Abstract
More than one-third of the calories consumed by U.S. and European populations contain acrylamide, a substance classified as a “probable human carcinogen” based on laboratory data. Thus, it is a public health concern to evaluate whether intake of acrylamide at levels found in the food supply is an important cancer risk factor. Mean dietary intake of acrylamide in adults averages 0.5 μg/kg of body weight per day, whereas intake is higher among children. Several epidemiological studies examining the relationship between dietary intake of acrylamide and cancers of the colon, rectum, kidney, bladder, and breast have been undertaken. These studies found no association between intake of specific foods containing acrylamide and risk of these cancers. Moreover, there was no relationship between estimated acrylamide intake in the diet and cancer risk. Results of this research are compared with other epidemiological studies, and the findings are examined in the context of data from animal models. The importance of epidemiological studies to establish the public health risk associated with acrylamide in food is discussed, as are the limitations and future directions of such studies.
Keywords: Acrylamide, intake, diet, cancer risk
INTRODUCTION
In April 2002, researchers from the Swedish National Food Administration first reported the finding of detectable levels of acrylamide in commonly consumed baked and fried foods (1). Acrylamide is known as a neurotoxin in humans and as a carcinogen in experimental studies, and it is classified as a “probable human carcinogen” by the International Agency for Research on Cancer (2). Thus, the discovery that the compound is found extensively throughout the food supply caused alarm that dietary acrylamide could be an important human cancer risk factor (3).
Rather than being a food contaminant, acrylamide forms naturally during cooking through a series of reactions, known as Maillard reactions, between an amino acid, primarily asparagine, and a reducing sugar such as fructose or glucose. Acrylamide formation begins at temperatures around 120 °C and peaks at temperatures between 160 and 180 °C (4). Thus, acrylamide is formed during frying, roasting, and baking and is not typically found in boiled or microwaved food.
Acrylamide is ubiquitous in the human diet, and more than one-third of the calories we take in each day come from foods with detectable levels of acrylamide (5). The highest levels appear in fried and roasted potato products and in cereal products such as breads, crackers, and breakfast cereals (Table 1). Because of the high temperatures used for roasting of the beans, coffee and cocoa also have moderately high levels of acrylamide (6).
Table 1.
Estimated Acrylamide Concentration for Several Food Groups: Swedish National Food Administration (128)
| acrylamide concentration (μ/kg) | ||
|---|---|---|
| food group | median | min—max |
| potato crisps (U.S., chips) | 1200 | 330–2300 |
| French fries | 450 | 300–1100 |
| pan-fried potatoes | 300 | |
| biscuits and crackers | 410 | <30–650 |
| crisp breads | 140 | <30–1900 |
| breakfast cereals | 160 | <30–1400 |
| corn chips | 150 | 120–180 |
| soft breads | 50 | <30–160 |
| Coffee | 25 | 8–0 |
Given the potential burden of disease associated with dietary sources of acrylamide, we sought to address whether the amount of acrylamide in the human diet is sufficient to be an important cancer risk factor. We present findings from our epidemiological studies to date and discuss these results in light of other epidemiological and animal studies.
DIETARY ACRYLAMIDE EXPOSURE IN HUMANS
Estimated dietary acrylamide intake in populations has been calculated by national food administrations for several countries (Table 2). For adults, estimated average intakes range from approximately 0.3 to 0.6 μg/kg of body weight (bw)/day. Children and adolescents tend to eat more acrylamide on a per body weight basis. This may be due to a combination of children’s higher caloric intake relative to body weight as well as their higher consumption of certain acrylamide-rich foods, such as French fries and potato crisps (7).
Table 2.
