Abstract
Background:
Cognitive impairment is one of the most common consequences of multiple sclerosis (MS), yet there is a shortage of data regarding how cognition changes during the life span of individuals with MS. This information is of increasing importance given the growing proportion of older adults with MS.
Objective:
To study possible changes in cognitive function in correlation with increasing age in individuals with MS.
Methods:
Participants (n = 129) were recruited and a priori allocated into one of three age groups (young, middle-aged, and older). All participants completed the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) during a single laboratory testing session. The BICAMS measures cognitive processing speed as well as verbal and visuospatial learning and memory.
Results:
A multivariate analysis of variance indicated that cognitive function significantly differed by age group, and these differences were not explained by amount of physical activity, years of education, years since diagnosis, or race. Older adults displayed significantly worse cognitive processing speed than young and middle-aged adults. The older and middle-aged adults also demonstrated significantly worse visuospatial learning and memory than the younger adults. Effect sizes indicated that cognitive processing speed and verbal learning and memory were more affected in late adulthood than early adulthood, whereas visuospatial learning and memory was affected similarly in early and late adulthood.
Conclusions:
Older adults with MS demonstrated significant impairments in cognitive function compared to young and middle-aged adults with MS. Future studies should determine the predictors of cognitive decline in this age cohort.
Keywords: multiple sclerosis, cognitive impairment, older adults, aging
Cognitive impairment is one of the most prevalent and life-altering consequences of multiple sclerosis (MS), occurring in more than 70% of individuals with the disease (Benedict et al, 2006; Chiaravalloti and DeLuca, 2008; Rao et al, 1991). Cognitive deficits manifest both early and late in the disease but generally progress over time (Chiaravalloti and DeLuca, 2008). The impairment is seen across multiple cognitive domains; the most frequently affected are cognitive processing speed, learning, and memory (Benedict et al, 2006; Chiaravalloti and DeLuca, 2008; Rao et al, 1991). Cognitive impairment has been associated with depression, increased rate of unemployment, difficulty completing daily activities, and reduced quality of life in individuals with MS (Campbell et al, 2017; Chiaravalloti and DeLuca, 2008; DeLuca et al, 2015; Goverover et al, 2007; Prakash et al, 2008).
The demographic landscape of the MS population is changing; the prevalence of older adults with MS is rapidly growing, making MS a “life span disease” (Marrie et al, 2010). The life span presence of MS is likely associated with better approaches for early and late disease management (eg, disease-modifying therapy) and parallels the demography of the general population (ie, there is an emerging “graying” within the MS population). Indeed, recent evidence indicates that the prevalence of MS in the United States is highest in individuals between 55 and 64 years, followed closely by those aged 65 to 74 years (Wallin et al, 2019).
Older adults with MS encounter a largely novel situation; namely, the intersection of disease progression and aging. Because research in MS has predominantly been focused on the young and middle-aged populations (Amato et al, 2013; Prakash et al, 2008), the effect of these two processes together on cognitive function is largely unknown. There is evidence that older adults (≥60 years) with MS experience a worsening of cognitive processing speed, learning, and memory compared to age-matched individuals without MS (Bodling et al, 2009; Bollaert and Motl, 2017; Bollaert et al, 2017; Roy et al, 2017, 2018); however, it is unclear how cognitive function changes with age in individuals with MS. Preliminary evidence suggests that older adults with MS experience greater cognitive deficits than younger adults with MS (Bodling et al, 2009; Roy et al, 2017). However, these two studies pooled data from several different studies that were not designed to specifically examine the effect of age on cognitive performance in individuals with MS. Additionally, the sample distribution was either disproportionately biased toward young and middle-aged adults than older adults (Bodling et al, 2009), or not indicated at all (Roy et al, 2017). Moreover, one of the studies (Bodling et al, 2009) tested only one cognitive domain using a method for which the validity has not been established for individuals with MS, whereas the other study (Roy et al, 2017) reported predicted values, based on regression equations, where the total variance explained by each model was admittedly low (R2 < 0.25). Given these findings, there is a clear need for research that focuses on age-related changes in cognitive performance in individuals with MS, as was just highlighted in a recent publication in Nature Reviews Neurology (Vaughn et al, 2019). A detailed description of the pattern of cognitive decline in aging individuals with MS could have important implications for the management of cognitive dysfunction in this fast-growing population with MS.
