Abstract
In the past decade, tumour flare reaction (TFR) was considered as a new side effect associated with immunomodulatory agents (IMiDs) and as a condition of chronic lymphocytic leukaemia (CLL). However, this phenomenon is also observed with immune checkpoint inhibitors in solid tumours. It is still poorly understood and its incidence is underestimated. TFR has been associated with morbidity, therefore, early recognition and management of patients with TFR is critical. An exhaustive literature research between 1985 and 2016 was performed using PubMed; American Society of Clinical Oncology and American Society of Hematology abstracts reporting TFR or pseudoprogression were identified. The incidence of TFR in CLL ranged from 28 to 58%. Tumour response in patients treated beyond progression was reported in 9.7% with ipilimumab, 10% with nivolumab, 6.7 and 12% with pembrolizumab, and in renal cell carcinoma 69% with nivolumab. Rare life-threatening or fatal cases were reported; symptoms were usually mild. Studies showed that treating patients beyond progression yielded tumour responses, considering TFR as predictive of response. Treatment with immunomodulatory agents is associated with TFR, often misinterpreted as progression. Therefore, the identification of appropriate clinical benefit criteria and the use of immune-related response criteria in prospective trials for a better understanding are compulsory.
Keywords: cancer, haematological malignancies, immunotherapy, pseudoprogression, tumour flare reaction
Introduction
In the past decade, tumour flare reaction (TFR) was considered as a new side effect associated with immunomodulatory agents (IMiDs) (thalidomide and lenalidomide) [1,2]. It was believed that TFR is a specific side effect of chronic lymphocytic leukaemia (CLL). However, TFR is also observed in solid tumours treated with immune checkpoint inhibitors (ICIs) [3]. Several cases of flare reaction with hormonotherapy and haematologic malignancies and manifestations of TFR possibly mimicking disease progression have been reported [4,5].
Lenalidomide induces a TFR suggestive of an immune-mediated inflammation in CLL; a concurrent decrease in absolute lymphocyte count in these patients led to the hypothesis that TFR is rather an immune reaction phenomenon instead of a disease progression [6,7].
Several studies have indicated that TFR may predict better responses, although no differences in progression-free survival were shown [8,9].
Method of literature research
A literature research using the following keywords: cancer, chronic lymphocytic leukaemia, haematological malignancies, ICIs, IMiDs, lenalidomide, nivolumab, pseudoprogression, solid tumours, tumour flare reaction, TFR was performed in PubMed as well as for the American Society of Clinical Oncology and American Society of Hematology abstracts covering TFR.
Tumour flare reaction definition
TFR corresponds to an increase in a lesion size related to treatment, simulating a progression of the disease (Table 1) [10].
Table 1.
Tumour flare definition according to National cancer institute-common toxicity criteria for adverse events v 3.0 grading scale [10]
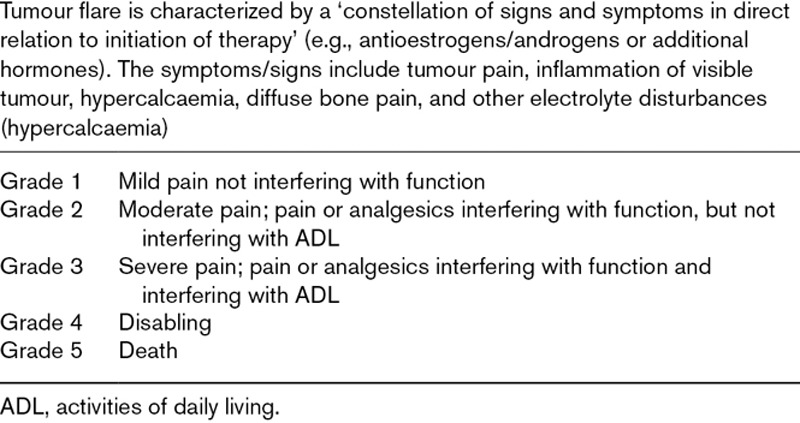
Tumour flare with IMiDs, and immune checkpoint inhibitors
In CLL, TFR resulting from immunomodulatory drugs (1) presents clinically with painful lymph nodes sometimes spleen enlargement, and can be accompanied by fever, rash and clear lymphocytosis and (2) and as an acute inflammatory reaction that primarily involves tumour-bearing sites [2,7].