Estimated Daily Acrylamide Intake through Diet in Different Countries
| country | survey | age group | mean daily intake (μg/kg of bw) | 95th percentile (*, 90th %) |
|---|---|---|---|---|
| France Germany |
L’enquête nationale de consommation alimentaire INCA (29) National Consumption Survey (30) |
adults, 15+ | 0.5 | 1.1 |
| adults, 25+ | 0.6 | |||
| children, 4–6 | 1.2 | |||
| The Netherlands | National Food Consumption Survey (28) | 1–97 | 0.4 (median) | 1.2 |
| children, 1–6 | 1.0 (median) | 1.7 | ||
| Sweden | National Food Administration Food Survey (19)) | adults, 18–74 | 0.45 | 1.03 |
| United States | MRCA 1982–1988 (31) | 2+ | 0.48 (MRCA) | 0.91* (MRCA) |
| CSFII 1994–1996 and 1998 (31) | 0.37 (CSFII) | 0.81* (CSFII) | ||
| children, 2–5 | 1.26 (MRCA) | 2.33* (MRCA) | ||
| 1.00 (CSFII) | 2.15* (CSFII) |
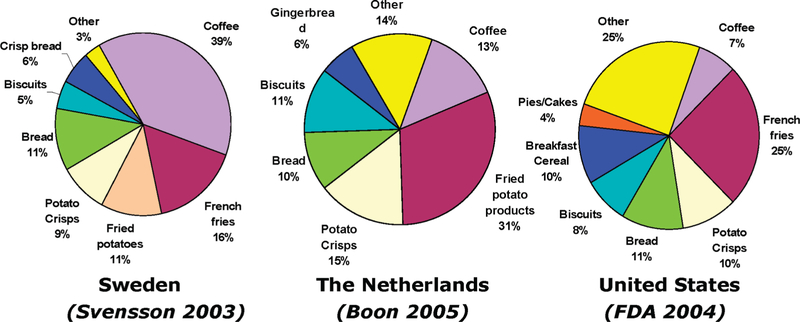
The foods that contribute the most to acrylamide intake vary across countries according to national dietary patterns (Figure 1). Generally, potato products, breads, and coffee are important contributors across populations.
Figure 1.
Contribution of food items to dietary acrylamide intake in Sweden, The Netherlands, and the United States.
Acrylamide is contained in a diverse set of foods that contribute significantly to the total micro- and macronutrient composition of the diet. High levels of acrylamide are formed in French fries and potato chips, which tend also to contain high levels of saturated fats. In the United States, foods that contain acrylamide contribute 38% to total daily energy intake, 47% to total daily iron intake, and 42% to total daily folate intake among adults (5). Eliminating acrylamide formation in an individual food item or group would have minimal effect on total exposure, even for foods with high concentrations of acrylamide. For example, preventing acrylamide formation in breads and bakery products would reduce the population mean intake from 0.43 to 0.34 μg/kg/day in the United States (5).
To put the acrylamide intake data in context of risk, one can extrapolate data from high-dose acrylamide animal feeding studies using mathematical models and predict the potential cancer risk associated with low-dose dietary acrylamide exposure in humans. On the basis of animal studies in the 1980s (8), the U.S. Environmental Protection Agency (9) estimated a cancer slope factor of 4.5 × 10−3, which implies per 10000 in the population an additional 18 cases at an intake of 0.4 μg/kg of bw/day and 45 cases at 1.0 μg/kg of bw/day. Dybing and Sanner (10) reported a lower cancer slope factor of 1.3 × 10−3 per μg/kg of bw/day based on mammary gland adenomas, which they selected as the most sensitive single tumor site. Using dietary intake data from Norway, this slope factor implies an upper bound lifetime cancer risk estimate from dietary acrylamide of 5 additional cases per 10000 women at the median intake and 11 additional cases per 10000 at the 90th percentile of intake. The Joint FAO/WHO Expert Committee on Food Additives (11) estimated a cancer slope factor of 3.3 × 10−4 per μg/kg/day also based on total mammary tumors.
Key consideration should go into interpreting risk assessment models, which depend on several important assumptions. The models yield divergent results at very low doses, and uncertainty surrounds these estimates, which assume linearity at low doses. The animal studies also used doses of acrylamide at levels 3–5 orders of magnitude greater than typical dietary human exposures, which makes the uncertainty about the dose-response at low doses important to consider.
