Abstract
Background.
Heart failure with preserved ejection fraction (HFpEF) is often manifested as impaired cardiovascular reserve. We sought to determine if conducted vasodilation, which coordinates microvascular resistance longitudinally to match tissue metabolic demand, becomes compromised in HFpEF. We hypothesized that the metabolic vasodilator adenosine facilitates, and that inhibition of adenosine kinase (ADK) augments conducted vasodilation for a more efficient myocardial perfusion and improved left ventricle (LV) diastolic function in HFpEF.
Methods.
We assessed conducted vasodilation in obese ZSF1 rats that develop LV diastolic dysfunction, and is used to model human HFpEF. Additionally, conducted vasodilation was measured in arterioles isolated from the right atrial appendages of HFpEF patients.
Results.
We found a markedly reduced conducted vasodilation both in obese ZSF1 rats and in HFpEF patients. Impaired conducted vasodilation was accompanied by increased vascular ADK expression. Isolated rat and human arterioles incubated with adenosine (10 nM) or ADK inhibitor, ABT-702 (0.1 μM) both displayed augmented conducted vasodilation. Treatment of obese ZSF1 rats with ABT-702 (1.5 mg/kg, i.p. for 8-week) prevented LV diastolic dysfunction, and in a crossover design augmented conducted vasodilation and improved LV diastolic function. ABT-702 treated obese ZSF1 rats exhibited reduced expression of myocardial carbonic anhydrase 9 and collagen, surrogate markers of myocardial hypoxia.
Conclusions.
Upregulation of vascular ADK mitigates adenosine-facilitated conducted vasodilation in obese ZSF1 rats and in HFpEF patients. We propose that pharmacologic inhibition of ADK could be beneficial for therapeutic augmentation of conducted vasodilation, thereby improving tissue perfusion and LV diastolic function in HFpEF.
Subject terms: Coronary Artery Disease, Basic Science Research, Coronary Circulation, Endothelium/Vascular Type/ Nitric Oxide, Vascular Biology
INTRODUCTION
Increasing number of patients with heart failure (HF) are diagnosed with heart failure with preserved ejection fraction (HFpEF).1, 2 HFpEF is a poorly understood clinical condition, which is accompanied by left ventricle (LV) diastolic dysfunction with high cardiovascular morbidity and mortality rates.3 HFpEF is often viewed as a syndrome of impaired cardiovascular reserve but the mechanistic underpinnings of specific pathologic changes are poorly described and understood; therefore, the options for treatment are limited and frequently ineffective. Recently, it has been proposed that abnormalities in the coronary microcirculation contribute to the development of LV diastolic dysfunction in HFpEF.4-6 Indeed, coronary flow reserve, an estimate of vasodilator capacity of coronary resistance arteries, was shown to be reduced in HFpEF patients, suggesting impaired myocardial perfusion behind LV diastolic dysfunction.6
Coronary circulation is tightly regulated by metabolic needs of the myocardium and therefore efficient coupling of perfusion with metabolism is governed by several important microvascular regulatory mechanisms that include conducted or spreading arteriolar dilation. Conducted vasodilation describes propagation of signaling events in vascular endothelial and smooth muscle cells in a retrograde direction to increase upstream blood flow in the feeding artery.7-9 We previously demonstrated that human coronary arterioles exhibit conducted vasodilation.10 Goto et al. reported that spontaneously hypertensive rats display impaired conducted vasodilation in mesenteric arteries.11 Mechanisms that modulate conducted vasodilation and potential changes that could underlie impairment in this microvascular regulatory mechanism is incompletely understood. In addition, whether conducted vasodilation becomes compromised in HFpEF, and whether it contributes to the development of LV diastolic dysfunction remain largely unknown.
Adenosine is an important metabolic vasodilator and critical regulator of myocardial perfusion.12, 13 Administered exogenously adenosine efficiently dilates human coronary arterioles.14 Adenosine is quickly metabolized to adenosine monophosphate by adenosine kinase (ADK), which is regarded as one of the principal regulators of endogenous adenosine concentration.15 Under hypoxia, ADK activity is reduced, which leads to a rapid rise in tissue adenosine levels.16 A previous study has shown that pharmacological inhibition of ADK exerts cardioprotective effect in the hypertrophic rodent heart.17 These aforementioned studies and considerations led us to raise the hypothesis that conducted vasodilation becomes compromised in HFpEF and that adenosine or pharmacological inhibition of ADK augments conducted vasodilation for a more efficient coupling of tissue perfusion and metabolism that result in improved LV diastolic function.
MATERIAL & METHODS
The data that support the findings of this study are available from the corresponding author upon request.
Obese diabetic Zucker fatty/spontaneously hypertensive heart failure F1 hybrid (ZSF1) rat model of HFpEF
Institutional Animal Care and Use Committee at Medical College of Georgia approved all animal protocols described in this study, and is in compliance with the ARRIVE guidelines.18, 19 Eight-week old male lean ZSF1 (n=29), obese ZSF1 (n=29) underwent bi-weekly echocardiographic evaluation. Subgroup of lean and obese ZSF1 rats (4 animals in each groups) received the ADK inhibitor, 4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]pyrimidine (ABT-702 dihydrochloride, 1.5 mg/kg, i.p, TOCRIS) two times a week or injections of the vehicle (2.8% DMSO in saline) for eight weeks followed by a crossover treatment in the obese ZSF1 groups only for additional eight weeks. Mean arterial pressure was measured in anesthetized rats (Isoflurane; 1.5%) using a physiological pressure transducer inserted into the left carotid artery.
