Abstract
Purpose
This study’s purpose is to examine whether a peer coaching intervention is more effective in improving clinical outcomes in diabetes when enhanced with e-Health educational tools than peer coaching alone.
Methods
The effectiveness of peer coaches who used an individually tailored, interactive web-based tool (iDecide) was compared with peer coaches with no access to the tool. 290 Veterans Affairs patients with A1c>8.0% received a six-month intervention with an initial session with a fellow patient trained to be a peer coach followed by weekly phone calls to discuss behavioral goals. Participants were randomized to coaches who used iDecide or to coaches who used non-tailored educational materials at the initial session. Outcomes were A1c (primary), blood pressure, and diabetes social support (secondary) at six and 12 months.
Results
255 participants (88%) completed six month- and 237 (82%) 12 month- follow-up. 98% were men, and 63% were African American. Participants in both groups improved A1c (>−0.6%, p<.001) at six months and maintained these gains at 12 months follow-up (>−0.5%, p<.005). Diabetes social support was improved at both 6 and 12 months (p<.01). There were no changes in blood pressure.
Conclusions
Clinical gains achieved through a volunteer peer coach program were not increased by the addition of a tailored e-Health educational tool.
Introduction
Low-income urban African American adults with diabetes on average experience more barriers to diabetes self-management and worse clinical outcomes than non-Latino white adults.1,2 While disparities among adults with diabetes who receive care at Veterans’ Affairs (VA) health systems are less than in other health systems,3 African American Veterans disproportionately receive care at VA facilities in which all patients on average have worse risk factor control than other VA facilities.4 The success of therapies for diabetes depends both on patients being prescribed appropriate medications and on their adhering to these medications and other recommended self-management behaviors—following diet and exercise regimens; self-monitoring; and coping with the rigors of living with diabetes. Yet, providers in under-resourced inner-city health systems face barriers to initiating and intensifying medication regimens, and many patients face barriers to effective diabetes self-management. These barriers on the patient side include poor understanding of diabetes and its treatments; lack of self-confidence and/or motivation to manage diabetes well; and unassertiveness in office visits with providers due to fear of being labeled as difficult.5-8 Compounding these issues, many adults with diabetes lack self-management support from families and friends, 9 and health systems lack resources to fill this gap and are unable to extend sustained support between periodic clinic visits.
Peer support among patients has been found to be an effective intervention to help address these barriers. “Peer support” is defined as “support from a person who has experiential knowledge of a specific behavior or stressor and similar characteristics as the target population.”10 Information and support from peers who share a common ethnic and socio-economic background may be particularly effective in minority groups who for historical reasons often have greater distrust of the formal health care system.11 In prior randomized controlled trials (RCTs) in safety net health systems, peer support interventions have resulted in improved diabetes management and clinical outcomes12-17 compared to comparison groups.
One potential limitation of peer support and other programs that shift self-management support tasks from health care professionals is that peer supporters by definition lack substantial medical and other content knowledge. To increase the potential impact of peer support programs in health care, a key next step is to test whether providing peer supporters with engaging, evidence-based educational tools to facilitate their medication and self-management discussions with patients enhances the effectiveness of such programs. Such tools, especially when individually tailored to each person based on characteristics unique to the patient 18,19 have been found in RCTs to improve outcomes in diabetes20-22 and other conditions.18,19,21,23-25 Software programs now enable tailoring to occur in real time and can easily be embedded into portable e-Health web applications, further increasing the scalability of such approaches. Yet, to date few such tools have been developed that can be deployed by nontraditional health care supporters such as peer supporters with low-income adults who disproportionately have low health and computer literacy.26
To address this gap in knowledge, the research team developed a personally tailored, interactive diabetes medication and self-management decision aid (iDecide) that can be delivered on tablet computers with 3G access. An earlier version of iDecide had been designed for salaried Community Health Workers (CHWs) to use with low-income, urban Latino and African American adults with diabetes. In a RCT, participants were randomized to CHWs who used iDecide or to CHWs using non-tailored, standard print materials. Those randomized to CHWs who used iDecide had greater improvements with satisfaction and decreased diabetes distress but no differences in other self-reported outcomes than those assigned to CHWs using print materials.27,28 The salaried CHWs in that evaluation, however, were very experienced and had received extensive training in behavioral counseling and in diabetes management. Since many health care systems in low-resource settings do not have trained CHWs or other outreach workers and often have burdened health care staff, it is important to investigate whether automated tailored e-Health programs such as iDecide are helpful in assisting less experienced CHWs, other lay health workers, or diabetes patients volunteering to be peer coaches. Accordingly, the current trial in a low-resource health care system tested whether peer coaching enhanced with e-Health educational tools (iDecide) is more effective then peer coaching alone among patients with poor glycemic control.
