Abstract
Intratumoral hypoxia extremely limits the clinic applications of photodynamic therapy (PDT). Endoperoxides allow thermally releasing singlet oxygen (1O2) in a defined quantity and offer promising opportunities for oxygen-independent PDT treatment of hypoxic tumors. However, previous composite systems by combining endoperoxides with photothermal reagents may result in unpredicted side effects and potential harmful impacts during therapy in vivo. Herein, we de novo design an all-in-one polymer carrier, which can photothermally release 1O2. The strategy has been demonstrated to effectively enhance the production of 1O2 and realize the photodamage in vitro, especially in hypoxic environment. Additionally, the polymer carrier accumulates into tumor after intravenous injection via the enhanced permeation and retention effects and accelerates the oxygen-independent generation of 1O2 in tumors. The oxidative damage results in good inhibitory effect on tumor growth. Realization of the strategy in vivo paves a new way to construct photothermal-triggered oxygen-independent therapeutic platform for clinical applications.
1. Introduction
Photodynamic therapy (PDT) has become an emerging noninvasive and selective cancer therapeutic modality, in which light triggers energy transfer between triplet excited states of photosensitizers and molecular oxygen to generate cytotoxic singlet oxygen (1O2) leading to apoptotic cell death [1–3]. It has been used for age-related macular degeneration, viral infection, atherosclerosis, and malignant cancers [4, 5]. However, intratumoral hypoxia severely limits its clinic applications owing to insufficient generation of 1O2. Furthermore, the consumption of oxygen during PDT treatment aggravates the hypoxic environment, further limiting the therapeutic outcome [6, 7]. To address this problem, oxygen-sufficient materials or oxygen-independent photosensitizers to generate reactive oxygen species are developed [8–19].
Polycyclic aromatic hydrocarbons are the most reliable organic compounds to cleanly supply 1O2 in a defined quantity without side reaction [20–24]. They can trap 1O2 yielding endoperoxides (EPOs) and release 1O2 upon elevating the temperature, which provides a new and powerful concept in 1O2 delivery for the treatment of hypoxic tumors. Till now, only few reports have developed the composite systems by combining EPOs with photothermal reagents (such as gold nanorods) to release 1O2 in cancer cells [25, 26]. Although they realized photothermal-triggered oxidative damage of cancer cells in in vitro experiments, the composite structures may result in unpredicted side effects and potential harmful impacts on biological environments during therapy in vivo. Moreover, the utilization of gold nanorods substantially increases the difficulty of clearance from the body and leads to long-term toxicity towards healthy tissues and organs [27]. In this regard, it is highly desirable to de novo design an all-in-one strategy without the above-mentioned concerns.
Herein, we propose a novel all-in-one polymer carrier (P1), composed of 1,4-dimethylnaphthalene (DMN), aza-BODIPY (B1), and hydrophilic polyethylene glycol (PEG) (Figure 1(a)), in which, DMN is able to deliver 1O2 into hypoxic tumors via reversible transformation between the naphthalene and endoperoxide forms, while B1 serves as not only an excellent organic photothermal agent, but also potential imaging reagents in vivo because of good physiological stability and near-infrared absorption and luminescence [28]. Taking advantages of oxygen-independence during the 1O2 generation process, the 1O2 loaded polymer P1-SO can be a good candidate to overcome the resistance of PDT caused by tumor hypoxia. In in vivo experiments, P1-SO was injected into tumor-bearing mice through tail vein; it accumulated in the tumor tissues, owing to the enhanced permeation and retention (EPR) effect. Moreover, laser irradiation on the P1-SO-injected mouse significantly restrained the growth of tumors, resulted from the oxidative damage towards hypoxic tumor. These results highlighted the excellent therapeutic effect of the designed all-in-one polymeric 1O2 carrier.
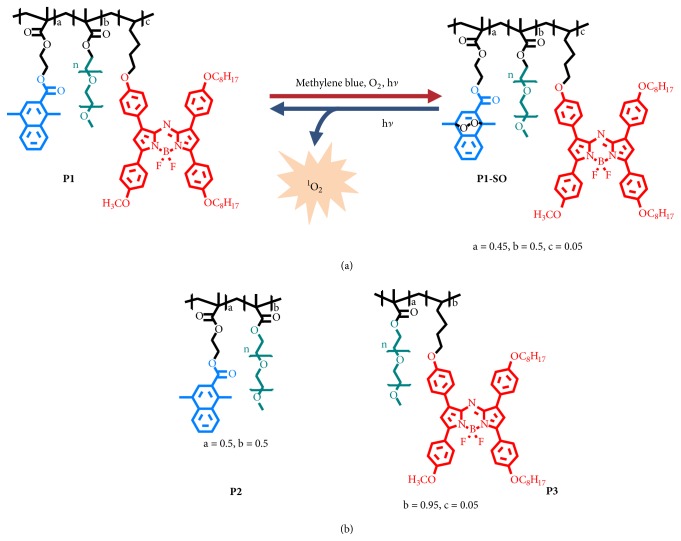
Figure 1.
Scheme illustration of the polymeric carrier serving as a PDT platform in vivo. (a) Mechanism of capture and release of 1O2 by the polymeric carrier, a = 0.45, b = 0.5, and c = 0.05. (b) The structures of P2 (a = 0.5, b = 0.5) and P3 (b = 0.95, c = 0.05).
