Background:
The effectiveness of oral emtricitabine (FTC)/tenofovir (TFV) disoproxil fumarate–based HIV pre-exposure prophylaxis (PrEP) depends on adherence. Pharmacologic measures help interpret patterns and predictors of PrEP adherence.
Setting:
We analyzed data from the subsample of men who have sex with men enrolled in HPTN 067/ADAPT in Bangkok, Thailand, and Harlem, NY, U.S.
Methods:
After a 5-week directly observed therapy period, participants were randomized to daily, time-driven, or event-driven PrEP. Follow-up occurred at weeks 4, 12, and 24 after randomization. Plasma and hair FTC/TFV levels indicated short- and long-term PrEP use, respectively. Electronic pill bottle data (Wisepill) were collected weekly. Pearson correlation coefficients between PrEP use measures were calculated; linear mixed models assessed predictors of plasma and hair drug concentrations.
Results:
Among 350 participants (median age: 31 years, interquartile range: 25–38), 49.7% were from Harlem, half had less than college education, and 21% reported heavy alcohol use. In multivariable models, being enrolled in Harlem, being in non–daily arms, and having less than college education were associated with lower hair FTC/TFV concentrations; heavy alcohol use was associated with higher concentrations. Similar results were found for plasma concentrations by site and arm, but older age and greater number of sex partners were associated with higher concentrations. Hair and plasma FTC/TFV concentrations were moderately correlated with Wisepill data (r ≥ 0.29) across visits.
Conclusions:
In HPTN067, plasma, hair, and Wisepill data correlated with one another and served as complementary adherence measures. Site, arm, education, age, alcohol, and sexual behavior influenced patterns of adherence.
Key Words: pre-exposure prophylaxis, HIV prevention, men who have sex with men, biomarkers, hair levels, plasma levels
INTRODUCTION
Daily oral emtricitabine (FTC)/tenofovir (TFV) disoproxil fumarate (TDF)–based pre-exposure prophylaxis (PrEP) has demonstrated efficacy in preventing HIV transmission and is being scaled up among high-risk populations, including men who have sex with men (MSM). Randomized trials of PrEP, delivered either daily or on-demand, reported reductions in HIV incidence (between 44% and 86% in intervention groups), which correlated strongly with objective adherence measures.1–7 Moreover, in trials where pharmacologic measures identified low overall PrEP adherence (eg, FEM-PrEP, VOICE), the efficacy of the intervention was nil.8,9 PrEP demonstration projects have shown the effectiveness of PrEP for HIV prevention in more “real-world” settings and, whereas some participants seem to be more adherent to PrEP when they know the product is effective, not all choose to initiate or adhere during risk periods.10–13 PrEP delivery programs must now focus on understanding PrEP pill-taking patterns and improving PrEP adherence to maximize its effectiveness among high-risk populations.7
PrEP adherence monitoring is crucial for accurately interpreting PrEP effectiveness and identifying individuals who may benefit from additional adherence support. However, PrEP delivery projects vary in the adherence metrics used, despite limitations of each. For example, self-reported adherence is subject to recall or social desirability bias and may considerably overestimate PrEP use.14–16 Electronic monitoring devices, such as Wisepill (Wisepill Technologies, Cape Town, South Africa), and medication event monitoring systems overcome some of these biases but do not measure actual drug ingestion or drug levels in target tissues.14,17 PrEP drug levels in various biomatrices represent different periods of drug exposure18,19 and have shown utility in PrEP studies. Plasma drug concentrations represent a small window of exposure (up to 1 week before sample collection, depending on the assay) and could be susceptible to intraindividual variability and “white-coat effects,” whereby participants take doses just before a clinic visit without consistent use, although this issue was not observed in previous PrEP clinical trials.20–22 PrEP drug levels in hair samples (which are noninvasive to collect and inexpensive to store) provide data on long-term drug exposure (weeks to months) and have been used in several PrEP studies.23–25
Combining and comparing metrics of PrEP adherence (electronic and pharmacologic) will further assess their utility, characterize predictors and patterns of short- and long-term PrEP dosing, and inform future PrEP delivery and adherence interventions. An analysis found that the combination of plasma and hair data had a higher ability to discriminate between low, moderate, and high levels of adherence than the use of a single PrEP exposure measure.26 In our study, we compared Wisepill data on PrEP dosing with plasma and hair FTC and TFV concentrations in a sample of 350 HIV-uninfected MSM participating in a PrEP delivery trial in Thailand and the United States (U.S.). This analysis provides a unique contribution to the literature by (1) longitudinally examining correlations between these PrEP adherence measures across a large number of study visits, over 3 PrEP dosing strategies, and among 2 high-risk populations and (2) exploring predictors of both recent and long-term PrEP dosing assessed via plasma and hair drug concentrations, respectively.
