Abstract
Objective:
Social support may have a positive impact on health outcomes for patients and caregivers, but the extent to which social support and health outcomes are interrelated for both is unknown. We examine the dyadic interrelationships between social support and health among cancer patients and their caregivers.
Methods:
Lung and colorectal cancer (CRC) patient and caregiver dyadic data were obtained from the Cancer Care Outcomes Research and Surveillance Consortium. Patients and caregivers self-reported sociodemographic, social support, and caregiving characteristics at 5-(n=218 lung; n=222 CRC) or 12-months post-diagnosis (n=198 lung; n=290 CRC). Structural equation modeling was used to examine actor-partner interdependence models (APIM) of lung and CRC dyads at 5- and 12-months post-diagnosis.
Results:
At 5-months post-diagnosis, no interdependence between patient and caregiver social support was detected for CRC or lung dyads (all p>0.05). At 12-months post-diagnosis, no interdependence was detected for CRC dyads (all p>0.70); lung dyads showed complete interdependence, indicating patient social support is associated with better caregiver self-reported health (β=0.15, p<0.001), and caregiver social support is associated with better patient self-reported health (β=0.18, p<0.001).
Conclusion:
Social support has positive impact on patient and caregiver perceived health across the cancer trajectory, and these effects may differ by cancer site and time. Future research and translational efforts are needed to identify effective ways to bolster both patient and caregiver social support and to determine critical moments for intervention.
Background
The number of cancer survivors is increasing, and it is estimated that cancer survivors currently make up about 5% of the population (16.5 million people).1 In 2017, there were an estimated 1.6 million newly diagnosed cases of cancer in the U.S., and many of these newly diagnosed patients receive substantial support from one or more caregivers who voluntarily provided support without compensation.1 It is estimated that of the 39.8 million Americans providing care to an adult for any reason, 2.8 million of these people were providing care to an adult whose primary health problem was cancer.2 As care continues to shift to outpatient and home settings, many caregivers report high burden and feel unprepared to meet the many demands of caregiving.3 This highlights the fact that support in all forms is critical for cancer patients and their caregivers. Social support, a multidimensional construct that consists of tangible, emotional, and informational domains of support,4,5 is imperative to study within the patient and caregiver context to further understand and design supportive care interventions to bolster health outcomes for patients and caregivers.
Few studies assess the dyadic nature of social support perceived by the patient and caregiver on health-related outcomes. A study of gastrointestinal cancer patients and caregivers found that one’s emotional support alleviates loneliness, leading to better mental health outcomes; however, no interrelationships between patients’ and caregivers’ emotional support and outcomes were found6. A study7 of caregivers and patients with advanced breast, colorectal, lung, or prostate cancer, one’s social support was positively associated with their own exercise and diet and interrelationships varied over time; specifically, caregiver social support at baseline was associated with patient exercise and diet at three months, but patient social support at three months was only associated with caregiver diet at six months post-intervention. In a study8 of distress and coping among cancer patients and caregivers, perceived social support was found to be positively associated with hope for their future while the interrelationship showed a negative association. While these findings support decades of research on the role of social support as it pertains to either the patient or the caregiver in terms of burden, mental, and physiological health outcomes, as well as the importance of exploring such relationships across time, findings in terms of the interrelationships of patient and caregiver social support remain unclear. Across studies, social support has been operationalized in different ways in relation to different health outcomes (i.e. lonlinesss, mental health, diet and exercise, hope) in groups of individuals with heterogeneous cancer sites. This issue is evidenced by a review9 of social support and cancer progression that describes how different operationalizations of social support impact outcomes differently across cancer types over time leading to an inconclusive literature. Since the role of social support on health-related outcomes likely differs across the cancer continuum by site, the discordance in findings could be attributable to pooling across different cancer sites and points along the cancer continuum to understand the role of social support across a wide array of health outcomes.