Patients with HFpEF
All protocols were performed as approved by the Institutional Review Board at Medical College of Georgia. HFpEF patients were clinically diagnosed with the signs and/or symptoms of heart failure and had an ejection fraction (EF) >45%, as determined by echocardiography. We excluded heart failure patients with reduced EF (HFrEF, EF <45%) from this study. Human coronary arterioles were isolated from right atrial appendages obtained from adult patients who underwent open-heart surgery requiring cardiopulmonary bypass. The de-identified, surplus atrial surgical specimens were accompanied by limited patient health information, including basic demographics, clinical data and medication history. Clinical data included and indicated if the HFpEF patient was clinically diagnosed with the signs and/or symptoms of heart failure and had an ejection fraction (EF) >45%, as determined by echocardiography. Heart failure patients with reduced EF (HFrEF, EF <45%) were excluded from this study. Patient characteristics are shown in Table 1.
Table 1.
Patient demographics, diseases, and medications
| HFpEF | Control | P | |
|---|---|---|---|
| n (%) | 6 | 9 | |
| Male | 3 (50) | 4 (44) | 1.00 |
| Age (years) | 53 ± 19 | 62 ± 4 | 0.32 |
| Body weight (kg) | 86 ± 21 | 87 ± 20 | 0.97 |
| BMI (kg/m2) | 30 ± 5 | 31 ± 6 | 0.67 |
| Systolic Blood Pressure (mmHg) | 135 ± 28 | 138 ± 15 | 0.82 |
| Diastolic Blood Pressure (mmHg) | 79 ± 11 | 72 ± 13 | 0.31 |
| Ejection Fraction (%) | 57 ± 9 | 63 ± 8 | 0.20 |
| Serum glucose (mg/dL) | 136 ± 63 | 101 ± 37 | 0.30 |
| Underlying disease, n (%) | |||
| Heart failure | 6 (100) | 0 (0) | 0.0002* |
| Type 2 diabetes | 2 (33) | 4 (44) | 1.00 |
| Hypertension | 6 (100) | 9 (100) | 1.00 |
| Hyperlipidemia | 4 (67) | 5 (56) | 1.00 |
| Coronary artery disease | 4 (67) | 8 (89) | 0.53 |
| Peripheral vascular disease | 1 (17) | 1 (11) | 1.00 |
| COPD | 1 (17) | 2 (22) | 1.00 |
| Medications, n (%) | |||
| Aspirin | 3 (50) | 7 (78) | 0.33 |
| Lipid lowering | 5 (83) | 7 (78) | 1.00 |
| Insulin | 1(17) | 0 (0) | 0.40 |
| Oral antidiabetic | 1(17) | 2 (22) | 1.00 |
| Beta blocker | 4 (67) | 6 (67) | 1.00 |
| ACE inhibitor | 4 (67) | 4 (44) | 0.61 |
| Diuretic | 4 (67) | 4 (44) | 1.00 |
| Anticoagulant | 3 (50) | 4 (44) | 1.00 |
| Calcium channel blocker | 1 (17) | 1 (11) | 1.00 |
| Surgical procedure, n (%) | |||
| CABG | 4 (67) | 8 (89) | 0.53 |
| Valve replacement | 2 (33) | 2 (22) | 1.00 |
n, number of HFpEF and control patients studied, categorical variables also indicate percentages. For continues variables mean ± SD are shown.
indicates statistical difference. All categorical variables were examined by Fisher’s exact test and continuous variables were assessed by Student’s t-test between the two patient groups. BMI, body mass index; COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme; CABG, coronary artery bypass grafting.
Echocardiographic assessment of LV function
Using echocardiography (VEVO 2100 digital ultrasound microimaging system, VisualSonics) we assessed LV dimensions at the parasternal short-axis view in anesthetized (0.5-2% inhaled isoflurane) rats. Mitral flow velocity tracings (peak velocity of early (E), late (A) mitral inflow, and deceleration time (DT) of early filling of mitral inflow) were obtained with pulsed-wave Doppler above the mitral leaflets to evaluate diastolic function.
Videomicroscopic assessment of conducted vasodilation
Using a similar method we have established earlier,10, 20, 21 conducted vasodilation was measured in isolated, cannulated and pressurized (710 mmHg) arteries after focally confined delivery of vasodilators. We used rat gracilis skeletal muscle arteries (~125-200 μm in diameter and 1,500 μm in length) and human coronary arterioles (~100 μm in diameter and 1,500 μm in length). Acetylcholine (ACh, 100 μM, in rat arteries, or bradykinin (BK, 100 μM, in human arteries) was ejected from a glass micropipette using pneumatic micro ejection system (10–40 kPa, 100 ms duration). Changes in diameter were measured with digital videomicroscopy at local application site, and at remote sites, 500 and 1,000 μm away from stimulation. Conducted vasodilation was assessed again after incubation of the isolated arteries with adenosine (10 nM for 30 minutes) or ABT-702 (0.1 μM for 30 minutes).
Histology and immunohistochemistry
Paraffin sections prepared from paraformaldehyde-fixed human heart and rat gracilis muscle were immunolabeled with rabbit polyclonal anti-ADK (Abcam, #ab38010, 1:100) and Cy5-conjugated anti-rabbit secondary antibody (Jackson-Immonoresearch, 1:250). DAPI was used for nuclear staining. Structured illumination microscopy (SIM-Apotome, AxioImagerM2, CarlZeiss) was used for immunofluorescent detection. Sections were stained with Masson’s Trichrome and collagen fibers were quantified using ImageJ color deconvolution, and collagen area was presented as percentage of cardiomyocyte area.
