Abstract
Objective:
Despite viral suppression and immune response on antiretroviral therapy (ART), people with HIV infection experience excess mortality compared to uninfected individuals. The Veterans Aging Cohort Study (VACS) Index incorporates clinical biomarkers of general health with age, CD4 count, and HIV-1 RNA to discriminate mortality risk in a variety of HIV positive populations. We asked whether additional biomarkers further enhance discrimination.
Design and Methods:
Using patients from VACS for development and from the Antiretroviral Therapy Cohort Collaboration (ART-CC) for validation, we obtained laboratory values from a randomly selected visit from 2000-2014, at least one year after ART initiation. Patients were followed for 5-year, all-cause mortality through September 2016. We fitted Cox models with established predictors and added new predictors based on model fit and Harrell’s c-statistic. We converted all variables to continuous functional forms and selected the best model (VACS Index 2.0) for validation in ART-CC patients. We compared discrimination using c-statistics and Kaplan-Meier plots.
Results:
Among 28,390 VACS patients and 12,109 ART-CC patients, 7,293 and 722 died respectively. Nadir CD4, CD8, and CD4:CD8 ratio did not improve discrimination. Addition of albumin, white blood count (WBC), and body mass index (BMI), improved c-statistics in VACS from 0.776 to 0.805 and in ART-CC from 0.800 to 0.831. Results were robust in all 9 ART-CC cohorts, all lengths of follow-up and all subgroups.
Conclusion
VACS Index 2.0, adding albumin, WBC, and BMI to version 1.0 and using continuous variables, provides improved discrimination and is highly transportable to external settings.
Keywords: albumin, BMI, cohort study, comorbidity, mortality, prognostic index, validation
Introduction
With antiretroviral treatment (ART), people with HIV infection (PWH) typically achieve viral suppression, leading to increasing CD4 count. However, their health remains compromised compared with demographically similar individuals without HIV [1-4]. Traditional HIV biomarkers (CD4 count and HIV-1 RNA) are no longer sufficient for clinical management and research. The Veterans Aging Cohort Study (VACS) Index, a validated, generalizable risk index [5], employs routine clinical data to provide a summary of overall disease burden. Higher scores indicate increasing risk of all-cause mortality, hospitalization [6], medical intensive care admission [6], cardiovascular disease [7], fragility fractures [8] and cognitive compromise [9, 10]. The original Index (version 1.0) includes age, CD4, HIV-1 RNA and general health biomarkers (hemoglobin, alanine and aspartate transaminases, platelets, creatinine and hepatitis C virus [HCV] sero-status). Adding these biomarkers to an index restricted to age, CD4 and HIV-1 RNA substantially improved discrimination (c-statistic: 0.78 vs 0.72) [5].
Although widely used, VACS Index 1.0 has limitations. It categorizes predictors to simplify calculation and interpretation, limiting its ability to detect small changes. While discrimination (how well those who die are distinguished from those who do not) is better than other risk indices in common use [11-14] adding predictors might further improve discrimination. Blood pressure, cholesterol and smoking did not improve VACS Index 1.0 [15], but team clinicians suggested other variables shown to be associated with poor outcomes. These include: nadir CD4, CD8, CD4:CD8 ratio [16, 17], albumin [18-21], white blood count (WBC) or absolute neutrophil count (ANC) [22, 23], and body mass index (BMI) [24, 25].
We aimed to 1) develop an improved VACS Index (2.0), 2) externally validate using data from European and North American cohorts participating in the Antiretroviral Therapy Cohort Collaboration (ART-CC), and 3) evaluate generalizability among important subgroups.
Methods
Development of VACS Index 2.0
We developed VACS Index 2.0 using patients from VACS, a cohort of all HIV-infected US military veterans in Veterans Health Administration (VA) care [26]. For this analysis, eligible patients were at least 18 years old, initiated ART between 1996 and 2014, and had a visit between 2000 and 2014. We excluded 2,782 individuals who had negative HCV RNA (at any time during the study period) after previously having detectable HCV RNA, because they may have received treatment for HCV infection or spontaneously cleared the virus. Few patients were treated for HCV prior to availability of direct acting antivirals (DAA) starting in 2014 and there is not yet long-term follow-up for those treated with DAAs. We obtained all laboratory values and BMI for a given individual for each visit date, at least one year after ART initiation. Values obtained prior to the visit date were allowed to carry forward for up to 180 days, resulting in complete information for 75% of visits. In sensitivity analysis, allowing values to carry forward for one year, 87% of visits had complete data. We randomly selected a visit date for each patient from among those with complete data to represent a typical patient in care. In addition to outpatient data, laboratory results obtained during hospitalization were included to provide a wider range of values. We only included one random day per hospitalization in the visit pool to avoid over-representation in the sampled visit days. Patients were followed up to five years for all-cause mortality until September 30, 2016. Ascertainment of deaths of VA patients is excellent [27, 28].
We first replicated the previously published VACS Index (1.0) by fitting a Cox model in the newly derived dataset using categorical predictors (age, CD4 count, HIV-1 RNA and laboratory measurements of hemoglobin, aspartate and alanine transaminases (AST, ALT), platelets, creatinine, and HCV status). Composite markers of liver and renal injury were calculated. FIB-4 is a validated indicator of liver fibrosis [29]. Estimated glomerular filtration rate (eGFR) is a validated indicator of impaired renal function based on the CKDEPI equation [30]. HCV infection status was based on detectable plasma HCV-RNA (85%), positive antibody test (10%), or documented diagnosis (5%). Once testing HCV positive, patients were assumed to remain positive (since we excluded treated patients). For comparison, we also modeled VACS Index 1.0 predictors as continuous variables, as described below.
We then evaluated additional candidate variables, one at a time and in combination, using Akaike’s information criterion (AIC, lower is better) for model fit and Harrell’s c-statistic (range 0.5 to 1.0, higher is better) for discrimination. We used categorical variables with 10-levels for each predictor, with equal number of deaths in each category. We fitted Cox models and plotted coefficients of categorized variables by median of each category. Categories were refined to assess shape of the curve, maintaining at least 100 deaths per level. We determined an appropriate continuous functional form for each variable including quadratic, cubic, and natural log terms to account for U-shaped associations. Extreme values were replaced with the 1st or 99th percentile to avoid undue influence; most variables were centered at the median. Splines were used if a suitable polynomial form was not found. Once a candidate final model was developed, we left out one variable at a time to see if any predictor could be dropped without affecting model fit and discrimination.
To create scores, we used regression coefficients, estimated in this sample, for VACS Index 1.0 (original index, categorical variables) and VACS Index 2.0 (additional predictors, continuous variables). We applied regression equations to each patient using their lab values and the model coefficients to create linear predictors for each index, which were then scaled to create scores of approximately 0 to 100. To illustrate in a clinically meaningful way, we calculated scores using a range of plausible values (between lowest and highest included in the model) for each predictor, while setting all others to the median. The range of scores showed which predictors had the greatest influence.
