Abstract
Background:
Biofilms represent a complex milieu of matrix-enclosed microorganisms, which can significantly contribute to the pathology of chronic wounds. In this study, we compare the activity of three commercial antimicrobial wound-care solutions, Vashe® (HOCl-based), PhaseOne® (HOCl-based), and Sulfamylon® (mafenide acetate), for their in vitro activity against bacterial and fungal biofilms.
Methods:
Reference and clinical isolates of 6 Gram-negative bacterial species (36 total strains), 3 Gram-positive bacteria (21 strains), and 3 Candida species (9 strains) were used to create biofilms. Various working concentrations of the 3 antiseptic agents were incubated with the biofilms in microwell plates; they were monitored from 1 minute to 24 hours to compare bacterial and fungal viability through colony forming unit (CFU) analysis.
Results:
Vashe® and PhaseOne® displayed excellent bactericidal and fungicidal activity, whereas Sulfamylon® demonstrated minimal activity against the biofilms tested. With the exception of C. albicans, all biofilms were eliminated at either 1 or 10 minutes using Vashe® and PhaseOne® solutions. In most cases, mafenide was unable to eliminate both bacterial and fungal biofilms, even with 24 hours of treatment.
Conclusions:
Biofilms represent a major clinical challenge, with no clear consensus for treatment of chronic wounds or prosthetic devices. Our results suggest that hypochlorous acid-based wound solutions such as Vashe® and PhaseOne® are more efficacious than mafenide in eliminating bacterial and fungal biofilms. Further studies are necessary to investigate and compare the in vivo efficacy of these products in clinical care.
INTRODUCTION
Many bacterial and fungal species can proliferate by two distinct modes of growth: as planktonic (free-living) organisms or surface-associated biofilms. While both lifestyles are clinically relevant, the latter presents unique challenges for medical management (1). Biofilms comprise matrix-enclosed microbial populations that adhere to each other and external surfaces as organized communities. The structural barrier of extracellular matrix, as well as the reduced growth-rate of biofilm-associated organisms, render these organisms less susceptible to antibiotic and antiseptic therapy (2). Biofilm development proceeds in vivo through a series of regulated steps, with the underlying molecular mechanisms differing from species to species. In general terms, the stages of biofilm formation include: attachment of planktonic organisms to a surface/interface; elaboration of extracellular matrix components (often polysaccharides and/or proteins, depending on the species); expansion and maturation as microcolonies; and (ultimately) re-mobilization of planktonic organisms from the biofilm, ensuring further dissemination (1). Preventing biofilm development throughout these stages—along with eliminating existing communities, an even greater challenge—is imperative for combating biofilm infections.
Overall, it is estimated that biofilms account for over 80% of total bacterial/fungal infections in clinical practice, including ~65% of nosocomial infections (3, 4). Perhaps not surprisingly, the cost of care for these infections is thought to exceed $1 billion annually in the United States alone (2, 5). Chronic, non-healing wound infections are among the most notorious biofilm-driven pathologies, causing significant morbidity and mortality around the globe. Wound-associated biofilms are often polymicrobial nature, with a combination of bacterial and fungal species (6-8). For instance, in one survey of 915 culture-specimens from chronic wounds, 23% of mixed cultures included a fungal component (6). Unfortunately, the paucity of literature on simultaneously managing bacterial and fungal species within chronic wounds limits the development of evidence-based therapeutic strategies.
An additional challenge lies in the absence of universal methods for assessing antibiofilm activity in the laboratory. Unlike with planktonic bacteria and fungi, reference guidelines have not been formalized for the antimicrobial susceptibility testing of biofilms, and various methodologies are encountered throughout the literature (9, 10). The situation is further complicated for evaluating the antibacterial/antifungal activity of antiseptic compounds (as opposed to antibiotics), such as those found in topical wound-care solutions. Standardized clinical testing protocols have not been developed with antiseptics for either planktonic organisms or biofilms. Despite these hurdles, quantifying and comparing the antimicrobial activity of different wound-care products remain valuable endeavors.
In this context, we have evaluated three common wound-care solutions for their in vitro microbicidal activity (i.e. quantitative killing of pre-formed biofilms) against biofilms of diverse wound-associated pathogens, including bacteria and fungi. Specifically, we assessed Vashe®, PhaseOne®, and mafenide acetate (5%) against various laboratory strains (from the American Type Culture Collection – ATCC) and clinical isolates. Vashe® and PhaseOne® both employ dilute stabilized hypochlorous acid (HClO) as the active agent (Vashe®: HClO – ≤0.033%, NaCl – 0.4%, pH 2.5–6.75; PhaseOne®: HClO – 0.025%, NaCl – <10%, pH 3.5–6.5) (11, 12). These three agents have not been systematically compared to one another for their killing activity on the diverse range of biofilms considered here, and we hypothesized that Vashe® and PhaseOne® would demonstrate broader anti-biofilm activity than mafenide—in particular against fungal species—given reports of the latter’s limited antifungal activity, as well its dominant bacteriostatic rather than bacteriocidal mode of action (ostensibly, see Discussion below) against bacteria (13).