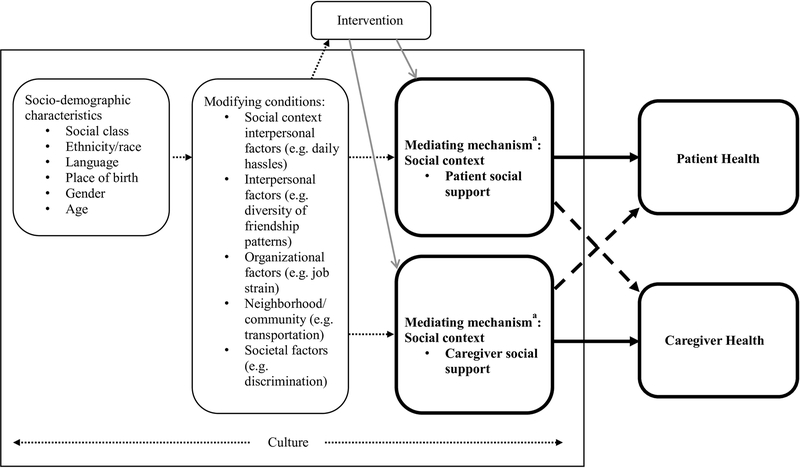
The Social Contextual Framework10 provides a basis for understanding the role of social support on health outcomes. This framework posits that socio-demographic characteristics and modifying factors independently impact outcomes, while mediating factors, such as social support, may be intervened upon to bolster health outcomes. This framework also takes into consideration overlaping social contexts and how the social context for one person may be related to and have an impact on the social context of another. Figure 1 illustrates how the Social Contextual Framework has been adapted to explore the role and interrelationships of patient and caregiver social support on health within a broader context. If such a relationship can be established then efforts to develop interventions that improve the quality and accessibility of social support should be pursued. To better understand the role of social support on health outcomes in the dyadic context, and how these factors may differ across disease sites and time since diagnosis, we propose to explore the following questions with a sample of CRC and lung cancer patients and their caregivers using the actor-partner interdependence modeling (APIM) approach:
RQ(1): Are patient and caregiver associations interrelated in the informal cancer caregiving context?
RQ(2): Do these associations differ by disease site (CRC or lung cancer) and
RQ(3): time since patient diagnosis (about 5 and 12 months post-diagnosis)?
Figure 1: Adapted Social Contextual Framework Illustrating the Role of Social Support on Health Outcomes in a Dyadic Context.
Note: aThis framework has been adapted to emphasize the role of social support on health outcomes in the patient-caregiver dyad. Please refer to the original framework10 for additional mediating outcomes in the social context (social norms, organizational environment) and individual factors (self-efficacy, attitudes/beliefs, intentions to perform behavior, and skills). Bold outlines and paths emphasize the dyadic analysis in this study; bold solid arrows indicate actor effects and bold dashed arrows indicate partner effects.
Methods
Data source
Data are from patients with lung or colorectal cancer, as well as data from patients’ primary informal caregiver, collected by the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium. Detailed information about the CanCORS study protocols, published elsewhere,11,12 is available at https://www.cancors.org/public. Briefly, the CanCORS consortium consisted of seven study sites ascertaining patients from cancer registries (five sites) or a healthcare system (two sites) in the United States between 2003–2005. Patients were eligible for the study if they were 21 years of age and older with newly diagnosed invasive non-small cell or small-cell lung cancer or adenocarcinoma of the colon or rectum. Patient data was collected approximately at 5-months post-diagnosis with verbal consent and phone interviews, and 12-months post-diagnosis from medical records, providers, and self-administered surveys. Caregiver data included two cross-sectional samples of caregivers who were identified by the patients either 5-months post-diagnosis (n=825) or 12-months post-diagnosis (n=802). Caregivers were invited to participate via mail (including a cover letter that provided informed consent information, self-administered questionnaire, information about the study, a postage-paid return envelope, and a $20 incentive). Caregivers completed the self-administered questionnaire on average 7.3 or 15.6 months since the survivors’ diagnosis, depending on which round of the survey they were originally invited to participate (baseline or follow-up). The analyses of de-identified survey data from CanCORS was approved and exempted by the Office of Human Subjects Research Protections at the National Institutes of Health (#13165).
Of the 440 patient-caregiver dyads who provided complete data at 5-months post-diagnosis for this study, 222 were CRC dyads and 218 were lung cancer dyads. At 12-months post-diagnosis, 488 dyads provided complete data, 290 were CRC dyads and 198 lung cancer dyads. The 5- and 12-month samples of caregivers are independent of each other.