In solid tumours, tumour flare or pseudoprogression which mimics progression on imaging was observed with ICIs included nivolumab in various tumour types, occasionally associated with tumour flare. It was referred to the apparent increase in tumour burden or the occurrence of new lesions that sometimes may precede antitumour effects, resulting from T-cells infiltrating the tumour site until a sufficient immune response develops [3].
Clinical evidence for therapy-related TFR: Pseudoprogression has been reported in brain tumour imaging, especially for high-grade gliomas. It has been observed in about 30% of patients after a combination of chemotherapy and radiotherapy; pseudoprogression after radiotherapy was observed in about 15% of patients [11].
In 60% of all cases, pseudoprogression occurred mainly within the first 3 months after completing treatment [12–14].
Patients with methylated O6-methylguanine-DNA-methyltransferase show pseudoprogression more frequently, particularly with temozolomide [12,15,16].
Tumour flare reaction in lymphoid malignancies
An overview of TFR in lymphoid malignancies is provided in Table 2.
Table 2.
Tumour flare with IMiDs in lymphoid malignancies
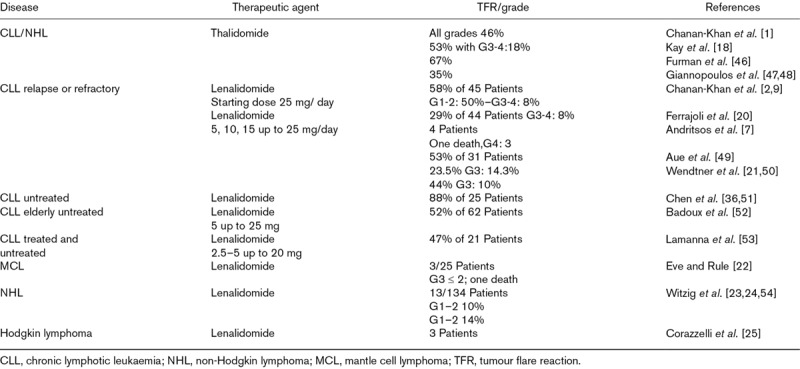
Early clinical trials testing thalidomide in patients with pretreated CLL reported a substantial number of patients experiencing tumour flare and other toxicities leading to poor accrual and premature closure [17,18]. Several subsequent small phase II studies showed similar limited benefits of thalidomide with a high incidence of tumour flare [1,17,19].
Chanan-Khan et al. [2] reported that 58% of CLL patients who received 25 mg/day of oral lenalidomide for 21 days through 28-day-cycles developed TFR (50% developed grade 1–2 reactions, and 8% developed grade 3–4 reactions). Ferrajoli et al. [20] observed that 28% of 35 CLL patients who were treated with a dose of 10 mg/day of lenalidomide and then with an increased dose of 25 mg/day developed TFR. Andritsos et al. [7] described unacceptable toxicities in 4 patients treated with lenalidomide.
TFR developed in 44% (10% G3) of CLL patients [21]; similar results were reported in other CLL studies (Table 2).
Three out of 25 patients with mantle cell lymphoma (MCL) and who were treated with lenalidomide developed TFR. Two had a mild TFR, however, the third patient died [22].
In non-Hodgkin lymphoma (NHL), TFR was observed both in relapsed or refractory indolent NHL and aggressive NHL [23,24]. In Hodgkin lymphoma, TFR was described in three patients [25].
Tumour flare reaction with Immunotherapy in solid tumours
An overview of TFR reported with immunotherapy in solid tumours is provided in Table 3.
Table 3.
Tumour flare with immunotherapy in solid tumours
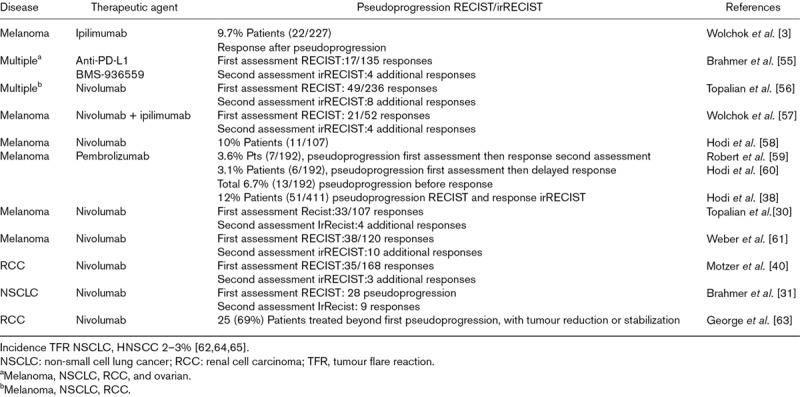
Treatment of various tumour types with ICIs such as nivolumab has sometimes been associated with « tumour flare [43,26–32].