Research Design
The current study is a parallel, two-armed randomized controlled trial of a six-month peer coach program in which peer coaches either did or did not have access to a tailored interactive computer-based tool (iDecide) ().
Description of Site
The study was conducted at the John D. Dingell VAMC in FY11. Approximately 65% of Veterans who receive their care there are African American, with most living in Detroit.
Patient Selection, Recruitment and Randomization
From September 2014 to September 2016 patients were identified from the EHR who met one of the following criteria within the prior 12 months: (1) Two outpatient visits or one hospitalization with a diabetes-related ICD-9 code; or (2) at least one prescription for a glucose control medication; and 3) an A1c of at least 8.0% if age < 70 or at least 8.5% if age 70+ within the 3 months prior to enrollment. Patients were excluded if ICD-9 diagnostic codes indicated that they had an active substance abuse disorder or serious psychiatric illness (bipolar disorder, dementia, schizophrenia, or personality disorders). Potentially eligible patients’ names were sent to their primary care providers to identify any patients who they did not recommend inviting to participate in the study. An invitation letter was then sent to eligible patients, with a follow up call by a research associate. Patients who agreed to participate were scheduled to complete written informed consent and baseline assessments. Baseline survey data were entered directly onto the iPad so survey data could be used for tailoring in the iDecide program. Variable block sizes were programmed into the computer randomization process, precluding prediction of treatment assignments by study staff. Patients and research staff were blinded to randomization results through completion of all baseline measures up to the start of the intervention. Data assessors remained blinded to group assignment throughout the study. Institutional Review Board approval was obtained from the relevant VA boards.
Peer Coach Selection, Training, and Assessment of Fidelity
Potential peer coaches were identified by first generating a list of patients through the electronic health record (EHR) who met the above criteria for having diabetes mellitus and who had poor glycemic control in the past (A1c≥8.0%) but whose most recent A1c in the prior 6 months was <8.0%. Prior research has found greater effectiveness of peer coaches who themselves have struggled with diabetes control.14,16,29,30 Letters were sent to these patients followed by a telephone call by a member of the research team to further describe the program, the roles of peer coaches, and to elicit interest in and capacity to serve as a volunteer peer coach. Interested peer coaches completed informed consent and also completed all study assessments. Two-hour initial trainings for 74 new peer coaches were held over the study period. For peer coaches in both arms, the initial training focused on key Motivational Interviewing (MI)-based communication skills, including open-ended questions, rolling with resistance, eliciting ‘change-talk’, and helping participants define a longer-term behavioral goal and specific short-term steps to reach that goal (‘action planning’). The same training approach was employed that had been used for peer coaches in an earlier RCT in which participants in the peer coach arm had significantly improved A1c at the end of the six-month study period more than participants randomized to usual care or to receive financial incentives. [14] Peer coaches in the iDecide arm participated in an additional one-hour training session on how to navigate the iDecide tool that was delivered on iPads. All coaches learned how to place calls using a computer-supported telephone platform. Peer coaches were encouraged to use that system to make all calls, thereby enabling research data collection on frequency and duration of telephone calls.
1.5 hour monthly meetings were held among peer coaches in both groups to allow them to discuss how their calls were going and provide booster follow-up training on communication strategies and MI reinforcement. Fostering a sense of community and exchange among the peer coaches themselves was an important strategy to maintain coach morale and interest in continuing to serve as coaches. Nearly all coaches regularly attended these monthly group meetings.
To assess fidelity and provide booster training and support, study team members trained In MI observed a random sample of the initial face-to-face sessions. Coaches also completed a self-assessment checklist on a sample of their telephone calls. All but three of the recruited coaches completed at least one six-month term as coaches, and 38 completed two or more. Eleven participants themselves became coaches after completing the six-month program. While their role as volunteers was emphasized to tap into their feelings of altruism, peer coaches received small stipends to ‘cover expenses’ that were distributed at the monthly meetings. Peer coaches elected to have 1-5 assigned patients at any one time, with a mean of four over the course of the study.
Intervention Elements Participants in both Groups Received
Participants in both groups had an initial face-to-face session of approximately 2 hours with their assigned coach. At the end of this session, the peer coach helped the participant identify a behavioral goal related to their diabetes care and specific action steps for the next week to meet that goal (action plan). The coach encouraged the participant to keep a copy of the action plan they developed with the participant at the end of the session and list of questions and concerns to discuss with their health care providers generated by the discussion. The peer coaches were instructed to call each of their peer partners at least once a week to check in on how they were doing, ask about how their action plans were going, offer encouragement, and if necessary help their peer partners brainstorm about solutions to barriers they were facing meeting their action steps, set a new action step if they fully met their prior week’s goal, try a different action step, or set a new goal. To place calls, the coaches dialed a toll-free number and then indicated which of their assigned mentees they were calling. The system then placed the call. If the peer coach did not make an initial call within the first 10 days using the computer telephone system, a research associate called them to offer support. In subsequent weeks, the peer mentor received outreach reminder calls from staff if they did not call one of their assigned peers over a 2-week period.
