The microbiota of the upper respiratory tract (URT) protects the host from bacterial pathogenic colonization by competing for adherence to epithelial cells and by immune response regulation that includes the activation of antimicrobial and (anti-)inflammatory components. However, environmental or host factors can modify the microbiota to an unstable community that predisposes the host to infection or inflammation. One of the URT diseases most often encountered in children is otitis media (OM).
KEYWORDS: Haemophilus influenzae, Lactobacillus, microbiome, Moraxella catarrhalis, otitis media, probiotics, Streptococcus pneumoniae
SUMMARY
The microbiota of the upper respiratory tract (URT) protects the host from bacterial pathogenic colonization by competing for adherence to epithelial cells and by immune response regulation that includes the activation of antimicrobial and (anti-)inflammatory components. However, environmental or host factors can modify the microbiota to an unstable community that predisposes the host to infection or inflammation. One of the URT diseases most often encountered in children is otitis media (OM). The role of pathogenic bacteria like Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the pathogenesis of OM is well documented. Results from next-generation-sequencing (NGS) studies reveal other bacterial taxa involved in OM, such as Turicella and Alloiococcus. Such studies can also identify bacterial taxa that are potentially protective against URT infections, whose beneficial action needs to be substantiated in relevant experimental models and clinical trials. Of note, lactic acid bacteria (LAB) are members of the URT microbiota and associated with a URT ecosystem that is deemed healthy, based on NGS and some experimental and clinical studies. These observations have formed the basis of this review, in which we describe the current knowledge of the molecular and clinical potential of LAB in the URT, which is currently underexplored in microbiome and probiotic research.
INTRODUCTION
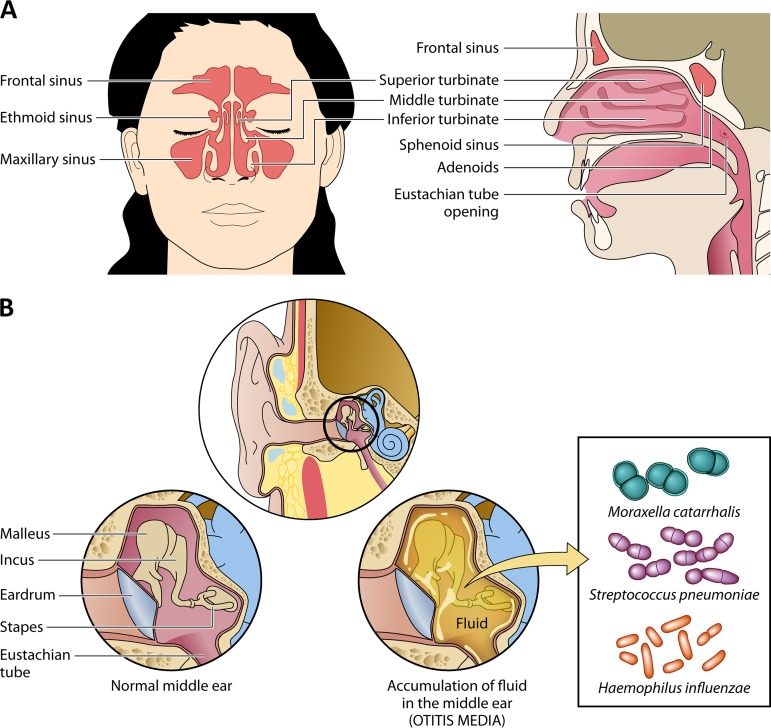
Various physical, chemical, and infectious agents can enter the human body via the upper airways, and this causes humans to be prone to upper respiratory tract (URT) diseases. The URT consists of the anterior nares, nasal passages, paranasal sinuses, the nasopharynx and oropharynx, and the portion of the larynx above the vocal cords (1). In children, the most common URT infection is otitis media (OM). OM encompasses a spectrum of disease conditions characterized by accumulation of fluid in the middle ear cavity and inflammation of the middle ear cleft (Fig. 1).
FIG 1.
Anatomy of the nasal cavity and characteristics of otitis media (OM). (A) Anatomy of the nasal cavity, depicting the connections between the different niches. (B) In healthy conditions, the middle ear is filled with air, while OM is characterized by the presence of fluid in the middle ear and the inflammation of the middle ear cleft. Dysfunction of the eustachian tube prevents the middle ear fluid from draining normally and, thus, creates an ideal environment for bacterial growth and the development of inflammation. The three main pathogens in (A)OM are Moraxella catarrhalis, Streptococcus pneumoniae, and Haemophilus influenzae, but other pathogens are emerging, such as Turicella and Alloiococcus, especially for more chronic forms of OM (see the text).
OTITIS MEDIA
Risk Factors for Otitis Media
A common pathway to all forms of OM is impaired function of the eustachian tube and inflammation of the middle ear (2–4). This is illustrated by the increased prevalence of middle ear effusion in children with an inherent anatomical abnormality causing dysfunction of the muscles involved in eustachian tube opening, such as children with cleft palate or Down syndrome (5, 6). Eustachian tube dysfunction may also be caused by congestion and inflammation of the mucosal lining (e.g., following an URT infection) or by mechanical obstruction from enlarged adenoids (7). As a consequence, accumulation of middle ear fluid can occur, creating an ideal environment for bacterial growth and the development of inflammation.
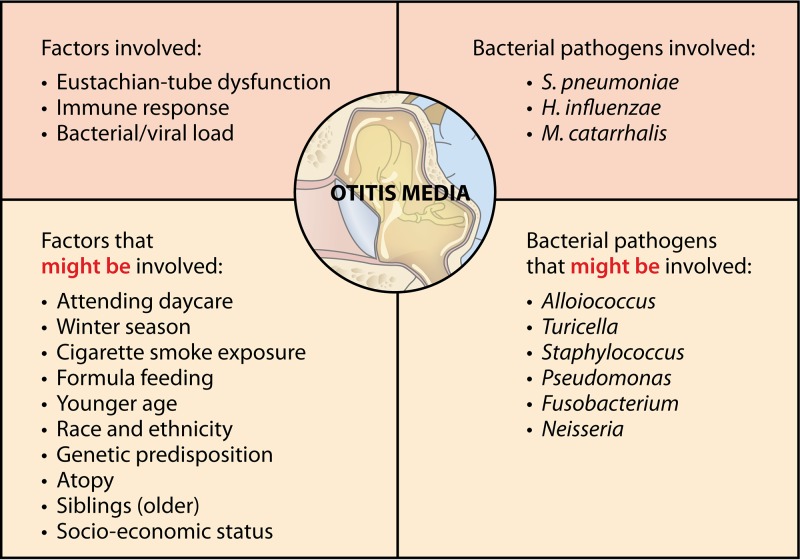
As it is a multifactorial condition, anatomical, host-related, and environmental factors play a role in OM (Fig. 2) (3, 8). Host factors that increase the risk for OM are, for instance, younger age, genetic predisposition, race and ethnicity, immunodeficiency, and laryngopharyngeal reflux. Environmental factors that have a negative influence on OM, on the other hand, are winter season, formula feeding or limited breastfeeding, exposure to cigarette smoke, low socioeconomic status, presence of older siblings, day care attendance, and pacifier use (as reviewed by Schilder et al. [3]). Although many factors are thus suggested to be involved, the pathogenesis is not yet fully understood.
FIG 2.
Factors and pathogens involved in OM pathogenesis. Based on data from Rovers et al. and Schilder et al. (3, 12) and the data presented in Table 2.
Different Forms of Otitis Media and Their Incidences
Different forms can be distinguished in OM. In this review, the widely used definitions as defined by Bluestone and by Schilder et al. (3, 9) are used (Table 1). Acute OM (AOM) is generally defined as the rapid onset of acute infection within the middle ear, characterized by signs and symptoms such as otalgia and fever. Otitis media with effusion (OME) is characterized by inflammation of the middle ear without signs or symptoms of acute infection and accompanied by the accumulation of fluid. Middle ear effusion (middle ear fluid [MEF]) is a liquid in the middle ear which may be serous, mucoid, or purulent. The duration of the effusion may range from less than 3 weeks (acute) to 3 weeks up to 2 to 3 months (subacute) or more than 3 months (chronic). Fluid in OME may persist in the middle ear cavity following an episode of AOM or result from eustachian tube dysfunction caused by a URT infection. It is the most common cause of hearing impairment in childhood, and resolution of hearing loss is the main treatment goal for OME (10). Chronic otitis media (COM) is defined as chronic inflammation (≥3 months) of the mucosa and submucosa of the middle ear and may result in changes not only to the mucosa and submucosa but also to the tympanic membrane (e.g., chronic suppurative otitis media [CSOM]) and ossicles. COM is the most severe form of OM but is very uncommon in developed countries (11).