In patients receiving immunotherapy, tumour flare or the appearance of new lesions may precede antitumour effects, resulting from T-cells infiltrating the tumour site until a sufficient immune response develops [3]. When assessed by RECIST criteria, TFR occurring with immunotherapy was considered as disease progression and generally led to treatment discontinuation before the potential clinical benefit of the treatment was fully realized [33]. Treatment beyond the first progression was allowed in patients with favourable tolerance and clinical benefit, some of them experienced subsequent tumour response (Table 3).
Tumour flare reaction with endocrine therapies
Flare reactions from tamoxifen are also known in the treatment of metastatic breast and advanced endometrial cancers [4].
During the initial period of treatment with medroxyprogesterone acetate in patients with hormone-resistant prostatic cancer, a marked clinical flare reaction, mainly with bone pain exacerbation was observed [5].
Time to occurrence/management: In CLL, TFR is mostly observed in early therapy phases and it is more common in previously untreated patients with bulky disease, advanced stages and moderate renal impairment. When using high starting doses of lenalidomide, TFR onset has been reported within hours after the first dose, with a median time of 6 days (range 0–56), and a median time to resolution of 14 days [95% confidence interval (CI), 10–26] [9]. This phenomenon was also observed within the first cycle of lenalidomide in relapsed MCL patients [22,34].
In CLL clinical trials with thalidomide, high TFR incidence led to poor accrual and premature closure [17].
TFR is associated with morbidity and may be severe and life-threatening, requiring hospitalization as reported in four patients with relapsed/refractory CLL and who received lenalidomide at a starting dose of 25 mg [7]. Even if rare cases of life-threatening or fatal TFR exist, symptoms were usually mild, and responded to anti-inflammatory therapy.
In a study, using 20 mg prednisone during the first 5 days and 10 mg for another 5 days as prophylaxis decreased severity, but not the incidence [2,35]. In another trial, one third of all patients were treated with lower doses of prednisone (25–50 mg for 5–10 days); in these patients, TFR was common, but mild in severity [36].
In clinical trials, TFR was monitored early after treatment initiation, during the first cycle and at each dose escalation. Adequate management of TFR relies on analgesics, nonsteroidal anti-inflammatory drugs, antihistamines and corticosteroids [20].
Although steroids modulate the timing and severity of TFR, CLL chemotherapy-naive patients demonstrate a high frequency of TFR compared with pretreated patients. The frequency of TFR appears to be lower when lenalidomide was used in combination. Furthermore, rituximab administered prior to lenalidomide may act as a debulking agent, thus reducing the rate and severity of TFR [37].
Biological implications of tumour flare reaction and challenges with therapeutic evaluation
In CLL, studies have shown that lenalidomide induces a TFR suggestive of immune-mediated inflammation [6,7]. Andritsos et al. [7] reported laboratory evidence that B-cell activation of the tumour cells may contribute to the development of tumour flare in vivo. Pretreatment and posttreatment evaluation of lymph nodes, infiltration of Ki67 and CD3-positive, CD8-positive, granzyme B-positive T cells increases.
This reaction has been reported in other B-cell malignancies, such as MCL, and Hodgkin lymphoma illustrating the contribution of upregulation by B-cell activation, T-cell activation and other innate immune effector cells to the mechanism of action of lenalidomide [22,25].
In-vitro findings indicate that the in-vivo antileukaemic activity of lenalidomide is not likely to be due to direct cytotoxicity on B-CLL cells [2].
Lenalidomide induced the expression of costimulatory ligands (CD86, CD80 and CD40) on B-CLL cells both in vitro and in vivo [7]. Upregulation of these ligands is a critical step in engaging an immune response. This rapid, robust and inflammatory nature of TFR suggests the involvement of the immune system dependent on natural killer cell function and then maintained by the rapid recruitment and proliferation of T cells [6].