Measures
Perceived social support is a composite variable of the tangible (e.g. “How often do you have someone to help you if you are confined to bed”; four items) and emotional/informational (e.g. “someone to give you good advice about a crisis”; five items) subscales of the MOS social support survey.5 Participants responded to each item using a scale of 1=None of the time to 5=All of the time. Responses were averaged, with higher scores indicating greater perceived social support. Although this variable represents only two components of social support, we refer to this variable as “perceived social support.”
Self-reported health was measured using a single item (“In general, how would you say your health is now?”) on a scale of 1 (poor) to 5 (excellent). This item has been validated across populations and disease sites as an independent predictor or mortality13.
Demographic variables
Several patient and caregiver demographic and health-related variables were explored in preliminary analyses as potential covariates of self-reported health. Covariates collected from both patients and caregivers were: age, gender (male/female), educational attainment, race (recoded as white/other), and marital status (married or living with partner/other). Additional patient information included SEER summary stage at diagnosis and self-reported treatment modalities (surgery, radiation, and/or chemotherapy). Caregivers indicated hours spent providing care per week and their comorbidities (0–8 Charlson Index).
Statistical Analyses
To determine the covariates significantly associated with self-reported health, statistically significant and meaningful (+/− 0.20) correlations between patient or caregiver covariates and respective self-reported health were identified. Covariates of patient and caregiver self-reported health were further refined using hierarchical multiple regression models that predicted either patient or caregiver self-reported health across cancer sites at either 5- or 12-months post-diagnosis. Dyads were analyzed according to cancer site and time point to more accurately explore the differences by disease site due to the cross-sectional nature of the caregiver survey. Statistically meaningful correlates of patient self-reported health at 5- and 12-months post-diagnosis for patients with CRC or lung cancer were added into their respective hierarchical regression models by time-point and disease site. The first block contained demographic characteristics (age, race, marital status, education, gender) and the second block contained cancer characteristics of stage at diagnosis and sum of treatment modalities. A similar process was used for caregiver models, where the first block contained demographic characteristics (age, race, marital status, educational attainment and gender); the second block contained health characteristics (Charlson Comorbidity Index); and the third block contained care-provision characteristics (hours spent providing care per week). For a full explanation of this process, please see supplement.
APIM allows for a concurrent evaluation of one dyad member’s predictor variables on their own outcome (actor effect) and on the other dyad member’s outcome (partner effect). APIM analyses were conducted using a structural equation modeling approach with full information maximum likelihood estimation and 5000 bootstrap resampling procedures to generate bias-corrected confidence intervals in Mplus version 8.1. Separate models were tested for colorectal and lung cancer dyads at 5-months and 12-months post-diagnosis, for a total of four APIM models. Patient and caregiver self-reported health was regressed on to their own perceived social support, respectively (actor effect), as well as on to their counterpart’s perceived social support (partner effect). The models controlled for patient and caregiver covariates identified in the hierarchical regressions described above. As models were tested and fit, nonsignificant covariates were removed after careful conceptual and statistical consideration to better serve the exploratory and hypothesis-generating nature of this study. Models considered to fit the observed data were indicated by a non-significant chi-square statistic, a root mean squared error of approximation (RMSEA) of less than or equal to 0.05, a Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI) of greater than 0.90.
Results
Most caregivers and care recipients were over the age of 50 years and non-Hispanic white. Patients were primarily male, and caregivers were primarily female. Table 1 provides demographic and key variable characteristics of patients and caregivers by cancer site and time that were assessed in the APIMs.