Western immunoblot
Heart samples were homogenized in radio-immunoprecipitation assay buffer (20 μL) and were mixed with 5x Laemmli sample buffer, then loaded for gel electrophoresis. After transfer to PVDF membrane and blotting, membranes were incubated with rabbit polyclonal anti-ADK antibody (Abcam, #ab38010, 1:1,000) followed by horseradish peroxidase-labeled secondary antibody. Protein expression was detected by chemiluminescence autoradiography. GAPDH (Santa Cruz, sc-25778, 1:5,000) was used to normalize for loading variations and to calculate ADK to GAPDH ratios.
RT-qPCR
Total RNA was extracted from fast-frozen skeletal muscle artery using Trizol (Invitrogen Life Technologies, Carlsbad CA) and assessed for RNA purity using NANO Drop. Complementary DNA was synthesized from 1 μg purified RNA using oligo(dT) primers and the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Life Technologies, Carlsbad CA). Primers were designed for ADK, carbonic anhydrase IX (CAIX) and for the house-keeping gene acidic ribosomal phosphoprotein P0 (RPLO). The relative gene expression of individual target genes was calculated using the 2−∆∆ct method.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism Software. Data were drawn to analyses after tested for and met normality using Kolmogorov-Smirnov test. The Fisher’s exact test was used for categorical variables between patient-group comparisons. Data comparisons between groups repeatedly over time were analyzed by two-way repeated measures of ANOVA followed by Tukey post-hoc test (two tailed test, confidence level = 95%). One way ANOVA with Tukey post-hoc test or Student’s t-test was used to compare two or more groups, as appropriate. As indicated in Table and Figure descriptions data are expressed either as mean±SEM, mean±SD or box-and-whisker plots, in which the minimum, the 25th percentile, the median, the 75th percentile, and the maximum are presented, and + indicates the mean of values. P<0.05 was considered statistically significant.
RESULTS
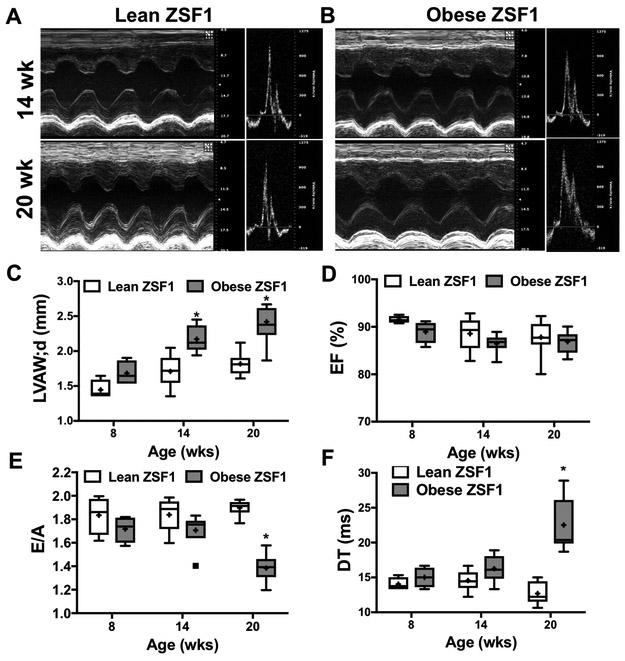
The obese ZSF1 rat, an established rodent model of human HFpEF22, 23 genetically develops obesity, hypertension and diabetes, which is accompanied by LV diastolic dysfunction, increased lung weight and exercise intolerance.24 We found that obese ZSF1 rats had a significantly higher body weight, adiposity, elevated blood glucose levels, when compared to age-matched, lean control ZSF1 rats (Supplementary Table 1). Both lean and obese ZSF1 rats display a similarly elevated mean arterial blood pressure (Supplementary Table 1). We found that obese ZSF1 rats, as early as 14 weeks of age, display increased LV weight and LV wall thickness as well as augmented right ventricular weight (Figure 1 and Supplementary Table 1). Note that by 20 weeks of age obese ZSF1 rats developed LV diastolic dysfunction, as indicated by decreased E/A ratio and increased deceleration time (DT) (Figure 1 E,F and Supplementary Table 1). The observed LV diastolic dysfunction developed with a preserved LV systolic function in the obese ZSF1 rats, as indicated by maintained fractional shortening and ejection fraction throughout the observation period (Figure 1D and Supplementary Table 1).
Figure 1. Obese ZSF1 rats develop LV diastolic dysfunction.
Representative echocardiogram images of 14 and 20 weeks old lean (A) and obese (B). Panels C-F: Summary data show selected echocardiogram parameters in lean and obese ZSF1 rats at 8, 14 and 20 weeks of age (for complete data see Table 2). Left ventricular anterior wall thickness during diastole (LVAW;d); Ejection fraction (EF); Peak velocity of early/late mitral inflow ratio (E/A); Deceleration time (DT) of early filling of mitral inflow.
* indicates P < 0.05 lean versus obese ZSF1 rats.