Treatment with ICIs such as nivolumab in various solid tumours has been associated with TFR [26,27,30-32,38. With ICIs, TFR is believed to be an immune activation into the tumour, potentially causing tumour growth or new lesions to appear upon imaging, while the immune system is priming for an antitumour response [3].
Immunologic treatment may induce the infiltration of immune cells and inflammation of the tumour, which results in increased tumour size by objective measures [3,33]. Alternately, the growth of pre-existing lesions or the appearance of new lesions can occur after administration of immunotherapy, as the process of immune activation may potentially be delayed. The tumour may grow transiently during the period of immune activation and before an effective antitumour response occurs [33].
Di Giacomo et al. [39] reported that some patients with melanoma treated with ipilimumab, a mAb against cytotoxic T-lymphocyte–-associated antigen-4, experienced initial increased size of tumour lesions, confirmed by biopsy as inflammatory cell infiltrates or necrosis, with subsequent tumour burden decrease.
Treatment beyond first RECIST-defined progression was investigated in a phase 2 of nivolumab in patients with metastatic renal cell carcinoma who tolerated nivolumab and exhibited clinical benefit [40]. Half of these patients treated beyond first progression experienced subsequent tumour reduction in target lesions.
Other studies assessing nivolumab in melanoma and nonsmall cell lung cancer, showed a response in a subset of patients treated beyond first progression [30-32]. Similar findings were reported in patients with melanoma treated with ipilimumab and with pembrolizumab [28,38].
Therefore, pseudoprogression represents a real challenge for clinicians, because it is not captured by conventional imaging and RECIST criteria.
These findings have prompted the development of immune-related response criteria to capture these unconventional response patterns, including requirement of confirmation of progression on two consecutive scans at least 4 weeks apart, and inclusion of new lesion measurements to the total tumour burden [3,41,42].
Discussion
Both solid tumours and haematologic malignancies are able to induce an immune response that can regulate their growth; this is known as tumour immunogenicity [43,44].
A new concept called ‘pseudoprogression’ has emerged, making response evaluation difficult. Using RECIST, tumour flare with immunotherapy may be considered as disease progression and may lead to treatment discontinuation before the clinical benefit of treatment is fully realized [33]. Therefore, initial progression may not indicate therapeutic failure.
Radiological features of TFR have proven to be challenging in clinical trials and in clinical practice setting, because it is difficult to differentiate between pseudoprogression and true progression, with imaging largely relying on the tumour size. Furthermore, conventional imaging and RECIST criteria may underestimate the benefit in a subgroup of treated patients, because immunotherapy works differently as compared with cytotoxics.
When evaluating the response to immunotherapy, even if uncommon, pseudoprogression should be considered until disease progression can be confirmed [27]. Histologic confirmation is not always possible. However, close monitoring using performance status, cancer-related symptoms and tumour burden at the time of progression may allow to differentiate between symptomatic and asymptomatic progression [3,45].
While being asymptomatic in most patients, TFR can be observed in a context with or without clinical deterioration. In many trials, to avoid discontinuing effective therapy, patients who presented with a clinically good performance status were allowed to remain on treatment in the case of a new or growing area of disease.
Conclusion
In conclusion, treatment with immunomodulatory agents is associated with TFR, more frequent with haematologic malignancies; TFR is less common in solid tumours.
TFR is poorly understood and as it is not captured by RECIST, its incidence is still underestimated. Thus, it is likely to be misinterpreted as progression. For this reason, after a first radiologic progression, the use of clinical symptoms may be a helpful indicator. Treatment continuation may be supported in patients with clinical benefit, unless progressive disease is confirmed by subsequent evaluations.