Validation of VACS Index 2.0
We validated VACS Index 2.0 using data from ART-CC (described elsewhere [31]), an international collaboration combining data on PWH from Europe and North America. Eligible cohorts contributed data on laboratory values of interest and reported at least 40 deaths in such patients. Included cohorts were randomly assigned a letter from A through I for anonymity. Patients and laboratory values were selected using the same approach as described for VACS patients, but without any limitation of values obtained during hospitalization (hospitalization dates were not available). The proportion of visit dates with complete information varied between 5% and 82% by cohort. Those with linkage to an electronic health record (EHR) had more complete data. In sensitivity analysis we compared discrimination between cohorts with at least 50% completeness to those with less than 50%.
Using VACS Index scores as predictors we compared performance in VACS and ART-CC (overall and by cohort). We evaluated discrimination using c-statistics, hazard ratios per 5-unit increase in VACS Index 2.0 score in Cox models, and Kaplan-Meier (KM) plots by decile of risk (customized for VACS and ART-CC to have equal number of deaths per decile). We evaluated discrimination at varying lengths of follow-up (30 days, 90 days, 6 months, 1, 2, 3, 4 and 5 years) using fixed weights from 5-year outcome models developed in VACS.
Performance across subgroups
Finally, development and validation datasets were combined to evaluate performance in subgroups [women; those with HIV-1 RNA<500 copies/mL; HCV co-infected patients; and low-risk patients (age <50 years, CD4 ≥200 cells/mm3 and HIV-1 RNA ≤500 copies/mL)]. Those not meeting criteria for low-risk were categorized as high-risk. We calculated c-statistics and mortality rates in patients defined as low- and high-risk as a function of VACS Index 2.0 score.
We used SAS version 9.4 (SAS Institute, Cary, NC, USA) for all analyses, except that calculation of Harrell’s c-statistic used Stata version 14 (Stata Corp., College Station, TX, USA). Institutional review boards from each cohort approved analysis of routinely collected data.
Results
Half the randomly selected visit dates were in 2010 and later (Table 1). Among 28,390 VACS patients there were 7,293 deaths (7.2 per 100 person-years (PY)); 39% occurred in the first year of follow-up. Median time on ART at the random visit date was 4.2 years; subsequent median follow-up was 4.1 years. Among 12,109 ART-CC patients there were 722 deaths (2.0 per 100 PY, ranging 1.2 to 4.5 by cohort); 44% occurred in the first year. Median time on ART was 4.2 years, median follow-up was 3.2 years. Compared to ART-CC, VACS patients were older (median 53 vs 43 years), more likely to be male (98% vs 74%) and more likely to have initiated ART before 1999 (Table 1). VACS patients were less likely than ART-CC patients to be virally suppressed (76% vs 88%) or defined as low-risk (24% vs 60%).
Table 1.
Characteristics of patients at a randomly selected visit date between 2000 and 2014, after a minimum of 1 year of antiretroviral therapy, in the development sample (VACS) and validation sample (ART-CC).
| VACS (N = 28390) |
ART-CC (N = 12109) |
|||
|---|---|---|---|---|
| Random visit date | ||||
| 2000-2004 | 6587 | (23) | 1307 | (11) |
| 2005-2009 | 7753 | (27) | 4744 | (39) |
| 2010-2014 | 14050 | (49) | 6058 | (50) |
| ART Initiation | ||||
| 1996-1998 | 7929 | (28) | 1696 | (14) |
| 1999-2002 | 6454 | (23) | 3282 | (27) |
| 2003-2007 | 6510 | (23) | 3958 | (33) |
| 2008-2014 | 7497 | (26) | 3173 | (26) |
| Years on ART | ||||
| Median (IQR) | 4.2 | (2.2-7.6) | 4.2 | (2.2-7.4) |
| Age (years) | ||||
| Median (IQR) | 52 | (46-59) | 43 | (36-49) |
| Male | 27696 | (98) | 8972 | (74) |
| Race | ||||
| White | 11576 | (41) | 6840 | (56) |
| Black | 13722 | (48) | 1403 | (12) |
| Hispanic | 2225 | (8) | 255 | (2) |
| Other/unknown | 867 | (3) | 3611 | (30) |
| CD4 cell count (cells/ul) | ||||
| Median (IQR) | 435 | (249-643) | 500 | (335-690) |
| HIV-1 RNA <= 500 copies/mL | ||||
| 21561 | (76) | 10650 | (88) | |
| Hemoglobin (g/dl) | ||||
| Median (IQR) | 14.0 | (12.8-15.1) | 14.3 | (13.0-15.3) |
| FIB-4 | ||||
| <1.45 | 15782 | (56) | 8994 | (74) |
| 1.45-3.25 | 9722 | (34) | 2459 | (20) |
| >3.25 | 2886 | (10) | 656 | (5) |
| eGFR (ml/min) | ||||
| Median (IQR) | 90 | (73-105) | 101 | (87-113) |
| Hepatitis C infection | 5523 | (19) | 1803 | (15) |
| Albumin (g/dl) | ||||
| Median (IQR) | 4.0 | (3.7-4.3) | 4.3 | (4.0-4.5) |
| White blood count (k/ml) | ||||
| Median (IQR) | 5.5 | (4.3-6.9) | 5.8 | (4.7-7.2) |
| Body mass index, kg/m2 | ||||
| Median (IQR) | 25.3 | (22.4-28.7) | 24.2 | (21.7-27.2) |
| Low-risk* | 6907 | (24) | 7303 | (60) |
Age <50 years, CD4 >= 200, and HIV-1 RNA <= 500
In VACS (development) data, model fit and discrimination improved with addition of CD4:CD8 ratio, BMI, albumin and WBC, individually and in combination, compared to VACS Index 1.0 (Appendix Figure 1). However, removal of CD4:CD8 ratio from the candidate final model did not decrease performance so it was dropped. Prediction was not improved with addition of nadir CD4 or CD8 count. WBC and ANC were highly correlated (r = 0.87) and performed equally well, but WBC was more widely available. The final VACS Index 2.0, using continuous variables, included all original variables (age, CD4 count, HIV-1 RNA, hemoglobin, FIB-4, eGFR, and HCV status) plus albumin, WBC, and BMI. Polynomial forms were found for all variables except eGFR which was modeled using splines (Appendix Table 1). Extending last value carried forward time to one year provided <3% additional visit dates or deaths, and all estimates were similar to those obtained using 180 days in the main analysis.
