimilation following combined radiation and chemotherapy in patients with locally unresectable pancreatic carcinoma. Cancer. 1982;50:2189–2192. [DOI] [PubMed] [Google Scholar]
- 11.Halloran CM, Cox TF, Chauhan S, et al. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11:535–545. [DOI] [PubMed] [Google Scholar]
- 12.Partelli S, Frulloni L, Minniti C, et al. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44:945–951. [DOI] [PubMed] [Google Scholar]
- 13.Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut. 1998;42:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts KJ, Schrem H, Hodson J, et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB (Oxford). 2017;19:859–867. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Muñoz JE, Nieto L, Iglesias-Garcia J. Pancreatic enzyme replacement therapy and nutritional advice are associated with longer survival in patients with unresectable pancreatic cancer (PC). Pancreas. 2013;42:1347 abstract. [Google Scholar]
- 16.Gubergrits N, Malecka-Panas E, Lehman GA, et al. A 6-month, open-label clinical trial of pancrelipase delayed-release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther. 2011;33:1152–1161. [DOI] [PubMed] [Google Scholar]
- 17.Safdi M, Bekal PK, Martin S, et al. The effects of oral pancreatic enzymes (Creon 10 capsule) on steatorrhea: a multicenter, placebo-controlled, parallel group trial in subjects with chronic pancreatitis. Pancreas. 2006;33:156–162. [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb DC, Lehman GA, Vasileva G, et al. Pancrelipase delayed-release capsules (Creon) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: a double-blind randomized trial. Am J Gastroenterol. 2010;105:2276–2286. [DOI] [PubMed] [Google Scholar]
- 19.Hendifar AE, Petzel MQB, Zimmers TA, et al. Pancreas cancer-associated weight loss. Oncologist. 2019;24:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, et al. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: a randomized, three-way crossover study. Aliment Pharmacol Ther. 2005;21:993–1000. [DOI] [PubMed] [Google Scholar]
- 21.de la Iglesia-García D, Huang W, Szatmary P, et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut. 2017;66:1354–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkin JA, Hall JA, Liao Q, et al. Mo1245—effect of Creon therapy on exocrine pancreatic insufficiency symptoms and coefficient of fat absorption associated with chronic pancreatitis. Gastroenterology. 2018;154(suppl 1): S-719.abstract. [DOI] [PubMed] [Google Scholar]
- 23.Zdenkowski N, Radvan G, Pugliese L, et al. Treatment of pancreatic insufficiency using pancreatic extract in patients with advanced pancreatic cancer: a pilot study (PICNIC). Support Care Cancer. 2017;25:1963–1971. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Hirano K, Isayama H, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: a prospective cohort study. Pancreas. 2017;46:341–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