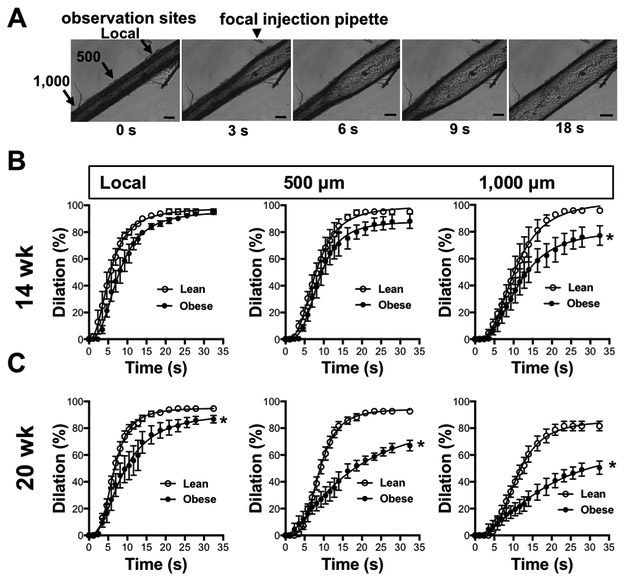
Next, conducted vasodilation was examined before (14-week) and after (20-week) the development of LV diastolic dysfunction in obese ZSF1 rats. Conducted vasodilation was assessed in isolated skeletal muscle (m. gracilis) arteries of the rat. As depicted on Figure 2A conducted vasodilation was initiated by focal application of acetylcholine (ACh, 100 μM). Figure 2B and 2C show that arteries of obese ZSF1 rats display impaired conducted vasodilation, which becomes further reduced by 20 weeks of age (Figure 2 B, C), at the time of onset of LV diastolic dysfunction.
Figure 2. Impaired conducted vasodilation in obese ZSF1 rats.
A; Representative time lapse images show conducted vasodilation in an isolated pressurized skeletal muscle artery of the obese ZSF1 rat. Panels B and C: Summary data of conducted vasodilation in skeletal muscle artery in response to focally applied acetylcholine at local and upstream sites (500 and 1,000 μm) over time in lean and obese ZSF1 rats at age of 14 weeks (B; n=4-4) and 20 weeks (C; n= 5-6). * indicates P < 0.05 lean versus obese ZSF1 rats.
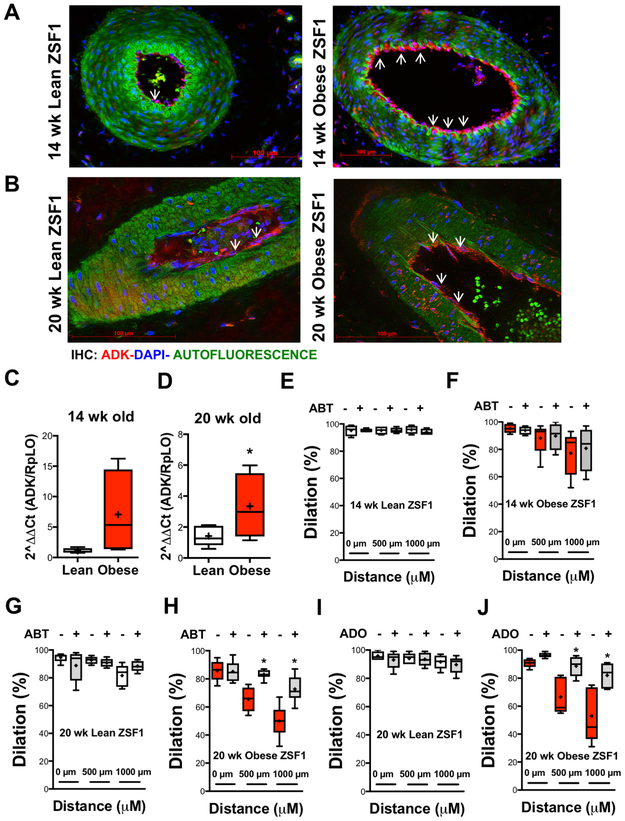
ADK expression is upregulated in arteries of obese ZSF1 rats
Using immunohistochemistry and qPCR we found an increased ADK expression in gracilis muscle arteries in obese ZSF1 rats, at both 14 and 20 weeks of age (Figure 3A-3D). Next, we pharmacologically assessed the role of vascular wall-expressed ADK in affecting conducted vasodilation. The selective ADK inhibitor, ABT-702 significantly augmented conducted vasodilation in 20 weeks old obese ZSF1 rats (Figure 3H), while no significant effect was observed in 14-week old obese ZSF1 rats and also lean controls (Figure 3E-3G). Incubation of arteries with low, non-vasoactive concentration of adenosine (10 nM) also augmented conducted vasodilation in obese ZSF1 rats (Figure 3J), but not in arteries of lean controls (Figure 3I). No significant changes were observed in vascular expression of adenosine A1, A2A, A2B and A3 receptors between the two groups of animals (Supplementary Figure 1).
Figure 3. Obese ZSF1 rats exhibit increased expression of vascular ADK.
Representative immunofluorescence images of gracilis muscle artery in lean and obese ZSF1 rats at 14 (A) and 20 (C) weeks of age (ADK in red:, DAPI in Blue: Green is autofluorescence). RT-qPCR analysis of ADK mRNA expression in skeletal muscle artery at 14 (B, n=4) and 20 (D, n=5) weeks of age in lean and obese ZSF1 rats. E-F panels: Summary data of local and conducted vasodilation in lean and obese ZSF1 rats before and after exposure to ABT-702 (0.1 μM) at 14 (E-F) and 20 (G-H) weeks of age.
* indicates P < 0.05.