TABLE 1.
Overview of different types of OM and their definitions
| OM type | Definitiona |
|---|---|
| Otitis media (OM) | A spectrum of disease conditions characterized by accumulation of fluid in the middle ear cavity and inflammation of the middle ear cleft |
| Acute otitis media (AOM) | The rapid onset of acute infection within the middle ear, characterized by signs and symptoms such as otalgia and fever |
| Otitis media with effusion (OME) | Inflammation of the middle ear without signs or symptoms of acute infection and accompanied by accumulation of fluid |
| Chronic otitis media (COM) | Chronic inflammation (≥3 mos) of the mucosa and submucosa of the middle ear; may result in changes not only to the mucosa and submucosa but also to the tympanic membrane and ossicles |
| Chronic suppurative otitis media (CSOM) | Chronic inflammation (≥3 mos) of the middle ear and mastoid mucosa with a nonintact tympanic membrane (perforation or ventilation tube) and persistent ear discharge |
Most children experience at least one episode of AOM (12), with a peak period of occurrence between 6 and 12 months. Recent monitoring data indicate that 46% of U.S. children have already suffered at least one episode of AOM before their first birthday (13). Typically in OME, a bimodal distribution in prevalence occurs, with a first peak around 2 years and a second peak around 5 years of age (14). It represents the most common form of OM in young children, with a point prevalence of ca. 20% (12).
Antibiotics in Otitis Media
In childhood, OM is a leading cause of antibiotic prescription (15). The rates of antibiotic prescription for AOM vary from 56% in the Netherlands to 95% in the United States (16, 17). However, contrary to what these numbers suggest, clinical practice guidelines first recommend a focus on pain relief without prescribing antibiotics, since spontaneous healing without complications is often observed and antibiotics only have a slight effect on pain in AOM (18). Depending on the age of the child and the severity of symptoms, however, antibiotics may be indicated to treat AOM according to published guidelines (4). As recently shown by a Cochrane Review (19), the use of oral antibiotics to treat OME has been associated with both benefits and harms, since it is associated with an increased chance of complete resolution at various follow-up times but these children are more likely to experience side effects like diarrhea, vomiting, or skin rash. Furthermore, the impact of antibiotics on hearing is unclear and there is no evidence that antibiotics are associated with fewer ventilation tube insertions.
Microbial Etiology of Otitis Media
Both viruses and bacteria are implicated in the pathogenesis of AOM; however, less is known about fungi. In children between 6 months and 3 years of age, about 90% of episodes of AOM are associated with a viral URT infection (20–22). The resulting inflammation of the epithelium in the nasopharynx and eustachian tube creates a negative middle ear pressure and promotes movement of bacteria and/or viruses into the middle ear, where they can cause infection. The risk of developing AOM after a viral URT infection has been related to the number of pathogens colonizing the nasopharynx. Half of the children carrying the three main AOM pathogens, Streptococcus pneumoniae, nontypeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis, develop AOM after a viral URT infection, compared to only 10% if none of these pathogens are present (23). The degrees of dominance of these otopathogens during OM have undergone dynamic changes since the introduction of the pneumococcal conjugate vaccines. A drop in the detection of S. pneumoniae was observed, while there appears to be an increase in the prevalence of M. catarrhalis. H. influenzae, however, appears to remain a dominant pathogen (24).
Via culture-dependent data, S. pneumoniae, NTHi and M. catarrhalis have long been described as the three main pathogens related to all other forms of OM as well (12), but next-generation-sequencing (NGS) approaches where the microbiome in diseased subjects is compared with the microbiome in healthy subjects have recently highlighted that other bacteria can be involved, as discussed in the next paragraphs. Viruses, commonly detected via immunological and molecular techniques, also play a role in OM. Studies indicate that the influenza A virus (20), respiratory syncytial virus (25), human rhinovirus (26), and adenovirus (20) could predispose to bacterial infection in AOM. These viruses can create changes in eustachian tube functioning by initiating inflammation (27), altering the biochemical and rheological properties of airway mucus (22), and compromising the mucociliary clearance (22, 28). Furthermore, by upregulating the expression of eukaryotic receptors, viruses can increase bacterial adherence and colonization (22, 29). To map the community of viruses and bacteriophages (i.e., the virome) via NGS approaches, standard 16S rRNA gene amplicon sequencing is not appropriate. Shotgun sequencing, dedicated DNA extraction, and other related protocols are needed, and these approaches are less widely adopted. Recently, the human respiratory virome is gaining more interest (30–35); however, to the best of our knowledge, no metagenomic URT virome data are yet available for children suffering from OM. Similarly, little is documented about the community of URT fungi (i.e., mycobiome) present during OM. Similar to the situation for the virome, different sequencing methods are needed to investigate the mycobiome, such as targeting of the internal transcribed spacer (ITS) regions of the rRNA locus for sequencing (36). Unfortunately, again, no mycobiome data are available for OM. Presumably if viruses or fungi were the primary cause of the infection, they would have been identified already. It is reasonable, however, that some specific viruses or fungi interact with the important pathogens to facilitate their infection and have always been underestimated. There is thus a clear need for more dedicated metagenomic studies that will give a better global overview of the total URT microbial community (bacteria, viruses, bacteriophages, and fungi). Such knowledge might be interesting for new therapies, as targeting important bystanders or cofactors might help to resolve the disease.
Since the information about the URT virome and mycobiome is limited, we will focus in this review on the bacterial microbiome and the potential bacterial interactions between OM pathogens and beneficial bacteria.
THE BACTERIAL MICROBIOME OF OTITIS MEDIA PATIENTS
The relationship between bacterial community composition in the URT, risk of pathogen colonization, and OM symptoms is increasingly being studied via culture-independent approaches like NGS, which is currently the main technique used for investigating microbial communities. NGS approaches certainly have their limitations in rather low-biomass niches like the respiratory tract, including the presence of inhibitors and contaminants, the difficulty in discriminating between live and dead bacteria, the short read lengths, and the lack of information about viruses and fungi and about absolute microbial numbers. However, these culture-independent approaches have still revealed novel insights on potential pathogenic and beneficial bacteria, as will be discussed below. It should be noted, however, that most approaches only identify the bacteria on the genus level, while pathogenicity is expressed at the strain level. This makes the distinction between commensal and potentially pathogenic species challenging. Furthermore, inconsistencies in microbiome studies can be due to differences in disease parameters, geographical location (37), sampling, storage, DNA extraction (38), sequencing approach (e.g., the targeting of different hypervariable regions of the 16S rRNA gene, indicated with V plus a number), and bioinformatic analysis (Table 2), among others, that can all favor and/or underestimate certain species. The next paragraphs aim to map the current knowledge about the bacterial microbiome differences between AOM, OME, and COM.
TABLE 2.
Overview of some of the most pioneering URT and OM microbiome studies
| Focus or OM type | Sample type(s) | Disease-associated bacteriaa | Sequencing method | No. of subjects (country) | Age(s) (mos or as indicated), no. of subjects/group | Reference |
|---|---|---|---|---|---|---|
| Focus | ||||||
| Respiratory microbiota in healthy infants | Nasal swabs | Haemophilus, Streptococcus | 454 sequencing, V5-V7 region | 60 (The Netherlands) | 1.5–24 | 41 |
| Nasal aspirates | Moraxella, Streptococcus, Haemophilus | Illumina MiSeq, V4 region | 234 (Australia) | 2–12 | 52 | |
| OM type | ||||||
| AOM | Nasal swabs | S. pneumoniae, Haemophilus, Rothia, Actinomyces | 454 sequencing, V1-V2 region | 108 (USA) | 6–78 | 59 |
| Nasal swabs | Moraxellaceae, Streptococcaceae, Pasteurellaceae | 454 sequencing, V3-V5 region | 153 (Switzerland) | 0–24 | 60 | |
| Nasal swabs | S. pneumoniae, H. influenzae, M. catarrhalis | 454 sequencing, V1-V2 region | 240 (USA) | 3–36 | 15 | |
| MEF aspirates | S. pneumoniae, H. influenzae, M. catarrhalis, T. otitidis, S. auricularis | Illumina MiSeq, V4 region | 79 (Finland) | 5–42 | 61 | |
| Nasal swabs | Moraxella, Haemophilus, Streptococcus | Illumina MiSeq, V4 region | 139 (USA) | 1–12 | 282 | |
| MEF aspirates, middle ear aspirates, ear canal swabs, nasal swabs | Haemophilus, Turicella, Alloiococcus, Staphylococcus | Illumina MiSeq, V3-V4 region | 196 (Australia) | 0–60 | 64 | |
| OME | MEF aspirates, adenoid and tonsil tissue samples | Pseudomonadaceae, Streptococcaceae, Fusobacteriaceae, Pasteurellaceae | 454 sequencing, V3-V4 region | 1 (USA) | 96 | 65 |
| MEF aspirates, adenoid swabs, nasal swabs | Alloiococcus, Haemophilus, Streptococcus, Moraxella | Illumina MiSeq, V1-V3 region | 11 (Australia) | 3–10 yrs | 66 | |
| MEF aspirates, adenoid swabs | Alloiococcus, Haemophilus, Moraxella, Staphylococcus, Streptococcus, Pseudomonas, Corynebacterium | Illumina MiSeq, V3-V4 region | 18 (Australia) | 1–16 yrs | 67, 68 | |
| Middle ear, adenoid, and tonsil swabs | Fusobacterium, Haemophilus, Neisseria, Porphyromonas | Illumina MiSeq, V3-V4 region | 10 (New Zealand) | 2–10 yrs | 70 | |
| COM | Middle ear swabs, mastoid swabs | Haemophilus, Staphylococcus, Alloiococcus | Illumina MiSeq, V3-V4 region | 46 (New Zealand) | 6 mos–87 yrs | 72 |
| MEF aspirates | Haemophilus, Moraxella, Turicella | Illumina MiSeq, V4 region | 55 (USA) | 0–24, 25 subjects; >24, 30 subjects | 71 | |
Bacteria were significantly more abundant in this group, as indicated by the authors.