METHODS
Study Design and Participants
The HIV Prevention Trials Network (HPTN) 067/Alternative Dosing to Augment PrEP Pill Taking (ADAPT) study was a phase 2, randomized, open-label clinical trial of daily versus intermittent oral FTC/TDF-based PrEP conducted from 2011 to 2014. The study enrolled MSM in Bangkok, Thailand, and Harlem, NY, U.S., with the main study findings described previously.27 Although this study also enrolled transgender women in Bangkok and Harlem and cisgender women in Cape Town, South Africa, as has been reported elsewhere,27,28 this particular analysis is restricted to MSM participants to minimize variability in risk factors.29,30 Participants were eligible for enrollment if they were HIV-antibody–negative, 18 years and older, literate in English or Thai and able to consent, reported sex with a man in the past 6 months, and had at least 1 risk factor for HIV in the 6 months before enrollment, including sex with more than 1 male partner, history of sexually transmitted infection, transactional sex, or sex without a condom with an HIV-infected partner or a partner of unknown status.
Participants completed a 5-week directly observed therapy period and were then randomly assigned to 1 of 3 open-label FTC/TDF PrEP dosing regimens: daily (1 tablet every day); time-driven (1 tablet twice a week, plus a post-sex dose); and event-driven (1 tablet both before and after sex). Regimens were assigned in a 1:1:1 ratio for each site, and participants, staff, and investigators were all aware of treatment assignments. After randomization, participants received a 1-month supply of PrEP with the Wisepill device and counseling about their dosing regimen.27,28 Follow-up visits occurred every 4 weeks until week 24 after randomization, with 30-day PrEP refills provided at each visit.
Data Collection
During enrollment and follow-up visits, participants underwent HIV testing and received HIV counseling, adherence support (Next Step Counseling), and PrEP refills. Blood and hair samples were collected at 4-, 12-, and 24-week visits for retrospective analysis of drug concentrations, which were not reported to participants or used in adherence counseling.
Participants completed computer-assisted self-interviewing surveys at all visits to collect data on demographics, HIV risk perception, knowledge, attitudes, and beliefs about PrEP, drug and alcohol use, and depression, along with facilitators and barriers to adherence. These questions were developed based on knowledge of the study sites and previous PrEP trials. Alcohol use was measured using the validated Alcohol Use Disorders Identification Test (AUDIT) scale (range 0–40), in which a sum score ≥8 indicates heavy alcohol use.31
Measures of PrEP Use
Daily PrEP use was measured using data from Wisepill electronic monitoring devices. Participants were asked to complete weekly in-person or phone interviews with trained staff who reviewed each recorded device opening and asked the participant to confirm whether the Wisepill device opening was reflective of an ingested dose (rather than a curiosity opening, device refill, or pocket dose). Weekly interview data were used to adjust Wisepill data by correcting dates and times for doses removed and taken later, doses removed but not taken, and doses taken from a container other than the Wisepill (“Wisepill-prompted weekly interviews”). Weekly interview data were used as a measure of PrEP use if no Wisepill data were available, and participants were also asked about dates, times, and types of sex acts during these interviews.
Blood was collected and processed for plasma, and FTC/TFV levels were quantified via liquid chromatographic–tandem mass spectrometric analysis within the Clinical Pharmacology Analytical Laboratory (Johns Hopkins University, Baltimore, MD).32 The lower limits of quantification for both FTC and TFV in plasma are 0.31 ng/mL. For hair collection, approximately 50–100 stands of hair were cut as close as possible to the scalp in the occipital region, placed in aluminum foil, and stored at room temperature until shipped to the University of California, San Francisco Hair Analytical Laboratory. The proximal section of the hair was chopped into 1- to 2-mm segments (corresponding to dosing in the previous month) and FTC/TFV concentrations quantitated via liquid chromatographic–tandem mass spectrometric.33 Hair levels were normalized by weight, and lower limits of quantification for TFV and FTC concentrations were 0.002 and 0.02 ng/mg, respectively.25 Plasma and hair assays were validated in accordance with the U.S. Food and Drug Administration bioanalytical guidelines and have been approved by the Division of AIDS (DAIDS) Clinical Pharmacology and Quality Assurance program in the DAIDS at the National Institutes of Health.34
Statistical Analyses
Descriptive statistics summarized sample characteristics. FTC and TFV drug concentrations were log-transformed in all analyses by taking the natural logarithm of the concentration. Pharmacologic measures below the lower limits of quantification were set equal to that limit before log transformation.
Pearson correlation coefficients assessed correlations between Wisepill and pharmacologic measures at the 3 follow-up visits when plasma and hair samples were collected. In the primary correlation analysis, we not only included Wisepill and continuous drug concentration values from participants in all arms together but also conducted sensitivity analyses restricting the sample to each arm separately. We compared the total number of device openings from the Wisepill data in the previous week to plasma drug concentrations. We compared Wisepill data in the previous month to hair drug concentrations. Correlation analyses were restricted to the sample of participants with Wisepill, plasma, and hair data available at a given time point, and those missing at least one measure were excluded.
Four linear mixed models with random intercepts assessed predictors of log-transformed plasma and hair FTC/TFV drug concentrations while accounting for repeated measures. In our primarily models, plasma and hair drug concentrations were parameterized as continuous outcomes, rather than using a cutoff to represent adherence thresholds defined in daily dosing studies, and served as proxy measures for recent PrEP dosing and longer-term PrEP use, respectively. Study visit, arm (daily, time-driven, and event-driven), site (Harlem, Bangkok), sexual activity (number of sex acts in the previous 3 months), and interaction terms for arm and sexual activity were included in all models based on a priori knowledge that these factors were related to PrEP dosing. We assessed several additional covariates for inclusion in the models, including age, education, marital status, drug use, alcohol use, height, weight, serum creatinine, depression, sex without a condom, and perceived HIV vulnerability and used a forward stepwise selection method to choose the maximum covariate set for all 4 models. Covariates were added to the multivariable model if they demonstrated P values <0.10. We back-transformed regression model covariates to determine fold-effects, defined as the fold change in drug concentration associated with a 1-unit increase in the predictor. We also conducted a secondary analysis examining covariates associated with hair TFV concentrations indicative of about 4 PrEP doses per week (TFV ≥0.023 ng/mL as established in the STRAND study).25 This analysis was restricted to participants in the daily arm only.