Table 1:
Patient and Caregiver Characteristics at 5- and 12-months Post-diagnosis
| 5-months | 12-months | |||
|---|---|---|---|---|
| CRC (N=222 dyads) |
Lung (N=218 dyads) |
CRC (N=290 dyads) |
Lung (N=198 dyads) |
|
| Patients | N (%) | |||
| Treatment Received | ||||
| Surgery | 202 (91) | 110 (51) | 223 (77) | 98 (50) |
| Radiation | 36 (16) | 89 (41) | 40 (14) | 56 (28) |
| Chemotherapy | 144 (65) | 151 (69) | 152 (52) | 92 (47) |
| Sum of treatment modalities | ||||
| mean ± SD | 1.72 ± 0.68 | 1.61 ± 0.66 | 1.43 ± 0.89 | 1.24 ± 0.96 |
| SEER Summary Stage | ||||
| 0, 1 or local | 53 (25) | 67 (31) | 67 (23) | 76 (38) |
| 2 | 54 (25) | 25 (12) | 78 (27) | 16 (8) |
| 3 or regional | 74 (33) | 70 (32) | 94 (32) | 60 (30) |
| 4 or distant | 34 (16) | 53 (24) | 36 (12) | 37 (19) |
| Perceived social support | ||||
| mean ± SD | 4.54 ± 0.71 | 4.50 ± 0.71 | 4.46 ± 0.73 | 4.48 ± 0.71 |
| Self-reported health | ||||
| mean ± SD | 3.29 ± 1.06 | 3.06 ± 1.07 | 3.22 ± 1.08 | 2.95 ± 1.09 |
| Caregivers | ||||
| Education | ||||
| <High School | 23 (10) | 27 (12) | 29 (10) | 18 (9) |
| High School | 58 (26) | 74 (34) | 54 (19) | 55 (28) |
| Some College | 98 (44) | 78 (36) | 114 (39) | 77 (39) |
| 4-yr degree | 28 (13) | 26 (12) | 55 (19) | 29 (15) |
| Graduate degree | 13 (6) | 11 (5) | 37 (13) | 16 (8) |
| Comorbidities | ||||
| mean ± SD | 0.70 ± 1.25 | 0.68 ± 1.23 | 0.77 ± 1.28 | 0.92 ± 1.39 |
| Perceived social support | ||||
| mean ± SD | 3.65 ± 1.02 | 3.65 ± 1.02 | 3.80 ± 0.96 | 3.69 ± 0.96 |
| Self-reported health | ||||
| mean ± SD | 3.25 ± 1.00 | 3.37 ± 0.91 | 3.22 ± 0.89 | 3.24 ± 0.93 |
Note: Descriptive statistics provided in Table 1 are for the variables used in the Actor-partner Interdependence Models. Please see the supplemental material for full sample demographic characteristics.
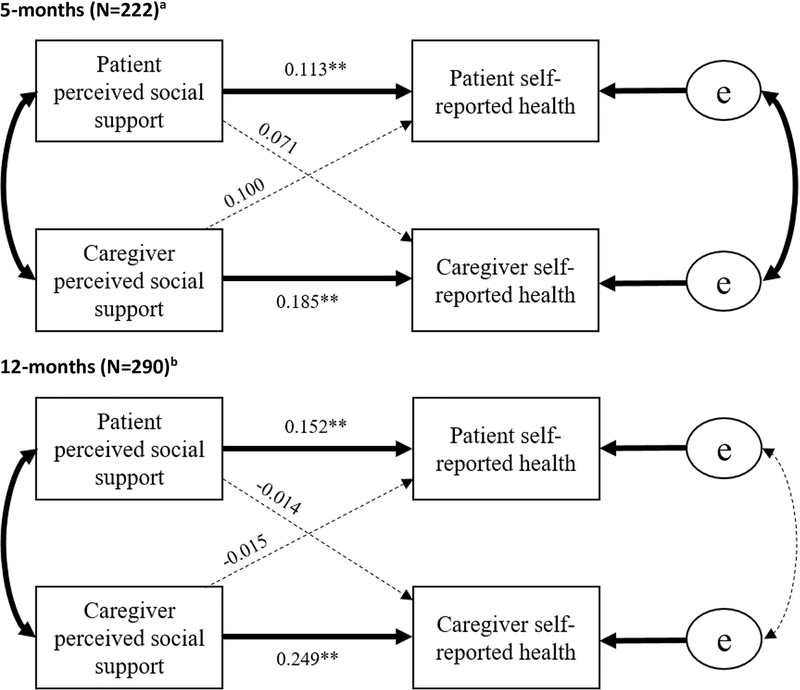
CRC dyads at 5-months post-diagnosis
This model exhibited an actor-only effect such that the largest predictor of both patients’ and caregivers’ self-reported health was their own perceived social support (β=0.113, p<0.01 and β=0.185, p<0.01, respectively). No significant partner effects were detected. For CRC dyads at 5-months post-diagnosis, the resultant model fit the data well (X2(14)=10.93, p=0.62, RMSEA=0.01 (CI:0.0–0.06), CFI=1.0, TLI=1.0), and explained 5% of the variance in patient self-reported health and 15% of the variance in caregiver self-reported health.