To avoid a discontinuation of a potentially effective therapy, assessments should be based on clinical symptoms, imaging and biomarkers. The use of irRECIST in prospective trials is needed for a better diagnostic and understanding of TFR while data should be collected through prospective clinical trials, to characterize this phenomenon more effectively.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chanan-Khan A, Miller KC, Takeshita K, Koryzna A, Donohue K, Bernstein ZP, et al. Results of a phase 1 clinical trial of thalidomide in combination with fludarabine as initial therapy for patients with treatment-requiring chronic lymphocytic leukemia (CLL). Blood. 2005; 106:3348–3352 [DOI] [PubMed] [Google Scholar]
- 2.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006; 24:5343–5349 [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009; 15:7412–7420 [DOI] [PubMed] [Google Scholar]
- 4.Brooks BJ, Jr, Lippman ME. Tamoxifen flare in advanced endometrial carcinoma. J Clin Oncol. 1985; 3:222–223 [DOI] [PubMed] [Google Scholar]
- 5.Fosså SD, Urnes T. Flare reaction during the initial treatment period with medroxyprogesterone acetate in patients with hormone-resistant prostatic cancer. Eur Urol. 1986; 12:257–259 [DOI] [PubMed] [Google Scholar]
- 6.Chanan-Khan AA, Chitta K, Ersing N, Paulus A, Masood A, Sher T, et al. Biological effects and clinical significance of lenalidomide-induced tumour flare reaction in patients with chronic lymphocytic leukaemia: in vivo evidence of immune activation and antitumour response. Br J Haematol. 2011; 155:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andritsos LA, Johnson AJ, Lozanski G, Blum W, Kefauver C, Awan F, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008; 26:2519–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aue G, Njuguna N, Tian X, Soto S, Hughes T, Vire B, et al. Lenalidomide-induced upregulation of CD80 on tumor cells correlates with T-cell activation, the rapid onset of a cytokine release syndrome and leukemic cell clearance in chronic lymphocytic leukemia. Haematologica. 2009; 94:1266–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanan-Khan A, Miller KC, Lawrence D, Padmanabhan S, Miller A, Hernandez-Illatazurri F, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011; 117:2127–2135 [DOI] [PubMed] [Google Scholar]
- 10.NIH. National Cancer Institute, DCTD Division of Cancer Treatments & Diagnosis. Common terminology Criteria for Adverse Events v3.0 (CTCAE). 2006. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Accessed 23 September 2018]
- 11.Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014; 15:11832–11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol. 2011; 32:1978–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009; 22:633–638 [DOI] [PubMed] [Google Scholar]
- 14.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009; 9:241–246 [DOI] [PubMed] [Google Scholar]
- 15.Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009; 27:1275–1279 [DOI] [PubMed] [Google Scholar]
- 16.Bulik M, Kazda T, Slampa P, Jancalek R. The diagnostic ability of follow-up imaging biomarkers after treatment of glioblastoma in the temozolomide era: implications from proton MR spectroscopy and apparent diffusion coefficient mapping. Biomed Res Int. 2015; 2015:641023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinblatt DL, Johnson J, Niedzwicki D, Rizzieri DA, Bartlett N, Cheson BD. Phase II study of thalidomide in escalating doses for follicular (F-NHL) and small lymphocytic lymphoma (Sll): CALGB study 50002. Blood. 2004; 104:3284 [Google Scholar]
- 18.Kay NE, Shanafelt TD, Call TG, Wu W, Laplant BR. N9986: a phase II trial of thalidomide in patients with relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 2009; 50:588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurenti L, Piccioni P, Tarnani M, De Padua L, Garzia M, Efremov DG, et al. Low-dose thalidomide in combination with oral fludarabine and cyclophosphamide is ineffective in heavily pre-treated patients with chronic lymphocytic leukemia. Leuk Res. 2007; 31:253–256 [DOI] [PubMed] [Google Scholar]
- 20.Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008; 111:5291–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendtner CM, Hillmen P, Mahadevan D, Bühler A, Uharek L, Coutré S, et al. Final results of a multicenter phase 1 study of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2012; 53:417–423 [DOI] [PubMed] [Google Scholar]
- 22.Eve HE, Rule SA. Lenalidomide-induced tumour flare reaction in mantle cell lymphoma. Br J Haematol. 2010; 151:410–412 [DOI] [PubMed] [Google Scholar]
- 23.Witzig TE, Vose JM, Kaplan HP, Wolf JL, Pietronigro D, Takeshita K, et al. Early results from a phase II study of lenalidomide monotherapy in relapsed/refractory indolent non-hodgkin’s lymphoma. Blood. 2006; 108:2482 [Google Scholar]
- 24.Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011; 22:1622–1627 [DOI] [PubMed] [Google Scholar]