In this cross-sectional study, we divided participants into groups of young (20–39 years), middle-aged (40–59 years), and older (60–79 years) adults with MS and examined whether cognitive processing speed, verbal learning and memory, and visuospatial learning and memory differed as a function of increasing age. We included education and physical activity as covariates in the final analyses because existing evidence links these factors to age-related differences in cognitive performance (Bollaert and Motl, 2017; Morrison and Mayer, 2017; Sandroff et al, 2014; Sumowski et al, 2010). We hypothesized that cognitive performance would be comparatively significantly worse in the older MS group across all three domains. We further hypothesized that the differences in cognition between the middle-aged and older groups (ie, late adulthood) would be greater than the differences in cognition between the younger and middle-aged groups (ie, early adulthood).
METHODS
Participants
We recruited participants by posting flyers in the community, running advertisements through the National MS Society, and conducting targeted searches of a university database of individuals with MS who had previously been treated by the local MS center. Individuals who were interested in participating in the study were asked to call our laboratory, and following a detailed description of the study procedures, prospective participants were screened for the following inclusion criteria: (a) aged 20 to 79 years; (b) diagnosis of MS; (c) relapse free over the past 30 days; (d) ambulatory with or without assistance; and (e) willingness to complete all of the testing procedures. Immediately upon enrollment in the study, the participants were categorized by self-reported age into predetermined age groups of young (20–39 years), middle-aged (40–59 years), and older (60–79 years) adults (Klaren et al, 2016).
Cognitive Performance
All of the participants completed the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS; Benedict et al, 2012), which consists of three neuropsychological tests of cognitive processing speed, verbal learning and memory, and visuospatial learning and memory. This assessment provides an approximation of the cognitive performance of individuals with MS. The three tests include the oral-response version of the Symbol Digit Modalities Test (SDMT; Smith, 1982), the first five learning trials of the California Verbal Learning Test—Second Edition (CVLT–II; Delis et al, 2000), and the first three learning trials of the Brief Visuospatial Memory Test—Revised (BVMT–R; Benedict, 1997).
The SDMT is recognized as a particularly sensitive, reliable, and valid measure of cognitive processing speed (Benedict et al, 2017). The test presents participants with a series of symbols and requires them to say aloud the digit that pairs with each symbol according to a key that is displayed at the top of the page. We instructed the participants to work as quickly as possible, and we recorded the total number of correct responses given within 90 seconds for each one.
The first five learning trials of the CVLT–II measure verbal learning and memory (Delis et al, 2000). In this test, a list of 16 words are read aloud by an examiner, and participants are instructed to immediately recall as many of the words from the list as possible, in any order. We repeated this process for four additional trials, using the same word list. The primary CVLT–II outcome is the summed number of correct responses from trials one through five, out of a possible 80 points.
The first three learning trials of the BVMT–R measure visuospatial learning and memory (Benedict, 1997). For each trial, six figures are presented on a display for 10 seconds. After the display is removed, participants are asked to draw the figures as accurately as possible from memory. Three trials are provided. For each trial, each figure receives a score of 0, 1, or 2 based on the accuracy and location of the drawn figure (out of a total of 12 points per trial). The primary BVMT–R outcome is the summed score across three trials, out of a possible 36 points.
Other Measures
All of the participants completed a general demographics questionnaire that included information about their age, race, education, years since diagnosis, and type of MS. Participants also completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ; Godin and Shephard, 1997), which provides a valid, subjective measure of physical activity in individuals with MS (Godin, 2011; Motl et al, 2006; Sikes et al, 2019). In the GLTEQ, participants report the number of bouts (≥15 minutes in duration) of mild, moderate, and strenuous physical activity they engaged in over the course of a typical week. The recorded number of bouts of mild, moderate, and strenuous physical activity are multiplied by weights of 3, 5, and 9, respectively. We scored the GLTEQ as a health contribution score by including the sum of the moderate and strenuous scores only.