When scores were calculated across a plausible range: age and albumin had the greatest influence. To illustrate, age 30 corresponds to 32 points and age 75 corresponds to 59 points, for a range of 27 points. An albumin of 2.0 g/dl corresponds to 65 points and 5.0 g/dl corresponds to 39 points, for a range of 26 points (Appendix Table 2). CD4 count (10-900 cells/ul, 23 points), HIV-1 RNA (1.3-5.0 log10 copies/mL, 18 points), FIB-4 (0.5-7.5, 20 points), BMI (15-35 kg/m2, 20 points), hemoglobin (9-16 g/dl, 16 points), and eGFR (0-180 ml/min, 16 points) were also influential on total score. In contrast HCV (yes or no, 6 points) was the least influential, as in VACS Index 1.0.
VACS Index 2.0 scores were 10 points higher in VACS (median 51, interquartile range 39-66) than in ART-CC (41, 33-52), with little variation by cohort except for Cohort C (35, 27-46). Scores were approximately normally distributed, but slightly right skewed (means: VACS, 54, ART-CC, 44). Mortality hazard ratios per 5-point increment of score were 1.31 (95% confidence interval [CI], 1.30-1.31) in VACS and 1.37 (1.35-1.39) in ART-CC with little variation by cohort (range 1.34 to 1.41) (Appendix Table 3). In VACS data, the c-statistic increased from 0.779 (95% CI 0.774, 0.784) for VACS Index 1.0 to 0.786 (0.781, 0.791) using VACS Index 1.0 predictors as continuous variables. The c-statistic further increased to 0.805 (0.800, 0.810) after addition of albumin, WBC, and BMI (VACS Index 2.0). Corresponding c-statistics in ART-CC data were 0.800 (0.782, 0.818) for VACS Index 1.0; 0.808 (0.790, 0.825) for continuous VACS 1.0 predictors and 0.831 (0.814, 0.847) for VACS Index 2.0. C-statistics improved in all 9 ART-CC cohorts (Figure 1a). In cohorts with at least 50% completeness in the visit pool and in those with less than 50% completeness, the c-statistic was greater with VACS Index 2.0, with no separation in confidence intervals comparing completeness. At all follow-up intervals VACS Index 2.0 had greater discrimination than 1.0 (Figure 1b and 1c). As expected, c-statistics were greater for shorter follow-up. Additionally, improvement from VACS Index 1.0 to 2.0 was greatest for shorter follow-up.
Figure 1.
Discrimination of 5-year, all-cause mortality, for VACS Index 1.0 (left) and VACS Index 2.0 (right): a. VACS, ART-CC and individual ART-CC cohorts. LT50 = ART-CC, complete data available for less than 50% of eligible, GE50= ART-CC, complete data available for at least 50% of eligible; b. VACS; c. ART-CC
KM plots by decile of risk (Figure 2, Appendix Table 4) in VACS showed better separation with VACS Index 2.0 compared to 1.0. While VACS Index 1.0 deciles 6 and 7 overlapped until 1 year, VACS Index 2.0 deciles were all distinct around 6 months of follow-up. Survival at 5-years for extreme deciles expanded from 13-92% with VACS Index 1.0 to 8-93% with VACS Index 2.0. In ART-CC, with only one-tenth as many deaths, curves were less distinct, but also showed improvement with VACS Index 2.0. The range of 5-year survival expanded from 35-97% with VACS Index 1.0 to 25-98% with 2.0. Similar patterns were seen with 1-year survival. In both VACS and ART-CC median survival was less than a year for those in the highest VACS Index 2.0 decile. Based on above findings we combined VACS and ART-CC data to look at subgroups.
Figure 2.
Kaplan-Meier plots for all-cause mortality by decile of risk according to VACS Index 1.0 and VACS Index 2.0, in development sample, VACS (a and b) and validation sample, ART-CC (c and d). Further detail available in Appendix Table 4.
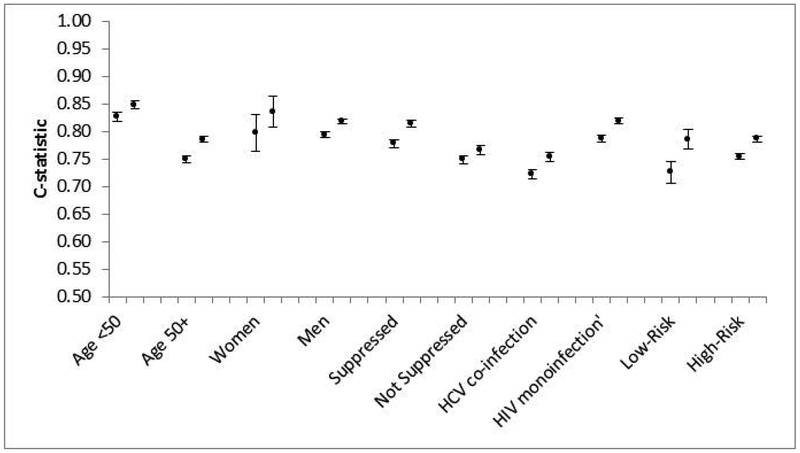
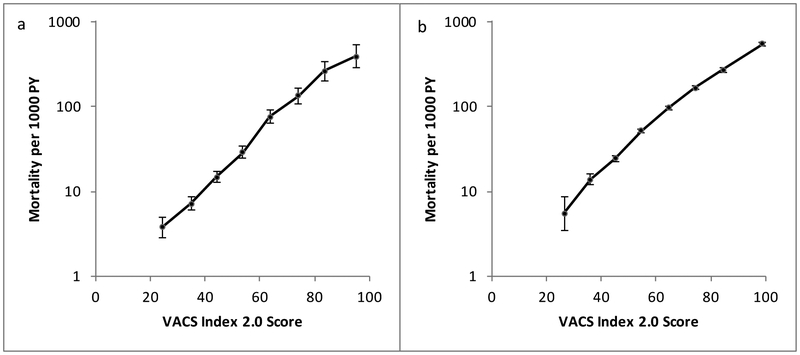
Combined data demonstrated higher c-statistics for VACS Index 2.0 than 1.0 for all subgroups (Figure 3): age <50 (0.85, 0.83), age 50+ (0.79, 0.75), men (0.82, 0.79), women (0.84, 0.80), suppressed virus (0.82, 0.78), unsuppressed virus (0.77, 0.75), HIV mono-infected (0.82, 0.79) and HCV co-infected (0.75, 0.72) and patients defined as low-risk (0.79, 0.73) and high-risk (0.79, 0.76). Mortality rates in both low-risk and high-risk patients had strong and similar associations with VACS Index 2.0 score (Figure 4).
Figure 3.
Discrimination of 5-year, all-cause mortality, for VACS Index 1.0 (left) and VACS Index 2.0 (right), in combined VACS and ART-CC data subgroups. Low-Risk = age <50 years, CD4 count ≥ 200 cells/μl, and HIV-RNA ≤ 500 copies/mL. High-Risk = all others.
Figure 4.
All-cause mortality rates during 5 years of follow-up by VACS Index 2.0 score. a. Low risk patients (age <50 years, CD4 ≥200 cells/ml, HIV-1 RNA ≤500 copies/mL), b. High risk patients (all others).