2. Results
2.1. Design, Synthesis, and Characterization of Polymers
1,4-Dimethylnaphthalene was selected as the 1O2 carrier, owing to the high capability to trap 1O2. Structural modification at the C2-position of the naphthalene rings allows easily controlling of 1O2 release. Aza-BODIPY (B1), which has strong NIR absorption and emission, was chosen as both the photothermal agent and imaging dye. Hydrophilic polyethylene glycol was introduced in the polymer to improve the water solubility and EPR effect. The monomer DMN-acryl has been synthesized according to the previous work [26]. The monomer B1 was synthesized in four steps (See in the Supplementary Material). Firstly, compounds 3 and 5 were obtained through an aldol/dehydration reaction and then Michael addition reaction. Compound 6 was directly transformed by 3 and 5. B1 was obtained by BF2 chelation step of 6. P1-P3 were synthesized via radical polymerization. To balance the capacity for loading 1O2 and guarantee the thermal effects of the polymer, the molar ratios of DMN and B1 in a polymer molecule were fixed to 45% and 5%, respectively. Endoperoxides of DMN and 1O2 loaded polymer (P1-SO) were formed in the presence of a commercial photosensitizer, methylene blue (MB), under the irradiation, and then MB could be removed easily by dialysis. The polymer dots were obtained by self-assembly due to their amphiphilic structures with hydrophobic DMN and B1 units and hydrophilic PEG as side chain.
The model polymers P2 and P3 were also obtained (Figure 1(b)). The detailed synthesis procedures of the polymer dots with DMN and aza-BODIPY pendants were illustrated in supporting information. The monomers and polymers were characterized by NMR spectra, gel permeation chromatography (GPC), transmission electron microscope (TEM) images, dynamic light scattering (DLS), and matrix-assisted laser desorption ionization time of light mass spectrometry (MALDI-TOF MS).
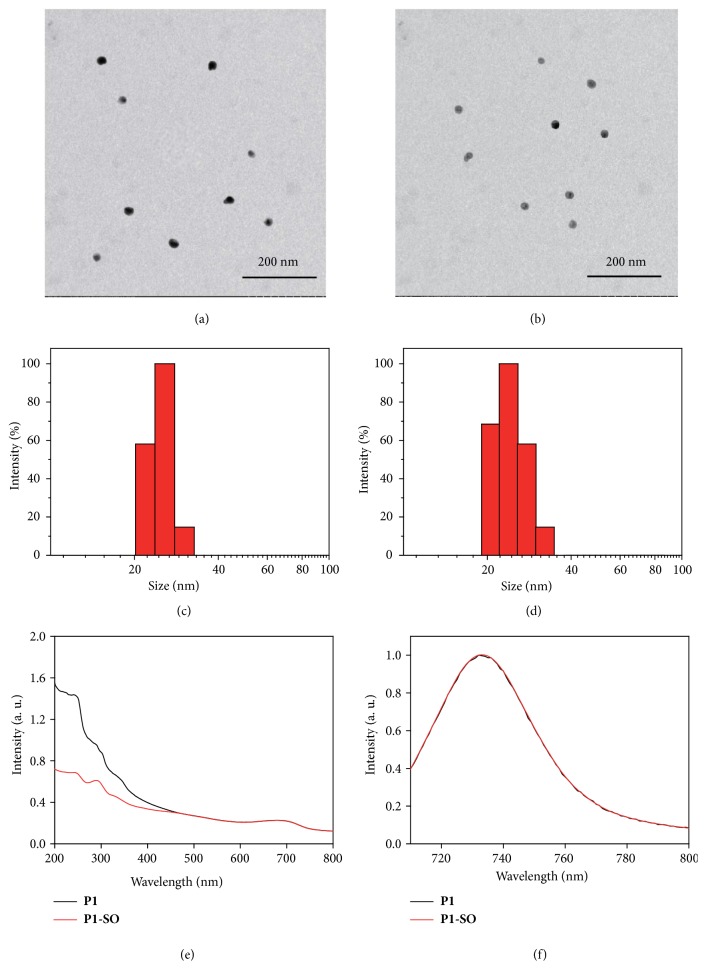
The number-average molecular weights (Mn) of P1-P3 were 19674, 21024, and 16024, respectively. The polydispersity index (Mw/Mn) of P1-P3 was 1.20, 1.31, and 1.24, respectively. A uniform spherical morphology of P1 dots was clearly revealed by TEM images (Figure 2(a)). The diameters of P1 dots were estimated to be around 20 nm. DLS indicated that the P1 dots were well-dispersed in water and had an average hydrodynamic size of 23 nm (Figure 2(c)), which contributed to be enriched in tumor tissues via EPR effect [29]. After loading 1O2, the results of TEM and DLS of P1-SO dots remain almost the same as those of P1 dots (Figures 2(b) and 2(d)), demonstrating that capture of 1O2 did not change the morphology, particle size, or dispersity of the polymer dots.
Figure 2.
Morphology, diameter, and photophysical properties of P1 and P1-SO. TEM images of P1 (a) and P1-SO (b). Size distribution of P1 (c) and P1-SO (d) by DLS. Absorption spectra (e) and emission spectra (f) of P1 and P1-SO in DMSO.
2.2. Photophysical, Photothermal, and Photodynamic Properties of P1-SO Dots
The photophysical properties of P1 and P1-SO dots were investigated by UV-Vis absorption and emission spectra. As illustrated in Figure 2(e), the absorption spectra of P1 dots displayed a strong absorption at 243 nm and a weak absorption at 689 nm, which were consistent with the absorption of DMN and B1 monomer, respectively. After capture of 1O2, the destruction of conjugated structure of DMN leaded to a sharp decrease at 243 nm in the UV-Vis absorption spectra [26]. In addition, P1 dots and P1-SO dots showed almost the same emission maxima at 730 nm (Figure 2(f)), which was attributed to the luminescence of B1 (see in the Supplementary Material) and negligibly affected by the 1O2 loading.