Development of the Healthy URT Microbiome in Children
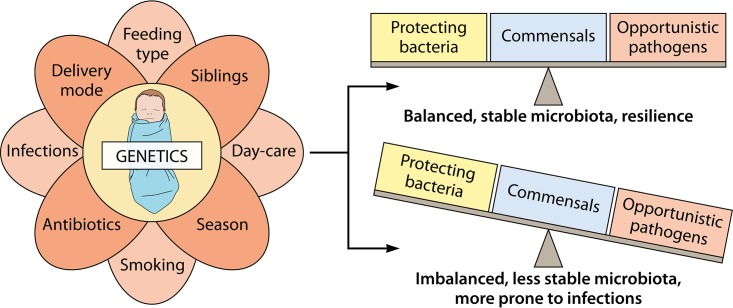
The microbiome of the URT is variable over time and depends on several, often environmental factors (Fig. 3) (1, 39). As the nose and nasopharynx are interconnected with the middle ear cavity, the microbiota of these niches can influence the middle ear microbiota (Fig. 1) and will be discussed in this paragraph as well. Already after 1 day of life, Bosch et al. (40) observed that the URT microbiota shifts to a Streptococcus viridans-predominated profile. After 6 months, a change toward a Corynebacterium pseudodiphteriticum/propinquum-, Dolosigranulum pigrum-, M. catarrhalis/nonliquefaciens-, S. pneumoniae-, and H. influenzae-dominated community or a mixed community with these bacteria was observed. In total, 11 nasopharynx microbiota profiles (termed clusters) were identified using Illumina MiSeq sequencing (V4 region), which confirmed earlier results (41). Biesbroek et al. (41) also noticed associations between certain taxa and microbiota stability during the first 2 years of life. Less-stable profiles contained a high abundance of Haemophilus and Streptococcus. In contrast, an early presence and high abundance of Moraxella and Corynebacterium/Dolosigranulum in the first period of life was associated with a more stable pattern (41), which was confirmed later on by the same researchers (40, 42) using Illumina MiSeq sequencing (V4 region) as well. In addition, Santee and colleagues (43), using a 16S rRNA PhyloChip sequencing approach focusing on the V5 region, observed an association between an enrichment of Moraxella nonliquefaciens in the nasopharynx of American children and acute sinusitis. The facts that, on one hand, early colonization of Moraxella is associated with a stable microbial pattern and, on the other hand, M. nonliquefaciens is enriched in children suffering from acute sinusitis highlights that association with health and disease should be studied at the strain or species level, since different strains and species have different virulence characteristics (44). This is not always possible with the currently available NGS approaches, especially not with amplicon sequencing, although pipelines such as the Divisive Amplicon Denoising Algorithm 2 (DADA2) that take into account genuine amplicon sequence variants (45) and shotgun sequencing approaches are an important step forward. Moreover, full-gene 16S rRNA gene sequencing analysis on the PacBio system could provide microbiome data at the species level in future microbiome analysis (46).
FIG 3.
Factors influencing the respiratory microbiota and/or bacterial density. First colonization in early life takes place during birth. The mode of delivery (natural versus Caesarian section) largely influences the microbial community in the newborn’s respiratory tract. Afterwards, the dynamics and evoluation of the microbiota are driven by many other environmental factors, such as feeding type, having older siblings or not, attending day care, the season, growing up in an environment with smokers, taking antibiotics, and having infections. Together with the host’s genetics, which influences the bacterial density in the nasopharynx, the microbiota can develop toward a balanced, stable microbiota where resilience, i.e., the ability of the host to remain healthy even when exposed to a stress, occurs. Conversely, the microbiota can also develop toward a community that is imbalanced, less stable, and more prone to infections and inflammation. The figure is based on data from references 1, 3, 15, 42, and 52.
As already mentioned, host and environmental factors play an important role in the maturation of the URT microbiome (Fig. 3). First of all, the mode of delivery seems to have a significant effect on the URT microbiota directly after birth. Indeed, a longitudinal study organized in the United States by Bosch et al. (40) has followed 102 children in the first 6 months of life and analyzed the bacterial DNA from nasopharyngeal swabs via Illumina MiSeq sequencing of the V4 variable region of the 16S rRNA gene. These authors observed that children who were delivered vaginally versus by Caesarean section carried a URT microbiota resembling, respectively, the maternal vaginal or skin microbiota directly after birth. This study confirmed earlier observations about the relationship between the mode of delivery and the baby’s microbiota by Dominguez-Bello et al. (47), where babies were only sampled immediately after birth and their microbiota was compared with the microbiota of different niches of the mother’s body via 454 pyrosequencing of the V2 variable region. Children born by Caesarian section showed diminished colonization with commensals like Corynebacterium and Dolosigranulum (40). The latter result was also observed in children with limited breast feeding (42). The members of the Dolosigranulum genus are rather unexplored lactic acid bacteria (LAB) belonging to the family of Carnobacteriaceae, while the Corynebacterium genus includes pathogenic species which are involved in diseases like diphtheria (48) and pneumonia (49), as documented for skin commensals with an inflammatory potential depending on the context (50). Both Dolosigranulum and Corynebacterium are gaining more interest recently, as they seem to be prevalent members in the nose and nasopharynx microbiota of healthy adults (51).
Next to the mode of delivery and feeding type, antibiotic use, host genetics, season, cohabiting with siblings, antibiotic use, attending day care, and exposure to cigarette smoke have an influence on the microbiome of children as well (15, 52–57). However, only a small number of studies with different sampling and sequencing methods have been performed on these topics.
The Bacterial Microbiome in Acute Otitis Media (AOM)
Taking the influence of all these (environmental) factors described above into account, it is not surprising that the URT microbiota balance can be easily disturbed, resulting in health issues such as OM. Investigations into the relationship between the microbiota of the middle ear and OM are, however, encountering some limitations, since it is difficult to obtain clinical samples from healthy control subjects, as getting access to the middle ear is only ethical when medical problems occur. Considering the fact that several URT niches are interconnected (Fig. 1) and these microbiotas can influence each other, the microbiome results investigated via the sampling of several of these URT niches are discussed in this section. Moreover, recent data indicate that microbiota composition in the nasopharynx could predict duration of AOM with tympanostomy tubes even better than MEF microbiota (58).
In one of the first NGS approaches on AOM, Laufer et al. (59) investigated nasal swabs of 108 children with and without AOM via 454 sequencing (V1-V2 region). The authors observed a relationship between the presence of S. pneumoniae, one of the main OM pathogens, and a less diverse (i.e., the number of different species in an environment) and less even (i.e., how close in population size each species in an environment is) microbial community. Furthermore, the presence of Haemophilus, Rothia, and Actinomyces was associated with an increased risk of AOM. In contrast, a potentially protective microbiota consisting of bacterial species such as Corynebacterium, Dolosigranulum, Propionibacterium, Lactococcus, and Staphylococcus was associated with a decreased risk of pneumococcal colonization and AOM. The same research group subsequently performed an analysis of nasal swabs of 240 children that also took the use of antibiotics in the 6 months before sampling into account (15). The mean levels of the AOM-associated taxa Rothia and Actinomyces were higher in children that received antibiotics in the past 6 months. Of interest for potential probiotic applications, Lactococcus, Anoxybacillus, and members of the family Enterobacteriaceae appeared negatively associated with colonization by each of the three classical bacterial AOM pathogens M. catarrhalis, S. pneumoniae, and H. influenzae and with AOM in children who used antibiotics in the past 6 months (15). However, such an association does not necessarily imply a causal relation between these potential probiotic taxa and health. Therefore, additional experimental evidence is necessary, as will be further discussed below in more detail. Hilty et al. (60) observed that the nasopharyngeal microbiota of children suffering from AOM more frequently contained bacteria from the families of Moraxellaceae, Streptococcaceae, and Pasteurellaceae, in agreement with the three major AOM pathogens. Although it is impossible to discuss pathogenicity and beneficial properties at the family level, these taxa are known to contain many common URT pathogens. In contrast, taxa which potentially contain more beneficial commensals, such as Staphylococcaceae, Flavobacteriaceae, Carnobacteriaceae, and Comamonadaceae, were less prevalent in AOM patients than in the control children (Table 2).