All analyses were conducted using SAS 9.4 (Cary, NC).
Ethical Statement
Institutional review board approvals were obtained locally in Harlem and Bangkok and via the U.S. Centers for Disease Control and Prevention. The study protocol was reviewed by the National Institutes of Health DAIDS before implementation. Participants provided written informed consent in their preferred language. The protocol was registered at ClinicalTrials.gov (identifier NCT01327651).
RESULTS
Participant Characteristics
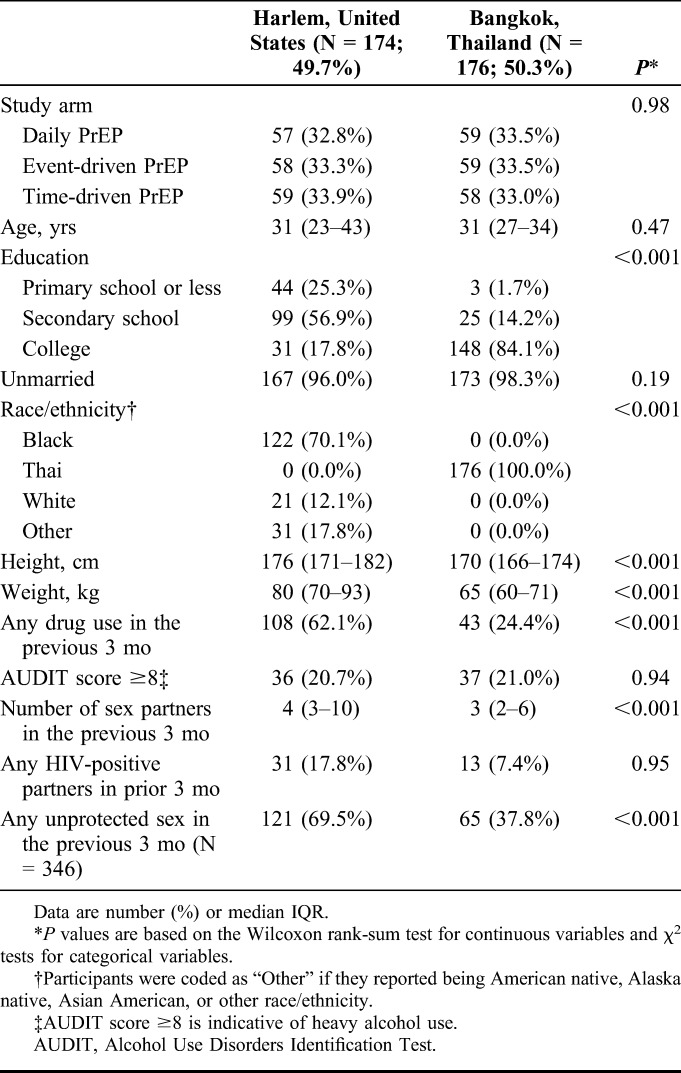
The HPTN 067 study randomized 350 MSM participants, including 174 (49.7%) in Harlem and 176 (50.3%) in Bangkok (Table 1). A total of 116 (33.1%) participants were randomized to the daily PrEP arm, and 117 (33.4%) were assigned to each of the intermittent dosing arms. The median age at enrollment was 31 years [interquartile range (IQR) 25–38 years]. Approximately, 143 (82.2%) participants at the Harlem site and 28 (15.9%) participants at the Bangkok site received less than a college education (P value <0.001). Over half of the participants in Harlem reported substance use in the 3 months before enrollment (N = 108; 62.1%), compared with only 24.4% of Bangkok participants (N = 43; P value <0.001). More participants in Harlem reported having any unprotected sex in the previous 3 months than in Bangkok (69.5% versus 37.8%, P value <0.001).
TABLE 1.
Enrollment Characteristics Among MSM Randomized in the HIV Prevention Trials Network (HPTN) 067 Study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014
Wisepill data indicate that participants took PrEP differently according to the study arm, as expected (P value <0.001). For example, participants in the daily dosing arm took a median of 6.0 doses/wk (IQR 3.7–6.7), compared with 1.4 doses/wk in the event-driven arm (IQR 0.7–2.4) and 2.2 doses/wk in the time-driven arm (IQR 1.8–2.8).