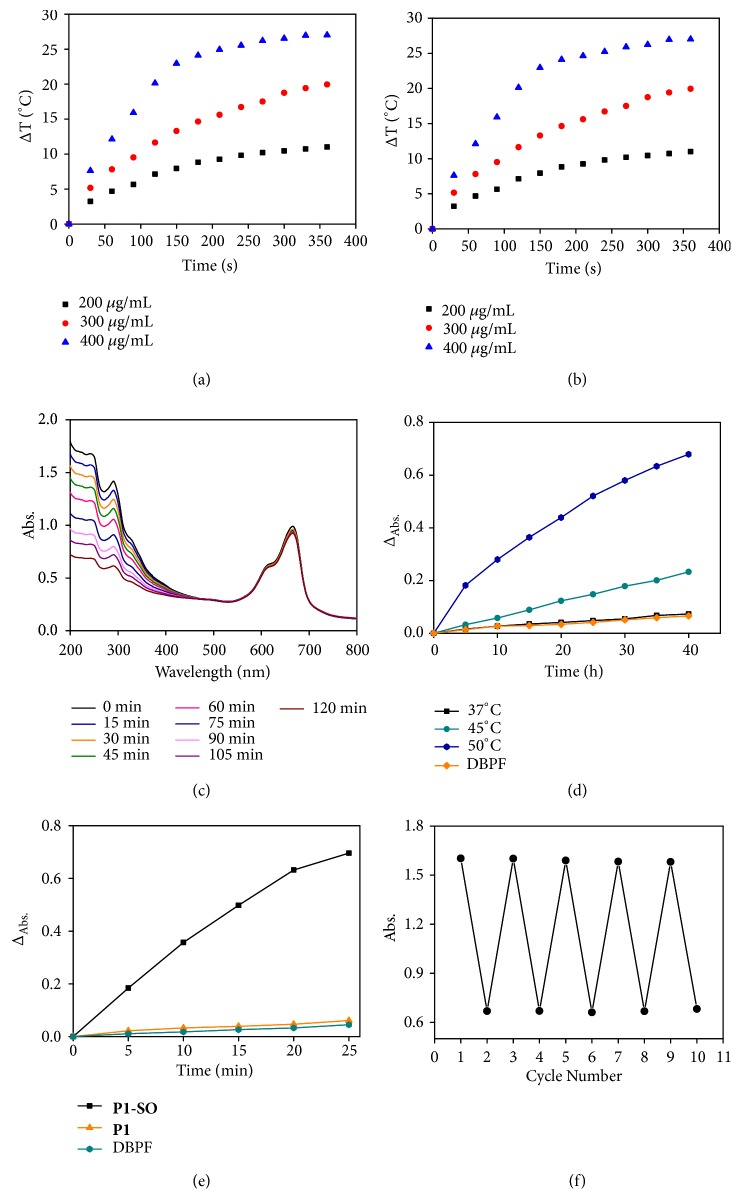
Thermal effects were the key factor to trigger the releasing of 1O2. Therefore, the photothermal performance of monomer B1, P1, and P1-SO was carried out via thermal infrared imager (FLIR E40). The temperature change was recorded at different concentration in dimethylsulfoxide (DMSO) under continuous exposure to irradiation (690 nm, 400 mW/cm2). As shown in in the Supplementary Material and Figures 3(a) and 3(b), the increasing concentration resulted in the elevation of temperature. After irradiating for 360 s, the temperatures increased by 21.5°C, 20.0°C and 19.8°C for B1 (40 μM), P1 (300 μg/mL), and P1-SO (300 μg/mL), respectively, demonstrating that the polymer containing B1 units displayed good photothermal effects.
Figure 3.
Photothermal effects, capture, and release of 1 O 2 of P1 and P1-SO. (a) and (b) Temperature elevation of P1 and P1-SO at different concentrations under irradiation (690 nm, 400 mW/cm2). (c) Absorption spectra of the mixture of P1 (300 μg/mL) and MB (10 μM) under different irradiation time (660 nm, 4 mW/cm2). (d) ΔAbs of DPBF under different temperatures in mixture solution of P1-SO and DBPF. (e) ΔAbs of DPBF in the mixture solution of P1-SO or P1 and DBPF under irradiation (690 nm, 400 mW/cm2). (f) The cycle number of the capture and release of 1O2. ΔAbs = At − A0, At was the absorption at different irradiation time and A0 was the absorption without irradiation.
To investigate the 1O2 capture ability of P1, MB was added to the DMSO solution of P1. As shown in Figure 3(c), when the mixture solution was irradiated by a 660 nm laser (4 mW/cm2), the absorption band at 243 nm decreased gradually with the extension of irradiation time, but the absorption of MB at 665 nm remained unchanged, indicating that 1O2 sensitized by MB was captured by P1 to form P1-SO. After removing MB, the abilities of 1O2 release of P1-SO were studied at different temperatures (Figure 3(d)). When the temperature was kept at 37°C, rare 1O2 was released according to the negligible response of the absorption band of the 1O2 indicator, 1,3-diphenylisobenzofuran (DPBF). In contrast, a sharp decrease of absorption band was observed when the temperature was increased to 50°C, indicating of a large amount of 1O2 generated. These results demonstrated that elevated temperature facilitated the release of 1O2. Moreover, to investigate the release of 1O2 under photothermal stimulation, the generation of 1O2 of P1-SO was measured under irradiation of laser (690 nm, 400 mW/cm2) in air and hypoxia environment that was produced by bubbling with nitrogen gas (Figure 3(e)). In the DMSO solution of P1-SO and DPBF, obvious decrease of the absorption band ascribed to DPBF was observed in both air and hypoxia environments, indicating that the oxygen levels had negligible influence for the 1O2 release. On the other hand, in the control group, when P1 was used instead of P1-SO, it was hardly able to generate 1O2 even in air since 1O2 was not trapped in the DMN units and heavy atom-free B1 had no ability to generate 1O2. Furthermore, to demonstrate the reversibility of the capture and release of 1O2 for P1-SO, we added MB to the DMSO solution containing P1 and exposed the mixture under light irradiation (660 nm, 4 mW/cm2). The absorption band at 243 nm was decreased after the light irradiation (660 nm, 4 mW/cm2) for 120 min owing to the capture of 1O2. When the light irradiation (690 nm, 400 mW/cm2) was prolonged for 25 min, the absorption band at 243 nm was increased because of the1O2 releasing. MB was not removed in this process. The reversible performance was displayed for 5 cycles (Figure 3(f)). The model polymer P2 also had ability to trap 1O2 to form P2-SO, but P2-SO was unable to release 1O2 under irradiation owing to the lack of photothermal agents (see Figures - in the Supplementary Material), while P3 could neither trap 1O2 nor generate 1O2 (see in the Supplementary Material).