EPIDEMIOLOGY STUDIES OF DIETARY ACRYLAMIDE AND CANCER RISK
Epidemiology is the study of the distribution and determinants of disease in a population (12), and a well-designed study can provide meaningful data to examine key research questions with respect to the public’s health. In response to the public health alarm raised by acrylamide in foods, we undertook four epidemiological studies on acrylamide intake and cancer risk using existing data from Swedish population-based case-control and cohort studies (13–17). In a cohort study, individuals are classified according to their level of exposure, they are observed over time for the development of the disease of interest, and the frequency of disease is compared across categories of exposure. A case-control study is an efficient approach to a cohort study, whereby cases are first ascertained and then a random sample of the population that gave rise to the cases is selected as disease-free controls. Cases and controls are then queried about past exposures. Cohort studies generally have the added strength that the assessment of exposure occurs before the development of disease, and therefore the recall of that exposure is not influenced by disease status, as is possible with case-control studies.
The study design for the four studies is summarized in Table 3. Each was a population-based study in Sweden, which relied on use of population health registers. In each, we estimated dietary acrylamide exposure through information collected from study participants’ food frequency questionnaires (FFQ). The FFQ included questions on the usual intake of many of the foods with detectable acrylamide levels consumed in the Swedish diet, including fried potato products (chips, French fries, fried potatoes), coffee, crisp and soft breads, cereals, cakes and biscuits, pancakes, and meatballs. Individuals were asked about how often they ate each of the foods, as well as portion size. We collected data on the mean acrylamide concentrations per kilogram of food from the Swedish National Food Administration acrylamide database and calculated a summary measure of dietary acrylamide for each individual using the following formula, summing over all acrylamide-containing food items on the questionnaire: AA intake = Σ (food item (g/serving) × median acrylamide (μg/kg)/1000) × servings/day.
Table 3.
Epidemiological Studies of Dietary Acrylamide Intake and Cancer Risk
| dietary acrylamide intakea | |||||||
|---|---|---|---|---|---|---|---|
| cancer site | design | population | sample size | mean (SE) | range | >1 μkg/day (%) | published |
| colon, rectum | case-control | Swedish men and women | 591 CRC, 538 controls | 34.0 (0.6) | 10.6–125.2 | 1,3 | Mucci et al. (13, 14) |
| bladder kidney |
263 133 |
||||||
| renal | case-control | Swedish men and women | 379 cases, 353 controls | 27.6 (0.6) | 4.0–182.1 | 1.1 | Mucci et al. (15) |
| colon, rectum | cohort | Swedish women | 589 cases 832000 person-years |
24.6 (0.04) | 5.6–309.1 | 1.5 | Mucci et al. (17) |
| breast | cohort | Swedish women | 49259 667 cases |
25.9 (0.08) | 1.0–214.8 | 0.4 | Mucci et al. (16) |
Estimated intake among controls (case-control study) or the study cohort (cohort study).
The mean intake of acrylamide across the studies ranged from 25 to 35 μg/day (Table 3), in line with other populations (1, 10, 18–20). In the studies including both men and women, dietary intake of acrylamide was higher among men compared to women. Fewer than 2% of the population consumed diets with levels >1 μg/kg of bw/day.
Our initial study (13) examined the relationship between dietary exposure to acrylamide and large bowel, bladder, and kidney cancer using data from an existing population-based case-control study of men and women in Sweden (21). An updated analysis including additional data on coffee consumption was published soon afterward (14). Information on dietary habits in the five years prior to study was assessed through a semiquantitative FFQ of 88 food items.