Discussion
VACS Index 2.0 had better discrimination than 1.0 in development (VACS) and external validation (ART-CC) data. This was achieved by study design; treating all predictors as continuous; and adding albumin, WBC, and BMI. Improved discrimination was evident across a variety of important subgroups, varying length of follow-up and across ART-CC cohorts. Improved discrimination was evident beyond c-statistics. Compared to VACS Index 1.0, KM plots comparing deciles of 2.0 showed better separation of mortality risk during the first 6-12 months of follow-up, that persisted across the 5-year follow-up. In both low- and high-risk patients there was a strong and consistent gradient of higher mortality with increasing score. Improved discrimination of VACS Index 2.0 was shown to be transportable to other settings [32].
Thus, VACS Index 2.0 can be used as a measure of disease burden for risk adjustment and/or as an outcome for clinical research. With automated calculation and risk interpretation by way of smartphone apps, online calculators, or decision support modules in EHRs, it can also be incorporated in medical decision making.
Generalizability of VACS Index 2.0 was likely enhanced by our study design. Because we started follow-up from a randomly selected date, the index was designed around a typical patient in care, rather than optimizing for some fixed point in clinical management. Including laboratory values obtained during hospitalization increased the range of severity of illness represented in model development data.
VACS Index 2.0 predictors are continuous, offering important advantages over the thresholds in VACS Index 1.0. For example, on the day a patient turns 50 the VACS Index 1.0 score increases by 12 points, translating to roughly 40% increased risk of mortality. While this risk is accurate in aggregate for those aged 50-64 years, no individual would experience such an abrupt change. VACS Index 2.0 models this change in risk smoothly across ages. Thresholds in VACS Index 1.0 limited investigator’s ability to use the index as an outcome to detect change from baseline to end of observation. With continuous variables more subtle changes in risk can be detected, enhancing suitability for longitudinal patient management.
Addition of albumin, WBC, and BMI enhanced discrimination of the index, and provided interesting insights. After age, albumin is the single most important marker of general health in the model. Low serum albumin may be associated with multiple HIV-related conditions (e.g. poor nutritional status, inflammation, nephropathy, and liver disease). We suspect that albumin is particularly important as an added indication of liver disease, which is increasingly common among those aging with HIV. In VACS Index 1.0 liver injury was only ascertained with FIB-4 and an indicator for HCV infection. Albumin measures liver synthetic function, thus enhancing detection of injury. We chose not to include hospitalization as a predictor because we want to use the index to predict future hospitalization. Also, hospitalization can be considered a downstream event in the causal pathway between VACS Index components and death. Inclusion would obfuscate associations with validated predictors. Finally, varying reasons for hospitalization have different associations with mortality.
VACS Index 2.0 is a stronger predictor than 1.0. Despite having similar ranges of scores, the hazard ratio for 5-year, all-cause mortality increased from 1.221 (1.216-1.227) per 5 points with VACS Index 1.0, to 1.307 (1.300-1.314) per 5 points with VACS Index 2.0. VACS Index 2.0 is better able to identify high-risk patients within 6 months of follow-up. In the 10th decile on KM plots, estimated 6-month survival in VACS patients decreased from 61% with VACS Index 1.0 to 51% with VACS Index 2.0. In ART-CC this change was 74% to 59%.
Interestingly, VACS Index 2.0 had higher discrimination in validation (ART-CC) than in development (VACS). This was also observed in validation of VACS Index 1.0 in ART-CC [5]. There are several possible explanations. First, follow-up time in ART-CC was shorter. All else equal, proximal deaths are easier to predict than distant deaths. Second, ART-CC subjects are younger and discrimination is slightly better among those under 50 years. Finally, the index is not designed to detect risk of unnatural deaths, such as suicide, accident, or overdose. Such deaths are more common in veteran populations [33, 34].
In prognostic modelling, important subgroups may be underrepresented, such as women in VACS. Therefore, it is important to demonstrate discrimination within these groups. We found superior discrimination with VACS Index 2.0 in all subgroups (including women) and among each of the nine participating cohorts in ART-CC. These observations offer strong evidence that improved discrimination of VACS Index 2.0 will generalize to new populations. It also suggests that the strong associations previously demonstrated with VACS Index 1.0 and biomarkers of inflammation [16, 35-37], hospitalization and medical intensive care unit admission [38], myocardial infarction [7], neurocognitive performance [9, 10], and fragility fractures [8, 39] will hold for 2.0.
Of note, improvement in discrimination from VACS Index 1.0 to 2.0 was unusually large in cohort F, increasing from 0.790 (95% CI 0.744, 0.835) to 0.873 (0.841, 0.906). We think this is due to missing data leading to selection of sicker patients with higher short-term mortality. Only 5% of visits had complete data. According to cohort personnel, selecting people with both hemoglobin and albumin likely sampled some of the sickest subjects, likely to die over a short interval of time. In fact, 40% of deaths occurred in the first 6 months, 10% higher (absolute) than any other cohort. Increased discrimination from VACS Index 1.0 to 2.0 was greatest for shorter follow-up times (Figure 1c).
The original VACS Index has been increasingly used in a variety of research, public health, and clinical settings. Since March 2013, online calculators (https://vacs.med.yale.edu; https://www.mdcalc.com/veterans-aging-cohort-study-vacs-index)) have been accessed >80,000 times. The index has been used as a risk adjuster in observational studies [25, 40]. Two ongoing NIH funded, alcohol intervention trials and the AIDS Clinical Trials Group use the VACS Index in randomized trials [41]. Independent groups are using the index as a measure of frailty or severity of illness [10, 36, 37, 42-50]. Additionally, the index is being used in surveillance. The Public Health-Seattle & King County, HIV/STD Program and the Washington State Department of Health use the index to monitor burden of disease among PWH. Several health systems have incorporated the index as a tool within their EHR for patient management. VACS Index 2.0 will enhance utility for all these applications.
An important limitation of VACS Index 2.0 is that we have not incorporated prognostic implications of HCV cure. Although patients treated for HCV were excluded from development sample, and most follow-up in validation sample is before widespread availability of DAAs, treatment of HCV may still have influenced our findings. In future work we hope to address this limitation once adequate mortality data are available among PWH treated for HCV co-infection. Another limitation is that we could only consider nadir CD4 as observed within the VA EHR, without being sure it is truly the lowest prior to ART initiation Missing data may also be a concern. We only randomly selected visit dates when patients had complete data within the prior 180 days. Nonetheless we found consistent results across all cohorts regardless of the proportion of visits with complete data. Finally, we have yet to conduct analyses determining calibration of VACS Index 2.0. As with the original index, we plan to conduct this analysis in an even broader array of cohorts in the coming months.