Pharmacological inhibition of ADK improves LV diastolic dysfunction in obese ZSF1 rats
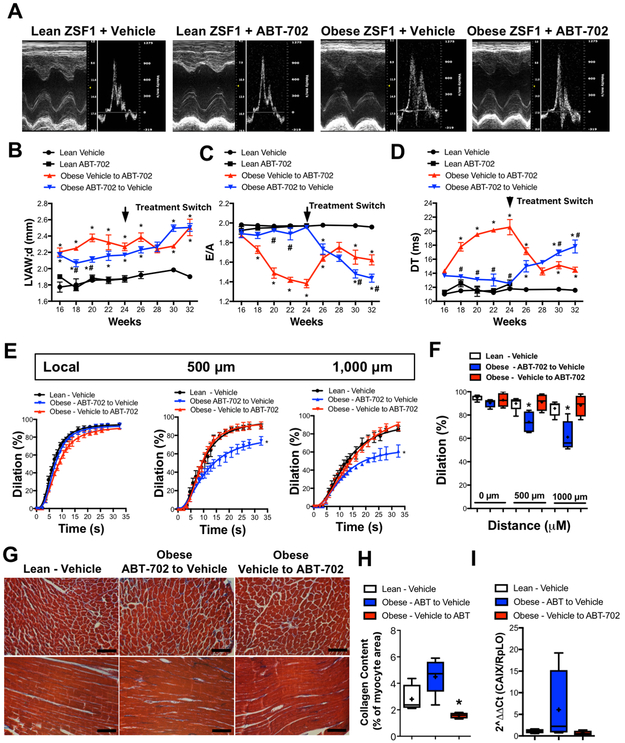
In order to examine the in vivo effects of pharmacologic ADK inhibition obese and lean ZSF1 rats were treated with the selective ADK inhibitor, ABT-702 (1.5 mg/kg, i.p, twice a week or vehicle)25, 26 for eight weeks, starting from 16 weeks of age, followed by a crossover treatment in the obese ZSF1 group for another eight week period, until 32 weeks of age of the animals. Figure 4B shows that obese ZSF1 rats had significantly increased LV wall thickness at age of 16 weeks when compared to controls, which remained significantly increased during the treatment period. We found that treatment of ABT-702 had no effects on LV wall thickness throughout the duration of the treatment, neither in control or in obese ZSF1 rats (Figure 4B and Supplementary Table 2). On the other hand, treatment with ABT-702 prevented the development of LV diastolic dysfunction, as indicated by maintained E/A ratio and DT values during the 8-week period that were not significantly different from those that were obtained in control animals (Figure 4C,D). Figure 4C and 4D panels also show that in a crossover treatment ABT-702 improved LV diastolic function (increased E/A and reduced DT ) in previously vehicle-treated obese ZSF1 rats. Interestingly, cessation of ABT-702 treatment caused deterioration of LV diastolic function (Figure 4C,D and Supplementary Table 2). Neither LV systolic function (EF and fractional shortening) nor systemic blood pressure was significantly affected by ABT-702 treatment in control and obese ZSF-1 rats (Supplementary Table 2).
Figure 4. Effects of ABT-702 treatment on cardiac and vascular parameters in lean and obese ZSF1 rats.
A: Representative echocardiogram images of lean and obese ZSF1 rats treated with ABT-702 or vehicle, at 24 weeks of age, before the treatment switch. Summary data show selected echocardiogram parameters in lean and obese ZSF1 rats (n=4 in each group) treated with ABT-702 or vehicle (for complete data see supplementary Table 2). B: Left ventricular anterior wall thickness during diastole (LVAW;d); C: Peak velocity of early/late mitral inflow ratio (E/A); D: Deceleration time (DT) of early filling of mitral inflow. Panels E and F: Summary data shows the time kinetics and maximally developed local and conducted vasodilation in gracilis muscle artery in response to focally applied acetylcholine in the above treatment groups. Panels G, H and I: Representative Masson’s Trichrome images (G) and summary data of quantification of collagen fibers (H) and mRNA expression of carbonic anhydrase 9 (CAIX, I) in the myocardium of aforementioned group of lean obese ZSF1 rats with vehicle or ABT-702 treatment. * indicates P < 0.05 compared to vehicle treated control lean ZSF1 rats. # indicates P < 0.05 within obese ZSF1 rat groups, i.e. Vehicle to ABT-702 versus ABT-702 to Vehicle groups.
Similar as above conducted vasodilation was also assessed in these treatment groups. Figure 4 panels E and F shows that those animals that were receiving subsequent ABT-702 treatment and had improved LV diastolic function (Vehicle to ABT-702 group) also had improved conducted vasodilation, compared to those obese ZSF1 rats that were on vehicle (ABT-702 to Vehicle group) and had developed impaired LV diastolic function at 32 weeks of age (Figure 4E,F).
Additionally, we measured surrogate markers of myocardial hypoxia and found increased carbonic anhydrase 9 (CAIX) expression and collagen content in the heart of vehicle-treated obese ZSF1 rats, when compared to lean controls (Figure 4G-I). Subsequent treatment with ABT-702 normalized myocardial expression of carbonic anhydrase 9 and resulted in reduced myocardial collagen content in obese ZSF1 rats (Figure 4G-I ).
ADK inhibition restores conducted vasodilation in patients with HFpEF
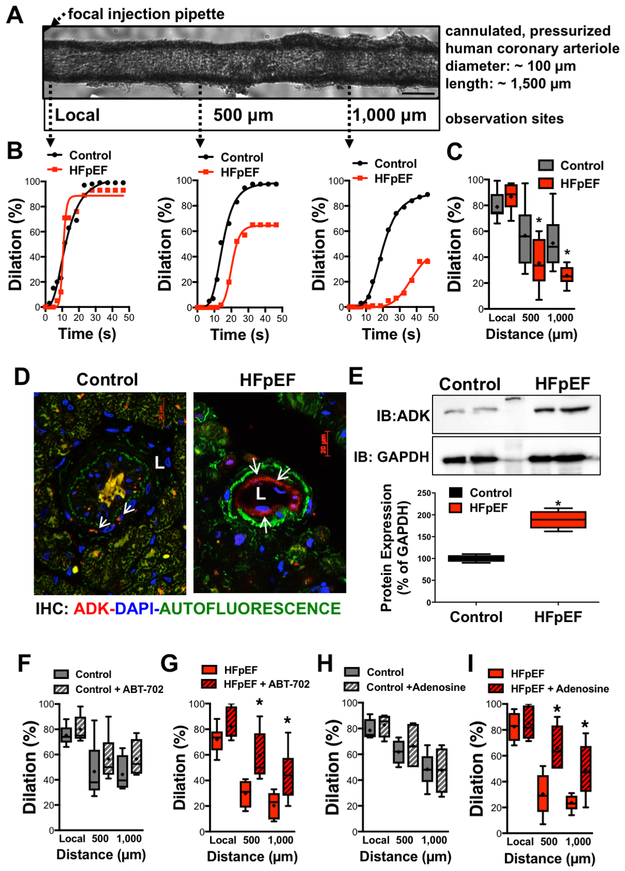
To provide clinical significance for our observation we also examined if conducted vasodilation is compromised in HFpEF patients. As we have shown previously,10 focal application of bradykinin induced conducted vasodilation, in isolated human coronary arteriole (Figure 5A,B). When compared to patients without heart failure, but suffering from similar comorbidities (Table 1) we found that conducted vasodilation was markedly reduced in HFpEF patients (Figure 5B,C).