MATERIALS AND METHODS
Bacterial and fungal strains.
Our study evaluated the in vitro efficacy of the above wound-care preparations against biofilms of 66 bacterial and fungal strains. These organisms included 3 Gram-positive bacterial species (21 strains), 6 Gram-negative bacterial species (36 total strains), and 3 fungal species (9 total strains). All clinical isolates were obtained from the Clinical Microbiology Laboratory at Vanderbilt University Medical Center (VUMC) as residual derivatives of routine patient care (utilized after patient de-identification; Vanderbilt IRB #171522). All strains are summarized in Table 1, with clinical isolates designated by VUMC in the name.
Bacterial biofilm formation.
Organisms were initially sub-cultured from freezer-stocks onto trypticase soy agar with 5% sheep blood and incubated aerobically at 35°C overnight. Liquid subcultures were prepared by inoculating a single colony into Brain Heart Infusion (BHI) broth, with aerobic overnight incubation at 35°C with 200-RPM orbital shaking. Cells were washed twice in sterile phosphate-buffered saline (PBS) and re-suspended in BHI with 1% glucose; bacterial density was adjusted to 0.5 McFarland (corresponding to ~107 CFU/mL). 100 μL were inoculated into individual wells of tissue-culture treated 96-well plates, which were incubated aerobically for 24 hours at 35°C. Wells were inspected microscopically and macroscopically to evaluate for confluent/adherent biofilm formation; they were gently washed three times with 1X PBS to remove non-adherent/planktonic cells.
Fungal biofilm formation.
Organisms were initially sub-cultured from freezer stocks onto Sabouraud dextrose agar (SDA) plates and incubated aerobically overnight at 30°C. Liquid subcultures were prepared by inoculating a single colony into Sabouraud dextrose broth (SDB), with aerobic overnight incubation at 30°C with 200-RPM orbital shaking. Cells were washed twice in sterile PBS and re-suspended in in Roswell Park Memorial Institute 1640 media with 3-N-morpholinopropanesulfonic acid sodium salt (MOPS); fungal density was adjusted to 0.5 McFarland (corresponding to ~106 CFU/mL). 100 μL were inoculated into individual wells of tissue-culture treated 96-well plates, which were incubated aerobically for 24 hours at 30°C. The remaining steps were performed as above with bacteria.
Addition of wound solutions.
Vashe® (SteadMed, Fort Worth, TX) and PhaseOne® (Integrated Healing Technologies, Franklin, TN) were obtained as the same commercially-available formulation utilized for patient care. Aqueous solutions of 5% mafenide acetate were obtained directly from the VUMC Compounding Pharmacy, the same formulations/lots used at our institution for clinical use. These original solutions of each agent (denoted as 100%) were further diluted in 1X PBS pH 7.4 to create 50% and 25% solutions; this diluent was intentionally chosen to mimic physiologic conditions, not those of the 100% formulation. 100 μL of each agent at 100%, 50%, and 25% were added directly the above wells with preformed biofilms. Control biofilms were inoculated with 1X PBS alone. Plates were incubated for 1, 10, and 60 minutes at room temperature, as well as for 24 h at 35°C. For one ATCC strain of each species, biofilm eradication experiments were conducted on three independent replicates to demonstrate intra-strain reproducibility, while a single replicate was performed on the larger quantity of clinical strains to demonstrate intra-strain robustness. For the triplicate experiments, at least two different lots of the respective wound-care solutions were used against each strain. Moreover, given the more limited shelf-life of mafenide, one of its replicates was performed after the solution had been stored for one month (room temperature, in the dark), to evaluate if its activity was commensurate with the activity of freshly compounded solutions. Indeed, for all tested strains, levels of mafenide killing were identical between freshly compounded and stored solutions. For all singlicate experiments, only freshly compounded mafenide was employed.
CFU analysis.
At each of the above incubation-times, bacterial/fungal viability was quantified by colony forming unit (CFU) assays, as described previously (14, 15). In brief, organisms within treated wells were suspended and homogenized, with 10-fold serial dilutions made in PBS. These were plated onto respective agars (BHI or SBD) and colonies were enumerated after overnight incubation. The calculated organism burden was expressed logarithmically in CFU/ml (with respect to the initial 100 μL volume of wound-care solution), with the corresponding untreated well (PBS-only) as a control.