All the results indicated that the photothermal effects of B1 could provide enough heat to trigger 1O2 release and P1-SO had excellent phototoxicity effects in solution. More importantly, oxygen is unnecessary in 1O2 release process of P1-SO compared to conventional photosensitizers, revealing enormous potential for improving the therapeutic effects of hypoxia-associative PDT.
2.3. Anticancer Investigation In Vitro
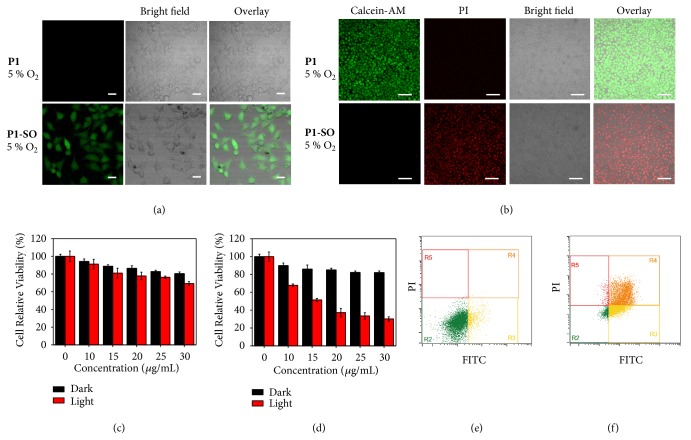
To demonstrate the feasibility of the polymer carrier to generate 1O2 in vitro, 2,7-dichlorifluoresceindiacetate (DCFH-DA), which can be oxidized to 2,7-dichlorofluorescein (DCF) by intracellular ROS in live cells, was utilized as a ROS tracer agent. The laser-scanning confocal luminescence microscopy was employed to investigate the ROS generation in HeLa cells. Upon irradiation at 690 nm, weak luminescence in the cells was observed under 21% (see in the Supplementary Material) and 5% oxygen concentration (Figure 4(a)) when the cells were incubated with P1. But bright green luminescence of DCF was exhibited in the cells incubated with P1-SO under 21% and 5% oxygen concentration. Without light irradiation, the generation of ROS hardly occurred in the cells incubated with P1-SO, since the physiological temperature (37°C) was relatively low so that it could not trigger the rapid release of 1O2 from endoperoxides (see in the Supplementary Material). The control group containing the cells only incubated with DCFH-DA displayed negligible luminescence in the absence and presence of irradiation at 690 nm (see in the Supplementary Material). These results demonstrated that the intracellular release of 1O2 from endoperoxides could be accelerated by photothermal effects of B1 in P1-SO.
Figure 4.
In vitro evaluation with P1 and P1-SO. (a) ROS generation in HeLa cells with DCFH-DA, cells were incubated with P1 or P1-SO for 2 h under 5% oxygen level under irradiation for 6 min (690 nm, 400 mW/cm2) (scale bar, 50 nm). (b) Calcein-AM and PI stained HeLa cells were incubated with P1 and P1-SO and then exposed under 690 nm laser irradiation (400 mW/cm2) for 6 min under 5% oxygen level (scale bar, 100 μm). (c) and (d) MTT assay of P1 and P1-SO under 5% oxygen level with and without irradiation. (e) and (f) Flow cytometry quantification of apoptosis of HeLa cells incubated with P1 and P1-SO under 5% oxygen level with 690 nm laser irradiation (400 mW/cm2).
The methyl thiazolyl tetrazolium (MTT) assay was used to evaluate the cytotoxicity of the polymer carrier towards HeLa cells. HeLa cells were incubated with different concentrations of P1 or P1-SO at 37°C for 24 h in dark. Low dark cytotoxicity of P1 and P1-SO were shown under 5% and 21% oxygen. When the P1 incubated cells were treated by 690 nm laser, the cell viability was relatively high for cells in 21% or 5% oxygen concentrations, indicating low toxicity of P1 (Figures 4(c) and in the Supplementary Material). Additionally, the cells incubated with 1O2 loading P1-SO showed relatively lower cell viability in 21% (see in the Supplementary Material) or 5% (Figure 4(d)) oxygen compared to the group of P1-treated cells under irradiation, revealing that the 1O2 release induced the oxidative damage and was the dominant reason to kill cells.
To study the therapy performance via cell apoptosis assay, Calcein-AM and PI were used to label the living and dead cells as indicators by staining the cytoplasm with green fluorescent AM and the nucleus with red fluorescent PI, respectively. The cells remained alive in dark. Under irradiation, the cells incubated with P1 remained alive even under 21% oxygen, whereas the cells incubated with P1-SO were dead under either 21% or 5% oxygen concentration (Figures 4(b) and in the Supplementary Material). Without irradiation, the cells treated with P1 or P1-SO mostly remained alive under 21% and 5% oxygen levels (see in the Supplementary Material). These results confirmed that the oxidative damage could be achieved by P1-SO in hypoxic cancer cells. To further determine the cell population at different stages of apoptosis, the flow cytometry experiments were performed (Figures 4(e) and 4(f) and in the Supplementary Material). Under irradiation by laser at 690 nm, the cells incubated with P1 were still alive but those incubated with P1-SO were dead after 6 hours under 5% oxygen condition (Figures 4(e) and 4(f)). All the results indicated that photothermal effect of P1-SO triggered the ROS generation and the oxidative damage dominated the therapeutic effects, encouraging us to investigate the potential application of P1-SO for cancer therapy in hypoxia environments.