Procedures
The procedures were approved by the University of Alabama at Birmingham’s Institutional Review Board, and all participants provided written informed consent to participate in the study. Participants attended a single testing session, during which they initially completed the demographics questionnaire and the GLTEQ; this was followed by administration of the BICAMS neuropsychological battery. The order of the BICAMS tests was kept consistent across all participants so that the SDMT was completed first, followed by the CVLT–II and then the BVMT–R.
Data Analysis
Data were analyzed using SPSS Statistics software (version 25). Descriptive statistics are presented in text and tables as M (SD) unless otherwise noted. We conducted a one-way ANOVA and χ2 tests to determine whether demographic and clinical characteristics differed with age group. We also compared between-group differences on cognitive performance outcomes using a multivariate analysis of variance (MANOVA), with all cognitive outcomes included as dependent variables. If there was a multivariate effect, we determined how each cognitive domain specifically differed by age group by examining the accompanying F ratios with each outcome. To determine if other factors accounted for the effect of age on cognitive performance, we conducted a multivariate analysis of covariance (MANCOVA) that controlled for education and physical activity (GLTEQ score), as these factors may partially account for differences in cognitive performance in individuals with MS (Bollaert and Motl, 2017; Morrison and Mayer, 2017; Sandroff et al, 2014; Sumowski et al, 2010). Additionally, we included any demographic or clinical characteristics (eg, race, disease type) that differed between the age groups as covariates in the analysis in order to confirm the between-groups differences on cognitive performance outcomes. To examine if differences in cognitive performance were greater in early (young to middle-aged) or late (middle-aged to older) adulthood, we calculated the effect size (Cohen’s d) between the young and middle-aged groups and between the middle-aged and older groups. The effect sizes were interpreted as small, moderate, or large based on values of 0.2, 0.5, and 0.8, respectively (Cohen, 1988).
RESULTS
Participant Characteristics
Of the 231 individuals who contacted the laboratory regarding participation in the study, 57 were uninterested in participating following a detailed description of the study; 174 were assessed for eligibility. Of those assessed for eligibility, two individuals were excluded based on the inclusion criteria, and 12 individuals declined to participate. Moreover, we were unable to contact 31 individuals after the initial screening to schedule them for participation. Participant demographics and clinical characteristics of the 129 individuals included in the study are presented in Table 1. As expected, age and years since diagnosis were significantly different between the three age groups. There was also a significant difference in race between the groups; that is, a greater proportion of young and middle-aged adults with MS were African American compared to older adults with MS. Conversely, sex, type of MS, education, and amount of physical activity (ie, GLTEQ score) were not significantly different between the age groups.
TABLE 1.
Demographic and Clinical Characteristics of Participants by Age Group
| Age Group |
|||||
|---|---|---|---|---|---|
| Young (n = 42) (20–39 years) |
Middle-aged (n = 45) (40–59 years) |
Older (n = 42) (60–79 years) |
Total (n = 129) |
P | |
| Age (years) | 33.1 (5.0) | 48.5 (5.7) | 65.8 (4.4) | 49.1 (14.2) | 0.001* |
| Sex (% female) | 78.6 | 73.3 | 73.8 | 75.2 | 0.83 |
| Race (% Caucasian) | 42.9 | 64.4 | 83.3 | 63.6 | 0.01* |
| Type of MS (% RRMS) | 88.1 | 88.9 | 83.3 | 86.8 | 0.80 |
| Years since diagnosis | 6.3 (5.1) | 12.3 (5.8) | 20.0 (8.6) | 12.8 (8.6) | 0.01* |
| Education (years) | 16.1 (2.5) | 16.4 (2.0) | 16.0 (2.4) | 16.2 (2.3) | 0.67 |
| GLTEQ score | 29.3 (27.3) | 22.5 (24.1) | 25.1 (24.8) | 25.5 (25.4) | 0.46 |
Data presented as M (SD) unless otherwise noted.