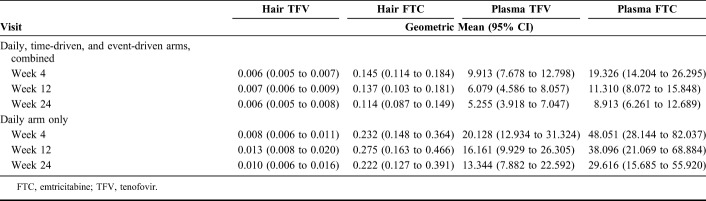
We had Wisepill data indicating the occurrence of at least 1 bottle opening for 344 (98.3%) participants over follow-up, including 344 from weeks 1–4, 335 from weeks 5–12, and 312 participants from weeks 13–24 visits. Plasma samples were collected and analyzed at 969 of 999 attended study visits (97.0%), including 100% of attended visits in Bangkok and 93.6% of attended visits in Harlem. Hair samples were collected at 827 study visits (519 visits in Bangkok and 308 visits in Harlem), with a 78.8% completion rate of sample collection reflecting acceptability and availability of hair for sampling. However, only a subset of these data were processed for analysis (N = 436 samples) because of prespecified cost restrictions for hair assays. The primary reason for missing hair samples was insufficient quantity of hair for sample collection, which was more prevalent in Harlem than in Bangkok. Retention rates were high in the cohort, with 88.0% of participants attending the 24-week visit, although retention differed by site (96.6% in Bangkok vs. 78.8% in Harlem). Geometric mean FTC and TFV concentrations were greater among participants in the daily arm than in the full sample and decreased over the duration of follow-up in both the full sample and among participants in the daily arm, indicating waning PrEP use over time (Table 2).
TABLE 2.
Geometric Mean Plasma and Hair Drug Concentrations Among MSM During Follow-up in the HIV Prevention Trials Network (HPTN) 067 Study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014
Correlations Between Measures of PrEP Use
Correlation analysis between Wisepill data, plasma FTC/TFV concentrations, and hair FTC/TFV concentrations was restricted to participants with all PrEP use measures at a given visit (151 participants at week 4; 125 at week 12; and 128 at week 24). Wisepill data were moderately correlated with hair and plasma concentrations of both drugs across all arms (correlation coefficients ranged from 0.29–0.61, all P values <0.05; Fig. 1). In general, the correlations between measures were weaker at the week-4 visit (range 0.29–0.47) than at the 24-week visit (range 0.37–0.61). Correlations between plasma and hair TFV levels were weaker than other reported correlations and did not increase substantially over time in comparison with correlations between Wisepill data and pharmacologic measures. Similar correlations were observed when restricting the analysis to participants in the daily arm only (range 0.24–0.70), the time-driven arm (range 0.24–0.58), and the event-driven arm (range 0.27–0.59).
FIGURE 1.
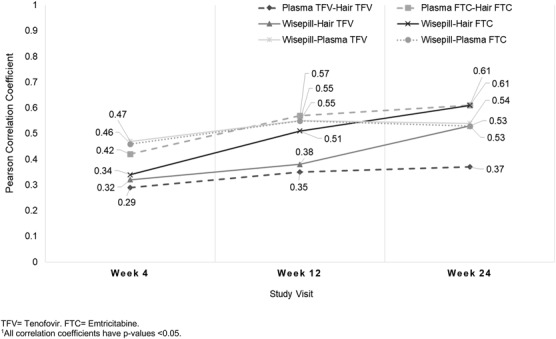
Pearson correlation coefficients between log-transformed hair and plasma drug concentrations and Wisepill data by follow-up visit, among MSM in the HIV Prevention Trials Network (HPTN) 067 study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014. FTC, emtricitabine; TFV, tenofovir. All correlation coefficients have P values <0.05.
Factors Associated With Pharmacologic Measures of PrEP Use
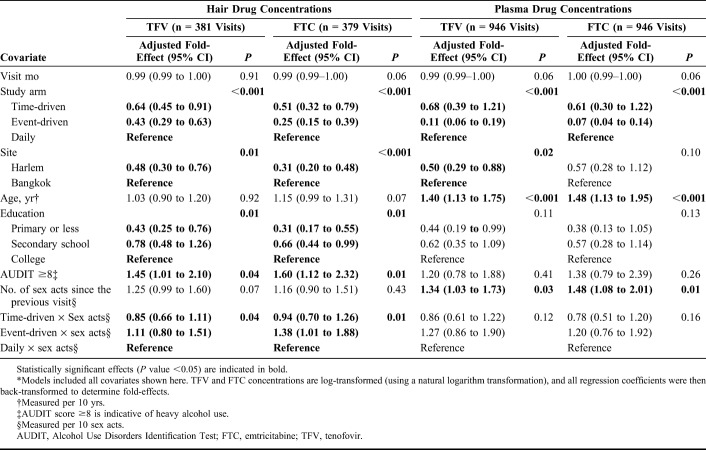
The analysis of factors associated with hair and plasma drug concentrations included data for 381 hair TFV, 379 hair FTC, 946 plasma TFV, and 946 plasma FTC samples, after excluding those with missing covariate data.
