Dear Editor-in-Chief,
Skeletal muscles that have been subjected to chronic mechanical overload typically present with a large increase in the total number of fibers per cross-section, and the review by Murach et al. proposes that fiber splitting could, at least in part, explain this effect (1). To support their position, the first figure of their manuscript uses data from Roy and Edgerton (2) to argue that if an increase in muscle fiber cross-sectional area and pennation angle occur simultaneously then there would be no net increase in the number of fibers per cross-section. However, their argument did not account for the 13% increase in muscle fiber length that was also reported by Roy and Edgerton (2). We believe that this was a critical oversight because, as illustrated below, subtle changes in fiber length could lead to a substantial increase in the both the number of fibers per cross-section and the overall cross-sectional area of the muscle.
Before explaining our position, we first want to state that we fully agree with Murach et al., in that models of chronic mechanical overload (e.g., synergist ablation) can induce a doubling in muscle mass within 1-2 weeks, whereas substantial changes in the fiber cross-sectional area typically occur on a much slower time scale (1-2 months). Importantly, however, it has also been shown that chronic mechanical overload can lead to a very rapid increase in fiber length. For instance, Aoki et al. (2009) reported that a ≈25% increase in both sarcomere number and fiber length can occur within 4 days after the onset of chronic stretch (3). Likewise, Williams and Goldspink (1973) reported that 7 days of chronic mechanical overload (via tenotomy) led to a 25% increase in the number of sarcomeres per fiber (4). Based on these studies, it seems reasonable to propose that the 13% increase in fiber length reported by Roy and Edgerton could have occurred within a matter of days after the onset of mechanical overload.
In figure 1 we describe the key elements of a geometric model that can be used to predict the architectural properties of skeletal muscle (5). As shown in figure 2, we used this model to assess what would happen if the plantaris muscle experienced a 13% increase in fiber length. The outcomes of our analysis revealed that, in the absence of any other changes (e.g., increase in fiber cross-sectional area, fiber splitting, etc.), a subtle 13% increase in fiber length would lead to a remarkable 52% increase in both the whole muscle cross-sectional area and the number of fibers per cross-section. Unfortunately, the majority of the studies in the field (including our own) have not measured fiber length. Yet, as recently summarized by Franchi et al. (2016), there are strong indications that even resistance-exercise in humans can lead to an increase in fiber length (6). Thus, it is our hope that this letter will help to raise awareness about the potentially profound role that changes in fiber length could play during various forms of mechanical load-induced growth.
Figure 1.
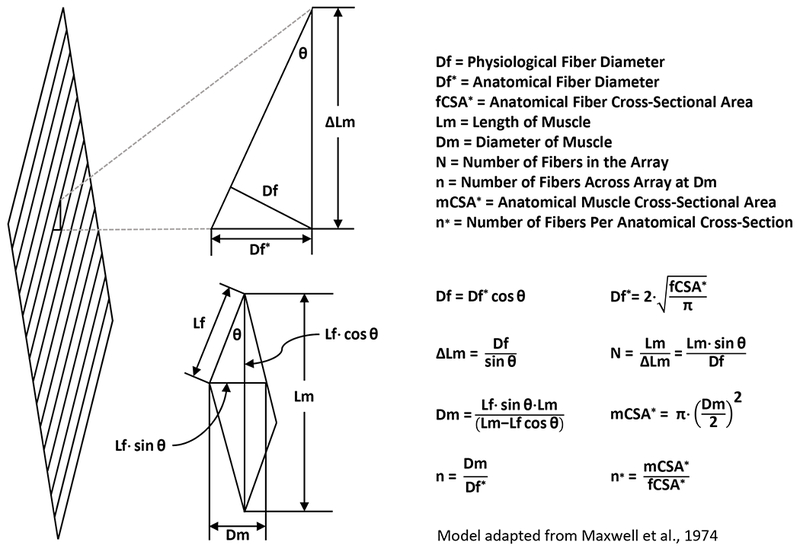
Geometric Model of Skeletal Muscle Architecture
Figure 2.
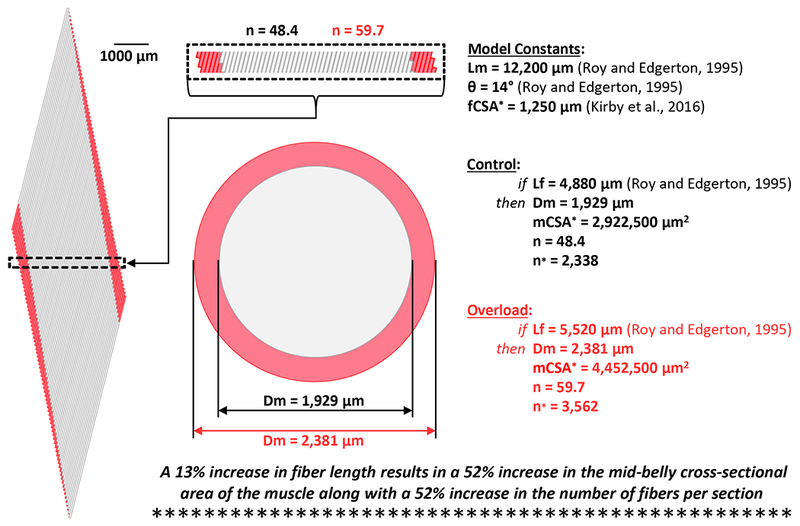
Scale Model of a Plantaris Muscle Undergoing a 13% Increase in Fiber Length
References
- 1.Murach KA, Dungan CM, Peterson CA, McCarthy JJ. Muscle Fiber Splitting Is a Physiological Response to Extreme Loading in Animals. Exercise and sport sciences reviews. 2019;47(2):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy RR, Edgerton VR. Response of mouse plantaris muscle to functional overload: comparison with rat and cat. Comp Biochem Physiol A Physiol. 1995;111(4):569–75. [DOI] [PubMed] [Google Scholar]
- 3.Aoki MS, Soares AG, Miyabara EH, Baptista IL, Moriscot AS. Expression of genes related to myostatin signaling during rat skeletal muscle longitudinal growth. Muscle Nerve. 2009;40(6):992–9. [DOI] [PubMed] [Google Scholar]
- 4.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- 5.Maxwell LC, Faulkner JA, Hyatt GJ. Estimation of number of fibers in guinea pig skeletal muscles. J Appl Physiol. 1974;37(2):259–64. [DOI] [PubMed] [Google Scholar]
- 6.Franchi MV, Atherton PJ, Maganaris CN, Narici MV. Fascicle length does increase in response to longitudinal resistance training and in a contraction-mode specific manner. Springerplus. 2016;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]