CRC dyads at 12-months post-diagnosis
The 12-month model exhibited an actor-only effect such that patients’ perceived social support was the largest predictor of their self-reported health (β=0.152, p<0.01) and caregivers’ perceived social support predicting caregiver self-reported health (β=0.249, p<0.01). No significant partner effects were detected. (Figure 2). This model fit the data well (X2(18)=21.78, p=0.84, RMSEA=0.03 (CI:0.0–0.06), CFI=0.93, TLI=0.95) and explained 4% of the variance in patient self-reported health and 15% of the variance in caregiver self-reported health.
Figure 2: Colorectal Cancer Dyads at 5-months and 12-months Post-diagnosis.
Note: Bold lines indicate significant paths
a 5-month models controlled for: patient stage at diagnosis on patient self-reported health and caregiver Charlson Comorbidities and education on caregiver self-reported health
b 12-month models controlled for: patient stage at diagnosis and sum of treatment modalities on patient self-reported health and caregiver Charlson Comorbidities and education on caregiver self-reported health.
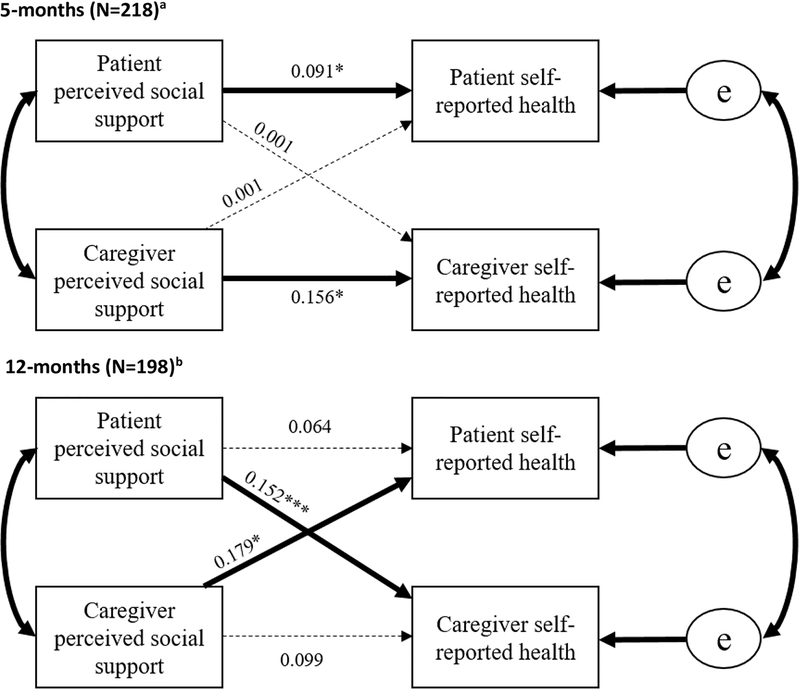
Lung cancer dyads at 5-months post-diagnosis
This model showed an actor-only effect such that patients’ perceived social support was the largest predictor of patient self-reported health (β=0.091, p<0.05), and caregivers’ perceived social support most strongly predicted their own self-reported health (β=0.156, p<0.05). No significant partner effects were detected. The resultant model fit the data well (X2(14)=20.67, p=0.11, RMSEA=0.047 (CI:0.00–0.09), CFI=0.890, TLI=0.914), and explained 4% of the variance in patient self-reported health, and 22% of the variance in caregiver self-reported health.
Lung cancer dyads at 12-months post-diagnosis
At 12-months post-diagnosis, lung cancer dyads exhibited a partner-effect, such that patients’ perceived social support was most strongly associated with caregivers’ self-reported health (β=0.152, p<0.001), while caregivers’ perceived social support was most strongly associated with patients’ self-reported health (β=0.179, p<0.05). This model fit the data well (X2(18)=26.01, p=0.10, RMSEA=0.047 (CI:0.00–0.085), CFI=0.904, TLI=0.930) and explained 10% of the variance in patient self-reported health (p<0.05), and 23% of the variance in caregiver self-reported health (p<0.01). No significant actor-effects were detected(Figure 3).
Figure 3: Lung Cancer Dyads at 5- and 12-months Post-diagnosis.