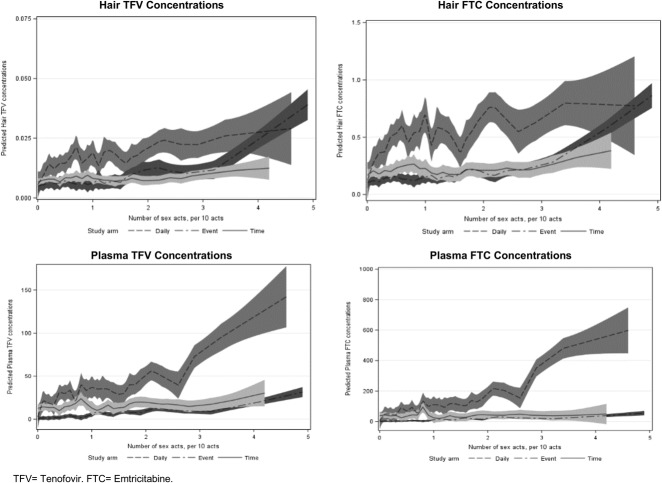
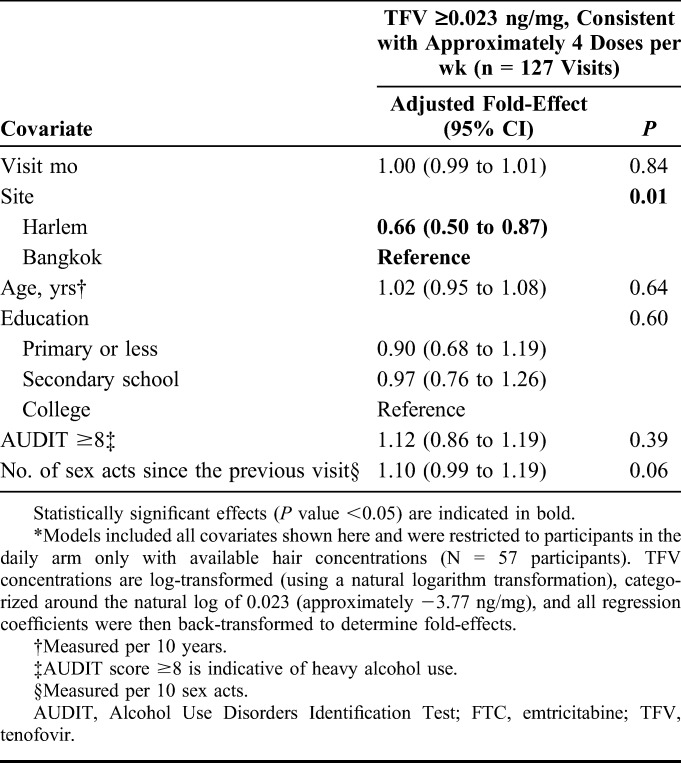
In multivariable models of hair concentrations, participants in the intermittent arms who reported zero sex acts had lower concentrations of log-transformed hair TFV and FTC during follow-up than those in the daily arm who reported zero sex acts (P value <0.001; Table 3) when controlling for other variables in the model. The number of sex acts did not influence hair drug concentrations among participants in the daily arm (the main effect for sexual activity was not statistically significant). However, we observed a statistically significant interaction effect between the study arm and number of sex acts. Specifically, as the number of reported sex acts increased, participants in the event-driven arm were estimated to have higher hair TFV and FTC concentrations than those in the daily or time-driven arms, indicating that those with high numbers of sex acts in the event-driven arm were taking PrEP more often than those reporting the same number of sex acts but in the daily dosing arm (top 2 panels, Fig. 2). Participants from Harlem had lower hair TFV and FTC concentrations than those from Bangkok (P values <0.001). Lower education status was negatively associated with hair FTC and TFV concentrations, and heavy alcohol use (AUDIT score ≥8) was associated with higher FTC/TFV concentrations. We found similar directions and magnitudes of association in our model assessing correlations between covariates and hair TFV levels ≥0.023 ng/mL (∼4 doses per week) among participants in the daily arm only (Table 4), but this analysis had limited power to detect statistically significant associations given the smaller number of included visits (N = 127; 29.1% with TFV levels ≥0.023 ng/mL and 70.9% with TFV levels <0.023 ng/mL).
TABLE 3.
Estimated Multivariable Associations of Demographic and Behavioral Covariates With Hair (N = 381) and Plasma (N = 946) Drug Concentrations Among MSM During Follow-up in the HIV Prevention Trials Network (HPTN) 067 Study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014*
FIGURE 2.
Interaction between the study arm and number of sex acts on predicted values for hair and plasma drug concentrations in multivariable models among MSM in the HIV Prevention Trials Network (HPTN) 067 study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014. FTC, emtricitabine; TFV, tenofovir. Figures show the predicted drug concentrations (y-axis) by number of sex acts (per 10 acts, x-axis) and study arm. 95% CIs around predicted values are shown with shading around each solid and dashed line.
TABLE 4.
Estimated Multivariable Associations of Covariates With Hair Drug Concentrations Consistent With Approximately 4 Doses/wk Among Men Who Have Sex With Men Assigned to the Daily Dosing Arm in the HIV Prevention Trials Network (HPTN) 067 Study in Bangkok, Thailand, and Harlem, New York, United States, 2011–2014*
In models of plasma concentrations, we similarly found that assignment to the intermittent arms was associated with lower FTC and TFV concentrations than assignment to the daily arm among participants who reported zero sex acts, and participants from Harlem had statistically significantly lower TFV concentrations than those from Bangkok, although the association between the site and FTC concentrations (Table 3) was not statistically significant. Age was statistically significantly associated with plasma concentrations. Specifically, every 10-year increase in age was associated with a 1.40-fold higher plasma TFV concentration [95% confidence interval (CI): 1.13 to 1.75; P value <0.001] and a 1.48-fold higher plasma FTC concentration (95% CI: 1.13 to 1.95; P value <0.001). The main effect for number of sex acts was associated with plasma concentrations in the model. Among participants in the daily arm, a 10-act increase in the number of sex acts was associated with a 1.34-fold higher plasma TFV concentration (95% CI: 1.03 to 1.73; P value 0.03) and a 1.48-fold higher plasma FTC concentration (95% CI: 1.08 to 2.01; P value 0.01). Unlike in the models of hair TFV and FTC concentrations, we did not observe statistically significant differences in the association between number of sex acts and plasma drug concentrations across the study arms; plasma concentrations were highest for participants in the daily arm regardless of the number of reported sex acts (bottom 2 panels, Fig. 2). We also did not find statistically significant effects of education or alcohol use on plasma concentrations.