Figure 5. Impaired conducted vasodilation in patients with HFpEF.
A: Representative image of an isolated pressurized human coronary arteriole. B: Representative traces show conducted vasodilation in human coronary arteriole in response to focally applied bradykinin, as measured at local and upstream sites (500 and 1,000 μm) over time in control and HFpEF patients. C: Box-and-whisker plots show summary data of local and conducted vasodilations in control (n=9) and HFpEF (n=6) patients. D: Representative immunofluorescence image of coronary arterioles in control and HFpEF patient (ADK in red:, DAPI in Blue: Green is autofluorescence, L: lumen of arteriole). E: Western immunoblot (IB) and summary data of ADK protein expression in control (n=4) and HFpEF patients (n=4). F-I panels: Summary data of bradykinin-induced local and conducted dilations in control and HFpEF patients before and after exposure to ABT-702 (F,G, n=4-5) or adenosine (H,I, n=3-4). * indicates P < 0.05.
Similar to that of observed in obese ZSF1 rats we found a prominent expression of ADK in the vascular endothelium in HFpEF patients (Figure 5D). Western immunoblot revealed an increased protein expression of ADK in atrial tissues obtained from HFpEF patients, when compared to controls (Figure 5E). Incubation with the selective ADK inhibitor, ABT-702 (0.1 μM) significantly augmented conducted vasodilation in coronary arterioles of HFpEF patients (Figure 5G). Similarly, exposing human coronary arterioles to low, non-vasoactive concentration of adenosine (10 nM) significantly increased conducted vasodilation in HFpEF patients (Figure 5I). Neither ABT-702 nor adenosine had significant effects on conducted vasodilation in control patients (Figure 5F,H).
DISCUSSION
Coronary microvascular dysfunction limits blood supply in the working myocardium, which leads to functional and structural impairment in the heart.27, 28 Recent clinical and experimental evidence indicate that abnormalities in the coronary microcirculation may contribute to the development of LV diastolic dysfunction in HFpEF.4-6, 29 The current study revealed a critical deficit in conducted vasodilation in HFpEF patients and in obese ZSF1 rats. In obese ZSF1 rats we observed a progressive decline in the magnitude of conducted vasodilation, which was further reduced at the onset of LV diastolic dysfunction. Comorbid conditions in HFpEF, such as hypertension, obesity and diabetes all impose a significantly greater hemodynamic and metabolic burden on the working myocardium, and with a diminishing microvascular reserve this could lead to relative or absolute myocardial ischemia. Indeed, chronic myocardial hypoperfusion is closely related to microvascular dysfunction; and that can be manifested as subendocardial inflammation and fibrosis, which was reported in obese ZSF1 rats.23 In this study we observed that impaired conducted vasodilation in the obese ZSF1 rats is accompanied by elevated levels of surrogate markers of myocardial hypoxia, including collagen and carbonic anhydrase 9. It is therefore plausible that impaired conducted vasodilation causes a mismatch between blood supply and metabolic demand, which could lead to relative or absolute hypoperfusion, and as such represents an early manifestation of microvascular dysfunction in HFpEF. The molecular mechanism that modulate conducted vasodilation and that could underlie these microvascular changes remain ill-defined in HFpEF.