Peer Support-Alone Group
At the initial session, the peer coach gave participants copies of two consumer-focused guides, “Pills for Type 2 Diabetes” and “Insulin for Type 2 Diabetes”31,32 and reviewed the guides with them. These Guides provide information on diabetes and summarize the effectiveness of currently available medication classes (oral and insulin) on A1c. They also include information on administration methods, average costs, medication side effects, risks of diabetes complications, suggested questions to discuss with health care providers and prompts to make notes of any questions for the doctor. The booklets include tables and graphs summarizing information. The educational content in these two guides was used in the iDecide tool, so this group received the same diabetes anti-hyperglycemic medication information provided in the iDecide tool.
Peer Support + iDecide Arm
At the initial session, participants randomized to this arm were guided by their coach through the personally tailored diabetes medication decision aid. The development process and content of the original iDecide program have been described in detail elsewhere.28 Briefly, Community-Based Participatory Research (CBPR) and User Centered Design (UCD) principles were used to iteratively develop and refine the iDecide tool. iDecide was delivered via tablet computers (e.g., an iPad connected to the internet), and enabled navigation by the peer coach and participant to selectively explore issues most important to the participant. iDecide was then adapted and pilot-tested for use by Veterans in VA.
The iDecide program consists of four main sections and includes the same content as the AHRQ Consumer Guides. The first section presents information and illustrative animations on how diabetes affects how glucose is processed in the body and how different medication classes, foods, and physical activity act to affect blood sugar. In the second section, participants view their own risk of diabetes complications (tailored based upon their baseline A1c) and can interactively change their A1c levels and see in pictographs how this changed their risk of different complications. In the third section, participants review their current diabetes medications and barriers to taking medications that they had identified on the baseline survey and engage with an interactive “issue card” to help elicit their preferences and priorities in terms of different medication characteristics (e.g. cost, likelihood of low blood sugar reactions, effect on weight, dosing schedules).33 In the fourth section, participants are prompted to set goals, develop a specific action plan to address identified barriers or other concerns, and generate specific questions and concerns to discuss with their doctor. Personal information from the baseline assessment is interwoven throughout the program (i.e., high-depth tailoring within sentences). Motivational Interviewing-based, tailored discussion prompts encourage autonomy-supportive coach-patient interactions at key points with open-ended questions and values exploration to help participants uncover their motivation, solve barriers to change, and develop an action plan.
The action plans and list of questions and concerns to discuss with their health care providers generated by the program and discussion were printed for the patient and peer coach to keep. Participants in the iDecide group also were given the link to the iDecide program with their personal information for them to access at their convenience throughout the intervention period and encouraged to continue to access the program as needed. The peer mentors then followed up with weekly computer-facilitated calls.
Outcome Measures
Changes in A1c from baseline to six months and from baseline to 12 months were examined. A1c data were also gathered from a third observational control arm, but of the patients randomized to that group only 30% had A1cs at 6- or 12-month follow-up recorded in the electronic health record (EHR), providing too few data points for comparison. As secondary outcomes, changes in systolic blood pressure control and reported diabetes-specific social support were compared. Point-of-service A1cs and blood pressure were assessed at baseline, 6 months, and 12 months. Trained staff members measured patients’ A1cs, using a Bayer DCA 2000+ analyzer. These assays have a test coefficient of variation (CV, measure of test precision) <5% as required by the National Diabetes Data Group and accurately measure A1c levels. Systolic blood pressure using an Omron® automatic blood pressure monitor with memory 34 and took the average of the two readings following American Heart Association guidelines.35 The Diabetes Support Scale (DSS) was used to assess perceived diabetes-specific support. This 12-item scale has excellent reliability (α> 0.90) and has been found in prior research to correlate with diabetes self-care behaviors and to be responsive to intervention effects. 13, 36 Each item consisted of a statement about support received in different areas of managing diabetes, with responses on a 6-point Likert scale from “Completely Disagree” to “Completely Agree”. The overall scale was scored from 0-100, with higher scores representing higher levels of social support.
Analysis
Because patients were clustered within patient coaches, the sample size was calculated to adjust for the possible correlation between members of the pairs (intraclass correlation, or rho). The sample size was calculated to provide 80% power to detect a difference between experimental groups of 0.5% in A1c with an alpha of .05, two-tailed.37 142 subjects were thus needed in each group (after attrition).