DISCUSSION
In this cohort of MSM participating in a trial to assess behavioral feasibility of non–daily PrEP dosing regimens, Wisepill and pharmacologic measures of PrEP use were moderately correlated and served as complementary indicators of PrEP exposure. Arm and site were significantly associated with both pharmacologic measures, whereas education and alcohol use were statistically significantly related to hair concentrations. Age and the number of sex acts were associated with plasma concentrations. These findings demonstrate that demographic and behavioral factors may impact participants' patterns of PrEP use in the immediate period before a study visit and during longer-term follow-up, which has important implications for future PrEP delivery programs focused on providing tailored adherence counseling within contexts of changing risk behaviors and PrEP decision-making.35 For example, high-risk younger individuals may require enhanced adherence counseling and support particularly during the time period between monthly or quarterly clinic visits.
Prior studies examining correlations between PrEP measures also found moderate associations between hair, plasma, and electronic data but used medication event monitoring systems caps to measure bottle openings rather than Wisepill.21,26,36,37 The correlations between Wisepill data and the pharmacologic measures in this cohort indicate that this electronic measure may accurately reflect steady-state PrEP dosing when pharmacologic data are not available. Of note, we relied on Wisepill data that incorporated participants' self-reported PrEP dosing, and the magnitude of the observed correlations between measures in our study may have been diminished by self-report bias, user errors, or Wisepill device errors.37 Correlations between Wisepill and plasma and hair concentrations increased over time in the study, indicating that either participants became more comfortable using the Wisepill device and/or self-reporting their PrEP use during weekly interviews or participants achieved steady-state drug concentrations after a longer follow-up period. By contrast, correlations between plasma and hair TFV concentrations did not increase over time. These findings may be partly explained by differences in participants' dosing in the period just before the study visit compared with the full 8- to12-week time period between study visits. In addition, TFV has a longer half-life than FTC in the plasma, which could exacerbate differences in plasma and hair drug concentrations as a result of different dosing patterns.18
Factors associated with PrEP use just before a study visit were older age and sexual behavior, whereas higher education and alcohol use were associated with long-term PrEP use during the follow-up period. A qualitative substudy with MSM in HPTN 067 found that participants described taking PrEP only during times when they were planning to have unprotected sex, when they had a high number of sexual partners, or when HIV concerns were on their minds.27,38 These data lend important context to the association we found between sexual behavior and recent PrEP use. Other studies have also found associations between PrEP adherence and the number of sex acts and sexual partners among men, indicating that individuals may be tailoring their PrEP use to perceived risk or to saliency of their HIV risk because of an upcoming study visit appointment.39–41 Recent data have shown that intermittent or on-demand PrEP may be efficacious for HIV prevention among MSM,1,42 and strategies to align PrEP use to periods of sexual risk may be appropriate in this population.41,43 Older age has been associated with increased PrEP adherence measured by plasma concentrations, self-reported adherence, electronic data, and pharmacy records among men across multiple settings.39–41,44,45 This finding could in part be explained by more white-coat adherence among older participants (because age was not associated with long-term adherence) and also by greater goal-directed behavior among older versus younger individuals.46–48
In our sample, higher education was related to longer-term PrEP use, which has been previously observed,49 and is plausible given that education may be an indicator of more stable individual contexts that promote adherence over a period of weeks to months rather than a contributor to PrEP dosing in the period just before a study visit.50–52 We did not observe a statistically significant main effect between long-term PrEP use and sexual behavior, implying that sexual behavior was not linked to long-term PrEP adherence among those in the daily dosing arm potentially because HIV risk perceptions, motivations to continue participating in the study, or other factors were more important in driving regular PrEP use in the month before a visit. However, we did see a significant interaction effect which reveals that those with high numbers of sex acts in the event-driven arm took PrEP more consistently throughout follow-up than those reporting the same number of sex acts but in the daily dosing arm. We also found that heavier alcohol use was associated with higher levels of long-term adherence, although we cannot rule out that alcohol use was associated with higher FTC/TFV concentrations due to pharmacokinetic effects. In the U.S. PrEP Demo Project, amphetamine use was associated with higher hair PrEP drug concentrations, which was believed to represent higher levels of adherence among MSM with higher-risk behavior.13,27 Heavy alcohol use is linked with unprotected sex among MSM, and HPTN 067 participants who drank alcohol regularly may have adjusted their PrEP use based on perceived HIV risk.53,54 Consistent with this hypothesis, in secondary data analyses, we found that having an AUDIT score ≥8 was associated with greater frequency of unprotected sex in this study sample although more work is needed to understand links between alcohol use and frequency of sexual activity, patterns of condom use, transactional sex, and HIV risk perceptions. An AUDIT score ≥8 is meant to capture individuals with regular heavy alcohol use rather than those who sporadically drink, and it is possible that heavy alcohol use and corresponding high-risk sexual behavior may have influenced HIV risk perceptions and longer-term PrEP use (as captured by hair drug concentrations) but did not have as strong of an impact on recent PrEP dosing and plasma drug concentrations. Similarly, in another phase II study of PrEP, substance use (khat and marijuana) was significantly associated with hair concentrations.38 Additional research is necessary to examine predictors of long-term PrEP dosing among high-risk populations, disentangle them from sexual behaviors and age that have been consistently shown to predict PrEP dosing shortly before study visits, and guide adherence approaches for consistent PrEP use.