Significant at P ≤ 0.05.
GLTEQ = Godin Leisure Time Exercise Questionnaire. MS = multiple sclerosis. RRMS = relapsing-remitting MS.
Cognitive Performance
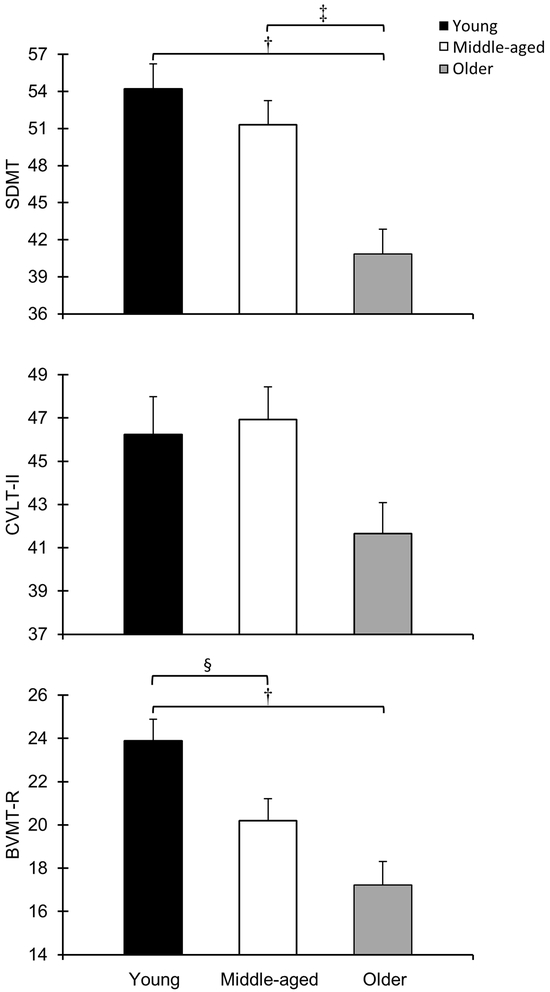
The participants’ performance on the BICAMS neuropsychological tests is presented in Table 2. Using Wilks’ statistics, the MANOVA indicated significant between-group differences (Λ = 0.79, F = 5.07, P ≤ 0.001) in cognitive performance; the individual F ratios indicated that cognitive processing speed (F = 10.31, P ≤ 0.001), verbal learning and memory (F = 3.09, P = 0.049), and visuospatial learning and memory (F = 10.04, P ≤ 0.001) significantly differed by age group (Figure 1). Post hoc tests with Bonferroni correction indicated that the older group demonstrated significantly slower cognitive processing speed than the young (P ≤ 0.001) and middle-aged (P = 0.003) groups, and the older (P ≤ 0.001) and middle-aged (P = 0.04) groups demonstrated significantly worse visuospatial learning and memory than the young group.
TABLE 2.
Mean Scores for Cognitive Performance by Age Group
| Age Group |
Effect Size (d value) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Young | Middle- aged |
Older | F | P | Young – Middle- aged |
Middle-aged – Older |
Young – Older |
|
| SDMT | 54.2 (12.94) |
51.3 (13.12) |
40.8 (12.97) |
10.31 | 0.00†‡ | 0.22 | 0.80 | 1.03 |
| CVLT–II | 46.2 (11.27) |
46.9 (10.08) |
41.7 (9.17) |
3.09 | 0.049* | 0.07 | 0.54 | 0.44 |
| BVMT–R | 23.9 (6.56) |
20.2 (6.78) |
17.2 (6.89) |
10.04 | 0.00†§ | 0.55 | 0.44 | 1.00 |
Data presented as M (SD).
Significant difference between age groups.
Significant difference between young and older groups.