International guidelines for analysis and reporting of clinical trials were followed.38 For bivariate relationships, t-tests were used to determine significant differences in mean values between groups and Pearson’s chi-squared tests to determine a significant difference between the expected frequencies and the observed frequencies between groups. To determine whether there was a significant change in outcomes overtime (i.e., from baseline to 6-month follow-up and from baseline to 12-months follow-up) within a group, t-tests were used to assess if the mean value of within-person changes in outcome was significantly different from zero. To assess group differences in within-person changes over time, multivariable, ordinary least squares (OLS) estimators were used with adjustments of age and insulin status which were significantly different between the two groups at baseline. To account for possible clustering of participant outcomes by peer coach, a sensitivity analysis adjusting for clustering by peer coach was conducted, with no change in results. Analyses were conducted using STATA 14.39 In alternative analyses, multiple imputation to impute missing values and assuming no improvement in A1c levels among those missing outcome values found no significant changes in the findings.
Results
Participant flow and baseline data
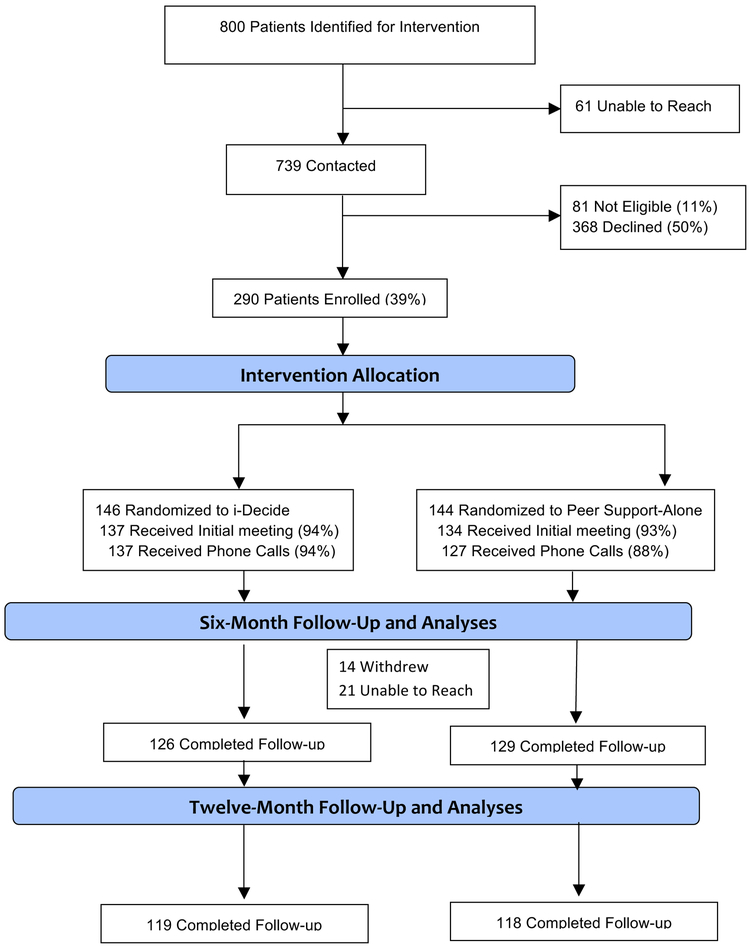
The CONSORT diagram in Figure 1 shows participant flow. Of 739 contacted patients, 290 (39%) were enrolled, 146 allocated to iDecide and 144 to the peer coaching-alone group. Participants’ baseline characteristics are reported in Table 1. There were complete outcome six-month data on 255 participants (88%) and complete 12-month data on 237 participants (82%).
Figure 1.