Note: Bold lines indicate significant paths
a 5-month models controlled for: patient stage at diagnosis on patient self-reported health and caregiver Charlson Comorbidities and education on caregiver self-reported health
b 12-month models controlled for: patient stage at diagnosis and sum of treatment modalities on patient self-reported health and caregiver Charlson Comorbidities and education on caregiver self-reported health.
Conclusions
This study assessed the dyadic associations between the social support environment on self-reported health for patients and caregivers in colorectal and lung cancer dyads at roughly five- and twelve-months post-diagnosis. The study builds upon the burgeoning literature on dyadic associations between patients with cancer and their caregivers by examining the role of the social support environment on the health of patients and caregivers at two distinct points along the cancer continuum. To our knowledge, this is the first study to assess the interrelated associations between social support and patient/caregiver health in the post-treatment setting. Study findings confirm the importance of patients’ and caregivers’ social support for their own health outcomes, and further suggest that for longer-term lung cancer dyads, social support is correlated with self-reported health across dyad members.
Our first research question sought to understand how patients’ and caregivers’ social support is associated with self-reported health outcomes and how individual’s social support may affect the health of the other. We only found one significant partner effect (lung cancer dyads at 12-months post-diagnosis); however, all cross-dyad associations between social support and health were positive, suggesting a consistent underlying trend. Previous literature has indicated the positive effects of social support on various facets of health, from financial strain, psychological well-being, and physiological health outcomes;29 this is the first study to show that the effects of social support may also spill over between cancer patients and their caregivers, particularly in some populations.
Our second and third research questions addressed how the benefits of social support operate across disease sites and time since diagnosis. The presence of actor effects was consistent across disease sites and time since diagnosis. In other words, the role of social support is similar for colorectal and lung cancer dyads and at 5-months post-diagnosis, in that one’s own perception of social support is most strongly associated with one’s own health outcomes. Conversely, partner effects varied across cancer site and time since diagnosis. Specifically, at 12-months post-diagnosis, lung cancer dyads exhibited a partner effect such that the social support perceived by the other was most strongly associated with one’s self-reported health.
There are several possible explanations for the partner effects in lung cancer dyads only at 12-months post-diagnosis. At the clinical level, treatment trajectory, symptom profile, and HRQOL for patients with lung cancer is markedly different than the trajectory of patients with CRC.14–17 Patients with lung cancer are often diagnosed at later stages, experience greater symptom burden and high mortality rates, relative to other cancers, such as CRC. At the individual level, self-stigma and shame have been negatively associated with patient quality of life and seeking medical and social support.18–26 Years of anti-tobacco campaigns and tobacco industry public comments have led many individuals to internalize blame for the connection between smoking and a lung cancer diagnosis.21,24,27 These messages overlook both mechanistic factors regarding the harmful constituents found in addictive cigarettes and the environmental context that encouraged smoking initiation, often leading to addiction.18,20,24 At the interpersonal level, many believe those with tobacco-related lung cancer are responsible for their disease21,22,24,26,28 and this belief is largely attributable to the decades of “smoker-shaming” messaging. Studies comparing social perceptions of lung to various other cancers have found that because of this perception, most people are less likely to endorse funding for lung cancer research in favor of funding for other cancer research.24,28 Self- and social stigma often exists in tandem with therapeutic nihilism, ultimately resulting in worse health outcomes.20 Over time, the impact of lung cancer stigma often leads to unsupportive relationships and resentment towards the patient for smoking-related issues, which is why having a stronger and more supportive social environment, as indicated by the partner effect, may be best suited to countering the impact of internalized and social stigmas around lung cancer.
Considering these clinical, individual, and interpersonal level factors within the Social Contextual Framework is a useful approach for future research as this framework maps which of these factors are modifying conditions (e.g. discrimination such as smoking stigma) and which factors are mediating mechanisms that may be intervened upon (e.g. social support). In addition, this framework posits that mediating factors, such as social support, influence health behaviors, which impact health outcomes. Evidence for this is found in a recent cluster-randomized control trial30 of a self-guided intervention relying on existing social networks and provision of emotional & informational support to target multiple risk behaviors. Results of this trial suggest that having more emotional and informational support (the social support constructs explored in our study) reduces risk behaviors.