The strengths of this study included the large sample with participants from 2 distinct geographic regions and the use of multiple measures of PrEP exposure. Overall retention rates were high, which minimized concerns about selection bias and differential attrition, but did differ across the 2 study sites. Limitations of this study included the lack of a gold-standard PrEP adherence measure, which prevented us from quantifying the magnitude of bias due to “white-coat effects,” “curiosity openings,” and “pocket dosing.” Although we were able to adjust our Wisepill data based on weekly interview data, the validity of these self-report data was potentially limited due to social desirability and recall bias. Although objective benchmarks of adherence have been established for pharmacologic measures in directly observed therapy studies,6,25 it was difficult to apply them to this work given important differences in expected PrEP dosing and definitions of adherence by the 3 randomized study arms. The HPTN 067 primary manuscript provides additional data on PrEP adherence and coverage of sex events for each dosing arm, and further analyses of patterns of adherence around recent sex events and using pharmacologic adherence measures are forthcoming.27 We were only able to analyze hair samples during 43.6% of 999 attended visits due to budget restrictions. Moreover, our results in this MSM cohort may not be generalizable to women or to all men or MSM, and the study only enrolled 5 transgender women, which prevented us from being able to examine correlates of PrEP adherence in this group. Finally, due to a low number of HIV seroconversions (only 1 acute HIV infection after randomization in a participant from Harlem), we could not examine associations between drug concentrations and HIV acquisition in the sample.
In conclusion, Wisepill, plasma, and hair data were moderately correlated with one another in this phase II PrEP trial, suggesting that these adherence measures can be combined to better understand PrEP exposure during roll-out. In particular, plasma measures may be useful for examining short-term adherence, whereas hair measures can assess longer-term PrEP adherence after a period of steady-state dosing is achieved. Age and sexual behavior may be related to recent PrEP dosing among MSM. Higher education and longer-term risk behavior (eg, alcohol use) may meaningfully influence steady patterns of PrEP use. Future research on electronic and pharmacologic measures of PrEP adherence should consider factors such as cost, feasibility, and acceptability of each metric in the study design. In addition to the measures presented here, additional pharmacologic measures could also be used to provide similar assessment of short-term (eg, urine) and long-term (eg, peripheral blood mononuclear cells, dried blood spots) PrEP use. Each objective measure comes with its own benefits and trade-offs for data collection, storage, cost, and interpretation. Our findings highlight a need for additional studies to continue exploring factors associated with recent and steady-state PrEP use, characterize patterns of adherence, and explore whether PrEP use aligns with sexual behaviors and risk perceptions among high-risk populations. These results support combining pharmacologic tools in future HIV prevention research both for outcome interpretation and to identify individuals in need of enhanced adherence support to improve PrEP effectiveness in key populations.
ACKNOWLEDGMENTS
The authors thank the individuals who participated in the study, and the teams at the Bangkok and Harlem study sites, and the HIV Prevention Trials Network that supported data collection and management for this work.
Footnotes
Supported by award numbers UM1-AI068619, UM1-AI068617, UM1-AI068613, and R01 AI118575, from the NIH [National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), and National Institute on Drug Abuse (NIDA)] to HPTN. This work was also supported in part by the Emory-CDC HIV/AIDS Clinical Trials Unit award number UM1AI069418 from the NIH (NIAID). J.V. was supported by the NIMH of the NIH (grant F31 MH113420). M.G. and P.B. were supported by 2R01 AI098472 from NIAID. Gilead Sciences donated study medication to the NIH to support this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, the National Institutes of Health, or the U.S. Centers for Disease Control and Prevention. The use of a trademark name herein does not indicate government endorsement.
Presented in part as a poster at the Conference on Retroviruses and Opportunistic Infections 2018 (CROI); March 4–7, 2018; Boston, MA.