RESULTS
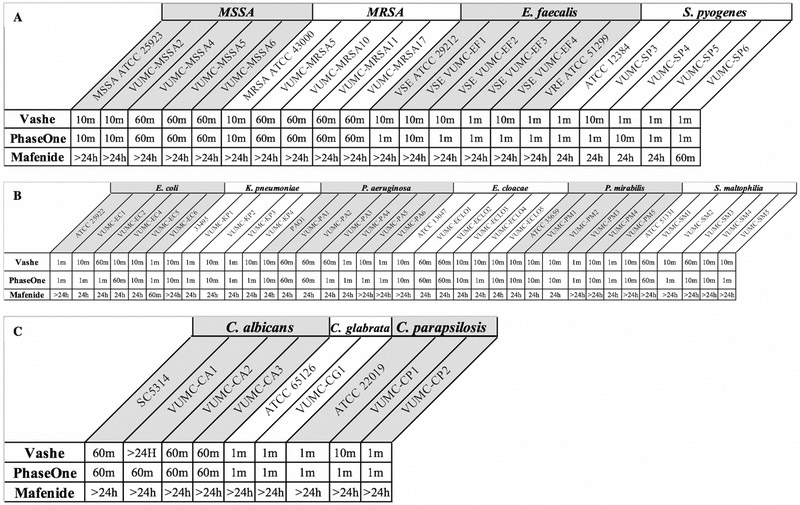
The observed time-dependant and concentration-dependant killing of bacterial/fungal biofilms by Vashe®, PhaseOne®, and mafenide are summarized in the following sections. Time-kill curves are included as Figures for several species, with full datasets provided in Appendix A. Overall, Vashe® and PhaseOne® demonstrated superior in vitro bactericidal and fungicidal activity compared to mafenide, in particular against fungal strains. Apart from Candida albicans, all biofilms (bacterial and fungal) were effectively sterilized (defined as no observed colonies by CFU analysis) at 10 minutes by Vashe® and PhaseOne® at each tested dilution. Among all species analyzed, the most dramatic killing was observed for Streptococcus pyogenes and C. glabrata, sterilized at 1 minute with even diluted Vashe® and PhaseOne®. In many cases, mafenide was only able to sterilize biofilms after 24 hours of exposure to the baseline concentration (denoted as ‘100%’ - 5% mafenide acetate), and sometimes not even under these conditions. The effective sterilization times for all three agents (at 100% concentration) and strains are summarized in Figure 1, with >24 hr indicting a failure to sterilize. Across all agents and species, similar trends in antimicrobial activity were observed for both common laboratory strains and clinical isolates, although some clinical isolated required longer exposure-times for the same killing effect.
Figure 1. Effective sterilization times for Vashe®, PhaseOne® and mafenide (at 100% concentration) against all strains.
A. Gram-positive strains; B. Gram-negative strains; C. Fungal strains. Note that: >24 hr indicate a failure to sterilize.
GRAM-POSITIVE SPECIES
Methicillin-resistant Staphylococcus aureus (MRSA).
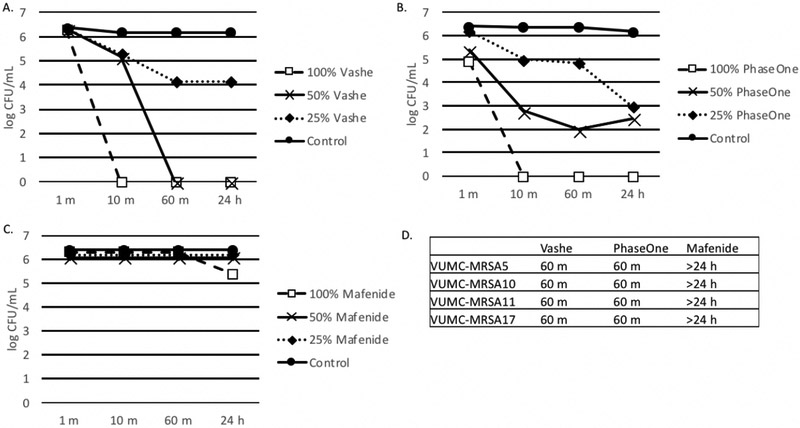
As depicted in Figure 2, 100% Vashe® and 100% PhaseOne® completely eradicated MRSA ATCC 43000 by 10 minutes, while diminished activity was observed against this strain for diluted concentrations. Vashe® and PhaseOne® demonstrated commensurate activity against all 4 clinical strains of MRSA, but with longer sterilization-times (60 minutes) than with ATCC 43000. Mafenide displayed no significant activity against both ATCC 43000 and all 4 clinical strains.
Figure 2. Effect of Wound Solutions on methicillin resistant S. aureus (MRSA).