In addition to the nasal and nasopharyngeal microbiota obtained via swab sampling, middle ear fluid (MEF) is also a specimen of interest for detailed microbiome analyses. Sillanpää et al. (61) investigated 90 MEF samples of 79 children between 5 and 42 months of age using a combination of nested PCR and Illumina MiSeq 16S rRNA gene amplicon sequencing (V4 region) and operational taxonomic unit (OTU) clustering. They observed dominance of S. pneumoniae in 14 samples (16%), H. influenzae in 15 (17%), and M. catarrhalis in 5 (5.6%), while the less well-known AOM pathogens Turicella otitidis and Staphylococcus auricularis dominated in two subjects each. For comparison, based on culture-dependent data, H. influenzae, S. pneumoniae, and M. catarrhalis were the pathogens detected in 22%, 19%, and 10% of the cases, respectively. This study thus showed that both culture-dependent and -independent techniques confirm that the three major AOM pathogens dominate MEF of children suffering from AOM but NGS can also point toward other emerging pathogens. T. otitidis and Alloiococcus otitidis are examples of such emerging pathogens. In the study of Sillanpää et al. (61), they were found in 5 (5.6%) and 3 (3.3%) MEF samples, respectively. Before, these pathogens were only occasionally reported to occur in AOM based on culture-dependent data (62, 63), but microbiome-based data revealing their relative abundance in OM patient samples are increasing. Also, in a more recent microbiome case-control study, T. otitidis and A. otitidis were detected in high abundances in the middle ear (relative abundances of 6.72% and 49.84% in MEF, respectively) and ear canal (relative abundances of 13.06% and 53.62%, respectively) of recurrent AOM patients (64). It should be noted, however, that this study used nasopharyngeal swabs of healthy controls to compare with and the relative abundances of both potential pathogens were very low in these nasal swab samples of both AOM patients and healthy controls. So although the study also identified T. otitidis and A. otitidis as emerging OM pathogens, it could not rule out the possibility of these strains belonging to the normal aural microbiota due to the high relative abundances in the ear canal.
The Bacterial Microbiome in OME and COM
Although less frequently than AOM, otitis media with effusion (OME) and chronic OM (COM) are also being characterized by NGS (Table 2). In one of the first studies in the field, Liu et al. (65) investigated the microbiota of tonsil, adenoid, and middle ear fluid specimens of one patient with COM via 454 sequencing (V3-V4 region). The study group saw overlapping communities in these three respiratory niches. The adenoids showed a more complex microbial profile, containing Pseudomonadaceae, Streptococcaceae, Fusobacteriaceae, and Pasteurellaceae, while the middle ear and tonsils were each dominated by just one family, Pseudomonadaceae and Streptococcaceae, respectively. This observation adds support to the assumption that the middle ear and the tonsil microbiota can originate from the adenoids (65). Subsequently, Jervis-Bardy et al. (66) provided a landmark study for OME, because they observed by Illumina MiSeq sequencing of the 16S rRNA V1-V3 region that OTUs from the classic AOM pathogens Streptococcus, Haemophilus, and Moraxella are also common in MEF, nasopharyngeal, and adenoid samples of 11 children with OME. Two follow-up studies also observed similarities between MEF and adenoids of OME patients (67, 68). However, an important difference from AOM appeared, namely, A. otitidis dominated the middle ear effusion microbiota (23% mean relative abundance), followed by Haemophilus (22%), Staphylococcus (11%), Corynebacterium (6%), Moraxella (5%), and Streptococcus (5%). These abundances were observed to be stable over time, as they did not change drastically after 1 year (68). Swabs of the adenoids, on the other hand, showed colonization by Haemophilus (25% mean relative abundance), Moraxella (14%), Streptococcus (13%), Fusobacteria (11%), and Neisseria (7%). Alloiococcus was inversely correlated with Haemophilus, found in greater relative abundance in unilateral effusion, and had a very low relative abundance in adenoid swabs (<1%) (67). In the external auditory canal, the same Alloiococcus was found to have the highest relative abundance (28%), followed by Staphylococcus (20.8%) and Pseudomonas (3.2%) (68). Thus, taken together, the current data suggest that dominance of A. otitidis is associated with OME, while dominance of M. catarrhalis, H. influenzae, and S. pneumoniae may favor AOM. Furthermore, the studies of Chan and colleagues suggest that the external auditory canal and adenoids can both act as bacterial reservoirs for middle ear infections (67, 68). As perforations in the tympanic membrane sometimes occur in AOM, this can indeed give a free pass to bacteria that normally reside in the external auditory canal to move to the middle ear cavity (69). In contrast to the research discussed above, the study of Johnston et al. (70) did not reveal significant similarity between the microbiota of the adenoids and that of the middle ear in children with OME via microbial network analysis. Using Illumina MiSeq sequencing of the 16S rRNA V3-V4 region, the researchers observed higher relative abundances of Haemophilus and Moraxella in adenoid tissue than in the middle ear, where Fusobacterium and Staphylococcus were the most abundant genera. Across the adenoids, tonsils, and middle ear, however, Fusobacterium, Haemophilus, Neisseria, and Porphyromonas were the most abundant sequences. Furthermore, Alloiococcus and Turicella were only found in the middle ear samples, but the external auditory channel was not included in the study. Thus, no consensus exists about the adenoids being a source for OM pathogens, also called the “pathogen reservoir hypothesis.”
With regard to chronic OM, 55 American children were sampled and 16S rRNA gene amplicon sequencing via the Illumina MiSeq Platform (V4 region) was performed, which resulted in different bacterial disease profiles. The six most abundant bacteria in the MEF samples of this study were Haemophilus (relative abundance 22.54%), Moraxella (11.11%), Turicella (7.84%), unclassified Alcaligenaceae (5.84%), Pseudomonas (5.40%), and Alloiococcus (5.08%), while Streptococcus accounted for 4.21% of the MEF bacterial reads (ranked as the 8th most abundant genus) (71). Neeff et al. (72) associated Haemophilus, Staphylococcus, and Alloiococcus with an increased risk of COM using Illumina MiSeq sequencing (V3-V4 region) in 24 patients with COM and 22 healthy controls. Higher relative abundances of Novosphingobium, Staphylococcus, Escherichia-Shigella, Burkholderia, and Propionibacterium were observed in the middle ear specimens of healthy controls.
Combination of NGS and (Translated) Koch’s Postulates Can Identify New Pathogens or Probiotics
In 1890, Robert Koch published his four criteria to establish a causative relationship between a microbe (pathogen) and a disease (73). These postulates, although they have their limitations (74), had an enormous influence in medical microbiology. They state, among other things, that a pathogen should be isolated from a diseased organism and cause disease when introduced in a healthy organism. The latter point is quite important in the current era of NGS approaches to study bacterial communities in health and disease. Bacteria such as A. otitidis and T. otitidis, for example, are now gaining attention in the etiology of OM due to their high abundance in diseased children. However, because insights into their pathogenesis and molecular pathogenic characteristics are currently lacking, their role as pathogenic drivers of the disease is still under debate. On the other hand, microbiome insights indicate that the original Koch’s postulates, which state that pathogens should not be found in healthy organisms, are not entirely valid for most opportunistic pathogens. Indeed, all OM pathogens, for example, can also be found in the URT of healthy persons, but generally in lower abundances (51).
Similarly, to identify new probiotic strains, defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” (75), knowledge about their increased prevalence and abundance in healthy persons is not sufficient. For this reason, we introduce possible translated “probiotic postulates,” based on Koch's postulates, for the search for next-generation probiotics. These translated “probiotic postulates” are based on comparative microbiome research combined with experiments to determine a causative relationship with improved health (76) and are suggested as follows: (i) the microorganism can be found in high abundance in healthy organisms and decreased abundance in the ones suffering from a disease; (ii) the microorganism can be isolated from a healthy organism and grown in pure culture; (iii) according to the definition of probiotics, the cultured organism should promote health when introduced into a diseased organism; and (iv) because probiotics are by definition administered as live microorganisms, it should be possible to reisolate these microorganisms from the healthy experimental host and identify them as being identical to the original specific causative agent. According to the research about the development of the healthy URT microbiome summarized above (15, 41, 42, 59), Dolosigranulum is currently a prime candidate as a next-generation probiotic. However, according to the definition of a probiotic and the translated “probiotic postulates,” further exploration of the beneficial functional potential of specific strains of this underexplored lactic acid bacterium is needed before they can be defined as probiotics.