Research and Clinical Implications
In the context of cancer, there are various clinical, individual, and interpersonal-level issues to consider and the importance of the social environment for patient and caregiver health is apparent across these levels. One way to leverage the social environment to improve health outcomes is to take a behavioral economics approach to engineering social incentives people may opt-in to, such as peer-to-peer support networks or turning healthy behavior or medication adherence into friendly competition, as appropriate.31 However, for social incentives to be effective, we need a better understanding of the various distal and proximal goals that govern everyday interactions and influence decision-making, acknowledging that the most valued goals (e.g. putting food on the table, paying rent) are not likely cancer-specific.32 The majority of social and environmental determinants of health exist outside of the healthcare system33 and these social milieus are where the majority of behavior change is promoted and sustained.34 Therefore, studies that explore how cancer care is prioritized in these social settings and how existing values may be leveraged for adherence to positive health behaviors, as well as how to integrate these interventions into the healthcare sector, is greatly needed in order to successfully engineer social incentives for better health outcomes. An example of one such intervention is the electronic Social Network Assessment Program (eSNAP)35, which is a tool that caregivers may access in the privacy of their home from a variety of electronic devices. eSNAP allows caregivers to visualize their support network by asking questions about available social resources across six categories of support and then provides a visual based on the entered responses that highlights strengths and weaknesses within the support network and offers resources to help strengthen areas of need.35 Such a tool may be easily implemented within the clinic setting by simply encouraging patients use of the tool and providing the link with a brief description of instructions and what the tool has to offer.
Study Limitations
Potential limitations should be acknowledged when considering the findings of this study. First, while this study assessed outcomes at 5- and 12-months post-diagnosis, each APIM consisted of unique samples of patients and caregivers and therefore cannot be considered a longitudinal study. Second, a composite measure of perceived social support was created using the available MOS items from the tangible and emotional/informational domains but did not include items from the positive social interaction or affective domains, thus precluding any direct comparisons to other studies using MOS. Third, we used a single-item measure of self-reported health, but future research that focuses on specific dimensions of health may further explicate the impact of social support on specific health outcomes in relation to the larger social context. Fourth, some patient socioeconomic status variables (i.e. patient education) were not included in the statistical models due to non-response on these items. In addition, the sample was predominantly white, and therefore did not allow us to explore differences across minority populations, thus this variable was dichotomized. The purpose of this study was to generate hypotheses regarding the interrelations of social support and health in a dyadic context, so future studies should explore the mechanisms that engender positive social support provision for better health outcomes to provide instructional guidance for interventions.
A better understanding of how decisions around cancer-related issues are influenced by more proximal concerns of everyday life will inform interventions that leverage social capital for better health outcomes for patients and caregivers. Such interventions have potential to complement traditional individual-level interventions that often focus solely on the patient/caregiver, or patient/caregiver-provider interactions, without burdening the healthcare sector. Considering that day-to-day interactions patients and caregivers have with their own social milieus far outnumber the interactions with the healthcare sector, interventions in this space have a better chance of self-sustaining and supporting better health outcomes in a way that counteracts the projected influx in demand on the healthcare system.
Supplementary Material
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to report.
References
- 1.American Cancer Society AC. Cancer Facts and Figures 2018. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf [accessed Oct 1, 2018].
- 2.National Alliance for Caregiving. Cancer Caregiving in the US: An Intense, Episodic, and Challenging Care Experience. Available from: https://www.caregiving.org/wp-content/uploads/2016/06/CancerCaregivingReport_FINAL_June-17-2016.pdf [accessed Oct 1, 2018].