A-C. Biofilms were formed in 100 μl BHI with 1% glucose. MRSA ATCC 43000 (panels A-C) or clinical strains of MRSA (panel D). Organisms were added to tissue culture treated plates and incubated for 24 h at 35°C. After 24 h biofilms were washed with 1X PBS three times and 100 μl, 50% or 25% Vashe (panel A), PhaseOne (panel B) or Mafenide (panel C) were added to the wells. Control wells contained 100 μl PBS. Plates were incubated for 1 m, 10 m, 60 m or 24 h. Wells were washed three times with PBS to remove the wound solutions and reconstituted with 100 μl H2O. Bacterial viability was monitored by the CFU assay. D. This data represents the minimum time to kill (CFU=0) clinical strains of MRSA with Vashe, PhaseOne or Mafenide. Bacteria that were not killed at 24 h are indicated by >24 h.
Panels A-D are representative of 3 independent experiments.
Methicillin-susceptible Staphylococcus aureus (MSSA).
Both Vashe® and PhaseOne® completely eradicated MSSA ATCC 25923 by 10 minutes at 100% and 50% concentrations. At 25% concentration, PhaseOne® displayed a faster onset of action compared to Vashe®, indicated by reduced CFU-counts at 10 and 60 minutes. 100% mafenide displayed modest activity against ATCC 25923, with a 2-log reduction in CFUs (relative to control) at 24 hours. Vashe® and PhaseOne® demonstrated commensurate activity against all four clinical strains of MSSA, while mafenide was unable to sterilize any clinical MSSA strains by 24 hours. See the Appendix - Figure 5 for a complete data summary.
Vancomycin-susceptible Enterococcus faecalis (VSE).
Both Vashe® and PhaseOne® sterilized VSE ATCC 29212 (at 100% and all dilutions), by 10 minutes and 1 minute respectively. Vashe® and PhaseOne® demonstrated commensurate activity against clinical strains VSE VUMC-EF1 and VUMC-EF3, with a minimum sterilization-time of 10 minutes for the 100% formulations. Strains VUMC-EF2 and VUMC-EF4 were more rapidly killed by PhaseOne® compared to Vashe®, at 1 minute and 10 minutes respectively. Mafenide displayed no significant activity against both ATCC 29212 and all 4 clinical strains. See the Appendix - Figure 6 for a complete data summary.
Vancomycin-resistant Enterococcus faecalis (VRE).
100% Vashe® and 100% PhaseOne® completely eradicated VRE ATCC 51299 by 1 minute. At 50% and 25% concentrations, PhaseOne® demonstrated a slightly faster onset of action than Vashe®. Undiluted mafenide displayed modest activity at 24 hours, with a ~3-log reduction in CFU/ml. See the Appendix - Figure 7 for a complete data summary.
Streptococcus pyogenes.
All three wound-care agents (at all dilutions) sterilized S. pyogenes ATCC 12384 by 24 hours. However, Vashe® and PhaseOne® demonstrated a dramatically more rapid effect than mafenide, requiring only 1 minute for sterilization. Vashe® and PhaseOne® demonstrated commensurate activity against VUMC-SP5 and VUMC-SP6, with a minimum sterilization-time of 1 minute for the 100% formulation. PhaseOne® displayed more rapid sterilization than Vashe® for VUMC-SP3 (1 minute versus 10 minute), although the trend was reversed for VUMC-SP4 sample. Finally, among clinical isolates, mafenide demonstrated rapid activity against VUMC-SP6 (sterilization at 60 minutes), while other strains required 24 hours. See the Appendix - Figure 8 for a complete data summary.
GRAM-NEGATIVE SPECIES
Escherichia coli.
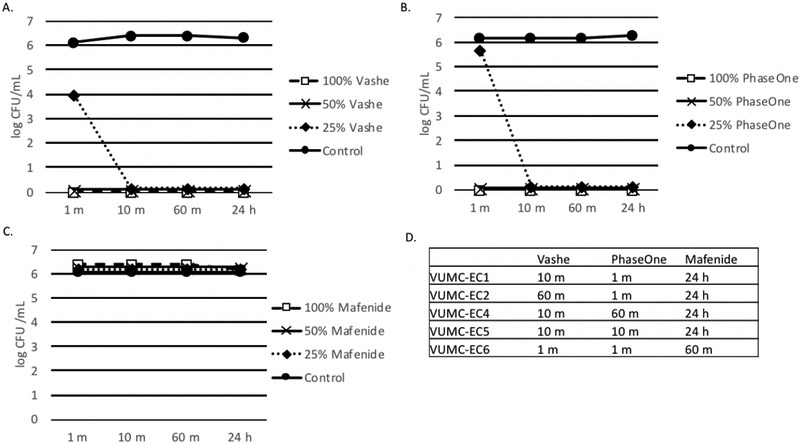
As depicted in Figure 3, Vashe® and PhaseOne® sterilized E. coli ATCC 25922 by 1 minute at both 100% and 50% concentrations. Some differences in sterilization time were observed for Vashe® and PhaseOne® for clinical E. coli isolates, although one agent was not uniformly more rapid than the other, and sterilization was observed consistently by 60 minutes in all cases. Mafenide demonstrated no observable activity against E. coli ATCC 25922 over the course of 24 hours, although the 100% formulation did sterilize clinical E. coli isolates after prolonged incubation times (24 hours for VUMC-EC1, VUMC-EC2, VUMC-EC3, VUMC-EC4, and VUMC-EC5; 60 minutes for VUMC-EC6).