CONSORT Flow Diagram
Table 1:
Baseline Characteristics of Participants
| Baseline Characteristics | (1) Peer Support only |
(2) Peer + iDecide |
P- value from test (1) = (2) |
|---|---|---|---|
| N | 144 | 146 | |
| age, mean (SD) | 62.1 (10.5) | 64.3 (9.7) | 0.07 |
| Gender | |||
| female | 2 (1.4%) | 5 (3.4%) | 0.26 |
| male | 142 (98.6%) | 141 (96.6%) | |
| Race | |||
| African American | 89 (61.8%) | 92 (63.4%) | 0.36 |
| White | 53 (36.8%) | 53 (36.6%) | |
| Other | 2 (1.4%) | 0 (0.0%) | |
| Work Status | |||
| Employed | 39 (27.1%) | 35 (24.5%) | 0.84 |
| Not Employed | 26 (18.1%) | 23 (16.1%) | |
| Retired | 69 (47.9%) | 72 (50.3%) | |
| Disabled | 10 (6.9%) | 13 (9.1%) | |
| Education Level | |||
| < High School | 8 (5.6%) | 4 (2.7%) | 0.45 |
| High School Graduate | 42 (29.2%) | 36 (24.7%) | |
| Some Tech or vocational | 10 (6.9%) | 13 (8.9%) | |
| Some college or more | 84 (58.3%) | 93 (63.7%) | |
| Number of People in Household | |||
| 1 | 37 (25.7%) | 50 (34.2%) | 0.14 |
| 2 | 59 (41.0%) | 62 (42.5%) | |
| 3-5 | 40 (27.8%) | 31 (21.2%) | |
| 6 or more | 8 (5.6%) | 3 (2.1%) | |
| Income | |||
| $0-$15,000 | 31 (21.5%) | 30 (20.7%) | 0.93 |
| $16,000-$30,000 | 37 (25.7%) | 44 (30.3%) | |
| $31,000-$55,000 | 31 (21.5%) | 28 (19.3%) | |
| $56,000 and above | 23 (16.0%) | 23 (15.9%) | |
| Prefer not to disclose | 22 (15.3%) | 20 (13.8%) | |
| Satisfied with VA Care | |||
| Completely Satisfied | 21 (14.6%) | 26 (17.8%) | 0.48 |
| Very Satisfied | 61 (42.4%) | 65 (44.5%) | |
| Somewhat Satisfied | 53 (36.8%) | 44 (30.1%) | |
| Not at all Satisfied | 6 (4.2%) | 10 (6.8%) | |
| Don’t need Support | 3 (2.1%) | 1 (0.7%) | |
| Baseline A1c, mean (SD) | 9.1 (1.7) | 9.1 (1.7) | 0.96 |
| Baseline Total Cholesterol, mean (SD) | 164.3 (51.5) | 162.2 (46.2) | 0.71 |
| Baseline HDL, mean (SD) | 41.8 (13.3) | 44.9 (13.4) | 0.04 |
| Baseline LDL, mean (SD) | 83.6 (35.9) | 85.4 (35.5) | 0.67 |
| Baseline Creatinine, mean (SD) | 1.2 (0.7) | 1.2 (0.4) | 0.25 |
| Statin | 95 (66.0%) | 88 (60.3%) | 0.31 |
| Number of years with diabetes, mean (SD) | 15.3 (9.9) | 15.0 (10.2) | 0.82 |
| Insulin use | 93 (64.6%) | 78 (53.4%) | 0.05 |
| Number of Oral Anti hyperglycemic meds, mean (SD) | 1.0 (0.9) | 1.1 (0.8) | 0.54 |
| Self-Rated Health Fair or Poor | 64 (44.4%) | 65 (44.5%) | 0.99 |
| Diabetes Management Fair or Poor | 72 (50.0%) | 73 (50.0%) | 1.00 |
| Satisfied with Social Support | |||
| Completely Satisfied | 23 (16.0%) | 23 (15.9%) | 0.96 |
| Very Satisfied | 49 (34.0%) | 50 (34.5%) | |
| Somewhat Satisfied | 43 (29.9%) | 39 (26.9%) | |
| Not at all Satisfied | 19 (13.2%) | 23 (15.9%) | |
| Don’t need Support | 10 (6.9%) | 10 (6.9%) | |
| Easy to get close to others | |||
| Strongly disagree | 10 (6.9%) | 17 (11.6%) | 0.55 |
| Disagree | 18 (12.5%) | 12 (8.2%) | |
| Slightly disagree | 5 (3.5%) | 3 (2.1%) | |
| Neither agree nor disagree | 6 (4.2%) | 8 (5.5%) | |
| Slightly agree | 17 (11.8%) | 15 (10.3%) | |
| Agree | 88 (61.1%) | 90 (61.6%) | |
| Strongly agree | 0 (0.0%) | 1 (0.7%) | |
| Systolic BP, mean (SD) | 138.2 (19.7) | 137.5 (16.8) | 0.73 |
| Diastolic BP, mean (SD) | 78.9 (9.8) | 78.9 (8.6) | 0.95 |
| Health Literacy, mean (SD) | 7.0 (1.8) | 7.1 (1.9) | 0.61 |
Primary Outcome of A1c
As Table 2 shows, participants in both groups had significant within-group improvements between baseline and six months and between baseline and 12 months in their A1c values. In the Peer Support-alone group, mean baseline A1cs of 9.07% improved to 8.39% at 6 months (−0.68%, P<0.001) and remained 8.55% (−0.54, P=0.004) at 12 months. Mean baseline A1c in the iDecide group was 9.08% at baseline and improved to 8.38% (−0.70, P<0.001) at six-months. At 12 months, mean A1c was 8.52% (−55, P=0.002). There were no significant between-group differences at six month or 12-month follow-up.