INFECTION MECHANISMS OF THE MAIN BACTERIAL OM PATHOGENS
Since both culture-dependent and culture-independent studies as reviewed above highlight S. pneumoniae, H. influenzae, and M. catarrhalis as key otitis media pathogens (77–80), we review their main pathogenesis mechanisms for host respiratory colonization and disease. These virulence mechanisms can be divided into three partially overlapping disease mechanisms: interactions with the nasopharyngeal epithelium, interactions with the host immune system, and formation of polymicrobial biofilms. Of note, since pathogenicity is strain specific, virulence factors can vary between distinct strains, which results in different grades of pathogenicity. However, molecular insights into virulence mechanisms will help in the study of probiotic mechanisms of interventions that could prevent or inhibit these key pathogenic steps as new alternative treatment strategies for OM. Probiotics, being living microorganisms expressing a multitude of effector molecules, use multifactorial mechanisms of actions that can all possibly target the virulence mechanisms of the pathogens, such as colonization, toxin production, inflammation, and biofilm formation. In the next paragraphs, the most commonly occurring virulence factors in the three main OM pathogens are discussed.
Interactions with Nasopharyngeal Epithelium
Impact on mucin and toxin production.
Before gaining access to the receptors of the epithelial cells, pathogenic invading bacteria must traverse the mucus layer of the nasopharynx. This layer consists of a mixture of water, ions, glycoproteins, proteins, and lipids and serves as an important defense mechanism of the host against invading pathogens (81). Moreover, the epithelial barrier is also important to keep a beneficial symbiosis in the host-microbiota relationship (1). The glycoproteins (with 70% to 80% O-linked glycosylation) in the mucus, also called mucins, are secreted by goblet cells. Although in healthy conditions the mucins help to protect the host mucosae, in diseased conditions like OM, the mucociliary clearance becomes ineffective and an excessive production of mucins will occur (82). Pathogens have developed multiple ways to overcome this mucus layer and get access to the epithelial cells more easily. S. pneumoniae, for instance, uses its neuraminidases (NanA and NanB) to cleave the layer and is helped by its capsule to prevent entrapment in the mucus (Fig. 4) (77, 83). However, at least 98 different capsule serotypes are known to date, while only a limited number of these serotypes are associated with colonization and disease (84). Protein D, on the other hand, is an outer membrane protein, present on the surface of all H. influenzae strains, which causes dysfunction of the nasopharyngeal cilia (85).
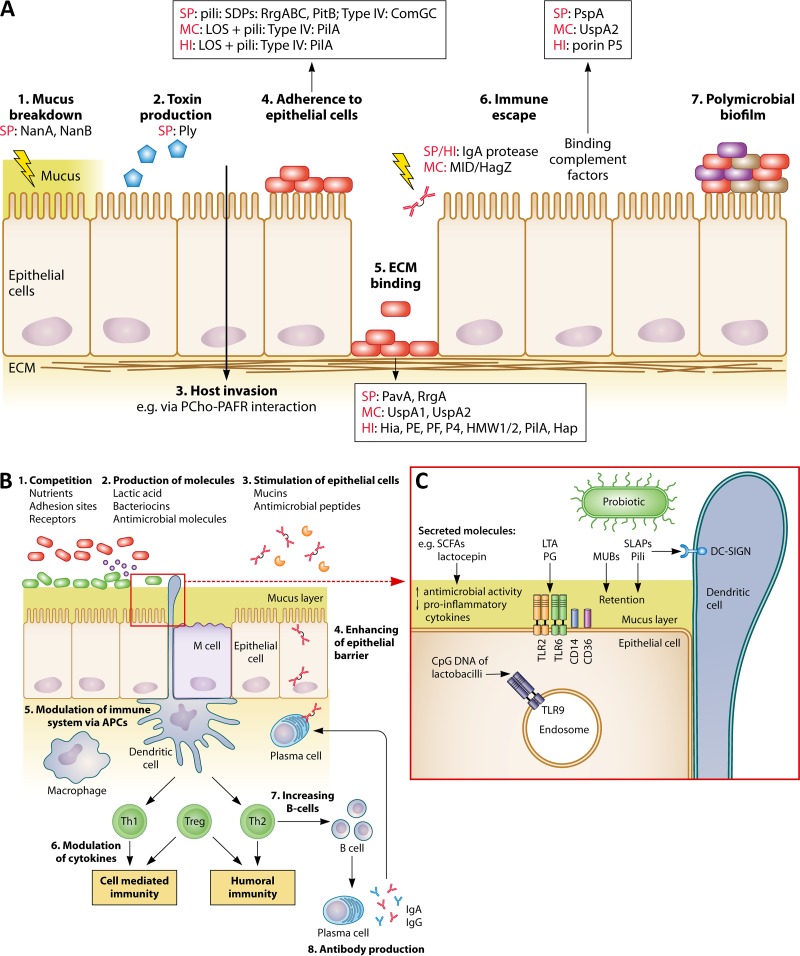
FIG 4.
Comparison between pathogenic and probiotic interactions with the nasopharyngeal epithelium and immune system. (A) Pathogens can interact with nasopharyngeal epithelium and host immune system via (1) breakdown of mucus; (2) production of toxins; (3) invasion of the host; (4) adhesion to the epithelium; (5) binding of ECM via microbial surface components recognizing adhesive matrix molecules (MSCRAMM); (6) escaping immune responses; and (7) the formation of a polymicrobial biofilm. Different frequently occurring pathogenic effector molecules are specified for each (A)OM pathogen. SP, S. pneumoniae; MC, M. catarrhalis; HI, H. influenzae; ECM, extracellular matrix; NanA/B, neuraminidases; Ply, pneumolysin; PCho, phosphorylcholine; PAFR, platelet-activating factor receptor; PavA, pneumococcal adhesion and virulence A; UspA, ubiquitous surface protein; Hia, H. influenzae adhesion; HMW1/2, high-molecular-weight molecules 1/2; Hap, Haemophilus adhesion protein; PspA, pneumococcal surface protein A. It should be noted that not all pathogenic strains or serotypes carry these effector molecules. (B) Postulated beneficial modes of action of URT probiotics. In agreement with beneficial activities in the gut, probiotics could also perform such activities in the URT by, for instance, (1) competition with pathogens for nutrients, adhesion sites, and receptors; (2) production of antimicrobial molecules, such as bacteriocins and lactic acid; (3) stimulation of epithelial cells to modulate mucin and antimicrobial peptide production; (4) enhancement of the epithelial barrier; (5) modulation of the immune system via APCs; (6) modulation of cytokine production; (7) stimulation of increased B-cell production; and (8) stimulation of antibody production. (C) Interaction of several probiotic effector molecules with their receptors localized on the epithelial/dendritic cells or endosomes. APC, antigen-presenting cells; LTA, lipoteichoic acid; MUB, mucus binding protein; PG, peptidoglycan; SCFA, short-chain fatty acids; SLAP, surface-layer-associated protein; Th1/2, T helper 1/2 cells; Treg, regulatory T cells. The figure is based on data described herein in “Infection Mechanisms of the Main Bacterial OM Pathogens” and obtained from references 174, 185, and 281.
In humans, more than 20 mucin genes have been identified (86). Upregulation of MUC5B, MUC5AC, and/or MUC4 is especially linked with OM (71, 86). Of note, the main pathogens that are involved in OM can upregulate MUC5AC (87–90). Furthermore, in a culture model of human middle ear epithelium, whole-cell lysates of the three pathogens induced upregulation of MUC2, MUC5AC, and MUC5B. (91). In mice, MUC5B appeared to be required for mucociliary clearance, for controlling infections in the airways and middle ear, and for maintaining immune homeostasis in mouse lungs, whereas MUC5AC was dispensable (92).
Pathogens can also attack nasopharyngeal epithelial cells by production of toxins. Current knowledge indicates that, of the three main OM pathogens, only S. pneumoniae produces such an exotoxin. Pneumolysin binds to cholesterol in cell membranes, forming oligomers and creating transmembrane pores (93). It is produced by almost all pneumococcal isolates and can decrease mucosal clearance in the upper airways (94–96). Haemophilus and Moraxella are Gram-negative bacteria that have lipooligosaccharides (LOS), or endotoxins, in their cell wall (79). Both M. catarrhalis and H. influenzae use them for adhesion, biofilm formation, and resistance to complement killing (79, 97–101). Not surprisingly, the presence of LOS is an important trigger for OM development in chinchilla models (98, 100).