Figure 3. Effect of Wound Solutions on E. coli.
A-C. Biofilms were formed in 100 μl BHI with 1% glucose. E. coli ATCC 25922 (panels A-C) or clinical strains of E. coli (panel D). Organisms were added to tissue culture treated plates and incubated for 24 h at 35°C. After 24 h biofilms were washed with 1X PBS three times and 100 μl 100%, 50% or 25% Vashe (panel A), PhaseOne (panel B) or Mafenide (panel C) were added to the wells. Control wells contained 100 μl 1X PBS. Plates were incubated for 1 m, 10 m, 60 m or 24 h. Wells were washed three times with PBS to remove the wound solutions and reconstituted with 100 μl H2O. Bacterial viability was monitored by the CFU assay. D. This data represents the minimum time to kill (CFU=0) clinical strains of E. coli with Vashe, PhaseOne or Mafenide. Bacteria that were not killed at 24 h are indicated by >24 h.
Panels A-D are representative of 3 independent experiments.
Klebsiella pneumoniae.
100% Vashe® and 100% PhaseOne® sterilized K. pneumoniae ATCC 33495 by 10 minutes. Both agents demonstrated commensurate activity against VUMC-KP1, VUMC-KP2, and VUMC-KP3. PhaseOne® killing of VUMC-KP4 biofilm was slightly more rapid than Vashe®, with a sterilization time of 1 versus 10 minutes. Although 100% mafenide was unable to completely sterilize ATCC 33495, it did successfully eradicate the clinical K. pneumoniae strains at 24 hours. See the Appendix - Figure 9 for a complete data summary.
Pseudomonas aeruginosa.
All three wound solutions (100%, plus one or both dilutions) sterilized P. aeruginosa ATCC BAA-47/PAO1 by 24 hours. However, Vashe® and PhaseOne® demonstrated significantly more rapid killing compared to mafenide. Commensurate activity was observed for Vashe® and PhaseOne® against VUMC-PA1, VUMC-2, VUMC-4, and VUMC-6, while PhaseOne® demonstrated more rapid activity against VUMC-PA3 & VUMC-PA5. 100% mafenide killing was significantly slower for all clinical strains, with 24 hours needed to sterilize VUMC-PA1, VUMC-PA2, VUMC-PA3, and VUMC-PA4; it was unable to sterilize VUMC-PA5 and VUMC-PA6. See the Appendix - Figure 10 for a complete data summary.
Enterobacter cloacae.
100% Vashe® and 100% PhaseOne® sterilized E. cloacae ATCC 13047 by 10 minutes, while Mafenide® proved ineffective even after 24 hours of exposure. For the clinical strains VUMC-ECLO1, ECLO2, ECLO3 and ECLO5 Vashe® and PhaseOne® had equivalent efficacy however, PhaseOne® displayed a faster onset of action compared to Vashe® for strain ECLO4 (1 minute compared to 10 minutes). Mafenide required a minimum time to kill of 24 hours against all five clinical strains. See the Appendix - Figure 11 for a complete data summary.
Proteus mirabilis.
100% Vashe® and 100% PhaseOne® sterilized P. mirabilis ATCC 35659, VUMC-PM1, VUMC-PM2 and VUMC-PM4 by 10 minutes, as well as VUMC-PM3 and VUMC-PM5 by 1 minute. Although mafenide could not sterilize VUMC-PM3 and VUMC-PM4, the 100% mafenide solution was able to sterilize the other P. mirabilis strains by 24 hours. See the Appendix - Figure 12 for a complete data summary.
Stenotrophomonas maltophilia.
100% Vashe® and 100% PhaseOne® sterilized S. maltophilia ATCC 51331 by 10 minutes. Both solutions demonstrated commensurate activity against VUMC-SM1 and VUMC-SM4; however, PhaseOne® displayed more rapid activity against VUMC-SM2, VUMC-SM3 and VUMC-SM5 compared to Vashe®. Mafenide demonstrated no observed activity against all strains of S. maltophilia tested. See the Appendix - Figure 13 for a complete data summary.
FUNGAL SPECIES
Candida albicans.