R.M.G. has been a site investigator for clinical trials funded by GlaxoSmithKline (GSK; managed by ViiV Healthcare) and Gilead Sciences to the Gladstone Institutes and the San Francisco AIDS Foundation. C.W.H. conducts research sponsored by ViiV Healthcare, GSK, and the Bill & Melinda Gates Foundation and is a consultant at TRI and the University of California, Los Angeles. K.R.A. received an unrestricted educational grant to the University of Michigan from Gilead Sciences (2015–2017). A.L. has led studies in which Gilead has donated drug. S.H.E. has done collaborative research studies with Abbott Diagnostics. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. [DOI] [PubMed] [Google Scholar]
- 5.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celum C, Baeten JM. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr Opin Infect Dis. 2012;25:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM, Haberer JE, Liu AY, et al. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr. 2013;63(suppl 2):S122–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Heffron R, Kidoguchi L, et al. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. Plos Med. 2016;13:e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates Open Res. 2017;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S79–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. [DOI] [PubMed] [Google Scholar]
- 16.Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62:e01710–e01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podsadecki TJ, Vrijens BC, Tousset EP, et al. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9:238–246. [DOI] [PubMed] [Google Scholar]
- 21.Hugen PWH, Langebeek N, Burger DM, et al. Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J Acquir Immune Defic Syndr. 2002;30:324–334. [DOI] [PubMed] [Google Scholar]
- 22.Nettles RE, Kieffer TL, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–1196. [DOI] [PubMed] [Google Scholar]
- 23.Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55:353–357. [PubMed] [Google Scholar]
- 24.Gandhi M, Murnane PM, Bacchetti P, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS. 2017;31:2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One. 2014;9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abaasa A, Hendrix C, Gandhi M, et al. Utility of different adherence measures for PrEP: patterns and incremental value. AIDS Behav. 2018;22:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the human immunodeficiency virus prevention trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekker L-G, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV. 2017;5:e68–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis Against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson PL, Kiser JJ, Gardner EM, et al. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babor TF, Higgins-Biddle JC, Saunders JB, et al. AUDIT: The Alcohol Use Disorders Identification Test, Guidelines for Use in Primary Care: World Health Organization; 2001. Available at: http://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf;jsessionid=97053AA2F4DA925B9A6D5367EF824127?sequence=1. Accessed December 1, 2018. [Google Scholar]
- 32.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Gandhi M, Greenblatt RM, et al. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun MA Spectrom. 2008;22:3401–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiFrancesco R, Rosenkranz SL, Taylor CR, et al. Clinical pharmacology quality assurance program: models for longitudinal analysis of antiretroviral proficiency testing for international laboratories. Ther Drug Monit. 2013;35:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxi SM, Liu A, Bacchetti P, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr. 2015;68:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musinguzi N, Muganzi CD, Boum Y, et al. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. AIDS. 2016;30:1121–1129. [DOI] [PubMed] [Google Scholar]
- 38.Franks J, Hirsch-Moverman Y, Loquere AS, et al. Sex, PrEP, and stigma: experiences with HIV pre-exposure prophylaxis Among New York city MSM participating in the HPTN 067/ADAPT study. AIDS Behav. 2018;22:1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan E, Moran K, Ryan DT, et al. Threefold increase in PrEP uptake over time with high adherence among young men who have sex with men in Chicago. AIDS Behav. 2018;22:3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu A, Glidden DV, Anderson PL, et al. Patterns and correlates of PrEP drug detection among MSM and transgender women in the Global iPrEx Study. J Acquir Immune Defic Syndr. 2014;67:528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haberer JE, Kidoguchi L, Heffron R, et al. Alignment of adherence and risk for HIV acquisition in a demonstration project of pre-exposure prophylaxis among HIV serodiscordant couples in Kenya and Uganda: a prospective analysis of prevention-effective adherence. J Int AIDS Soc. 2017;20:21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noret M, Balavoine S, Noret M, et al. Daily or on demand oral TDF/FTC for HIV pre-exposure prophylaxis: experience from an hospital-based clinic in France. AIDS. 2018;32:2161‐2169. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PL, García-Lerma JG, Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Curr Opin HIV AIDS. 2016;11:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Epps P, Maier M, Lund B, et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (PrEP) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77:272–278. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linnemayr S. HIV prevention through the lens of behavioral economics. J Acquir Immune Defic Syndr. 2015;68:e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant RM, Koester KA. What people want from sex and preexposure prophylaxis. Curr Opin HIV AIDS. 2016;11:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoagland B, Moreira RI, De Boni RB, et al. High pre-exposure prophylaxis uptake and early adherence among men who have sex with men and transgender women at risk for HIV Infection: the PrEP Brasil demonstration project. J Int AIDS Soc. 2017;20:21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson K, Biello K, Rosenberger JG, et al. The impact of social support and partner relationship dynamics on engagement in HIV care and antiretroviral treatment adherence among MSM in Latin America. AIDS Care. 2018;30:1406–1412. [DOI] [PubMed] [Google Scholar]
- 51.Bolsewicz K, Debattista J, Vallely A, et al. Factors associated with antiretroviral treatment uptake and adherence: a review. Perspectives from Australia, Canada, and the United Kingdom. AIDS Care. 2015;27:1429–1438. [DOI] [PubMed] [Google Scholar]
- 52.Musheke M, Bond V, Merten S. Individual and contextual factors influencing patient attrition from antiretroviral therapy care in an urban community of Lusaka, Zambia. J Int AIDS Soc. 2012;15(suppl 1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heath J, Lanoye A, Maisto SA. The role of alcohol and substance use in risky sexual behavior among older men who have sex with men: a review and critique of the current literature. AIDS Behav. 2012;16:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen VC, Myers HF, Ray L. The association between alcohol consumption and condom use: considering correlates of HIV risk among black men who have sex with men. AIDS Behav. 2015;19:1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]