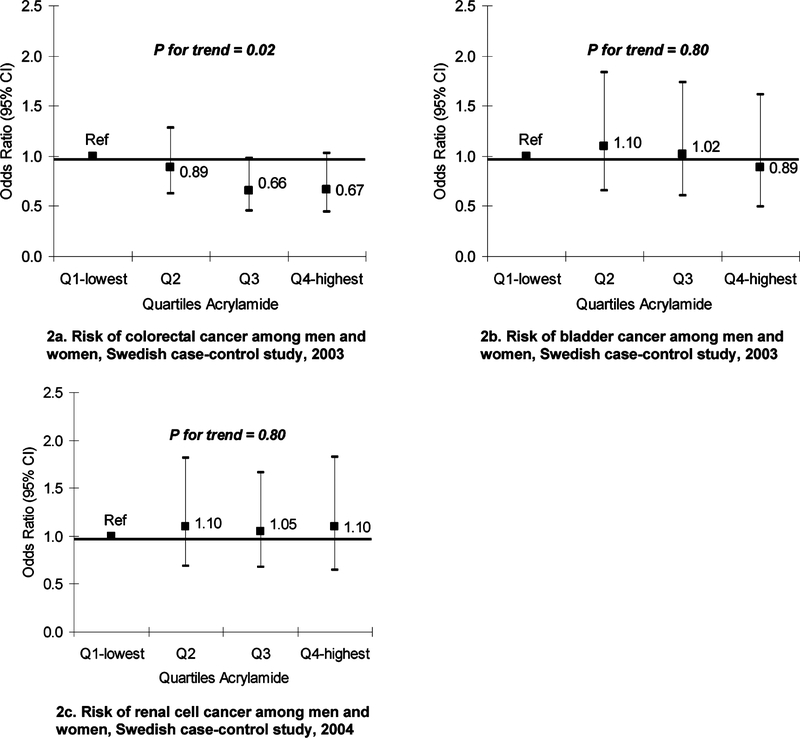
Among the controls in the study, the major dietary sources of acrylamide were crisp bread (28%), fried potato products (22%), and coffee (20%) (14). We used multivariate logistic regression models to study the associations between dietary acrylamide intake and cancer risk, adjusting for the known risk factors for colorectal, renal, and bladder cancer. There was no evidence that intake of food items with elevated levels of acrylamide was associated with cancer of the large bowel, kidney, or bladder. Likewise, there was no positive association between total dietary acrylamide intake and risk of these cancers (Figure 2). Smoking is an important source of acrylamide exposure, with 1–2 μg of exposure per cigarette smoked. We examined the association between dietary sources of acrylamide and cancer separately among smokers and nonsmokers, with no increased risk found for either group.
Figure 2.
Estimated acrylamide through diet and risk of cancers of the colon, rectum, kidney, bladder: results from case-control studies.
We then undertook a reanalysis of a larger case-control study of renal cell cancer using data from the Swedish component of an international collaborative population-based study (15). Incident cases of renal cell cancer (N = 379) were identified through regional cancer registries, and controls (N = 353) were randomly selected from the study base through the register of the total population and frequency matched on age and sex. Data were obtained through structured interviews, and an FFQ asked about intake of selected food items for five years prior to the cancer diagnosis. We undertook multivariate logistic regression to calculate odds ratios, as an estimate of the relative risk and 95% confidence intervals. We adjusted for many of the known risk factors for renal cell cancer in the models. We found no association between intake of foods with elevated acrylamide levels and risk of renal cell cancer and no increased risk for the highest compared to lowest quartiles of total intake (Figure 2c), even accounting for potential confounders. Again, there was no difference in the effect of acrylamide intake between smokers and nonsmokers.
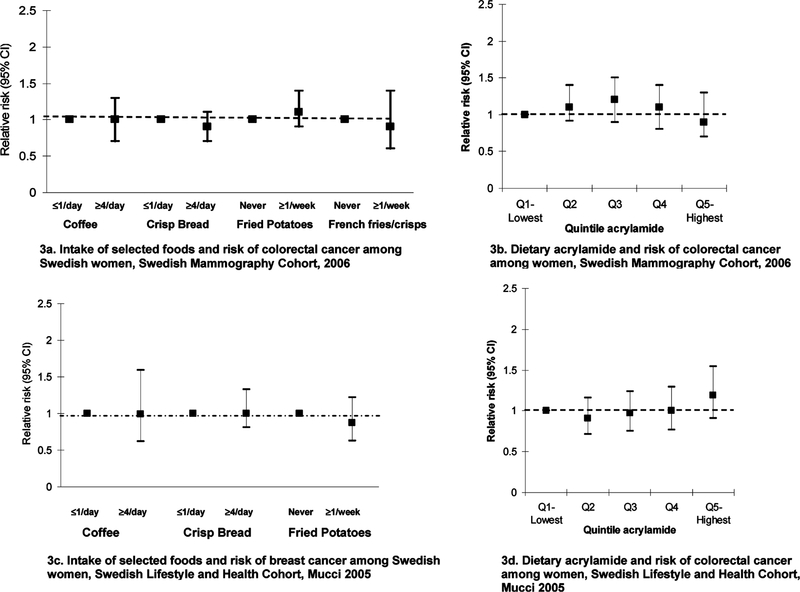
Case-control studies are potentially subject to recall and selection biases, particularly in studies of nutritional epidemiology. For this reason, prospective studies are considered to be a higher level of evidence. Thus, we have now conducted two prospective studies on acrylamide intake and cancer. The first study examined acrylamide and colorectal cancer risk among 61467 women in the Swedish Mammography cohort (17). Cancer incidence in the cohort over time was assessed through linkage with the Swedish Cancer Registry. During 16 years of follow-up, there were 504 incident colon and 237 rectal cancers among the women. We used Cox regression models to evaluate the association between intake of specific foods or estimated dietary acrylamide levels and time to cancer risk. In multivariate models, we adjusted for many of the known risk factors for colorectal cancer. There was no association between intake of foods that contain acrylamide and risk of either cancer (Figure 3a). After adjustment for potential confounders, the relative risk (RR) for the highest versus lowest quintile of dietary acrylamide intake (>31.4 versus <15.8 μg/day) was 0.9 [95% confidence interval (CI), 0.7–1.3] (Figure 3b).