Adhesion to epithelial cells and extracellular matrix (ECM).
As for most mucosal pathogens, adhesion to the nasopharyngeal epithelium is thought to be another key step in pathogenesis. Pili are long and thin proteinaceous protrusions of the cell surface present on specific Gram-positive and Gram-negative bacteria. Their molecular structure can be very diverse. Two types of sortase-dependent pili have been reported in S. pneumoniae (102–104). Type 1 pili are thermosensitive, as they are not induced in environments where the temperature is lower than 31°C (105), which suggests that the pathogen uses different virulence mechanisms in cooler anatomic sites, such as the nares, than in warmer sites, such as the nasopharynx/lungs. Both type 1 and type 2 pili have been shown to play an important role in adherence of S. pneumoniae to epithelial cells, although the corresponding host receptors are as yet unidentified (102, 104). Furthermore, S. pneumoniae uses the sortase-independent type IV pili for binding and internalization of exogenous DNA, which can lead to incorporation of new genetic material and resistance to antibiotics and vaccines (106). This type IV pilus is only assembled during bacterial competence (107), but its role in adhesion is unknown.
Type IV pili are also quite common in Gram-negative pathogens, with M. catarrhalis and H. influenzae expressing them as well to use for adhesion (108–111). Although the exact mechanism of adhesion is not yet unraveled, the pili of H. influenzae have been shown to bind the intercellular adhesion molecule 1 (ICAM-1) receptor (110), which is also used by other OM pathogens, such as the rhinovirus (112).
Adhesion to epithelial cells is also facilitated by the Haemophilus adhesion protein (Hap) (80, 113). Furthermore, β-glucan receptors on the surface of monocytic cells and macrophages are involved in the adherence and nonopsonic entry of NTHi, which does not express capsular polysaccharides (114). Moreover, phosphorylcholine (PCho) can be covalently attached through its phosphate group to the LOS of H. influenzae (80), similar to the way PCho can be bound to lipoteichoic acid (LTA) of S. pneumoniae (115). PCho enhances the bacterium’s survival in the respiratory tract, as it increases adherence and invasion (116–119).
Underneath the epithelial cells, the extracellular matrix (ECM) of the host appears to be a major target for colonization by the key OM pathogens, because they all contain microbial surface components recognizing adhesive matrix molecules (MSCRAMM) (Fig. 4) (120–126). These molecules bind fibronectin, fibrinogen, laminin, and/or collagen I and, thus, have an important function in host invasion.
Interactions with Host Immune System
Proinflammatory interactions in the host.
Once the OM pathogens have invaded and crossed the epithelial barrier, they interact with antigen-presenting cells (APCs) and stimulate them to secrete different cytokines, which play a pivotal role in the inflammatory responses. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), for example, have been thought to initiate the acute inflammatory response in OM (127). Moreover, in a chinchilla model, both IL-1β and TNF-α appear to regulate mucin production in a dose- and time-dependent way, especially the MUC5AC gene (128). IL-8, on the other hand, is an important attractant for neutrophils (129). Si et al. (2014) (130) observed increased mRNA levels of interferon gamma (IFN-γ), TNF-α, IL-1β, and IL-6, while protein analysis via enzyme-linked immunosorbent assay (ELISA) only recorded higher TNF-α and IL-1β concentrations in MEF samples of OME children compared to those of non-OME children. Similarly, ELISA of MEF samples of OME children showed a positive correlation between the concentrations of the proinflammatory cytokines IL-1 β, IL-6, IL-8, and TNF- α and the amounts of OM pathogens in the MEF (131).
Immune responses can be activated by specific pattern recognition receptors (PRRs), often Toll-like receptors (TLRs), which are found on epithelial cells, mast cells, dendritic cells, and other APCs. These receptors are trained to trigger host immune responses to bacterial ligands. In the middle ear mucosa of both OM and non-OM patients, TLR2, TLR4, TLR5, TLR6, and TLR9 are found at the mRNA and protein levels, but the correlation between expression levels and OM phenotype differs in different studies (130, 132). Interestingly, the outer membrane protein ubiquitous surface protein A1 (UspA1) of M. catarrhalis is able to inhibit the TLR2/NF-κB proinflammatory responses in the host (133). On the other hand, in H. influenzae-associated infections, the TLR2-interacting lipoproteins seem to be major triggers of the immune system (80). Moreover, both LTA of S. pneumoniae and PCho of S. pneumoniae and H. influenzae can also induce inflammation in the host via a TLR2-independent mechanism (77, 134, 135).
As already mentioned, pneumolysin is a very important virulence factor of most serotypes of S. pneumoniae, but it has also an effect on the host immune response. It can activate CD4+ T-cells by impairing the respiratory burst of phagocytic cells, by inducing production of chemokines and cytokines, by stimulating complement fixation, and by activating inflammation (77, 94, 136). However, some strains and serotypes have evolved mechanisms to evade the immune responses of the inflammasome (137).
Immune escape factors.
To protect themselves against the host’s adaptive immune defense, many pathogens directly target antimicrobial molecules or antibodies from the host. For instance, IgA1 proteases produced by S. pneumoniae and H. influenzae cleave human secretory antibodies like sIgA (Fig. 4) (78, 138), and the M. catarrhalis immunoglobulin D (IgD) binding protein/hemagglutinin (MID/Hag) binds soluble IgD (139). Furthermore, PCho protects H. influenzae against IgG binding and the human antimicrobial cathelicidin LL-37 (140, 141). In addition, extracellular DNA (eDNA) of H. influenzae can neutralize human β-defensin (HBD) (141–145), while the pneumococcal surface protein A (PspA) of pneumococci can bind the antibacterial lactoferrin (146). Furthermore, a camouflage strategy to protect against antibody recognition is reported to be used by the variable LOS of H. influenzae (147) and the orientation-switching lipoprotein P6 of S. pneumoniae (148). Pathogens can also evade the host’s immune system by, for instance, binding complement factors. The pneumococcal surface protein C (PspC or CbpA), UspA2 of M. catarrhalis, and the porin P5 of H. influenzae both prevent complement-mediated opsonization (80, 146, 149–154).
Polymicrobial Biofilm Formation
A pathogenesis mechanism that receives a lot of attention in COM and OME is mono- and polymicrobial biofilm formation by OM bacterial pathogens (Fig. 4) (110, 155–164). These studies indicate that the presence of biofilms causes OM episodes to recur more often. By investigating middle ear mucosa biopsy specimens of OME children with confocal scanning laser microscopy and fluorescence in situ hybridization (FISH), Hall-Stoodley et al. (155) observed the presence of all three main OM pathogens in the biofilms. M. catarrhalis, however, seemed to be present in polymicrobial infections more often than in monomicrobial infections (165). These observations suggest that other bacterial pathogens can facilitate persistence of and/or infection by M. catarrhalis. Indeed, although bacteria often compete with each other for, e.g., nutrients and receptors, in many cases they collaborate for the greater common good. The formation of an extensive exopolysaccharide or exopolymeric substance (EPS) matrix, for example, results in general protection of the inhabitants of the biofilm. Additionally, in a polymicrobial biofilm, β-lactam-resistant H. influenzae and M. catarrhalis can protect S. pneumoniae against β-lactam antibiotics, while S. pneumoniae, on its turn, protects the other two pathogens against macrolide killing (166, 167). Furthermore, Cope et al. (168) observed upregulation of type IV pili of H. influenzae and increased H2O2 production by S. pneumoniae when they were growing together in a biofilm. The exact functions of these molecules in a polymicrobial biofilm are, however, not yet clear.
POTENTIAL OF PROBIOTICS AGAINST OM AND THEIR MOLECULAR MECHANISMS
Among the bacteria that are more prevalent in healthy subjects than in OM patients are potential probiotics that can contribute to better ear and upper respiratory tract health. However, as we suggested by introducing the translated “probiotic postulates,” not only is higher abundance in healthy persons compared to diseased persons important, but also, a causative relationship with health-promoting effects should be demonstrated before a strain can be designated probiotic. Thus, for a microbial strain to be probiotic, its health benefits should first be shown in relevant in vitro and in vivo model systems and then ultimately be documented in clinical trials that can substantiate causal health relations for the specific probiotic applied. Of note, as mentioned above, LAB, which are widely applied as gastrointestinal probiotics, are also among the interesting probiotic candidates for the URT based on several NGS studies mentioned above (and summarized in Table 2); therefore, various examples will be given for this group of bacteria.