Adenosine is one of the most characterized metabolic vasoregulators, which mediates a prompt adaptive response to the increased metabolic demand, aiming to prevent relative or absolute myocardial ischemia/hypoxia. Under hypoxia intracellular adenosine levels rise, which is then released to the extracellular/interstitial space through nucleoside transporters,30 and induces vasodilation by activating vascular smooth muscle adenosine receptors (A2B and A2A). Interestingly, it has been posited that extracellular adenosine could induce vascular smooth muscle cell hyperpolarization through activation of potassium channels in the human coronary arteriole.31 Results from our present study suggest that endogenously produced adenosine could also act to amplify the hyperpolarization spread and hence augment conducted vasodilation for a more efficient coupling of tissue perfusion with metabolism. We propose that this normal, adenosine-induced metabolic vasoregulatory mechanism can become compromised in disease, due to an enhanced activity of adenosine metabolizing enzyme, ADK, what we found to be upregulated in HFpEF patients and obese ZSF1 rats. Notable, we demonstrate that administration of non-vasoactive concentration of adenosine or pharmacological inhibition of ADK with ABT-702 augments conducted vasodilation. The exact mechanisms by which ADK inhibition improves LV diastolic dysfunction in obese ZSF1 rats remain to be elucidated. Our results indicate a direct microvascular effect by endogenously produced adenosine. In support, we found that 8-week treatment with ABT-702 significantly reduced myocardial carbonic anhydrase 9 expression and collagen content in obese ZSF1 rats, which can be correlated with improved myocardial perfusion according to earlier observations.23, 29 It is unlikely that this fast (within two weeks) functional improvement in LV diastolic function is due to changes collagen-related myocardial stiffness in the obese ZSF1 rats. In contrast, reduced LV relaxation and increased stiffening of the myocardium in HFpEF seem independent from increased collagen deposition and has been mainly attributed to diminished NO-cGMP-PKG-mediated phosphorylation of cardiomyocyte titin.4, 5 Clinical trials, such as the NEAT-HFpEF trial of administering Isosorbide Mononitrate, a direct NO donor to HFpEF patients 32 and the RELAX Study evaluating the effect of sildenafil, a phosphodiesterase 5A inhibitor, to augment NO-cGMP-PKG signaling, produced somewhat disappointing results.33 Another recent clinical trial using neladenoson bialanate, a partial adenosine A1 receptor agonist also produced neutral outcome on exercise capacity in HFpEF patients.34 While the underlying mechanisms behind these negative results are not known, clearly therapeutic approaches other than restoring NO levels or directly activating adenosine receptors may be considered. Experimental evidence supports that in the diseased human heart coronary arteriolar vasodilation is adaptively maintained by mechanisms independent from that of NO, such as achieved by EDHF, hydrogen peroxide35 and cyclooxeganse-2-derived vasodilator prostanoids.21 The study by Ohta et al.31 suggest that endogenously produced adenosine may act as an EDHF. Upregulated ADK, the main adenosine metabolizing enzyme could underlie impaired EDHF effects by adenosine, hence mitigate conducted vasodilation and microvascular reserve, which can lead to organ dysfunction in HFpEF. Whereas pharmacological inhibition of ADK could potentiate endogenous adenosine effects and therefore may provide therapeutic benefit.
Limitations.
There are several limitations to our study. Small sample size in the ABT-702 treatment arm of the animal study, as well as limited number of patient and their selection without detailed assessment LV diastolic function carry significant limitations. It should be noted that our results provide no direct evidence for the mechanistic, causative link between impaired conducted vasodilation and LV diastolic dysfunction, which has yet to be elucidated in future studies. In addition, possible mechanisms independent from that of coronary microvascular dysfunction in leading to LV diastolic dysfunction cannot be excluded. For example, we cannot exclude the role of direct cardiomyocyte mechanisms, such as alterations in cardiomyocyte stiffness due to changes in phosphorylation of titin, which we did not evaluate. Other, non-cardiac causes, such as renal or skeletal muscle function could also affect LV diastolic function in the obese ZSF1 rats. In this regard, it should be noted that in the obese ZSF1 rat conducted vasodilation was assessed in skeletal muscle arterioles due to technical considerations. Therefore, the observed impairment as well as improvement in LV diastolic function after ADK inhibition can also be attributed to extra-cardiac systemic effects, such as changes in skeletal muscle vascular resistance and perfusion efficiency during effort, a possibility, which has yet to be elucidated in future studies.
In conclusion, our study identifies impaired conducted vasodilation as a novel mechanism by which microvascular dysfunction occurs in HFpEF and by which it could contribute to the development of LV diastolic dysfunction. We propose that unlike nitrates and phosphodiesterase 5A inhibitors, which non-selectively increase coronary blood flow, the inhibition of ADK could be a more effective approach, as it amplifies a key metabolic vasoregulatory mechanism by endogenous adenosine production for a more efficient coupling the metabolic demand with perfusion at the right time and place in the failing myocardium.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Conducted vasodilation coordinates vascular resistance longitudinally whereby it plays a critical role in efficient coupling of metabolic demand with tissue perfusion in the working myocardium.
This study is the first to demonstrate a critical deficit in conducted vasodilation in a rodent model of HFpEF, the obese ZSF1 rat, and also in HFpEF patients.
Notably, increasing endogenous adenosine levels via a pharmacological inhibition of adenosine kinase by ABT-702 amplifies conducted vasodilation and protects against the development of LV diastolic dysfunction in obese ZSF1 rats.
What are the clinical implications?
In HFpEF coronary microvascular dysfunction underlies LV diastolic dysfunction and impaired cardiovascular reserve, but the nature of underlying mechanisms remains largely unknown.
This study newly uncovers that adenosine, which is released during myocardial hypoxia, facilitates conducted vasodilation, but this effect becomes compromised in HFpEF due to the upregulated adenosine kinase.
Pharmacological targeting and inhibition of adenosine kinase, via amplifying conducted vasodilation, could improve myocardial perfusion and LV diastolic dysfunction in HFpEF.
Acknowledgments
Sources of Funding
The author’s studies are supported by awards from the National Institute of Aging (R01AG054651 to ZB), National Heart, Lung, and Blood Institute (F31 HL142183 to AD) and the American Heart Association (GRNT33680171 to ZB).
ABBREVIATIONS
- HFpEF
Heart failure with preserved ejection fraction
- ZSF1 rat
Obese diabetic Zucker fatty/spontaneously hypertensive heart failure F1 hybrid rat
- ADK
adenosine kinase
- EF
ejection fraction
- DT
Deceleration time
- ACh
Acetylcholine
- BK
bradykinin
- CAIX
carbonic anhydrase 9
Footnotes
Disclosures
The authors declare no competing interests.