Significant difference between middle-aged and older groups.
Significant difference between young and middle-aged groups.
BVMT–R = Brief Visuospatial Memory Test—Revised. CVLT–II = California Verbal Learning Test—II. SDMT = Symbol Digit Modalities Test.
FIGURE 1.
Cognitive performance by age group. Cognitive processing speed (top), verbal learning and memory (middle), and visuospatial learning and memory (bottom) significantly differed by age group. Error bars represent SEM. †Significant difference between young and older groups. ‡Significant difference between middle-aged and older groups. §Significant difference between young and middle-aged groups.
BVMT–R = Brief Visuospatial Memory Test—Revised. CVLT–II = California Verbal Learning Test—Second Edition. SDMT = Symbol Digit Modalities Test.
The follow-up MANCOVA indicated that race, years since diagnosis, education, and amount of physical activity did not account for the main effect of age on cognitive performance (Λ = 0.84, F = 3.39, P = 0.003). Table 3 presents the adjusted mean scores for cognitive performance by age group, controlling for race, years since diagnosis, education, and amount of physical activity.
TABLE 3.
Adjusted Mean Scores for Cognitive Performance by Age Group, Controlling for Race, Years Since Diagnosis, Education, and Amount of Physical Activity
| Age Group |
|||||
|---|---|---|---|---|---|
| Young | Middle-aged | Older | F | P | |
| SDMT | 54.9 (2.12) | 50.5 (1.76) | 42.5 (2.24) | 6.58 | 0.002†‡ |
| CVLT–II | 47.2 (1.76) | 46.1 (1.45) | 42.0 (1.85) | 1.88 | 0.157 |
| BVMT–R | 24.0 (1.10) | 19.8 (0.91) | 17.9 (1.16) | 6.30 | 0.003†§ |
Data presented as M (standard error).
Significant difference between young and older groups.
Significant difference between middle-aged and older groups.
Significant difference between young and middle-aged groups.
BVMT–R = Brief Visuospatial Memory Test—Revised. CVLT–II = California Verbal Learning Test—II. SDMT = Symbol Digit Modalities Test.
Regarding potential early- or late-adulthood differences in cognitive performance among individuals with MS (Table 2), effect sizes indicated a moderate difference between the young and middle-aged groups in visuospatial learning and memory (d = 0.55), whereas the differences between these groups for cognitive processing speed and verbal learning and memory were small (0.22 and 0.07, respectively). There was a large difference in cognitive processing speed between the middle-aged and older groups (d = 0.80) and a moderate difference in verbal (d = 0.54) and visuospatial (d = 0.44) learning and memory, respectively.
DISCUSSION
Cognitive deficits are common in individuals with MS (Chiaravalloti and DeLuca, 2008; Prakash et al, 2008); however, research focusing on cognitive impairment across the life span of individuals with MS (ie, aging with a disabling disease) has been limited. Indeed, as the prevalence of older adults with MS continues to rise (Wallin et al, 2019), there is an increasing need for research examining the effects of age on cognitive performance in individuals with the disease (Vaughn et al, 2019). This research is important because cognitive performance is known to decline with old age in healthy adults (Deary et al, 2009); thus, older individuals with MS are likely to experience more significant cognitive impairment as a combined effect of aging and disease progression.
Our results concerning the effect of age on cognitive performance in adults with MS indicated that multiple cognitive domains—cognitive processing speed (SDMT), verbal learning and memory (CVLT–II), and visuospatial learning and memory (BVMT–R)—differed by age group. Most noteworthy, the group of older adults with MS demonstrated poorer performance across all three domains compared to the young and middle-aged groups. Importantly, there were differences between individual age group comparisons for cognitive processing speed and visuospatial learning and memory after controlling for years since clinical diagnosis as a factor. This finding suggests that the effect of age on cognitive performance is not simply related to the number of years since clinical diagnosis, but rather it is independently related to age. The effect of age on cognitive processing speed and verbal learning and memory was greater in late adulthood (middle-aged to older) than early adulthood (young to middle-aged), whereas the effect of age on visuospatial learning and memory was similar in both early and late adulthood.