Possible Application Routes and Formulations for URT Probiotics
Although the URT mucosa is the target site and most health-promoting mechanisms of action of probiotics happen at that location, most of the human studies conducted are performed with orally administered probiotic LAB. Orally applied probiotics could benefit the URT via systemic immune effects, but it is also possible that orally ingested probiotics transfer to regions of the URT via the nasopharynx, since all these human body sites and their associated microbial niches are interlinked (1). Lactobacillus rhamnosus GG, for example, has also been shown to colonize the tonsils when administered in a dairy formulation containing 1010 CFU daily for 3 weeks (169). In addition, L. rhamnosus GG was recovered from adenoids (100% recovery by quantitative PCR [qPCR]) and middle ear fluid (MEF) (21% recovery by qPCR) after oral consumption in a dairy formulation for 3 weeks at ca. 1.6 × 1010 CFU/dose (170, 171).
Nasal applications on the other hand, have the advantage of promoting a more direct contact of the applied probiotics with the nasopharyngeal niche and pathogens. Furthermore, by using this delivery route, bacteria do not have to survive the stressful transit through the gastrointestinal tract for the systemic immune stimulation. In addition, the oronasopharyngeal cavity is more accessible and generally populated by a less complex and less dense microbiota than the gut, which makes nasal delivery an interesting alternative to the classical oral route. However, several other barriers emerge to which probiotics should be properly adapted, as further discussed below.
Both oral and nasal administration generally need a drying step in the formulation of the product in order to properly store the probiotics and increase shelf life, but drying can reduce the activity of the probiotic bacteria. Sufficient viability of the strains and preservation of their morphological and metabolic properties after drying are indispensable for probiotics, and consequently, specific pharmaceutical biotechnological strategies are needed. Several protective approaches, such as the addition of protective agents, accurate control of the drying process parameters, and prestressing the probiotics prior to drying, can be used to enhance the viability of strains (172). In addition, safeguarding the presence of cell surface molecules, such as pili, is crucial, as these molecules can be important for adherence to respiratory cells and immunological stimulation (173). However, in many cases, these specific characteristics of the probiotic products administered in clinical studies are unknown.
Clinical Studies with Topical Application of Probiotics
Currently, only a limited number of clinical trials have been performed with potential probiotics in relation to health benefits to the URT of the host (Table 3). Furthermore, the current data on the clinical efficiency of probiotics for OM are not univocal. Both oral and topical intake of probiotics has been explored in recent years (as reviewed in Marom et al. and Niittynen et al. [174, 175]). The oral administration route especially aims at enhancing immune responses systemically (mainly via the gastrointestinal immune cells). On the other hand, topical application of the probiotic strains directly in the URT, e.g., via a nasal spray, might be a better administration route to directly target the OM pathogens, but this has only been explored for a limited number of probiotic species so far. Some of the best documented LAB probiotics for topical application are alpha-hemolytic Streptococcus (AHS) bacteria (174). A combination of two strains of Streptococcus mitis and Streptococcus sanguis and one strain of Streptococcus oralis, all isolated from the eustachian tube opening of healthy children and able to inhibit growth of S. pneumoniae, was used in two Swedish studies. In the first one, 108 otitis-prone children were investigated after daily nasal administration of the AHS mixture (7.5 × 107 CFU per intake) or placebo for 10 consecutive days. The AHS treatment group experienced fewer recurrences of AOM than the placebo group as monitored for a 3-month period (176). However, the second study, which tested the same mixture of AHS (5 × 105 CFU per intake) in 43 children with recurrent OM for 4 months, did not see a difference in AOM recurrences and did not detect significant changes in the nasopharyngeal colonization of the children (177). This difference could be due to the smaller amount of streptococci administered in the latter study (Table 3). In addition, after their colonization, safety, and tolerability were investigated (178), a mixture of two other Streptococcus strains, Streptococcus salivarius 24SMB and S. oralis 89a, was tested in an Italian cohort of 267 children (179). A reduction in the reoccurrence of AOM was observed in all children using the spray, while only 50% of the children in the control group experienced fewer AOM episodes. Skovbjerg et al. (180) used lactobacilli in a similar study. They compared the administration of S. sanguinis NCIMB 40104, L. rhamnosus NCIMB 40564, or a placebo in 60 children with serous OM. In both treatment groups, ca. 50% of the children showed improvements or were cured (9/19 in the Streptococcus group and 9/18 in the Lactobacillus group), while this number decreased to only 18% (3/17) in the placebo group. The spray treatment did not alter the composition of the nasopharyngeal microbiota (although it was only monitored with culture techniques) or the cytokine patterns (IL-1β, IL-6, IL-8, and IL-10) in the middle ear fluid (180). More recently, S. salivarius 24SMB also showed promising results (181). Children who administered the strain in each nostril twice per day for 5 consecutive days during 3 consecutive months showed fewer episodes of AOM and received less antibiotics over a 6-month period. In addition, Mårtensson et al. (182) reported the successful nasal administration to healthy adults of promising Lactobacillus and Bifidobacterium strains, isolated from honeybees and proven to have antimicrobial activity against the important human URT pathogens Streptococcus pyogenes, Staphylococcus aureus, and Pseudomonas aeruginosa. The spray did not increase URT inflammation as tested with a cytokine microarray representing 30 cytokines/chemokines and mediators involved in type 1 and 2 inflammatory responses. Moreover, no adverse effects were observed after administration. Since ancient times, honey has been used to treat respiratory diseases and its medicinal properties have received considerable recognition in medicine (183). However, whether the gut lactobacilli of the honeybee are partially responsible for the antimicrobial and healing activities of honey remains to be substantiated.
TABLE 3.
Overview of some of the most pioneering clinical trials where the probiotic strains are applied directly to the URT
| URT disease | Probiotic strain(s) | Vehicle | No. of subjects | Dose and duration | Resultsa | Reference |
|---|---|---|---|---|---|---|
| OM | S. mitis, S. sanguis, S. oralis | Saline spray | 108 | 5 × 108 CFU/ml in 150-μl saline suspension per nostril 2×/day intranasally, 10 days/mo for 2 consecutive mos | Cured (↑), 42% in streptococcal group vs. 22% in placebo group (P = 0.02); recurrence of OM (↓), 40% vs. 51% (P = 0.04); nasopharyngeal microbiota change, ns | 176 |
| OM | S. mitis, S. sanguis, S. oralis | Saline spray | 43 | 107 CFU/ml in 50-μl saline suspension per nostril 1×/day intranasally for 4 mos | Episodes of OM, 44% in streptococcal group vs. 40% in placebo group (ns); nasopharyngeal microbiota change, ns | 177 |
| OM | S. sanguinis NCIMB 40104, L. rhamnosus NCIMB40564 | Saline spray | 60 | 5 × 109 CFU/ml in 100-μl saline suspension per nostril 2×/day for 10 days | Recovery, 7/19 patients in S. sanguinis group vs. 1/17 in placebo group (P < 0.05) and 3/18 patients in L. rhamnosus group (P = 0.60 compared with placebo group); nasopharyngeal microbiota change, ns | 180 |
| OM | S. salivarius 24SMB | Saline spray | 100 | 1011 CFU/ml in 50-μl saline suspension per nostril 2×/day intranasally 5 days/mo for 3 consecutive mos | Recurrence of OM (↓), children without OM, 30% in streptococcal group vs. 14.9% in placebo group (P = 0.076); antibiotic use (↓), 70% versus 83.0% (P = 0.13); recurrence of OM, 13.6% after colonization of S. salivarius 24 SMB vs. 42.8% without colonization | 181 |
| OM | S. salivarius 24SMB, Streptococcus oralis 89a | Saline spray | 267 | 109 CFU/dose, 2×/day intranasally, 1 wk/mo for 3 consecutive mos | Recurrence of OM (↓), 9.4% of children had the same number of AOM episodes in spray-treated group versus 68.5% in control group | 179 |
| General URT inflammation | Mixture of 9 Lactobacillus spp. and 4 Bifidobacterium spp. | Honey and pollen in water spray | 22 | 1011 CFU/ml in 200-μl suspension per nostril intranasally as 1 dose | No untoward effects; no significant difference in SNOT-22 scores obtained after challenge with LAB and sham treatment; nasopharyngeal microbiota change, ns | 182 |
↑, increase in active group; ↓, decrease in active group; nasopharyngeal microbiota change, difference in distribution of bacteria between active and placebo groups observed via culture-dependent techniques and/or PCR; ns, not significant.