In conclusion, VACS Index 2.0 is highly predictive of risk of all-cause mortality among those on treatment for HIV infection. With use of continuous variables, it is now better suited to application for individual patients. With addition of parameters readily obtained during routine clinical practice it is more discriminating than the original VACS Index. Its superior discrimination is robust across development and validation sets, among important clinical subgroups, and among individual cohorts.
Acknowledgements
We thank all patients, doctors, and study nurses associated with the participating cohort studies.
Appendix
Appendix Table 1.
VACS Index 2.0 Cox proportional hazards model, for 5-year, all-cause mortality, estimated in Veterans Aging Cohort Study, varying length of last value carried forward (LVCF).
| N deaths |
Main analysis LVCF 180 days 28390 7293 |
Sensitivity LVCF 1 year 28830 7479 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | PE | SE | χ2 | p | HR (95% CI) | PE | SE | χ2 | p | HR (95% CI) |
| Age (years), censored at 30-75, centered at (age-50) | ||||||||||
| X | 0.056 | 0.012 | 22 | <.0001 | 1.06 (1.03-1.08) | 0.058 | 0.012 | 24 | <.0001 | 1.06 (1.04-1.09) |
| X2 | −0.004 | 0.004 | 2 | 0.22 | 1.00 (0.99-1.00) | −0.006 | 0.004 | 3 | 0.11 | 0.99 (0.99-1.00) |
| X3 | 0.005 | 0.001 | 29 | <.0001 | 1.01 (1.00-1.01) | 0.005 | 0.001 | 30 | <.0001 | 1.01 (1.00-1.01) |
| CD4 cell count (cells/ml), censored at 0-1000, as ln (1000-CD4) | ||||||||||
| X | −0.056 | 0.025 | 5 | 0.03 | 0.95 (0.90-0.99) | −0.048 | 0.025 | 4 | 0.05 | 0.95 (0.91-1.00) |
| X2 | −0.153 | 0.023 | 46 | <.0001 | 0.86 (0.82-0.90) | −0.149 | 0.023 | 43 | <.0001 | 0.86 (0.82-0.90) |
| X3 | 0.024 | 0.002 | 94 | <.0001 | 1.02 (1.02-1.03) | 0.023 | 0.002 | 86 | <.0001 | 1.02 (1.02-1.03) |
| HIV-1 RNA (log copies/ml), censored at 1.3- 5.0, centered at (logVL - 2) | ||||||||||
| X | 0.513 | 0.033 | 247 | <.0001 | 1.67 (1.57-1.78) | 0.518 | 0.032 | 257 | <.0001 | 1.68 (1.58-1.79) |
| X2 | −0.422 | 0.041 | 109 | <.0001 | 0.66 (0.61-0.71) | −0.412 | 0.040 | 106 | <.0001 | 0.66 (0.61-0.72) |
| X3 | 0.098 | 0.011 | 77 | <.0001 | 1.10 (1.08-1.13) | 0.095 | 0.011 | 73 | <.0001 | 1.10 (1.08-1.12) |
| Hemoglobin (g/dl), censored at 9-16, centered at (14 - hemoglobin) | ||||||||||
| X | −0.134 | 0.011 | 141 | <.0001 | 0.88 (0.86-0.89) | −0.132 | 0.011 | 142 | <.0001 | 0.88 (0.86-0.90) |
| X2 | 0.026 | 0.006 | 16 | <.0001 | 1.03 (1.01-1.04) | 0.026 | 0.006 | 17 | <.0001 | 1.03 (1.01-1.04) |
| X3 | 0.005 | 0.001 | 10 | 0.002 | 1.01 (1.00-1.01) | 0.004 | 0.001 | 10 | 0.002 | 1.00 (1.00-1.01) |
| FIB-4, censored at .5-7.5 | ||||||||||
| X | 0.220 | 0.028 | 62 | <.0001 | 1.25 (1.18-1.32) | 0.213 | 0.028 | 59 | <.0001 | 1.24 (1.17-1.31) |
| X2 | −0.009 | 0.003 | 7 | 0.008 | 0.99 (0.99-1.00) | −0.008 | 0.003 | 7 | 0.0106 | 0.99 (0.99-1.00) |
| eGFR (ml/min), censored at 0-180,* | ||||||||||
| X1 | −0.031 | 0.028 | 1 | 0.28 | 0.97 (0.92-1.03) | −0.014 | 0.028 | 0 | 0.61 | 0.99 (0.93-1.04) |
| X2 | −0.077 | 0.045 | 3 | 0.0917 | 0.93 (0.85-1.01) | −0.107 | 0.045 | 6 | 0.0174 | 0.90 (0.82-0.98) |
| X3 | 0.106 | 0.027 | 16 | <.0001 | 1.11 (1.06-1.17) | 0.131 | 0.026 | 25 | <.0001 | 1.14 (1.08-1.20) |
| X4 | 0.133 | 0.034 | 15 | 0.0001 | 1.14 (1.07-1.22) | 0.093 | 0.033 | 8 | 0.0054 | 1.10 (1.03-1.17) |
| Hepatitis C co-infection | ||||||||||
| Yes | 0.342 | 0.028 | 147 | <.0001 | 1.41 (1.33-1.49) | 0.350 | 0.028 | 160 | <.0001 | 1.42 (1.35-1.50) |
| Albumin (g/dl), censored at 2-5, centered at (albumin - 4) | ||||||||||
| X | −0.443 | 0.034 | 165 | <.0001 | 0.64 (0.60-0.69) | −0.467 | 0.034 | 189 | <.0001 | 0.63 (0.59-0.67) |
| X2 | 0.104 | 0.051 | 4 | 0.04 | 1.11 (1.00-1.23) | 0.141 | 0.050 | 8 | 0.01 | 1.15 (1.04-1.27) |
| X3 | 0.028 | 0.027 | 1 | 0.30 | 1.03 (0.98-1.08) | 0.055 | 0.026 | 4 | 0.04 | 1.06 (1.00-1.11) |
| White blood count (k/ml), censored at 2.5-11, centered at (WBC - 5.5) | ||||||||||
| X | 0.126 | 0.011 | 130 | <.0001 | 1.13 (1.11-1.16) | 0.125 | 0.011 | 132 | <.0001 | 1.13 (1.11-1.16) |
| X2 | 0.020 | 0.004 | 30 | <.0001 | 1.02 (1.01-1.03) | 0.021 | 0.004 | 35 | <.0001 | 1.02 (1.01-1.03) |
| X3 | −0.004 | 0.001 | 23 | <.0001 | 1.00 (0.99-1.00) | −0.005 | 0.001 | 27 | <.0001 | 1.00 (0.99-1.00) |
| Body mass index, kg/m2, censored at 15-35, centered at (BMI - 25) | ||||||||||
| X | −0.055 | 0.003 | 388 | <.0001 | 0.95 (0.94-0.95) | −0.055 | 0.003 | 407 | <.0001 | 0.95 (0.94-0.95) |
| X2 | 0.004 | 0.000 | 62 | <.0001 | 1.00 (1.00-1.01) | 0.004 | 0.000 | 62 | <.0001 | 1.00 (1.00-1.00) |
X1 = eGFR/10, X2 = (eGFR-35)/10, X3 = (eGFR-65)/10, X4 = (eGFR-115)/10.