Our cross-sectional study categorized individuals with MS into predetermined age groups (Klaren et al, 2016) and examined whether cognitive performance differed with age. Our results are consistent with previous research showing an effect of age on cognitive performance in individuals with MS (Bodling et al, 2009; Roy et al, 2017); however, compared to the previous studies, our study was designed to examine the pattern of cognitive decline that accompanies aging in individuals with MS. Moreover, our sample sizes were proportionate between the three age groups, which is important for the appropriate application and interpretation of the data analysis. We further demonstrated that the effect of age on cognitive performance was independent of other factors, including education and amount of physical activity, although these two factors have previously been reported to influence cognitive performance in individuals with MS (Bollaert and Motl, 2017; Morrison and Mayer, 2017; Sandroff et al, 2014; Sumowski et al, 2010).
The present results support our hypothesis that there is an overall age-related difference in cognitive performance in adults with MS; specifically, older adults with MS demonstrated worse cognitive performance compared to young and middle-aged adults with MS. The importance of age for cognitive performance in individuals with MS is further supported by the evidence here that cognitive processing speed and visuospatial learning and memory were significantly different between age groups after controlling for race, years since diagnosis, education, and amount of physical activity. The possibility that age itself is associated with cognitive performance in individuals with MS may have important implications for the management of cognitive impairment. Our results, therefore, suggest that age is an important factor that contributes to cognitive decline in individuals with MS. However, we are aware that there may be other contributing factors—such as comorbid conditions, prior cognitive impairment, medication effects, and lifestyle (such as sedentary vs active life, and nutrition)—that may influence the degree of cognitive decline in individuals with MS (Benedict and Zivadinov, 2011). These factors, both independently and in association with aging, should be the focus of future research examining cognitive performance in individuals with MS.
The difference in cognitive processing speed and verbal learning and memory across the age groups was much greater in late adulthood (middle-aged to older adults) than early adulthood (young to middle-aged adults). This finding suggests that older age may accelerate the decrease in cognitive performance specifically within these two domains. This pattern is consistent with findings from a longitudinal study on individuals with MS that reported that older adults exhibited greater cognitive decline over 8 years than younger adults (Bergendal et al, 2007). Taken together, these findings suggest that there may be a specific age threshold, or age range, after which cognitive decline is accelerated in adults with MS. Future research should attempt to identify the age range at which cognitive decline accelerates (ie, an age-specific threshold or tipping point) so as to optimize existing and develop novel treatment approaches (such as cognitive rehabilitation or exercise training [Sandroff and DeLuca, 2019]) that may delay age-related cognitive decline.
Compared to cognitive processing speed and verbal learning and memory, the deficit pattern for visuospatial learning and memory between age groups differed. Whereas the decrease in cognitive processing speed and verbal learning and memory observed from middle age to older adulthood was sudden, visuospatial learning and memory decreased in a more linear manner across the three age groups. It is important to distinguish the different patterns of cognitive decline among adults with MS because this pattern may have a considerable influence on therapeutic design. By establishing a descriptive age-related cognitive profile in individuals with MS, researchers and clinicians can begin to modify treatment approaches that delay, or even reverse, the cognitive decline associated with aging in this population (Sandroff and DeLuca, 2019).
The current study was not without limitations. The cross-sectional nature and correlation analysis of the study limit inferences regarding causality between age and cognitive domains. We believe, however, that our data will support any future longitudinal research to clarify the nature of age-related decline in cognitive performance in individuals with MS. The current study did not include an age-matched control (ie, a non-MS group for all age groups), which precludes interpretations of whether the findings are specific to MS or aging in general. However, a recent review (Vaughn et al, 2019) suggested that aging does not differentially affect cognitive decline in individuals with and without MS, which supports a more focused examination of the cognitive decline pattern in individuals with MS. There is some evidence suggesting an association between disability and cognitive performance (Chiaravalloti and DeLuca, 2008), although this relationship is not well understood. A formal assessment of disability using a test such as the Expanded Disability Status Scale was not performed in the current study; therefore, we were unable to control for this potential factor. However, there is a known association between MS disability status and years since diagnosis (Tullman, 2013); thus, by controlling for years since diagnosis here, we partly accounted for the role of disability that accumulates over time with MS. The current study used an established and valid measure of cognitive performance (BICAMS) across multiple domains; however, we were not able to consider all cognitive domains (eg, executive function, working memory); this is, therefore, something that future research should address.