Figure 3.
Intake of specific foods, estimated acrylamide through diet, and risk of cancers of the colon, rectum, and breast among women: results from cohort studies.
The second prospective study was the Swedish Women’s Health and Lifestyle Cohort to study the relationship between acrylamide exposure and breast cancer risk (16). This cohort consists of 43404 Swedish women who participated in a baseline questionnaire in 1991. They were prospectively followed through December 31, 2002, with linkage to the Swedish Cancer Registry and Death Registry, for a total of 490000 person-years of follow-up time and 667 incident breast cancer cases. Mean acrylamide intake in the total cohort was 25.9 μg/day. Coffee (54% of intake), fried potatoes (12%), and crisp bread (9%) were the largest contributors to acrylamide intake. We incorporated Cox regression models to evaluate the association between intake of specific foods or estimated dietary acrylamide levels and time to breast cancer risk, adjusting for many of the known risk factors for colorectal cancer. There was no association between breast cancer risk and intake of any specific foods high in acrylamide (Figure 3c). After adjustment for potential confounders, no significant increase in risk of breast cancer was seen among those with higher acrylamide intakes (Figure 3d). The relative risk of breast cancer in the highest versus lowest quintile of intake (≥34 versus <17 μg/day) was 1.19, with a 95% confidence interval of 0.91–1.55. The results were similar when we compared smokers and nonsmokers.
On the basis of the results of our studies, it appears that the amount of acrylamide typically consumed in diet is not associated with an excess risk of cancers of colon, rectum, bladder, kidney, or breast. These data are in line with those of Pelucchi et al. (22, 23) using existing data from Italian case-control studies conducted between 1991 and 2000 and including cancers of the oral cavity and pharynx (749 cases, 1772 controls), esophagus (395 cases, 1066 controls), larynx (527 cases, 1297 controls), large bowel (1225 colon and 728 rectum cases, 4154 controls), breast (2569 cases, 2588 controls), and ovary (1031 cases, 2411 controls). In these studies, controls were sampled from patients admitted to the same network of hospitals for cases of acute, non-neoplastic conditions. For all of the studied cancers, there was no evidence of a positive association between dietary intake acrylamide and any of the studied cancers. Similar to our reports, there was no difference in the associations among smokers and nonsmokers.