Table 2:
Intervention Effect on Change in A1c
| A1c level |
Within-person A1c change from baseline |
Between-group difference in within- person change from baseline |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peer Support Only |
Peer support +iDecide |
Peer Support Only | Peer support +iDecide |
||||||||||
| Time | N | Mean | N | Mean | N | Mean |
P- value |
N | Mean |
P- value |
N | Coef* | P- value* |
| Baseline | 144 | 9.07 | 146 | 9.08 | - | - | - | - | - | - | - | - | - |
| 6 months | 129 | 8.39 | 126 | 8.38 | 129 | −0.68 | <0.001 | 126 | −0.70 | <0.001 | 255 | −0.07 | 0.77 |
| 12 months | 118 | 8.55 | 119 | 8.52 | 118 | −0.54 | 0.004 | 119 | −0.55 | 0.002 | 237 | −0.04 | 0.87 |
adjusted for age and insulin at baseline
Table 3 shows results for systolic blood pressure and reported diabetes-specific social support. There were no significant changes in systolic blood pressure at any time point in either group. Significant within-group improvements were observed in self-reported diabetes-specific social support in both groups between baseline and six months and between baseline and 12 months.
Table 3:
Intervention Effect on Reported Diabetes-Specific Social Support*
| Baseline level |
Within-person change from baseline |
Between-group difference in within- person change from baseline |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peer Support Only |
Peer support +iDecide |
Peer Support Only | Peer support +iDecide |
||||||||||
| Time | N | Mean | N | Mean | N | Mean |
P- value |
N | Mean |
P- value |
N | Coef* |
P- value* |
| Baseline | 144 | 53.1 | 146 | 54.7 | - | - | - | - | - | - | - | - | - |
| 6 months | 129 | 56.8 | 126 | 58.7 | 129 | +3.4 | 0.009 | 126 | +4.0 | <0.001 | 255 | 0.3 | 0.98 |
| 12 months | 118 | 57.3 | 119 | 57.7 | 118 | +4.2 | 0.006 | 119 | +3.0 | <0.01 | 237 | 1.2 | 0.79 |
Adjusted for age and insulin at baseline
Diabetes-Specific Social Support Scale is scored from 0-100 with higher scores indicating higher levels of social support.
Discussion
Among this population of low-income predominantly African American and male Veterans with poor glycemic control, participants in both peer support interventions made significant improvements in their glycemic control at the end of the program, and gains were sustained at 12 months (six months after the program ended). The mean baseline systolic blood pressure was 138 mm Hg (slightly above recommended VA guidelines of 135 mm Hg), and there was no significant change at 6- or 12-months. Both groups reported significant improvements in diabetes-specific social support at 6- and 12-months. There were no between-group differences in any of these outcomes.
This study found that both peer support models were effective in improving A1c levels right after the interventions, and importantly, these gains were sustained six months after the programs’ conclusion. This finding is consistent with the large and growing body of evidence on the effectiveness of volunteer peer support interventions in improving diabetes outcomes.17,40 Although there was not a usual care comparison group, multiple prior RCTs comparing peer support not only to usual care16,41,42 but also to nurse care management13 and to financial incentives14 have shown A1c improvements of at least 0.5% greater in the peer support arms than in comparison arms. In prior diabetes RCTs in VA, participants in the usual care arm did not have significant decreases in their A1c levels, and in some cases had increases in their A1c over the six-month study period. [13, 14] The A1c improvements of >0.5% achieved at both six- and 12-months in both intervention groups in this study are both statistically and clinically significant. A mean difference in A1c level of 0.5% translates into an absolute 2.8% risk reduction in diabetes events over 10 years, or a number needed to treat of 36.43
Of note, these clinically significant and sustained gains were not further improved through use of an e-health educational tool in the initial face-to-face visit and its availability to participants throughout the intervention period. This study is among the first efforts to respond to the call for the testing of e-Health consumer health applications for use by nontraditional caregivers such as volunteer peer coaches with racial and ethnic minority and low-literate populations.26 The iDecide software used state of the art software to deliver personally tailored educational information to participants, thus possibly increasing its personal relevance, and enabled participants to engage actively with the delivered information (e.g., examining how changes in their own A1cs would affect their risks of different complications). Yet, in this study, participants in the peer support-alone group achieved equally substantial improvements in A1c as those in the iDecide group. This suggests that the ongoing supportive relationships between peer coaches and their assigned patients in both peer support arms were the most important active ingredient in the intervention’s success. This is good news for resource-constrained health systems that may lack the capacity to develop, continually update, and manage tailored e-health programs. Volunteer peer support programs can be important complements to over-burdened formal health care providers to improve the frequency and intensity of ongoing support between face-to-face clinic visits. Unlike most other tested diabetes management support programs, gains achieved over the six-month intervention period were sustained six months after the end of the program. Moreover, such programs that mobilize patients to help other patients could realistically be provided over sustained periods of time.