Appendix Table 2.
Range of plausible values and associated VACS Index 2.0 score, setting all other predictors to their median value.
| Predictor | Median | Range of plausible values* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||||
| Value | 52 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 |
| Score | ** | 32 | 38 | 41 | 43 | 44 | 45 | 47 | 49 | 53 | 59 |
| CD4 cell count (cells/ml) | |||||||||||
| Value | 435 | 10 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | 900 |
| Score | ** | 55 | 53 | 51 | 48 | 45 | 43 | 40 | 37 | 34 | 32 |
| HIV-1 RNA (log copies/mL) | |||||||||||
| Value | 1.7 | 1.3 | 1.5 | 1.8 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5 |
| Score | ** | 37 | 41 | 46 | 48 | 51 | 52 | 51 | 50 | 51 | 55 |
| Hemoglobin (g/dl) | |||||||||||
| Value | 14 | 9 | 9.5 | 10 | 10.5 | 11 | 12 | 13 | 14 | 15 | 16 |
| Score | ** | 58 | 58 | 57 | 55 | 54 | 51 | 47 | 44 | 42 | 42 |
| FIB-4 | |||||||||||
| Value | 1.34 | 0.50 | 1.00 | 1.45 | 2.00 | 3.25 | 4.00 | 5.00 | 6.00 | 7.00 | 7.50 |
| Score | ** | 41 | 43 | 45 | 47 | 51 | 53 | 56 | 58 | 60 | 61 |
| eGFR (ml/min) | |||||||||||
| Value | 90 | 0 | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 |
| Score | ** | 53 | 51 | 49 | 45 | 44 | 44 | 46 | 51 | 55 | 60 |
| Hepatitis C co-infection | |||||||||||
| Value | No | Yes | |||||||||
| Score | ** | 51 | |||||||||
| Albumin (g/dl) | |||||||||||
| Value | 4 | 2.00 | 2.25 | 2.50 | 2.75 | 3.00 | 3.25 | 3.5 | 4.00 | 4.50 | 5.00 |
| Score | ** | 65 | 62 | 59 | 57 | 54 | 52 | 49 | 44 | 41 | 39 |
| White blood count (k/ml | |||||||||||
| Value | 5.5 | 2.5 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Score | ** | 43 | 42 | 42 | 43 | 46 | 49 | 51 | 54 | 55 | 55 |
| Body mass index ( kg/m2) | |||||||||||
| Value | 25.3 | 15 | 17 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | 35 |
| Score | ** | 62 | 57 | 55 | 51 | 48 | 46 | 44 | 42 | 41 | 41 |
Clinically meaningful values between lowest and highest values used in development model.
Score = 44 when all values are set to their median and Hepatitis C is set to no.
Appendix Table 3.
Number at risk, number of deaths, distribution of VACS Index 2.0 scores, and all-cause mortality hazard ratio (HR) per 5 points, in the development sample (VACS) and validation sample (ART-CC), overall and by individual cohort (A-I).
| VACS Index 2.0 Score | Risk of all-cause mortality, per 5 points |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Deaths | Median | 25th | 75th | 1st | 99th | HR (95% CI) | |
| VACS | 28,390 | 7,293 | 51 | 39 | 66 | 15 | 111 | 1.31 (1.30-1.31) |
| ART-CC | 12,109 | 722 | 41 | 33 | 52 | 14 | 97 | 1.37 (1.35-1.39) |
| A | 1,011 | 40 | 41 | 31 | 52 | 14 | 91 | 1.41 (1.32-1.52) |
| B | 944 | 95 | 42 | 34 | 53 | 17 | 98 | 1.38 (1.31-1.44) |
| C | 1,872 | 112 | 35 | 27 | 46 | 11 | 93 | 1.37 (1.32-1.42) |
| D | 1,509 | 78 | 44 | 36 | 54 | 18 | 89 | 1.38 (1.31-1.45) |
| E | 863 | 73 | 42 | 33 | 54 | 15 | 104 | 1.34 (1.28-1.41) |
| F | 1,899 | 111 | 42 | 34 | 53 | 17 | 102 | 1.38 (1.33-1.43) |
| G | 2,231 | 120 | 42 | 34 | 54 | 16 | 94 | 1.40 (1.34-1.46) |
| H | 891 | 53 | 44 | 34 | 54 | 19 | 103 | 1.34 (1.27-1.42) |
| I | 889 | 40 | 41 | 33 | 50 | 17 | 95 | 1.40 (1.30-1.51) |
Appendix Table 4.
Number at risk, number of deaths, distribution of VACS Index 2.0 scores, and all-cause mortality hazard ratio (HR) per 5 points, in the development sample (VACS) and validation sample (ART-CC), overall and by individual cohort (A-I).