Our study showed that cognitive performance differs with age in individuals with MS, whereby older adults revealed significantly worse cognitive processing speed compared to young and middle-aged adults. Older and middle-aged adults with MS also showed significantly worse visuospatial learning and memory than young adults with MS. The overall pattern of decline differed with cognitive domain evaluated. That is, cognitive processing speed and verbal learning and memory were more affected in late adulthood, whereas visuospatial learning and memory were affected similarly in early and late adulthood. Establishing a descriptive profile of age and cognitive decline in individuals with MS will have implications for future treatment and rehabilitation strategies, especially considering the rapidly growing prevalence of MS in older adults.
Acknowledgments
Supported in part by a training grant (2T32HD071866-06) from the National Institutes of Health to J.F.B.
Glossary
- BICAMS
Brief International Cognitive Assessment for Multiple Sclerosis
- BVMT–R
Brief Visuospatial Memory Test—Revised
- CVLT–II
California Verbal Learning Test—Second Edition
- GLTEQ
Godin Leisure-Time Exercise Questionnaire
- MS
multiple sclerosis
- SDMT
Symbol Digit Modalities Test
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- Amato MP, Langdon D, Montalban X, et al. 2013. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol. 260:1452–1468. [DOI] [PubMed] [Google Scholar]
- Benedict RH. 1997. Brief Visuospatial Memory Test—Revised: Professional Manual. Odessa, Florida: Psychological Assessment Resources. [Google Scholar]
- Benedict RH, Amato MP, Boringa J, et al. 2012. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol. 12:55. doi: 10.1186/1471-2377-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Cookfair D, Gavett R, et al. 2006. Validity of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychol Soc. 12:549–558. [DOI] [PubMed] [Google Scholar]
- Benedict RH, DeLuca J, Phillips G, et al. 2017. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 23:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Zivadinov R. 2011. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. 7:332–342. [DOI] [PubMed] [Google Scholar]
- Bergendal G, Fredrikson S, and Almkvist O. 2007. Selective decline in information processing in subgroups of multiple sclerosis: an 8-year longitudinal study. Eur Neurol. 57:193–202. [DOI] [PubMed] [Google Scholar]
- Bodling AM, Denney DR, Lynch SG. 2009. Cognitive aging in patients with multiple sclerosis: a cross-sectional analysis of speeded processing. Arch Clin Neuropsychol. 24:761–767. [DOI] [PubMed] [Google Scholar]
- Bollaert RE, Balto JM, Sandroff BM, et al. 2017. Preliminary evidence for the effects of aging and multiple sclerosis on cognitive performance: an analysis based on effect size estimates. Exp Aging Res. 43:346–354. doi: 10.1080/0361073X.2017.1333820 [DOI] [PubMed] [Google Scholar]
- Bollaert RE, Motl RW. 2017. Physical and cognitive functions, physical activity, and sedentary behavior in older adults with multiple sclerosis. J Geriatr Phys Ther. doi: 10.1519/jpt.0000000000000163 [DOI] [PubMed] [Google Scholar]
- Campbell J, Rashid W, Cercignani M, et al. 2017. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J. 93:143–147. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. 2008. Cognitive impairment in multiple sclerosis. Lancet Neurol. 7:1139–1151. [DOI] [PubMed] [Google Scholar]
- Cohen J 1988. Statistical Power Analysis for the Behavioral Sciences (computer software) (2nd ed.). Hillsdale, New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- Deary IJ, Corley J, Gow AJ, et al. 2009. Age-associated cognitive decline. Br Med Bull. 92:135–152. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, et al. 2000. California Verbal Learning Test (CVLT–II). San Antonio, Texas: Psychological Corporation. [Google Scholar]