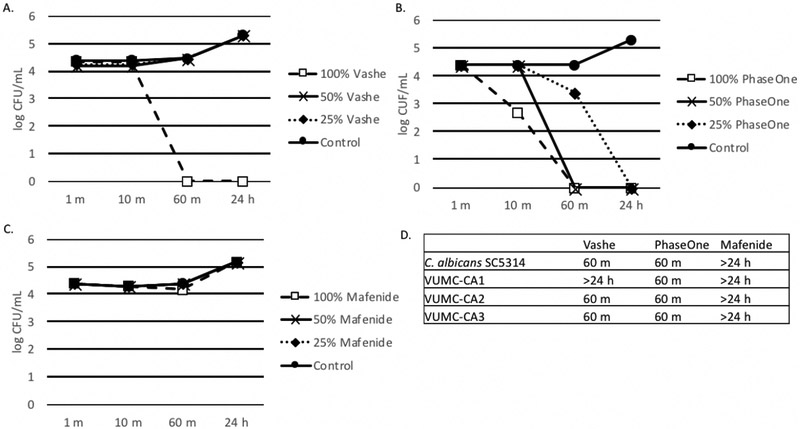
As depicted in Figure 4, 100% Vashe® and 100% PhaseOne® sterilized C. albicans ATCC SC5314 by 60 minutes, as well as clinical strains VUMC-CA2 and VUMC-CA3. However, diluted Vashe® failed to demonstrate killing of ATCC SC5314 over 24 hours, while killing of this strain was still observed (albeit more slowly) with diluted PhaseOne®. Interestingly, 100% Vashe® was unable to sterilize VUMC-CA1 even at 24 hours, while 60-minute sterilization of this strain was observed for PhaseOne®. Mafenide demonstrated essentially no activity against all C. albicans strain tested.
Figure 4. Effect of Wound Solutions on C. albicans.
A-C. Biofilms were formed in 100 μl RPMI with MOPS. C. albicans ATCC MYA-2876/SCS314 (panels A-C) or clinical strains of C. albicans (panel D). Organisms were added to tissue culture treated plates and incubated for 24 h at 35°C. After 24 h biofilms were washed with 1X PBS three times and 100 μl 100%, 50% or 25% Vashe (panel A), PhaseOne (panel B) or Mafenide (panel C) were added to the wells. Control wells contained 100 μl 1X PBS. Plates were incubated for 1 m, 10 m, 60 m or 24 h. Wells were washed three times with PBS to remove the wound solutions and reconstituted with 100 μl H2O. Bacterial viability was monitored by the CFU assay. D. This data represents the minimum time to kill (CFU=0) clinical strains of C. albicans with Vashe, PhaseOne or Mafenide. Yeast cells that were not killed at 24 hare indicated by >24 h.
Panels A-D are representative of 3 independent experiments.
Candida glabrata.
PhaseOne® (all dilutions) and Vashe® (all dilutions except 25%) sterilized C. glabrata ATCC 65126 and VUMC-CG1 by 1 minute. 100% Mafenide demonstrated only mild activity against ATCC 65126, without complete sterilization after 24 hours. See the Appendix - Figure 14 for a complete data summary.
Candida parapsilosis.
All tested concentrations of Vashe® and PhaseOne® sterilized C. parapsilosis ATCC 22019 within 60 minutes of exposure. PhaseOne® demonstrated more rapid activity against VUMC-CP1 compared to Vashe®; however, both agents demonstrate commensurate activity against VUMC-CP2. 100% Mafenide demonstrated partial activity at 24 hours against ATCC 22019 (~3-log CFU reduction); however, no activity was observed against VUMC-CP1 and VUMC-CP2. See the Appendix - Figure 15 for a complete data summary.
DISCUSSION
In this study, we compared the in vitro activity of Vashe®, PhaseOne® and mafenide on bacterial and fungal biofilms. Overall, Vashe® and PhaseOne® demonstrated excellent bactericidal and fungicidal activity. The observed differences between these two hypochlorite-based agents were minimal and we do not believe they imply any generalizable trends about the microbiologic/clinical scenarios in which one product would be favored over the other. By contrast, mafenide demonstrated lower in vitro killing activity across the board, in particular with Candida strains. Several mechanisms may underpin this minimal activity, including the possibility of insufficient mafenide penetration in the biofilms, not to mention the issue (inherent to clinical practice) of utilizing a putatively bacteriostatic topical agent against a condition like chronic wounds, where pre-existing biofilms (prior to application) contributes to overall pathology. In this regard, future experiments could compare mafenide activity against planktonic- and biofilm-phase growth of these same organisms (regardless, biofilms do remain the dominant mode of growth within actual wounds). The maximum concentration of mafenide employed here may also be insufficiently low at 5%, although this concentration was specifically chosen to reflect clinical reality, as 5% and 2.5% formulations are most commonly used for patient care. In fact, the mafenide solutions used here were provided by our institution’s compounding pharmacy, reflecting the same lots in concurrent clinical use.