More recently, a nested study was undertaken within The Netherlands Cohort Study on Diet and Cancer (NCLS), a prospective cohort of 62573 women (24). Using a case-cohort design, the authors included 1350 women with incidence breast cancer, 195 with ovarian cancer, and 221 with endometrial cancer diagnosed between 1986 and 1997. A random sample of the cohort was taken to increase efficiency in coding information from the FFQ and included 2438 women without prevalent cancer at baseline. In line with our study, the authors found no association between intake of acrylamide in the diet and risk of breast cancer. However, the authors observed that women with the highest intake of acrylamide, based on quintiles, had a 1.8 (95% CI = 1.1–29) times greater risk of ovarian cancer. In addition, although there was no association between acrylamide and endometrial cancer overall, the relative risk associated with the highest versus lowest intake was 2.0 (95% CI = 1.1–3.5) among women who were never smokers. These findings for ovarian cancer and endometrial cancer are in contrast with the observations by Pelucchi et al. (23). The NCLS study included a prospective assessment of diet prior to cancer diagnosis, which avoids the potential selection biases to which hospital-based studies may be subject. The cohort had long and complete follow-up, so loss to follow-up should not be a concern. It is perhaps noteworthy that in The Netherlands study population spiced ginger cake was the major source of acrylamide in the diet. The authors do not show the specific relative risk estimates for each of the major foods items with elevated acrylamide in relation to cancer, which would be useful in trying to disentangle acrylamide from other dietary components of spiced cake as predictors of risk. Still, these prospective data suggest that further research is warranted.
STRENGTHS AND LIMITATIONS OF EPIDEMIOLOGICAL STUDIES
Excluding the most recent findings on ovarian and endometrial cancer, researchers have pondered why the animal and human data on acrylamide and risk of diverge. There are several possible reasons that should be put in the light of strengths and limitations of epidemiological studies. One of the major strengths of an epidemiological study is that it allows a researcher to directly address whether acrylamide exposure in the range of intakes relevant to humans increases risk of human cancer within the relevant biologic model of interest. Such a study avoids the uncertainties of extrapolating data from animal models, where the dose of exposure is 1000–100000 times higher than what humans consume through diet. The mathematical models used to make low-dose extrapolations assume linear doses, and yield divergent results at very low doses (11). Moreover, the level of conversion of acrylamide to the more reactive compound glycidamide may be different at very low versus high doses, and cellular protective mechanisms such as DNA repair and apoptosis may effectively detoxify acrylamide and glycidamide at the lower doses (10, 11).
The source of acrylamide exposure between animals and humans differs, as animals were exposed to aqueous solutions of acrylamide. Using animal data to compare human exposures to acrylamide in food raises issues about the bioavailability of acrylamide in humans or rats through these different exposure methods. Acrylamide exposure may also produce different effects when delivered with a multitude of nutrients and other compounds in foods rather than as a single additive to water.
A well-designed and analyzed epidemiological study can detect the public health relevance between an exposure and cancer risk, but may not be able to exclude a very small increase in risk. The risk assessment models imply a relative risk of cancer of 1.006–1.05 for the highest dose of acrylamide of >70 μg/day to which <2% of adults in our studies are exposed (10, 25). Assuming the validity of these numbers, they imply relative risks that are so small that even large, well-designed epidemiological studies could not detect this effect. Mucci and Adami (26) calculate that a cohort of more than 2 million people would be needed to detect the relative risks estimated from animal studies. With such small estimated excess risk estimates, it is unlikely that epidemiological evidence will be able to prove or disprove an association. At the same time, such a small increase in risk is not a priority from a public health point of view. In addition, selection bias and recall bias are serious shortcomings of case-control studies, particularly for nutrition. However, prospective studies do not have these limitations, and the two prospective studies to date have also failed to find significant associations. All epidemiological studies that rely on self-reported FFQs are subject to misclassification of dietary intakes. It is difficult to use FFQs to measure acrylamide intake in particular. A single acrylamide value must be assigned to each survey item, and survey items often group foods that have quite different acrylamide levels. The resulting random misclassification of acrylamide exposure will bias relative risks toward the null value of 1. Finally, the epidemiological studies conducted to date have used a single assessment of exposure measured at midlife; it is possible that early life exposures to acrylamide are important.
CONCLUSIONS AND FUTURE DIRECTIONS
The epidemiological body of literature on acrylamide in the diet and cancer risk is accumulating, but there are additional research questions that need to be addressed. More prospective epidemiological studies in additional populations and additional cancer sites, in particular for ovarian and endometrial cancer, are needed. Moreover, there are variations in genes involved in acrylamide metabolism that could shed further light on the level of risk and the possible existence of higher risk subgroups of the population. Only one study to date has examined genetic polymorphisms in vitro (27), but the role of P450 genotypes, which may affect conversion of acrylamide to glycidamide, has yet to be studied.