2.4. Anticancer Investigation In Vivo
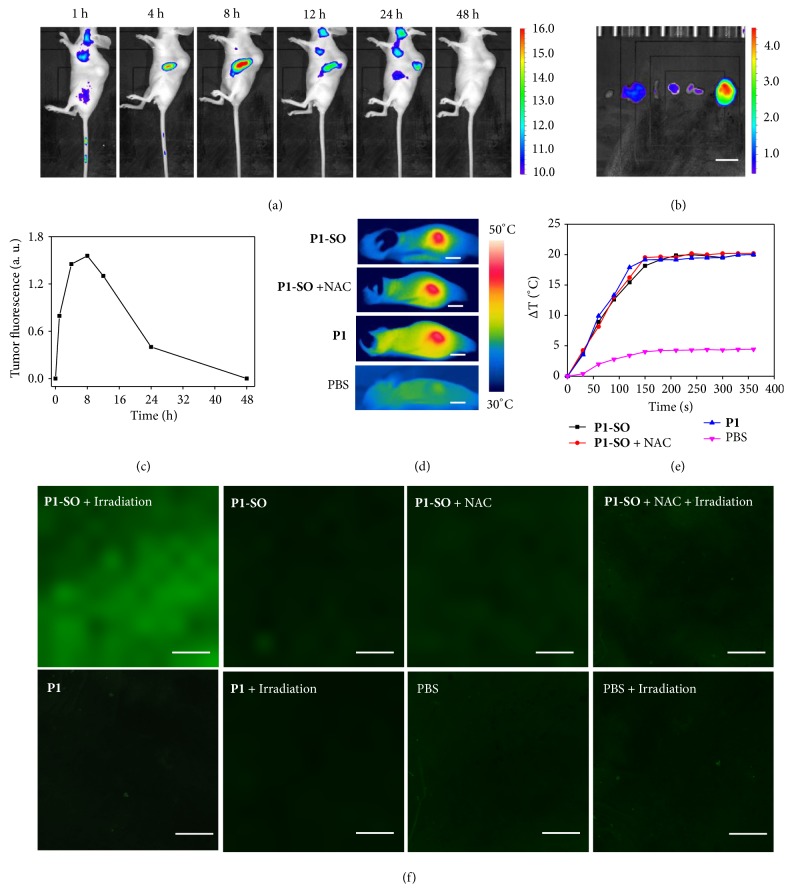
To test the in vivo behaviors of P1-SO, the polymer carrier was intravenously injected into the HeLa tumor-bearing mice, and then their biodistributions were evaluated at 1, 4, 8, 12, 24, and 48 h after injection (Figure 5(a)). After injection of P1-SO for 1 h, the gradually enhanced fluorescence at the tumors was observed. A maximized distribution was exhibited in tumor at 8 h after injection compared with other major organs (Figure 5(b)), demonstrating that the highest level of P1-SO was accumulated in tumor. After 48 h, no fluorescence was observed in tumor, revealing the metabolism of P1-SO with time prolonging (Figure 5(c)). Hence, the PDT treatments will be carried out at 8 h after injection of P1-SO. After the anticancer treatments, P1-SO can be eliminated from the body. These results indicated good capacity of the polymer dots to accumulate in tumor, owing to the EPR effect mediated by appropriate particle size.
Figure 5.
In vivo photothermal effects and generation of 1 O 2 of P1 and P1-SO. (a) In vivo fluorescence imaging of the HeLa tumor-bearing mouse at different time points after tail intravenous injection of P1-SO. (b) Fluorescence imaging of main organs after tail intravenous injection of P1-SO 8 h (scale bar, 1 cm). (c) The fluorescence intensity of tumor at different time. (d) Photothermal imaging of the mice bearing HeLa tumor treated with different treatments under irradiation (690 nm, 400 mW/cm2) (scale bar, 1 cm). (e) Temperature changes of mice tumors with different treatments under irradiation for 6 min (690 nm, 400 mW/cm2). (f) DCFH-DA staining at the tumors of mice with different treatments under irradiation for 6 min (690 nm, 400 mW/cm2) (scale bar, 250 μm).
To prove the photothermal effects of the polymer carrier in vivo, four groups of tumors bearing mice were investigated after 8 h after injection (Figure 5(d)). Upon irradiation at 690 nm (400 mW/cm2) by a FLIR camera, mice injected with P1-SO and P1 showed temperature increase to about 48°C in the tumor area. Similar temperature increase was also observed when the mouse was injected with P1-SO and N-acetyl-L-cysteine (NAC), which is a ROS-scavenger. In the control experiment where the mouse was injected with PBS, the temperature was almost kept at 37°C (Figure 5(e)). To investigate the ability of ROS generation in tumor, the tumor biopsies of mice with different treatments were studied by confocal imaging (Figure 5(f)). The in vivo generation of 1O2 from P1-SO under irradiation was observed using DCFH-DA as the indicator. After intravenous injection of P1-SO for 8 h, NAC was injected into the tumor. With a 690 nm laser irradiation (400 mW/cm2) for 6 min, no luminescence of DCF was found, and P1 treated tumor also displayed no 1O2 generation under irradiation. These results prove that P1-SO has strong ability to generate 1O2 in vivo under irradiation.