While not all biofilms are pathogenic – with many acting as commensals of physiologically colonized anatomic sites – various biofilms have been shown to impair the physiologic processes underlying wound healing, thus promoting the development of chronic wounds (16). Chronic wounds are characterized by a persistent inflammatory state with high levels of inflammatory cytokines, proteases (e.g. matrix metalloproteases and elastases), and neutrophils (17, 18). They are known to harbor a significantly higher proportion of biofilm-associated microorganisms compared to acute wounds (19-21). Historically, chronic wounds were considered the products of certain host factors, such as abnormal host genetics and/or comorbidities including repetitive trauma, malnutrition, or poor perfusion. However, in recent years there has been a shift away from the notion of a purely host-derived pathology to consider the role of microbial contributions, whereby organisms within biofilms manipulate the host response. In this context, the biofilm matrix is hypothesized to mask cardinal signs of infection such as pain, erythema, swelling, and heat (classically observed in chronic cutaneous wounds). Rather, predominate features include exudate formation, delayed healing, and a fluctuating course of exacerbation (16).
The clinical sequelae of a biofilm may vary in accordance with its individual microbial composition. In general, however, studies have emphasized that organisms within biofilms can be more challenging to treat with antimicrobial therapy than their planktonically growing counterparts (16, 22). Several features of bacterial and fungal biofilms may underlie this phenomenon, including: the inability of antimicrobial agents to penetrate the biofilm matrix; in situ inactivation of antimicrobial compounds; transfer of resistance genes among strains/species; the role of organism-to-organism communication (e.g. quorum sensing), regulating the expression of resistance/virulence and improved defences against innate and adaptive immunity, which often act in concert with pharmacologic therapy to clear an infection (1).
Current wound-management strategies are predicated upon the use of wound dressings, such as skin substitutes to regenerate damaged skin, antimicrobial dressings, and antimicrobial wound cleansing solutions. Wound solutions may be used to both directly irrigate the wound and saturate wound dressings. Of the three solutions examined in this study, both Vashe® and PhaseOne® are hypochlorous-based wound cleansing agents. Manufacturers of both agents claim that they exert their effects by physically disrupting biofilms and have bactericidal and fungicidal properties (23, 24), although published data to support these claims are limited (25, 26). Mafenide is another wound solution that is currently utilized at our institution’s burn unit. Mafenide is a sulphur-containing agent, mainly used under moist dressings over skin grafts in burn wounds. Its product insert claims bacteriostatic activity against Gram-negative and Gram-positive bacteria (27). Limited published communications have suggested some possible degree of bacteriocidal and/or antibiofilm activity (28), but robust data to these ends are lacking (further reinforced by our observations here).
One important caveat to this study, which we much emphasize, is the in vitro nature of the data. The non-physiologic surfaces tested here do not necessarily reflect in vivo oxygen/nutrient gradients—as well as the host inflammatory response—with downstream effects on microbial physiology and biofilm architecture. In general, an inherent challenge for biofilm research is the difficulty in analyzing and replicating their precise in vivo structures. So, while our results give cause for optimism on the biofilm-killing activity of Vashe® and PhaseOne®, they do not necessarily replicate the in vivo dynamics within infected wounds. They thus should not be used by themselves to inform patient-management decisions on the specific choice of commercial wound-care solution (Vashe®, PhaseOne® or mafenide).
As mentioned in the introduction, one of the challenges of conducting antimicrobial susceptibility testing on biofilm-phase organisms is the lack of standard methods for clinical use, either for systemic antibiotics or (even more so) topical antiseptics. By contrast, for traditional susceptibility testing of planktonic organisms, not only do international guidelines provide detailed reference protocols, but they also correlate the resultant data to pharmacologic parameters and clinical outcomes (9, 10). These in vitro-in vivo associations are what ultimately allow laboratory results to be interpreted as ‘clinically susceptible’ versus ‘clinically resistant’ for a given organism/agent. Unfortunately, this extensive framework does not yet exist for either biofilm susceptibility testing or evaluation of antiseptic compounds, although we hope the current data can contribute to the necessary foundation. In particular, we hope this work can segue into future studies with animal models of chronic wounds and ultimately into human studies.
CONCLUSION
Biofilms contribute significantly to morbidity in patients with chronic wounds, with no current treatment consensus among clinicians. Our results demonstrate that hypochlorous acid-based wound solutions (i.e. Vashe® and PhaseOne®) exert potent biofilm killing-activity in vitro, especially in comparison to mafenide. This study is the first to evaluate these three preparations in a direct comparison with one another against biofilm-stage bacterial and fungal growth. The methods and data likewise provide a framework for much-needed future studies on the topical antisepsis of biofilms. We hope the findings will encourage greater inquiry—both in vitro and in vivo—into the microbiologic and clinical efficacy of these and other wound-care products.