| N | Died |
30 days Survival |
Left |
Died |
6 months Survival |
Left |
Died |
1 year Survival |
Left |
Died |
5 years Survival |
Left |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VACS sample | |||||||||||||
| Overall | 28390 | 348 | 1706 | 2833 | 7293 | ||||||||
| Decile | |||||||||||||
| VACS Index 1.0 | |||||||||||||
| 1 | 10646 | 12 | 100% | 10634 | 113 | 99% | 10533 | 199 | 98% | 10447 | 732 | 92% | 5247 |
| 2 | 4763 | 17 | 100% | 4745 | 109 | 98% | 4653 | 220 | 95% | 4543 | 737 | 82% | 2325 |
| 3 | 3249 | 21 | 99% | 3228 | 122 | 96% | 3127 | 225 | 93% | 3023 | 723 | 75% | 1469 |
| 4 | 2239 | 27 | 99% | 2211 | 141 | 94% | 2097 | 233 | 90% | 2005 | 737 | 63% | 898 |
| 5 | 1864 | 28 | 98% | 1835 | 140 | 92% | 1722 | 260 | 86% | 1603 | 718 | 58% | 700 |
| 6 | 1449 | 29 | 98% | 1419 | 157 | 89% | 1291 | 264 | 82% | 1185 | 729 | 45% | 427 |
| 7 | 1268 | 30 | 98% | 1237 | 148 | 88% | 1119 | 242 | 81% | 1026 | 716 | 40% | 353 |
| 8 | 1083 | 45 | 95% | 1033 | 215 | 80% | 867 | 351 | 68% | 731 | 743 | 28% | 204 |
| 9 | 962 | 41 | 96% | 920 | 226 | 76% | 733 | 370 | 62% | 592 | 728 | 21% | 137 |
| 10 | 867 | 98 | 88% | 763 | 335 | 61% | 531 | 469 | 46% | 398 | 730 | 13% | 64 |
| VACS Index 2.0 | |||||||||||||
| 1 | 12381 | 10 | 100% | 12371 | 100 | 99% | 12281 | 185 | 99% | 12196 | 729 | 93% | 5586 |
| 2 | 4275 | 16 | 100% | 4259 | 96 | 98% | 4179 | 196 | 95% | 4079 | 729 | 81% | 2324 |
| 3 | 2853 | 24 | 99% | 2827 | 105 | 96% | 2747 | 220 | 92% | 2633 | 730 | 72% | 1405 |
| 4 | 2029 | 14 | 99% | 2014 | 108 | 95% | 1919 | 207 | 90% | 1821 | 730 | 61% | 878 |
| 5 | 1597 | 23 | 99% | 1573 | 116 | 93% | 1480 | 213 | 87% | 1383 | 729 | 51% | 593 |
| 6 | 1391 | 19 | 99% | 1371 | 140 | 90% | 1249 | 260 | 81% | 1130 | 729 | 44% | 437 |
| 7 | 1149 | 19 | 98% | 1128 | 175 | 85% | 974 | 305 | 73% | 844 | 729 | 33% | 279 |
| 8 | 1016 | 35 | 96% | 979 | 203 | 80% | 812 | 324 | 68% | 691 | 729 | 25% | 183 |
| 9 | 888 | 61 | 93% | 827 | 264 | 70% | 623 | 397 | 55% | 491 | 729 | 15% | 95 |
| 10 | 811 | 127 | 84% | 678 | 399 | 51% | 411 | 526 | 35% | 285 | 730 | 8% | 41 |
| ART-CC sample | |||||||||||||
| Overall | 12109 | 47 | 192 | 318 | 722 | ||||||||
| Decile | |||||||||||||
| VACS Index 1.0 | |||||||||||||
| 1 | 4824 | 2 | 100% | 4789 | 10 | 100% | 4443 | 23 | 99% | 3915 | 72 | 97% | 1398 |
| 2 | 2087 | 1 | 100% | 2065 | 8 | 100% | 1928 | 19 | 99% | 1745 | 64 | 95% | 694 |
| 3 | 1824 | 5 | 100% | 1800 | 16 | 99% | 1688 | 31 | 98% | 1539 | 82 | 94% | 610 |
| 4 | 1148 | 1 | 100% | 1138 | 10 | 99% | 1057 | 20 | 98% | 960 | 68 | 91% | 394 |
| 5 | 824 | 2 | 100% | 816 | 20 | 97% | 739 | 31 | 96% | 670 | 75 | 87% | 258 |
| 6 | 492 | 4 | 99% | 485 | 21 | 95% | 428 | 35 | 92% | 376 | 72 | 81% | 149 |
| 7 | 362 | 7 | 98% | 350 | 24 | 93% | 300 | 36 | 89% | 254 | 73 | 71% | 82 |
| 8 | 206 | 4 | 98% | 202 | 21 | 89% | 169 | 32 | 83% | 141 | 71 | 53% | 39 |
| 9 | 196 | 9 | 95% | 186 | 26 | 86% | 153 | 46 | 74% | 120 | 72 | 52% | 43 |
| 10 | 146 | 12 | 91% | 130 | 36 | 74% | 97 | 45 | 67% | 78 | 73 | 35% | 19 |
| VACS Index 2.0 | |||||||||||||
| 1 | 5838 | 1 | 100% | 5785 | 10 | 100% | 5356 | 27 | 99% | 4662 | 73 | 98% | 1559 |
| 2 | 2397 | 1 | 100% | 2379 | 10 | 100% | 2224 | 16 | 99% | 2051 | 72 | 95% | 865 |
| 3 | 1247 | 3 | 100% | 1240 | 14 | 99% | 1169 | 26 | 98% | 1070 | 72 | 92% | 489 |
| 4 | 884 | 1 | 100% | 876 | 12 | 99% | 812 | 24 | 97% | 755 | 71 | 89% | 335 |
| 5 | 618 | 4 | 99% | 609 | 12 | 98% | 557 | 29 | 95% | 501 | 73 | 83% | 197 |
| 6 | 359 | 1 | 100% | 355 | 19 | 94% | 305 | 28 | 91% | 267 | 73 | 69% | 83 |
| 7 | 311 | 3 | 99% | 302 | 22 | 92% | 255 | 38 | 86% | 213 | 72 | 69% | 84 |
| 8 | 190 | 6 | 97% | 182 | 20 | 89% | 159 | 33 | 81% | 133 | 72 | 52% | 46 |
| 9 | 151 | 11 | 92% | 139 | 29 | 80% | 109 | 44 | 69% | 88 | 71 | 37% | 15 |
| 10 | 114 | 16 | 85% | 95 | 44 | 59% | 61 | 53 | 51% | 47 | 73 | 25% | 15 |
Appendix Figure 1.