- 25.Corazzelli G, De Filippi R, Capobianco G, Frigeri F, De Rosa V, Iaccarino G, et al. Tumor flare reactions and response to lenalidomide in patients with refractory classic hodgkin lymphoma. Am J Hematol. 2010; 85:87–90 [DOI] [PubMed] [Google Scholar]
- 26.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008; 8:1. [PMC free article] [PubMed] [Google Scholar]
- 27.Hales RK, Banchereau J, Ribas A, Tarhini AA, Weber JS, Fox BA, Drake CG. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010; 21:1944–1951 [DOI] [PubMed] [Google Scholar]
- 28.Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol. 2012; 35:606–611 [DOI] [PubMed] [Google Scholar]
- 29.Hodi FS, Ribas A, Daud A, Hamid O, Robert C, Kefford R, et al. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014; 32Suppl 15S3006 [Google Scholar]
- 30.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014; 32:1020–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015; 372:2521–2532 [DOI] [PubMed] [Google Scholar]
- 33.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015; 33:3541–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013; 31:3688–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musial L, Miller KC, Tonelli A, Manochakian R, Padmanabhan S, Lawrence D, et al. Low-dose prednisone decreases the severity but not the frequency of lenalidomide associated tumor flare feaction (TFR) in chronic lymphocytic leukemia (CLL) patients. Blood. 2006; 108:4987 [Google Scholar]
- 36.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011; 29:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013; 31:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodi FS, Ribas A, Daud A, Hamid O, Robert C, Kefford R, et al. Patterns of response in patients with advanced melanoma treated with Pembrolizumab (MK-3475) and evaluation of immune-related response criteria (irRC). J Immunol Cancer. 2014; 2Suppl 3P103 [Google Scholar]
- 39.Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009; 58:1297–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015; 33:1430–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, Ramaiya NH. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012; 198:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol. 2015; 84:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012; 12:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015; 15:201–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxnard GR, Morris MJ, Hodi FS, Baker LH, Kris MG, Venook AP, Schwartz LH. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst. 2012; 104:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furman RR, Leonard JP, Allen SL, Rosenthal T, Gabrilove JL. Thalidomide alone or in combination with fludarbabine are effective treatments for patients with fludarabine-relapsed and refractory CLL. J Clin Oncol. 2005; 23Suppl 16S6640 [Google Scholar]
- 47.Giannopoulos K, Dmoszynska A, Kowal M, Wasik-Szczepanek E, Bojarska-Junak A, Rolinski J, et al. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia. 2009; 23:1771–1778 [DOI] [PubMed] [Google Scholar]
- 48.Giannopoulos K. Molecular and immunological effects of thalidomide in CLL. ASH Annual Meeting. 2008; 112:2092 [Google Scholar]
- 49.Aue G, Soto S, Valdez J, Arthur DC, Tian X, Wiestner A. Phase II trial of pulse dosed lenalidomide in previously treated chronic lymphocytic leukemia. Blood. 2010; 116:1383 [Google Scholar]
- 50.Wendtner CM, Mahadevan D, Stilgenbauer S, Frankfurt O, Bloor A, Bosch F, et al. Preliminary results of a phase 1/2, multi-center, open-label study (CLL- 001) investigating a stepwise dose-escalation schedule of lenalidomide in relapsed or refractory chronic lymphocytic leukemia. Blood. 2008; 112:2104 [Google Scholar]
- 51.Chen C. Long-term follow-up of a phase 2 study of single-agent lenalidomide in previously untreated, chronic lymphocytic leukemia. Blood. 2012; 120:abstract 718 [Google Scholar]
- 52.Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011; 118:3489–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamanna N, Heaney ML, Maslak PG, Jurcic JG, Rosenblat TL, Noy A, et al. A new paradigm in CLL: minimizing toxicity by using the minimum effective dose (MED) of lenalidomide for older patients with CLL. J Clin Oncol. 2012; 30Suppl 16S6609 [Google Scholar]
- 54.Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-hodgkin’s lymphoma. J Clin Oncol. 2009; 27:5404–5409 [DOI] [PubMed] [Google Scholar]
- 55.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369:122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. J Clin Oncol. 2014; 32Suppl 15S9002 [Google Scholar]
- 59.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014; 384:1109–1117 [DOI] [PubMed] [Google Scholar]
- 60.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016; 34:1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (checkmate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015; 16:375–384 [DOI] [PubMed] [Google Scholar]
- 62.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016; 2:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375:1856–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016; 17:956–965 [DOI] [PubMed] [Google Scholar]