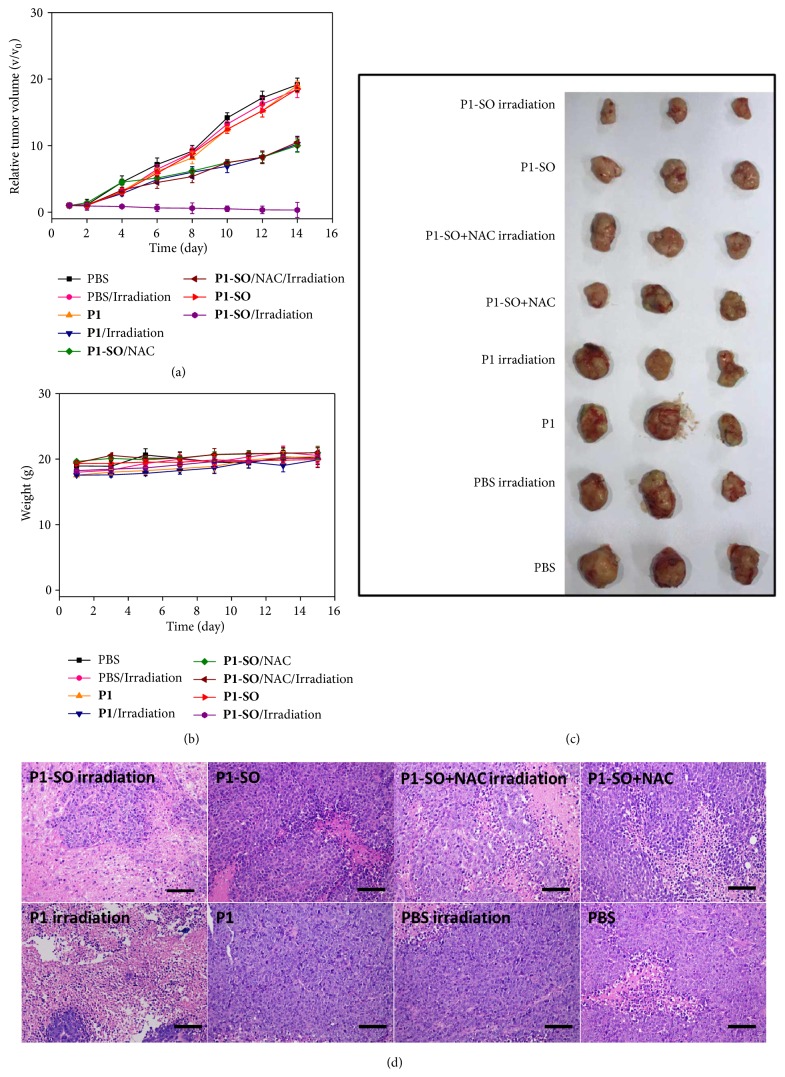
To investigate the in vivo PDT efficacy, P1-SO or P1 was intravenously injected into the mice bearing HeLa tumors of 100-300 mm3 in the presence or absence of NAC, followed by irradiation at 690 nm laser for 6 min (400 mW/cm2). The tumor size and weight of the mice were recorded every 2 days (Figures 6(a)–6(c)). As a control, the mice injected with PBS showed a 19-fold increase of tumor volumes regardless of irradiation, indicating that the irradiation of laser hardly had evident influence to the growth of tumors. Without irradiation, P1 and P1-SO treated mice also displayed similar tumor growth rate compared to those injected with PBS, demonstrating negligible anticancer efficacy of P1 and P1-SO due to their low dark cytotoxicity. When tumors were exposed to irradiation, P1 treated tumors showed a 10-fold increase of tumor volumes, but clear shrink of tumors was found on the mice injected with P1-SO after 2 weeks, indicating that the photothermal effect of B1 was unable to eliminate tumor but can trigger the release of 1O2 in P1-SO. Importantly, 1O2 generation by P1-SO caused irreversible oxidative damage towards tumor and inhibited the growth of tumor. To further prove the influence of 1O2 generation for tumor therapy, the ROSscavenger NAC was intratumorally injected into the P1-SO treated mice before therapy. The tumors did not stop growing and displayed 11-fold increases of tumor volumes after 14 days under irradiation. All the photographs of the mice after treatments were shown in in the Supplementary Material. These results demonstrated that the released 1O2 played a dominant role in the therapeutic process.
Figure 6.
In vivo treatments of P1 and P1-SO. (a) Relative tumor volume changes of mice with different treatments. Relative tumor volume was calculated by the (b) Body weight changes of mice with different treatments. (c) Photograph of the tumors extracted from the mice. (d) H&E-stained tumor sections harvested from mice after different treatments (scale bar, 100 μm).
To further evaluate the detail anticancer efficacy of P1 and P1-SO, the proliferation and morphology of the tumors and organs were investigated by hematoxylin & eosin (H&E) staining (Figure 6(d)). Firstly, obvious tumor damage was not observed in group PBS with or without irradiation. Few tumor cells were found in the group of P1-SO with irradiation, indicating an ideal ability to release 1O2 of P1-SO under irradiation. A small amount of tumor necrosis displayed in group P1-SO + NAC and P1 with irradiation owing to the photothermal effects. No distinct tumor damage was found in P1-SO or P1 without irradiation, which indicated that irradiation played a key role in the therapeutic process. These results demonstrated that tumor damage caused by P1-SO under irradiation was mainly attributed to oxidative damage with few photothermal effects. In addition, P1-SO exhibited negligible influence to the normal tissues (such as heart, liver, spleen, lung, and kidney) during the therapeutic process (see in the Supplementary Material).
3. Discussion
In this study, a novel all-in-one polymeric 1O2 carrier, which can rapidly release cytotoxic 1O2 in cancer cells under photothermal stimulation, was developed to overcome the restriction of hypoxic tumor during PDT process in vivo. The capture of 1O2 was attributed to the DMN units and the release of 1O2 was triggered by the photothermal effect of B1 under NIR light irradiation. The strategy has been demonstrated to effectively enhance the production of 1O2 in vitro and realize the photodamage to cancer cells, especially in hypoxic environments. Additionally, introduction of near-infrared excitable B1 facilitates the potential imaging-guided therapy in vivo. The polymer dots accumulate into tumor after intravenous injection via EPR effect and accelerate the oxygen-independent generation of 1O2. The oxidative damage towards tumor results in good inhibitory effect on tumor growth in vivo. The realization of this concept in vivo not only is a huge boost to the novel thermal-triggered PDT strategy, but also provides a valuable means to construct photothermal-triggered oxygen-independent therapeutic platform for clinical applications.
4. Materials and Methods
4.1. Materials
All reagents and starting materials were purchased from commercial sources and used without further purification. All aqueous solutions were prepared by using deionized water.