Model development in VACS Cohort comparing model fit using Akaike’s information criterion (AIC) and discrimination using Harrell’s c-statistic
Footnotes
Participating ART-CC cohorts: AIDS Therapy Evaluation Project Netherlands (ATHENA), Austrian HIV Cohort Study (AHIVCOS), Italian Cohort of Antiretroviral-Naive Patients (ICONA), Aquitaine Cohort (France), Swiss HIV Cohort Study (SHCS), VACH (Spain), South Alberta Clinical Cohort (Canada), Tennessee Center for AIDS Research Cohort (US), and the University of Washington HIV Cohort (US)
References
- 1.Wong C, et al. , Multimorbidity Among Persons Living with Human Immunodeficiency Virus in the United States. Clin Infect Dis, 2018. 66(8): p. 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg RS, et al. , Health-adjusted life expectancy in HIV-positive and HIV-negative men and women in British Columbia, Canada: a population-based observational cohort study. Lancet HIV, 2017. 4(6): p. e270–e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park LS, et al. , Association of Viral Suppression With Lower AIDS-Defining and Non-AIDS-Defining Cancer Incidence in HIV-Infected Veterans: A Prospective Cohort Study. Ann Intern Med, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Althoff KN, et al. , Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin. Infect. Dis, 2015. 60(4): p. 627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tate JP, et al. , An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS, 2013. 27(4): p. 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akgun KM, et al. , Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV infected Veterans. J. Acquir. Immune. Defic. Syndr, 2013. 62(1): p. 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salinas JL, et al. , Baseline, Time-Updated, and Cumulative HIV Care Metrics for Predicting Acute Myocardial Infarction and All-Cause Mortality. Clin Infect Dis, 2016. 63(11): p. 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Womack JA, et al. , Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin. Infect. Dis, 2013. 56(10): p. 1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquine MJ, et al. , The Veterans Aging Cohort Study (VACS) Index and Neurocognitive Change: A Longitudinal Study. Clin Infect Dis, 2016. 63(5): p. 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marquine MJ, et al. , The Veterans Aging Cohort Study Index is Associated With Concurrent Risk for Neurocognitive Impairment. J. Acquir. Immune. Defic. Syndr, 2014. 65(2): p. 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnino MW, et al. , APACHE II scoring to predict outcome in post-cardiac arrest. Resuscitation, 2013. 84(5): p. 651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards G, et al. , CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J Intensive Care Med, 2011. 26(1): p. 34–40. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, et al. , Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care, 2015. 43(2): p. 175–86. [DOI] [PubMed] [Google Scholar]
- 14.Kieszak SM, et al. , A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol, 1999. 52(2): p. 137–42. [DOI] [PubMed] [Google Scholar]
- 15.Tate J, Freiberg M, and AC J. Do Risk Factors for Cardiovascular Disease Improve VACS Index Prediction of All Cause Mortality? in 16th International Workshop on HIV Observational Databases (IWHOD) 2012. Athens, Greece. [Google Scholar]
- 16.Duffau P, et al. , Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS, 2015. 29(16): p. 2099–108. [DOI] [PubMed] [Google Scholar]
- 17.Trickey A, et al. , CD4:CD8 Ratio and CD8 Count as Prognostic Markers for Mortality in Human Immunodeficiency Virus-Infected Patients on Antiretroviral Therapy: The Antiretroviral Therapy Cohort Collaboration (ART-CC). Clin Infect Dis, 2017. 65(6): p. 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang J, et al. , Serum albumin and short-term risk for mortality and cardiovascular disease among HIV-infected veterans. Aids, 2013. 27(8): p. 1339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SH, et al. , Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses, 2006. 22(1): p. 14–21. [DOI] [PubMed] [Google Scholar]
- 20.Siedner MJ and Hunt PW, All About the Albumin? Prognostic Capacity of Serum Albumin in Patients With Treated HIV Infection. J Infect Dis, 2018. 217(3): p. 347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronit A, et al. , Serum Albumin as a Prognostic Marker for Serious Non-AIDS Endpoints in the Strategic Timing of Antiretroviral Treatment (START) Study. J Infect Dis, 2018. 217(3): p. 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunyer J, et al. , Longitudinal relation between smoking and white blood cells. Am J Epidemiol, 1996. 144(8): p. 734–41. [DOI] [PubMed] [Google Scholar]
- 23.Madjid M, et al. , Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol, 2004. 44(10): p. 1945–56. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, et al. , Relationship between Body Mass Index and Mortality in HIV-Infected HAART Users in the Women’s Interagency HIV Study. PLoS One, 2015. 10(12): p. e0143740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuh B, et al. , Weight change after antiretroviral therapy and mortality. Clin Infect Dis, 2015. 60(12): p. 1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fultz SL, et al. , Development and verification of a “virtual” cohort using the National VA Health Information System. Med. Care, 2006. 44(8 Suppl 2): p. S25–S30. [DOI] [PubMed] [Google Scholar]
- 27.Fisher SG, et al. , Mortality ascertainment in the veteran population: alternatives to the national death index. American Journal of Epidemiology, 1995. 141(3): p. 242–250. [DOI] [PubMed] [Google Scholar]
- 28.Sohn MW, et al. , Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr, 2006. 4: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterling RK, et al. , Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology, 2006. 43(6): p. 1317–1325. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, et al. , A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009. 150(9): p. 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May MT, et al. , Cohort profile: Antiretroviral Therapy Cohort Collaboration (ART-CC). Int J Epidemiol, 2014. 43(3): p. 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justice AC, Covinsky KE, and Berlin JA, Assessing the generalizability of prognostic information. Ann Intern Med, 1999. 130(6): p. 515–524. [DOI] [PubMed] [Google Scholar]
- 33.Simkus K, V.L., Pedlar D, Veteran Suicide Mortality Study (1976 to 2012), in Veterans Affairs Canada,. 2017. [Google Scholar]
- 34.Weiner J, et al. , Military veteran mortality following a survived suicide attempt. BMC Public Health, 2011. 11: p. 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justice AC, et al. , Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin. Infect. Dis, 2012. 54(7): p. 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams B, et al. , SCD14 and SCD163 Levels Are Correlated with VACS Index Scores: Initial Data from the Blunted Immune Recovery in CORE Patients with HIV (BIRCH) Cohort. AIDS Res Hum Retroviruses, 2016. 32(2): p. 144–147. [DOI] [PubMed] [Google Scholar]
- 37.Mooney S, et al. , Elevated Biomarkers of Inflammation and Coagulation in Patients with HIV Are Associated with Higher Framingham and VACS Risk Index Scores. PLoS One, 2015. 10(12): p. e0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akgun KM, et al. , Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit. Care. Med, 2013. 41(6): p. 1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin MT, et al. , Fracture prediction with modified-FRAX in older HIV-infected and uninfected men. J Acquir Immune Defic Syndr, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice AC, et al. , Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS, 2018. 32(6): p. 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tashima KT, et al. , Mortality among HIV+ Participants Randomized to Omit NRTIs vs. Add NRTIs in OPTIONS (ACTG A5241). 21st Conference on Retroviruses and Opportunistic Infections (CROI), 2014. [Google Scholar]
- 42.Robinson-Papp J and Sharma SK, Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDS., 2013. 27(10): p. 539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adeyemi O and Livak B, Higher Veterans Aging Cohort Study (VACS) index scores in HIV-positive adults with CD4 counts <200 cells/mm3 despite viral suppression. J. Acquir. Immune. Defic. Syndr, 2013. 63(2): p. e78–81. [DOI] [PubMed] [Google Scholar]
- 44.Furuya-Kanamori L, Kelly MD, and McKenzie SJ, Co-morbidity, ageing and predicted mortality in antiretroviral treated Australian men: a quantitative analysis. PLoS. One, 2013. 8(10): p. e78403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huggan PJ, et al. , Presentation and outcome amongst older Singaporeans living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): does age alone drive excess mortality? Ann. Acad. Med. Singapore, 2012. 41(12): p. 581–6. [PubMed] [Google Scholar]
- 46.Marquine MJ, et al. , The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) Index and neurocognitive function among HIV-infected persons. J Neurovirol, 2016. 22(4): p. 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escota G, et al. , The VACS Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses. 31(3): p. 313–7. [DOI] [PubMed] [Google Scholar]
- 48.Cohen MH, et al. , Gender-Related Risk Factors Improve Mortality Predictive Ability of VACS Index Among HIV-Infected Women. J Acquir Immune Defic Syndr, 2015. 70(5): p. 538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erlandson KM, et al. , Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J.Acquir.Immune.Defic.Syndr., 2013. 63(2): p. 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erlandson KM, et al. , Relationship of physical function and quality of life among persons aging with HIV infection. AIDS, 2014. 28(13): p. 1939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]