REFERENCES
- 1.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y and Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 2.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA and Roger VL. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16. [DOI] [PubMed] [Google Scholar]
- 3.Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540–3. [DOI] [PubMed] [Google Scholar]
- 4.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 5.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ and Hamdani N. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–24. [DOI] [PubMed] [Google Scholar]
- 6.Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, Shah SJ and Blair JEA. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;314:H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K and Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circulation Research. 2000;86:649–655. [DOI] [PubMed] [Google Scholar]
- 8.Segal SS and Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–70. [DOI] [PubMed] [Google Scholar]
- 9.Segal SS and Duling BR. Propagation of vasodilation in resistance vessels of the hamster: development and review of a working hypothesis. Circ Res. 1987;61:II20–5. [PubMed] [Google Scholar]
- 10.Feher A, Broskova Z and Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2014;306:H1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto K, Rummery NM, Grayson TH and Hill CE. Attenuation of conducted vasodilatation in rat mesenteric arteries during hypertension: role of inwardly rectifying potassium channels. J Physiol. 2004;561:215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroll K, Decking UK, Dreikorn K and Schrader J. Rapid turnover of the AMP-adenosine metabolic cycle in the guinea pig heart. Circ Res. 1993;73:846–56. [DOI] [PubMed] [Google Scholar]
- 13.Ledoux S, Runembert I, Koumanov K, Michel JB, Trugnan G and Friedlander G. Hypoxia enhances Ecto-5’-Nucleotidase activity and cell surface expression in endothelial cells: role of membrane lipids. Circ Res. 2003;92:848–55. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Terata K, Miura H, Toyama K, Loberiza FR Jr., Hatoum OA, Saito T, Sakuma I and Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol. 2005;288:H1633–40. [DOI] [PubMed] [Google Scholar]
- 15.Boison D Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev. 2013;65:906–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morote-Garcia JC, Rosenberger P, Kuhlicke J and Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–80. [DOI] [PubMed] [Google Scholar]
- 17.Peart JN and Gross GJ. Cardioprotection following adenosine kinase inhibition in rat hearts. Basic Res Cardiol. 2005;100:328–36. [DOI] [PubMed] [Google Scholar]
- 18.Kilkenny C, Browne WJ, Cuthill IC, Emerson M and Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C and Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ and Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63:1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A and Bagi Z. Increased cyclooxygenase-2 expression and prostaglandin-mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res. 2006;99:e12–7. [DOI] [PubMed] [Google Scholar]
- 22.Hamdani N, Franssen C, Lourenco A, Falcao-Pires I, Fontoura D, Leite S, Plettig L, Lopez B, Ottenheijm CA, Becher PM, Gonzalez A, Tschope C, Diez J, Linke WA, Leite-Moreira AF and Paulus WJ. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–49. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk CG, Oosterhuis NR, Xu YJ, Brandt M, Paulus WJ, van Heerebeek L, Duncker DJ, Verhaar MC, Fontoura D, Lourenco AP, Leite-Moreira AF, Falcao-Pires I, Joles JA and Cheng C. Distinct Endothelial Cell Responses in the Heart and Kidney Microvasculature Characterize the Progression of Heart Failure With Preserved Ejection Fraction in the Obese ZSF1 Rat With Cardiorenal Metabolic Syndrome. Circ Heart Fail. 2016;9:e002760. [DOI] [PubMed] [Google Scholar]
- 24.Lourenço AP, Leite-Moreira AF, Balligand J- L, Bauersachs J, Dawson D, de Boer RA, de Windt LJ, Falcão-Pires I, Fontes-Carvalho R, Franz S, Giacca M, Hilfiker-Kleiner D, Hirsch E, Maack C, Mayr M, Pieske B, Thum T, Tocchetti CG, Brutsaert DL and Heymans S. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. European Journal of Heart Failure. 2018;20:216–227. [DOI] [PubMed] [Google Scholar]
- 25.Elsherbiny NM, Ahmad S, Naime M, Elsherbini AM, Fulzele S, Al-Gayyar MM, Eissa LA, El-Shishtawy MM and Liou GI. ABT-702, an adenosine kinase inhibitor, attenuates inflammation in diabetic retinopathy. Life Sci. 2013;93:78–88. [DOI] [PubMed] [Google Scholar]
- 26.Kowaluk EA, Mikusa J, Wismer CT, Zhu CZ, Schweitzer E, Lynch JJ, Lee CH, Jiang M, Bhagwat SS, Gomtsyan A, McKie J, Cox BF, Polakowski J, Reinhart G, Williams M and Jarvis MF. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin- 3-yl)pyrido[2,3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties. II. In vivo characterization in the rat. J Pharmacol Exp Ther. 2000;295:1165–74. [PubMed] [Google Scholar]
- 27.Niccoli G, Scalone G, Lerman A and Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37:1024–33. [DOI] [PubMed] [Google Scholar]
- 28.Henderson KK, Turk JR, Rush JW and Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol. 2004;97:1159–68. [DOI] [PubMed] [Google Scholar]
- 29.Dryer K, Narang N, Gajjar M, Cheng JY, Lee M, Paul J, Nathan S, Shah AP, Gheorghiade M, Davidson CJ, Fearon WF, Shah SJ and Blair JEA. Coronary Microvascular Dysfunction in Patients With Normal and Abnormal Diastolic Function in Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2017;136. [Google Scholar]
- 30.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR and Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1004–13. [DOI] [PubMed] [Google Scholar]
- 31.Ohta M, Toyama K, Gutterman DD, Campbell WB, Lemaitre V, Teraoka R and Miura H. Ecto-5’-nucleotidase, CD73, is an endothelium-derived hyperpolarizing factor synthase. Arterioscler Thromb Vasc Biol. 2013;33:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E and Network NHFCR. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2015;373:2314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E and Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. Jama. 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah SJ, Voors AA, McMurray JJV, Kitzman DW, Viethen T, Bomfim Wirtz A, Huang E, Pap AF and Solomon SD. Effect of Neladenoson Bialanate on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. Jama. 2019;321:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M and Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.