4.2. Instruments
NMR spectra (1H: 400 MHz, 13C: 100 MHz) were recorded on a Bruker ACF400 spectrometer. Tetramethylsilane (TMS) was used to report chemical shifts. The number-average molecular weight (Mn) of the polymers was characterized in tetrahydrofuran (THF) by gel permeation chromatography (GPC) using polystyrene as standard. UV-visible absorption spectra were obtained via a Shimadzu UV-3600 UV/Vis/NIR spectrophotometer. Emission spectra were obtained with Edinburgh FL 920 spectrophotometer. The particle size and morphology of polymer dots were characterized by the transmission electron microscope (TEM, JEOL JEM-2100, 200 kV). The average hydrodynamic size and zeta potential of polymer dots were measured via dynamic light scattering (DLS) on a zeta particle size analyzer (Brookhaven 90Plus). Oxygen concentration was controlled by flow counters (HORIBA STEC, SEC-E40JS, 60 SCCM). The excitation light source used to generate 1O2 and photothermal effect were MW-GX-660/2000mW and MW-GX-690/2000mW laser. Temperature was measured by a thermal infrared imager (FLIR E40). The power density meter is VLP-2000 laser power meter. In vivo and vitro imaging were measured by small animals living fluorescence imaging system IVIS LUMINA K/IVIS LUMINA K. Cell viability was measured with an enzyme-linked immune sorbent assay (ELISA) reader. Confocal luminescence images were carried out by a laser-scanning confocal microscopy (Olympus Fluo view FV1000) equipped with 20× objective lens. Photographs of the mice were taken with a Cannon EOC 400D digital camera.
4.3. Animals and Tumor Model
The athymic female nude mice were purchased from Comparative Medicine Centre of Yangzhou University (Permit number: SCXK(SU)2017-0007). HeLa cells (about 106 per mouse) were injected into nude mice. The mice bearing HeLa tumors were treated when the tumor volumes were about 100 mm3.
4.4. In Vivo Therapy
24 mice were divided into 8 groups averagely. P1-SO, P1 (300 μg/L, 100 μL), or PBS were injected. After 8 h, the mice were exposed to a 690 nm laser (400 mW/cm2) for 6 min or not. The weight and tumor volumes were recorded every two days. Volume of tumors was calculated by equation: volume = length × width2/2. The relative tumor volume = v/v0, v was the tumor volume at different day, v0 was the tumor volume at first day. All the mice were sacrificed after treatments and tumors and main organs (heart, liver, spleen, lung, and kidney) were fixed by using 4% formalin solution for further histomorphological analysis.
Acknowledgments
This work was financially supported by the National Funds for Distinguished Young Scientists (61825503), National Natural Science Foundation of China (51473078, 61805122, and 21671108), National Program for Support of Top-Notch Young Professionals, Scientific and Technological Innovation Teams of Colleges and Universities in Jiangsu Province (TJ215006), Priority Academic Program Development of Jiangsu Higher Education Institutions (YX03001), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_0751).
Contributor Information
Qiang Zhao, Email: iamqzhao@njupt.edu.cn.
Wei Huang, Email: iamdirector@fudan.edu.cn.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Conflicts of Interest
The authors declare no competing financial interests.
Authors' Contributions
T. Huang, Q. Zhao, and W. Huang conceived and designed this work. T. Huang, M. Zhao, and Z. Feng performed the synthesis work. T. Huang and Q. Yu performed data collection and data analysis. M. Zhao performed in vivo experiments. M. Xie performed the in vitro experiments. T. Huang, M. Zhao, Q. Yu, S. Liu, Q. Zhao, and W. Huang analyzed the data and wrote the manuscript. Q. Yu and K. Y. Zhang revised the manuscript and provided some suggestions. All authors discussed the results and commented on the manuscript at all stages. Tianci Huang, Menglong Zhao, and Qi Yu contributed equally to this work.
Supplementary Materials
Figure S1: the UV-Vis absorption and emission spectra of B1. Figure S2: the photothermal properties of B1. Figure S3: the singlet oxygen trapping properties of P2. Figure S4: the singlet oxygen generation of P2 and P2-SO. Figure S5: the singlet oxygen generation of P3. Figure S6: the ROS generation of P1 and P1-SO under irradiation in vitro. Figure S7: the ROS generation of P1 and P1-SO without irradiation in vitro. Figure S8: the ROS generation without photosensitizers in vitro. Figure S9: the cytotoxicity of P1. Figure S10: the cytotoxicity of P1-SO. Figure S11: the PDT effects of P1 and P1-SO under irradiation in vitro. Figure S12: the PDT effects of P1 and P1-SO without irradiation in vitro. Figure S13: flow cytometry of PDT effects under hypoxia. Figure S14: photographs of the mice. Figure S15: H&E stained of the main organs.