This study has limitations. Although participants in the iDecide group were encouraged to access the program on their own after the initial session, the peer coaches only actively engaged participants with the iDecide tool at the initial face-to-face session. Future interventions should test whether integrating e-Health tools throughout a peer support self-management support intervention would lead to greater effectiveness than peer support without such tools. Second, the two models were tested in only one VA medical center among predominantly African American and male patients and thus the results may not generalize to other populations and settings. Third, although the peer coaches were blinded to the study’s hypotheses, the nature of the intervention prevented blinding to treatment group. Finally, this intervention focused exclusively on supporting patients to improve their diabetes self-management. A potentially more powerful intervention would also directly involve their primary care providers to encourage them to respond to identified concerns and to initiate or intensify medication.
In conclusion, the iDecide program did not contribute to improved outcomes beyond ongoing telephone-based support from fellow Veteran patients with diabetes who had received brief initial training and monthly booster support meetings. The significant improvements in glycemic control achieved through both peer support models, gains that were sustained six months after the conclusion of the intervention, further reinforces the power of peer support to improve social support and clinical outcomes in diabetes. A key challenge continues to be to explore ways peer support programs might be made even more effective in improving and sustaining gains in health outcomes.
Implications for Practice
This study also has important implications for diabetes educators and other health professionals who aim to educate and counsel patients to help them improve diabetes self-management behaviors. There is a great deal of attention now devoted to supplementing behavioral coaching with e-Health tools. Yet, this study’s results suggest that the most important element of effective education and counseling remains nurturing and sustaining strong, personal relationships based on trust and that extend ongoing social support.
Acknowledgments
Role of Funding SourcesThis research was supported a Grant from the VA HSR&D 12-412 and by Grant Number P30DK092926 (MCDTR) from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have no conflict of interest or financial disclosures. The funding sources had no role in the study design; data collection; administration of the interventions; analysis, interpretation, or reporting of data; or decision to submit the findings for publication.
Contributor Information
Michele Heisler, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, and Center for Clinical Management Research, Ann Arbor Veterans Affairs (VA) Healthcare System, Ann Arbor, Michigan; Department of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor, Michigan, and Michigan Center for Diabetes Translation Research (MCDTR), University of Michigan, Ann Arbor VA, Ann Arbor, Michigan; VA Corporal Michael J. Crescenz Medical Center and Center for Health Equity Research and Promotion and University of Pennsylvania Department of Internal Medicine, Philadelphia, Pennsylvania.
Hwajung Choi, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, and Center for Clinical Management Research, Ann Arbor Veterans Affairs (VA) Healthcare System, Ann Arbor, Michigan.
Rebecca Mase, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, and Center for Clinical Management Research, Ann Arbor Veterans Affairs (VA) Healthcare System, Ann Arbor, Michigan.
Judith A. Long, VA Corporal Michael J. Crescenz Medical Center and Center for Health Equity Research and Promotion and University of Pennsylvania Department of Internal Medicine, Philadelphia, Pennsylvania.
Pamela J. Reeves, John D. Dingell VA Medical Center, Detroit, Michigan.
References
- 1.Heisler M, Faul JD, Hayward RA, et al. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853–1860. [DOI] [PubMed] [Google Scholar]
- 2.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(5 Suppl):101S–56S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Veterans Affairs. VHA Office of Quality and Performance Report. http://vaww.pdw.med.va.gov/MeasureMaster/MMReport.asp. Accessed June 29, 2011.
- 4.Heisler M, Smith DM, Hayward RA, et al. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41(11):1221–1232. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LA, Beach MC, Johnson RL, et al. Delving below the surface. Understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21 Suppl 1:S21–S27. 1484840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979–987. 3483913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frosch DL, May SG, Rendle KA, et al. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030–1038. [DOI] [PubMed] [Google Scholar]
- 8.Peek ME, Quinn MT, Gorawara-Bhat R, et al. How is shared decision-making defined among African-Americans with diabetes? Patient Educ Couns. 2008;72(3):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosland AM, Kieffer E, Israel B, et al. When Is Social Support Important? The Association of Family Support and Professional Support with Specific Diabetes Self-management Behaviors. J Gen Intern Med. 2008;23(12):1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis CL. Peer support within a health care context: a concept analysis. Int J Nurs Stud. 2003;40(3):321–332. PMCID: Not Required. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong K, McMurphy S, Dean LT, et al. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23(6):827–833. PMCID: PMC2517897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghorob A, Vivas MM, De Vore D, et al. The effectiveness of peer health coaching in improving glycemic control among low-income patients with diabetes: protocol for a randomized controlled trial. BMC Public Health. 2011;11:208. PMCID: PMC3082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heisler M, Vijan S, Makki F, et al. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–515. PMCID: PMC4117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long JA, Jahnle EC, Richardson DM, et al. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156(6):416–424. PMCID: PMC3475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, et al. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care. 2011;34(9):1926–1931. PMCID: PMC3161298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thom DH, Ghorob A, Hessler D, et al. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med. 2013;11(2):137–144. PMCID: PMC3601392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Yang S, Sun K, et al. How to achieve better effect of peer support among adults with type 2 diabetes: A meta-analysis of randomized clinical trials. Patient Educ Couns. 2016;99(2):186–197. PMCID: Journal In Process. [DOI] [PubMed] [Google Scholar]
- 18.Neville LM, O’Hara B, Milat AJ. Computer-tailored dietary behaviour change interventions: a systematic review. Health Educ Res. 2009;24(4):699–720. PMCID:PMC2706490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133(4):673–693. PMCID: Not Required. [DOI] [PubMed] [Google Scholar]
- 20.Adams SY, Crawford AG, Rimal RN, et al. The effects of a computer-tailored message on secondary prevention in type 2 diabetes: a randomized trial. Popul Health Manag. 2009;12(4):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–1568. [DOI] [PubMed] [Google Scholar]
- 22.Wallace AS, Seligman HK, Davis TC, et al. Literacy-appropriate educational materials and brief counseling improve diabetes self-management. Patient Educ Couns. 2009;75(3):328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med. 2010;51(3-4):214–221. 2939185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacey D, Belkora J, Clay K, et al. 2012 Update of the IPDAS Collaboration Background Document. 2012; http://ipdas.ohri.ca/resources.html. Accessed March 25, 2019.