Supplementary Material
Footnotes
Financial Disclosures:
None
REFERENCES:
- 1.Percival SL, McCarty SM, Lipsky B. Biofilms and Wounds: An Overview of the Evidence. Adv Wound Care (New Rochelle). 2015;4(7):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. [DOI] [PubMed] [Google Scholar]
- 3.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–9. [DOI] [PubMed] [Google Scholar]
- 4.Health NIo. SBIR/STTR Study and Control of Microbial Biofilms 1999. [Available from: https://grants.nih.gov/grants/guide/pa-files/PA-99-084.html].
- 5.Archibald LK, Gaynes RP. Hospital-acquired infections in the United States. The importance of interhospital comparisons. Infect Dis Clin North Am. 1997;11(2):245–55. [DOI] [PubMed] [Google Scholar]
- 6.Dowd SE, Delton Hanson J, Rees E, Wolcott RD, Zischau AM, Sun Y, et al. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J Wound Care. 2011;20(1):40–7. [DOI] [PubMed] [Google Scholar]
- 7.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE. 2008;3(10):e3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis SJ. Chronic wound infection and antimicrobial use. Adv Skin Wound Care. 2008;21(11):531–40; quiz 41–2. [DOI] [PubMed] [Google Scholar]
- 9.CLSI. Performance Standards for Antimicrobial Susceptibility Testing 28th Edition. CLSI Supplement M100, Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 10.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts 4th Edition. CLSI Supplement M27, Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 11.Vitality Medical. Safety Data Sheet - Vashe® Wound Solution 2017. [Available from: https://www.vitalitymedical.com/pdf/SDSVasheWoundSolution.pdf].
- 12.NovaBay Pharmaceuticals, Inc. Material Safety Data Sheet 0.03% NeutroPhase Plus Skin and Wound Cleanser 2015. [Available from: http://neutrophaseus.com/wp-content/uploads/2013/12/L0049.04_MSDS_NeutroPhase-0-03.pdf].
- 13.Agostinho AM, Hartman A, Lipp C, Parker AE, Stewart PS, James GA. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J Appl Microbiol. 2011;111(5):1275–82. [DOI] [PubMed] [Google Scholar]
- 14.Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother. 2009;53(9):3914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harriott MM, Noverr MC. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob Agents Chemother. 2010;54(9):3746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care. 2008;17(8):333–41. [DOI] [PubMed] [Google Scholar]
- 17.Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg. 1998;25(3):341–56. [PubMed] [Google Scholar]
- 18.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–52. [DOI] [PubMed] [Google Scholar]
- 19.Harrison-Balestra C, Cazzaniga AL, Davis SC, Mertz PM. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol Surg. 2003;29(6):631–5. [DOI] [PubMed] [Google Scholar]
- 20.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. [DOI] [PubMed] [Google Scholar]
- 21.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354–9. [DOI] [PubMed] [Google Scholar]
- 22.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PhaseOne Skin and Wound Cleanser [Integrated Healing Technologies Wound Care web site]. 2017. Available at: http://ihtwoundcare.com/pdf/phaseone-factsheet.pdf. Accessed March 12, 2018.
- 24.Vashe Wound Solution Brochure [SteadMed Medical website] 2014. Available at: http://www.steadmed.com/wp-content/uploads/2016/11/Vashe-Wound-Cleansing-Final-final.pdf. Accessed March 12, 2018.
- 25.Day A, Alkhalil A, Carney BC, Hoffman HN, Moffatt LT, Shupp JW. Disruption of Biofilms and Neutralization of Bacteria Using Hypochlorous Acid Solution: An In Vivo and In Vitro Evaluation. Adv Skin Wound Care. 2017; (12):543–551. [DOI] [PubMed] [Google Scholar]
- 26.Brindle CT, Porter S, Bijlani K, Arumugam S, Matias R, Najafi R, Fisher J. Preliminary Results of the Use of a Stabilized Hypochlorous Acid Solution in the Management of Ralstonia Pickettii Biofilm on Silicone Breast Implants. Aesthet Surg J. 2018. May 15;38(suppl_2):S52–S61. [DOI] [PubMed] [Google Scholar]
- 27.Sulfamylon (Mafenide Acetate, USP) for 5% Topical Solution. [US Food and Drug Administration web site] 1998. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/1998/19832lbl.pdf. Accessed March 12, 2018.
- 28.Huang XQ, Xiang J, Song F, Huan JN. [Effects of topical agents for burns on Acinetobacter baumannii within biofilm]. Zhonghua Shao Shang Za Zhi. 2012;28(2):106–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.