References
- 1.Gross S., Gilead A., Scherz A., Neeman M., Salomon Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nature Medicine. 2003;9(10):1327–1331. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 2.Castano A. P., Mroz P., Hamblin M. R. Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovell J. F., Liu T. W. B., Chen J., Zheng G. Activatable photosensitizers for imaging and therapy. Chemical Reviews. 2010;110(5):2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 4.Fan W., Huang P., Chen X. Overcoming the Achilles' heel of photodynamic therapy. Chemical Society Reviews. 2016;45(23):6488–6519. doi: 10.1039/C6CS00616G. [DOI] [PubMed] [Google Scholar]
- 5.Obaid G., Broekgaarden M., Bulin A.-L., et al. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale. 2016;8(25):12471–12503. doi: 10.1039/C5NR08691D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z., Song J., Nie L., Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chemical Society Reviews. 2016;45(23):6597–6626. doi: 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin C. S., Lovell J. F., Chen J., Zheng G. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano. 2013;7(3):2541–2550. doi: 10.1021/nn3058642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W., Zhen Z., Wang M., et al. Red blood cell-facilitated photodynamic therapy for cancer treatment. Advanced Functional Materials. 2016;26(11):1757–1768. doi: 10.1002/adfm.201504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Cheng H., Jiang C., et al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nature Communications. 2015;6(1, article no. 8785) doi: 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C.-C., Chia W.-T., Chung M.-F., et al. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. Journal of the American Chemical Society. 2016;138(16):5222–5225. doi: 10.1021/jacs.6b01784. [DOI] [PubMed] [Google Scholar]
- 11.Chen H., Tian J., He W., Guo Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. Journal of the American Chemical Society. 2015;137(4):1539–1547. doi: 10.1021/ja511420n. [DOI] [PubMed] [Google Scholar]
- 12.Zhu W., Dong Z., Fu T., et al. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Advanced Functional Materials. 2016;26(30):5490–5498. doi: 10.1002/adfm.201600676. [DOI] [Google Scholar]
- 13.Bai J., Jia X., Zhen W., Cheng W., Jiang X. A facile ion-doping strategy to regulate tumor microenvironments for enhanced multimodal tumor theranostics. Journal of the American Chemical Society. 2018;140(1):106–109. doi: 10.1021/jacs.7b11114. [DOI] [PubMed] [Google Scholar]
- 14.Xu S., Zhu X., Zhang C., Huang W., Zhou Y., Yan D. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nature Communications. 2018;9(1):p. 2053. doi: 10.1038/s41467-018-04318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberto M. E., Pirillo J., Russo N., Adamo C. Theoretical exploration of type I/Type II dual photoreactivity of promising Ru(II) dyads for PDT approach. Inorganic Chemistry. 2016;55(21):11185–11192. doi: 10.1021/acs.inorgchem.6b01782. [DOI] [PubMed] [Google Scholar]
- 16.Gilson R. C., Black K. C. L., Lane D. D., Achilefu S. Hybrid TiO2–ruthenium nano-photosensitizer synergistically produces reactive oxygen species in both hypoxic and normoxic conditions. Angewandte Chemie International Edition. 2017;56(36):10717–10720. doi: 10.1002/anie.201704458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv Z., Wei H., Li Q., et al. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(II) photosensitizer through type i photochemical process. Chemical Science. 2018;9(2):502–512. doi: 10.1039/C7SC03765A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Yang D., Chen H., et al. Reduction-sensitive fluorescence enhanced polymeric prodrug nanoparticles for combinational photothermal-chemotherapy. Biomaterials. 2018;163:14–24. doi: 10.1016/j.biomaterials.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Shi H. F., Ma X., Zhao Q., et al. Ultrasmall phosphorescent polymer dots for ratiometric oxygen sensing and photodynamic cancer therapy. Advanced Functional Materials. 2014;24(30):4823–4830. doi: 10.1002/adfm.201400647. [DOI] [Google Scholar]
- 20.Klaper M., Linker T. New singlet oxygen donors based on naphthalenes: Synthesis, physical chemical data, and improved stability. Chemistry - A European Journal. 2015;21(23):8569–8577. doi: 10.1002/chem.201500146. [DOI] [PubMed] [Google Scholar]
- 21.Fudickar W., Linker T. Release of singlet oxygen from organic peroxides under mild conditions. ChemPhotoChem. 2018;2(7):548–558. doi: 10.1002/cptc.201700235. [DOI] [Google Scholar]
- 22.Fudickar W., Linker T. Why triple bonds protect acenes from oxidation and decomposition. Journal of the American Chemical Society. 2012;134(36):15071–15082. doi: 10.1021/ja306056x. [DOI] [PubMed] [Google Scholar]
- 23.Turan I. S., Yildiz D., Turksoy A., Gunaydin G., Akkaya E. U. A bifunctional photosensitizer for enhanced fractional photodynamic therapy: singlet oxygen generation in the presence and absence of light. Angewandte Chemie International Edition. 2016;55(8):2875–2878. doi: 10.1002/anie.201511345. [DOI] [PubMed] [Google Scholar]
- 24.Turro N. J., Chow M.-F., Rigaudy J. Mechanism of thermolysis of endoperoxides of aromatic compounds. activation parameters, magnetic field, and magnetic isotope effects. Journal of the American Chemical Society. 1981;103(24):7218–7224. doi: 10.1021/ja00414a029. [DOI] [Google Scholar]
- 25.Kolemen S., Ozdemir T., Lee D., et al. Remote-controlled release of singlet oxygen by the plasmonic heating of endoperoxide-modified gold nanorods: towards a paradigm change in photodynamic therapy. Angewandte Chemie. 2016;128(11):3670–3674. doi: 10.1002/ange.201510064. [DOI] [PubMed] [Google Scholar]
- 26.Lv W., Xia H., Zhang K. Y., et al. Photothermal-triggered release of singlet oxygen from an endoperoxide-containing polymeric carrier for killing cancer cells. Materials Horizons. 2017;4(6):1185–1189. doi: 10.1039/C7MH00726D. [DOI] [Google Scholar]
- 27.Yang X., Yang M., Pang B., Vara M., Xia Y. Gold nanomaterials at work in biomedicine. Chemical Reviews. 2015;115(19):10410–10488. doi: 10.1021/acs.chemrev.5b00193. [DOI] [PubMed] [Google Scholar]
- 28.Ge Y., O'Shea D. F. Azadipyrromethenes: from traditional dye chemistry to leading edge applications. Chemical Society Reviews. 2016;45(14):3846–3864. doi: 10.1039/C6CS00200E. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C., Liu L., Yang Q., Lv F., Wang S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chemical Reviews. 2012;112(8):4687–4735. doi: 10.1021/cr200263w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: the UV-Vis absorption and emission spectra of B1. Figure S2: the photothermal properties of B1. Figure S3: the singlet oxygen trapping properties of P2. Figure S4: the singlet oxygen generation of P2 and P2-SO. Figure S5: the singlet oxygen generation of P3. Figure S6: the ROS generation of P1 and P1-SO under irradiation in vitro. Figure S7: the ROS generation of P1 and P1-SO without irradiation in vitro. Figure S8: the ROS generation without photosensitizers in vitro. Figure S9: the cytotoxicity of P1. Figure S10: the cytotoxicity of P1-SO. Figure S11: the PDT effects of P1 and P1-SO under irradiation in vitro. Figure S12: the PDT effects of P1 and P1-SO without irradiation in vitro. Figure S13: flow cytometry of PDT effects under hypoxia. Figure S14: photographs of the mice. Figure S15: H&E stained of the main organs.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.