- 25.Wilkinson MJ, Nathan AG, Huang ES. Personalized decision support in type 2 diabetes mellitus: current evidence and future directions. Curr Diab Rep. 2013;13(2):205–212. 3593795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons MC, Wilson RF, Samal L, et al. Impact of consumer health informatics applications. Evidence report/Technology assessment No. 188. AHRQ. 2009. [PMC free article] [PubMed] [Google Scholar]
- 27.Heisler M, Choi H, Palmisano G, et al. Comparison of community health worker-led diabetes medication decision-making support for low-income Latino and African American adults with diabetes using e-health tools versus print materials: a randomized, controlled trial. Ann Intern Med. 2014;161(10 Suppl):S13–S22. PMCID: PMC4391371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson VA, Barr KL, An LC, et al. Community-based participatory research and user-centered design in a diabetes medication information and decision tool. Prog Community Health Partnersh. 2013;7(2):171–184. PMCID: PMC4117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskowitz D, Thom DH, Hessler D, et al. Peer coaching to improve diabetes self-management: which patients benefit most? J Gen Intern Med. 2013;28(7):938–942. PMCID: PMC3682027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piette JD, Resnicow K, Choi H, et al. A diabetes peer support intervention that improved glycemic control: mediators and moderators of intervention effectiveness. Chronic Illn. 2013;9(4):258–267. PMCID: PMC3830685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goei M, Schechtel M, Meyer S, et al. Premixed insulin for type 2 diabetes Effective Health Care. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 32.Robinson S, Rugge B, Schechtel M, et al. Pills for type 2 diabetes Effective health care. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 33.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167(10):1076–1082. [DOI] [PubMed] [Google Scholar]
- 34.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol. 2000;37(1):1–7. [DOI] [PubMed] [Google Scholar]
- 35.American Heart Association. Understanding Blood Pressure Readings. http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/KnowYourNumbers/Understanding-Blood-Pressure-Readings_UCM_301764_Article.jsp#.Wl-XSzYUnDA. Accessed January 15, 2018.
- 36.Barrera M Jr., Glasgow RE, McKay HG, et al. Do Internet-based support interventions change perceptions of social support?: An experimental trial of approaches for supporting diabetes self-management. Am J Community Psychol. 2002;30(5):637–654. PMCID: Not Required. [DOI] [PubMed] [Google Scholar]
- 37.Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action). J Health Serv Res Policy. 2000;5(1):12–16. PMCID: Not Required. [DOI] [PubMed] [Google Scholar]
- 38.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2011;9(8):672–677. [DOI] [PubMed] [Google Scholar]
- 39.2015. Stata: Release 14. Statistical Software. College Station, TX: StataCorp LP; [computer program]. [Google Scholar]
- 40.Fisher EB, Ballesteros J, Bhushan N, et al. Key Features Of Peer Support In Chronic Disease Prevention And Management. Health Aff (Millwood). 2015;34(9):1523–1530. PMCID: Journal In Process. [DOI] [PubMed] [Google Scholar]
- 41.Tang TS, Funnell MM, Sinco B, et al. Peer-Led, Empowerment-Based Approach to Self-Management Efforts in Diabetes (PLEASED): A Randomized Controlled Trial in an African American Community. Ann Fam Med. 2015;13 Suppl 1:S27–S35. PMCID: PMC4648139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang TS, Funnell M, Sinco B, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care. 2014;37(6):1525–1534. PMCID: PMC4030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]