Data collected on acrylamide intake from FFQs will be strengthened with collection of biomarkers of acrylamide exposure, which our group and others are undertaking, which will provide valuable information on the validity of using existing FFQs to study acrylamide-cancer associations.
No single study can provide conclusive evidence on the health effects of acrylamide in the diet. However, an accumulation of evidence through well-designed studies in multiple disciplines can shed light on this important public health concern.
In summary, our epidemiological data do not support acrylamide intake in the diet as an important public health factor for cancer risk. However, the foods that contain acrylamide also contain a diversity of other food nutrients. For example, high levels of acrylamide are found in chips and French fries, and these fried foods are associated with a higher risk of obesity and cardiovascular disease and should be consumed in moderation.
ACKNOWLEDGMENT
We recognize the study participants who made this research possible.
The research was supported in part by grants from the Department of Defense Breast Cancer Program (PI Adami DAMD 17-03-10760) and by grants from the Swedish Cancer Society. K.M.W. is supported in part by NIH/NCI Training Grant R25 (PI Stampfer CA098566).
LITERATURE CITED
- (1).Acrylamide in food, Swedish National Food Administration, 2002.
- (2).International Agency for Research on Cancer. Some Industrial Chemicals; Monographs on the Evaluation of Carcinogen Risk to Humans; IARC: Lyon, France, 1994. [Google Scholar]
- (3).Tareke E; Rydberg P; Karlsson P; Eriksson S; Tornqvist M Acrylamide: a cooking carcinogen. Chem. Res. Toxicol 2000, 13 (6), 517–522. [DOI] [PubMed] [Google Scholar]
- (4).Mottram DS; Wedzicha BL; Dodson AT Acrylamide is formed in the Maillard reaction. Nature 2002, 419 (6906), 448–449. [DOI] [PubMed] [Google Scholar]
- (5).Petersen BJ; Tran N Exposure to acrylamide: Placing exposure in context In Chemistry and Safety of Acrylamide in Food; Friedman M, Mottram D, Eds.; Springer Press: New York, 2005; pp 63–76. [DOI] [PubMed] [Google Scholar]
- (6).Stadler RH; Scholz G Acrylamide: an update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr. ReV 2004, 62 (12), 449–467. [DOI] [PubMed] [Google Scholar]
- (7).Dybing E; Farmer PB; Andersen M; Fennell TR; Lalljie SP; Muller DJ; Olin S; Petersen BJ; Schlatter J; Scholz G; Scimeca JA; Slimani N; Tornqvist M; Tuijtelaars S; Verger P Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol 2005, 43 (3), 365–410. [DOI] [PubMed] [Google Scholar]
- (8).Johnson KA; Gorzinski SJ; Bodner KM; Campbell RA; Wolf CH; Friedman MA; Mast RW Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol. Appl. Pharmacol 1986, 85 (2), 154–168. [DOI] [PubMed] [Google Scholar]
- (9).Integrated risk information system (IRIS): Acrylamide (CASRN 79–06–1), U.S. Environmental Protection Agency, 1993. [Google Scholar]
- (10).Dybing E; Sanner T Risk assessment of acrylamide in foods. Toxicol. Sci 2003, 75 (1), 7–15. [DOI] [PubMed] [Google Scholar]
- (11).FAO/WHO Expert Committee on Food Additives. Summary and Conclusions Report from 64th Meeting; FAO: Rome, Italy, 2005. [Google Scholar]
- (12).Rothman K; Greenland S Modern Epidemiology, 2nd ed.; Lippincott-Reven: Philadelphia, PA, 1998; pp 3–6. [Google Scholar]