- DeLuca GC, Yates RL, Beale H, et al. 2015. Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathol. 25:79–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G 2011. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. The Health & Fitness Journal of Canada. 4:18–22. [Google Scholar]
- Godin G, Shephard RJ. 1997. Godin Leisure-Time Exercise Questionnaire. Med Sci Sports Exerc. 29:S36–S38. [Google Scholar]
- Goverover Y, Genova HM, Hillary FG, et al. 2007. The relationship between neuropsychological measures and the Timed Instrumental Activities of Daily Living task in multiple sclerosis. Mult Scler J Exp Transl Clin. 13:636–644. [DOI] [PubMed] [Google Scholar]
- Klaren RE, Sebastiao E, Chiu C-Y, et al. 2016. Levels and rates of physical activity in older adults with multiple sclerosis. Aging Dis. 7:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie R, Yu N, Blanchard J, et al. 2010. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. 74:465–471. doi: 10.1212/WNL.0b013e3181cf6ec0 [DOI] [PubMed] [Google Scholar]
- Morrison JD, Mayer L. 2017. Physical activity and cognitive function in adults with multiple sclerosis: an integrative review. Disabil Rehabil. 39:1909–1920. doi: 10.1080/09638288.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, McAuley E, Snook EM, et al. 2006. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disabil Rehabil. 28:1151–1156. doi: 10.1080/09638280600551476 [DOI] [PubMed] [Google Scholar]
- Prakash RS, Snook EM, Lewis JM, et al. 2008. Cognitive impairments in relapsing-remitting multiple sclerosis: a meta-analysis. Mult Scler. 14:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, et al. 1991. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 41:685–691. [DOI] [PubMed] [Google Scholar]
- Roy S, Drake A, Snyder S, et al. 2018. Preliminary investigation of cognitive function in aged multiple sclerosis patients: challenges in detecting comorbid Alzheimer’s disease. Mult Scler Relat Disord. 22:52–56. doi: 10.1016/j.msard.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Roy S, Frndak S, Drake AS, et al. 2017. Differential effects of aging on motor and cognitive functioning in multiple sclerosis. Mult Scler J Exp Transl Clin. 23:1385–1393. doi: 10.1177/1352458516679036 [DOI] [PubMed] [Google Scholar]
- Sandroff BM, DeLuca J. 2019. Will behavioral treatments for cognitive impairment in multiple sclerosis become standards-of-care? Int J Psychophysiol. doi:org/ 10.1016/j.ijpsycho.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Sandroff BM, Klaren RE, Pilutti LA, et al. 2014. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J Neurol. 261:363–372. [DOI] [PubMed] [Google Scholar]
- Sikes EM, Richardson EV, Cederberg KJ, et al. 2019. Use of the Godin Leisure-Time Exercise Questionnaire in multiple sclerosis research: a comprehensive narrative review. Disabil Rehabil. 41:1243–1267. [DOI] [PubMed] [Google Scholar]
- Smith A 1982. Symbol Digit Modalities Test (SDMT). Manual (rev. ed.). Los Angeles, California: Western Psychological Services. [Google Scholar]
- Sumowski J, Wylie G, Gonnella A, et al. 2010. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology. 75:1428–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullman MJ. 2013. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 19:S15–S20. [PubMed] [Google Scholar]
- Vaughn CB, Jakimovski D, Kavak KS, et al. 2019. Epidemiology and treatment of multiple sclerosis in elderly populations.Nat Rev Neurol. 15:329–342. [DOI] [PubMed] [Google Scholar]
- Wallin MT, Culpepper WJ, Campbell JD, et al. 2019. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 92:e1029–e1040. doi: 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]