- 3.Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer. 2016;122:1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psycho Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 5.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
- 6.Secinti E, Rand KL, Johns SA, et al. Social correlates of mental health in gastrointestinal cancer patients and their family caregivers: Exploring the role of loneliness. Supp Care Cancer. 2018; DOI: 10.1007/s00520-018-4467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis KR, Janevic MR, Kershaw T, et al. (2017). Engagement in health-promoting behaviors and patient–caregiver interdependence in dyads facing advanced cancer: an exploratory study. J Behav Med. 2017; 40: 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldzweig G, Baider L, Andritsch E, Rottenberg Y. Hope and social support in elderly patients with cancer and their partners: an actor–partner interdependence model. Future Oncol. 2016; 12: 2801–2809. [DOI] [PubMed] [Google Scholar]
- 9.Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: a systematic review. J Psychosom Res. 2009;67: 403–415. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen G, Emmons K, Hunt MK, et al. Model for incorporating social context in health behavior interventions: applications for cancer prevention for working-class, multiethnic populations. Prev Med. 2003;37: 188–197. [DOI] [PubMed] [Google Scholar]
- 11.van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psycho-Oncol. 2011;20:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Chrischilles EA, Wallace RB, et al. Understanding cancer treatment and outcomes: the cancer care outcomes research and surveillance consortium. J Clin Oncol. 2004;22:2992–2996. [DOI] [PubMed] [Google Scholar]
- 13.Idler E, Benyamini Y. Self-rated health and mortality: A review of 28 studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 14.Akin S, Can G, Aydiner A, Ozdilli K, Durna Z. Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur J Oncol Nurs. 2010;14:400–409. [DOI] [PubMed] [Google Scholar]
- 15.Ediebah D, Coens C, Zikos E, et al. Does change in health-related quality of life score predict survival? Analysis of EORTC 08975 lung cancer trial. Brit J Cancer. 2014;110:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro LC, Zagar TM, Reeve BB. The prognostic value of pre-diagnosis health-related quality of life on survival: a prospective cohort study of older Americans with lung cancer. Qual Life Res. 2017;26:1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. Cancer. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 18.Carter‐Harris L Lung cancer stigma as a barrier to medical help‐seeking behavior: Practice implications. J Am Assoc Nurse Pra. 2015;27:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cataldo JK, Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncol. 2013;85:33–40. [DOI] [PubMed] [Google Scholar]
- 20.Chambers SK, Dunn J, Occhipinti S, et al. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328:1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp S, Marziliano A, Moyer A. Identity threat and stigma in cancer patients. Health Psychol. 2014; DOI: 10.1177/2055102914552281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebel S, Castonguay M, Mackness G, Irish J, Bezjak A, Devins GM. The psychosocial impact of stigma in people with head and neck or lung cancer. Psycho-Oncol. 2013;22:140–152. [DOI] [PubMed] [Google Scholar]
- 24.Riley KE, Ulrich MR, Hamann HA, Ostroff JS. Decreasing Smoking but Increasing Stigma? Anti-tobacco Campaigns, Public Health, and Cancer Care. AMA J Ethics. 2017;19:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steffen LE, Vowles KE, Smith BW, Gan GN, Edelman MJ. Daily diary study of hope, stigma, and functioning in lung cancer patients. Health Psychol. 2018;37:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss J, Yang H, Weiss S, et al. Stigma, self-blame, and satisfaction with care among patients with lung cancer. J Psychosoc Oncol. 2017;35:166–179. [DOI] [PubMed] [Google Scholar]
- 27.Raleigh ZT. A biopsychosocial perspective on the experience of lung cancer. J Psychosoc Oncol. 2010;28:116–125. [DOI] [PubMed] [Google Scholar]
- 28.Sriram N, Mills J, Lang E, et al. Attitudes and stereotypes in lung cancer versus breast cancer. PloS one. 2015;10:e0145715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. [DOI] [PubMed] [Google Scholar]
- 30.Greaney ML, Puleo E, Sprunck-Harrild K, Haines J, Houghton SC, & Emmons KM (2018). Social support for changing multiple behaviors: factors associated with seeking support and the impact of offered support. Health Education & Behavior, 45(2), 198–206. [DOI] [PubMed] [Google Scholar]
- 31.Asch DA, Rosin R. Engineering social incentives for health. NEJM. 2016;375:2511–2513. [DOI] [PubMed] [Google Scholar]
- 32.Kreuter M Getting to the roots of health inequity Paper presented at: Experience Summit; June 6, 2018, 2018; St. Louis, MO. [Google Scholar]
- 33.Gilson L, Doherty J, Loewenson R, Francis V. Challenging inequity through health systems. Final report of the Knowledge Network on health systems. 2007. [Google Scholar]
- 34.Bandura A Health promotion by social cognitive means. Health Edu Behav. 2004;31:143–164. [DOI] [PubMed] [Google Scholar]
- 35.Reblin M, Ketcher D, Forsyth P, et al. Outcomes of an electronic social network intervention with neuro-oncology patient family caregivers. J Neuro-Oncol. 2018;3:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.