- (13).Mucci LA; Dickman PW; Steineck G; Adami HO; Augustsson K Dietary acrylamide and cancer of the large bowel, kidney, and bladder: absence of an association in a population-based study in Sweden. Br. J. Cancer 2003, 88 (1), 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Mucci LA; Dickman PW; Steineck G; Adami HO; Augustsson K Dietary acrylamide and cancer risk: additional data on coffee [letter]. Br. J. Cancer 2003, 89 (4), 775–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mucci LA; Lindblad P; Steineck G; Adami HO Dietary acrylamide and risk of renal cell cancer. Int. J. Cancer 2004, 109 (5), 774–776. [DOI] [PubMed] [Google Scholar]
- (16).Mucci LA; Sandin S; Balter K; Adami HO; Magnusson C; Weiderpass E Acrylamide intake and breast cancer risk in Swedish women. JAMA-J. Am. Med. Assoc 2005, 293 (11), 1326–1327. [DOI] [PubMed] [Google Scholar]
- (17).Mucci LA; Adami HO; Wolk A Prospective study of dietary acrylamide and risk of colorectal cancer among women. Int. J. Cancer 2006, 118 (1), 169–173. [DOI] [PubMed] [Google Scholar]
- (18).Konings EJ; Baars AJ; van Klaveren JD; Spanjer MC; Rensen PM; Hiemstra M; van Kooij JA; Peters PW Acrylamide exposure from foods of the Dutch population and an assessment of the consequent risks. Food Chem. Toxicol 2003, 41 (11), 1569–1579. [DOI] [PubMed] [Google Scholar]
- (19).Svensson K; Abramsson L; Becker W; Glynn A; Hellenas KE; Lind Y; Rosen J Dietary intake of acrylamide in Sweden. Food Chem. Toxicol 2003, 41 (11), 1581–1586. [DOI] [PubMed] [Google Scholar]
- (20).Acrylamide, WHO/FAO, 2003.
- (21).Augustsson KSK; Jagerstad M; Dickman PW; Steineck G Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet 1999, 353, 703. [DOI] [PubMed] [Google Scholar]
- (22).Pelucchi C; Franceschi S; Levi F; Trichopoulos D; Bosetti C; Negri E; La Vecchia C Fried potatoes and human cancer. Int. J. Cancer 2003, 105 (4), 558–560. [DOI] [PubMed] [Google Scholar]
- (23).Pelucchi C; Galeone C; Levi F; Negri E; Franceschi S; Talamini R; Bosetti C; Giacosa A; La Vecchia C Dietary acrylamide and human cancer. Int. J. Cancer 2006, 118 (2), 467–471. [DOI] [PubMed] [Google Scholar]
- (24).Hogervorst JG; Schouten LJ; Konings EJ; Goldbohm RA; van den Brandt PA A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol. Biomarkers PreV. 2007, 16 (11), 2304–2313. [DOI] [PubMed] [Google Scholar]
- (25).Hagmar L; Tornqvist M Inconclusive results from an epidemiological study on dietary acrylamide and cancer Br. J. Cancer 2003,894, 774–775 (author reply pp 775–776). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Mucci LA; Adami HO The role of epidemiology in understanding the relationship between dietary acrylamide and cancer risk in humans In Chemistry and Safety of Acrylamide in Food; Friedman M, Mottram D, Eds.; Springer: New York, 2005; pp 39–47. [DOI] [PubMed] [Google Scholar]
- (27).Paulsson B; Rannug A; Henderson AP; Golding BT; Tornqvist M; Warholm M In vitro studies of the influence of glutathione transferases and epoxide hydrolase on the detoxification of acrylamide and glycidamide in blood. Mutat. Res 2005, 580 (1–2), 53–59. [DOI] [PubMed] [Google Scholar]
- (28).Boon PE; de Mul A; van der Voet H; van Donkersgoed G; Brette M; van Klaveren JD Calculations of dietary exposure to acrylamide. Mutat. Res 2005, 580 (1–2), 143–155. [DOI] [PubMed] [Google Scholar]
- (29).Acrylamide: Point D’Information 2. In Agence Francaise De Securite Sanitaire Des Aliments (afssa), 1999. [Google Scholar]
- (30).Assessment of acrylamide intake from foods containing high acrylamide levels in Germany. In Federal Institute for Risk Assessment (Germany), 2003. [Google Scholar]
- (31).DiNovi M; Howard D The updated exposure assessment for acrylamide JIFSAN 2004 Acrylamide in Food Workshop; U.S. Food and Drug Administration: Washington, DC, 2004. [Google Scholar]