As summarized above and in Table 3, although various clinical benefits have been reported, the randomized-controlled studies with probiotics do not all show efficacy. This could be explained by the fact that the probiotic strain applied was not optimally selected or, perhaps, administered (e.g., too low a dose or too short a duration) for the URT condition targeted or because most of the study participants (hosts) were not responsive to the selected probiotics. Detection methods, host genetics, too severe inflammation, or too severe microbiome dysbiosis could indeed influence (measured) responses to probiotic treatment, as also shown for gastrointestinal applications of probiotics, highlighting the need for patient stratification (e.g., see Claes et al. [184]). Therefore, it can be anticipated that knowledge about the molecular mechanisms of action of the probiotics in the URT, and better molecular knowledge of OM pathogenesis, will facilitate the selection of the most optimal probiotic strain for each condition and the subjects benefiting most from their application (as potential responders). In the next paragraphs, potential probiotic mechanisms of action against infection by OM pathogens are discussed. Although little is yet documented about the potential protective characteristics of nasopharyngeal probiotics, we have rationalized these mechanisms similarly as for the gastrointestinal tract (GIT) (185).
Properties That Can Be Rationalized To Be Important for URT Probiotics
Since most clinical studies with URT probiotics performed so far have been done with lactic acid bacteria (LAB), and since current microbiome studies also suggest a potential role for LAB (Table 2), potential mechanisms of action of probiotics will be explored here mainly for LAB (summarized in Table 4). Moreover, LAB have an advantage over other, less well studied health-related taxa, such as Corynebacterium (Table 2), because they have a long history of safe use (generally recognized as safe [GRAS] and qualified presumption of safety [QPS] status) in fermented foods, which is important for future applications.
TABLE 4.
Overview of some of the most important molecular mechanisms of probiotics
| Type of action | Mode of action | Molecule(s)/structure(s)/adjustment | Example(s) | Reference(s) |
|---|---|---|---|---|
| Competition | Adhesion sites and receptors | Pili or fimbriae | SpaCBA pili in L. rhamnosus GG | 173, 187 |
| Lectins | Llp-1 in L. rhamnosus GR-1 | 190 | ||
| Nutrients | General better adaptation | |||
| Environment | General better adaptation | |||
| Production of molecules | Acids | Lactic acid | General in LAB | 197, 200–202 |
| Proteins | Bacteriocins | Lactacin in L. acidophilus, plantacin in L. plantarum, nisin in L. lactis | 209, 210, 212 | |
| Others | H2O2 | In many LAB | 216 | |
| Quorum-sensing interaction | AI-2 in many LAB | 219 (for L. rhamnosus GG) | ||
| Stimulation of epithelial cells | Mucins | Unknown | L. rhamnosus GG | 197 |
| Antimicrobial peptides (defensins, lysozymes, cathelicidins, etc.) | Muramyl dipeptide motif | M-tri-Lys in L. salivarius | 240 | |
| Enhancement of epithelial barrier | Upregulation of tight junctions | Secreted soluble proteins | Msp1/p75 and Msp2/p40 in L. rhamnosus GG | 231 |
| Unknown | L. plantarum WCFS1 | 230 | ||
| Barrier repair | Secreted soluble proteins | Msp1/p75 and Msp2/p40 in L. rhamnosus GG | 232, 233 | |
| Modulation of immune system (via APCs, modulation of cytokines, increased B cells, antibody production) | MAMPs | Surface layer-associated proteins (SLAPs) | SlpA in L. acidophilus NCFM | 248 |
| Pili | SpaCBA pili in L. rhamnosus GG | 254, 255 | ||
| EPS | L. casei Shirota, L. plantarum | 261, 262 | ||
| LTA | In several LAB | 257–260 | ||
| CpG-rich DNA | In several LAB | 263 | ||
| Secreted effector molecules | Proteases | PrtP protease or lactocepin in L. paracasei | 264 | |
| Short-chain fatty acids (SCFAs) | In several LAB (direct or indirect through cross-feeding) | 266–269 | ||
Adaptation Mechanisms Rationalized for URT Probiotics
Considering the fact that most URT pathogens adhere strongly to the nasopharyngeal or middle ear epithelium, at least temporarily, during their infection process (as discussed in earlier paragraphs), it is reasonable to envisage that probiotics to be applied in the URT should be able to persist temporarily at the mucosa to compete with these pathogens, especially considering that nasal clearance is less than 20 min (186).
Selecting highly adherent probiotic strains is generally part of the screening platforms, although there is no consensus in the literature that gastrointestinal probiotics should be able to strongly adhere to the mucosa. Successful gastrointestinal probiotics, such as Lactobacillus rhamnosus GG, show a high capacity to adhere to human intestinal epithelial cells and mucus due to the presence of adhesive heteromeric SpaCBA pili (173, 187). More specifically, the tip pilin SpaC acts as a mucus binding protein (MUB). Whether the SpaCBA pili are also important for adherence to respiratory and nasopharyngeal epithelium cells is not known at present. In addition to pili, other sortase-dependent proteins (SDPs) could promote adherence of lactobacilli, as well as related potential probiotics, to the respiratory tract epithelium (188). For instance, we recently found indications for a novel type of sortase-dependent pili or fimbriae in the nasopharyngeal Lactobacillus casei AMBR2 strain (189). Other surface proteins that are linked to adherence to the host epithelium are lectins, i.e., proteins that bind carbohydrates with high specificity. For instance, the lectin-like protein 1 (Llp-1) of L. rhamnosus GR-1 has been shown to play a tissue-specific role in adhesion to vaginal epithelium (190) but not gastrointestinal and endocervical cells, suggesting that lectins could also mediate tissue-specific adhesion to the URT niche.
Being able to strongly adhere to the nasopharyngeal or middle ear epithelium will probably not be sufficient to efficiently compete with the OM pathogens and to sufficiently interact with the human host cells to confer beneficial effects. It can be hypothesized that the applied probiotics should also be able to adapt to the specific host nutritional environment and stress conditions of the URT. Indeed, the conditions in the gut and the URT are not comparable, as they differ substantially in oxygen level, pH, relative humidity, travel distance and time, temperature, etc. (1). The thickness of the EPS layer of L. rhamnosus GG, for example, has been shown in vitro to increase in a neutral pH (cf. URT) compared to its thickness in an acidic environment (cf. gut), which causes pili of L. rhamnosus GG to unfold and be more accessible for interaction with proteins (191), but whether this is also true in vivo remains to be substantiated. Further mechanistic studies are certainly needed to define the most important characteristics of candidate probiotic bacteria in the URT. At present, a standard model is lacking for in vitro URT adhesion assays (192), but several cell lines are used, such as A549 lung epithelial cells (193), Calu-3 human bronchial cells (194), FaDu hypopharyngeal cells (195), Detroit 562 pharyngeal cells (124), and CCl-23 laryngeal cells (196). In contrast to the interaction with the gut epithelium, mucosal adhesion of lactobacilli to the nasopharyngeal epithelium has not been extensively studied. However, by in vitro assays, Guglielmetti et al. (195) observed that Lactobacillus helveticus MIMLh5 was able to adhere to FaDu hypopharyngeal carcinoma cells and antagonize the typical sore-throat pathogen S. pyogenes. The model gastrointestinal probiotic L. rhamnosus GG has also been shown to inhibit the adherence of S. pneumoniae to the laryngeal cell line CCL-23 in a time- and dose-dependent way (196) and to significantly decrease the adhesion of M. catarrhalis to Calu-3 human bronchial cells (197).
The reduced pH stress (pH 6.3 and 7 in nasal cavity and nasopharynx, respectively), lower temperature, and higher oxygen level (1) in the URT compared to those in the GIT can be hypothesized to favor other probiotics than the classical GIT ones. At present, the available nutrients and other stress factors in the URT are not well characterized, but it can be rationalized that the probiotics will have to adapt to low concentrations of free carbohydrates and iron (198), as well as to the presence of antimicrobial molecules in the mucus, such as lysozyme, lactoferrin, and PLUNC (palate, lung, and nasal epithelial clone) proteins (199). For instance, our recently isolated L. casei AMBR2 strain from the nasopharynx is catalase positive (while most other Lactobacillus species are catalase negative), suggesting a role for catalase in adaptation to the oxidative environment of the URT (189). Indeed, URT lactobacilli will have to withstand other stresses than in the GIT: they will not necessarily have to resist gastric digestive enzymes and bile acid stress, unless immunomodulatory effects are also aimed for via the gastrointestinal immune system.
Probiotic Mechanisms Rationalized for URT Probiotics
Direct antimicrobial actions against OM pathogens.
In addition to competition for adhesion sites, probiotics can directly inhibit pathogens by producing antimicrobial molecules, such as lactic and acetic acid, bacteriocins, and hydrogen peroxide, in their microenvironment (Table 4) (185). These molecules can inhibit both Gram-positive and Gram-negative bacteria, but of course, the most active mechanism will depend on the exact pathogen(s) that are targeted by probiotic application. Organic acids like lactic and acetic acid can mainly be inhibitory against Gram-negative bacteria, since their undissociated form can enter the bacterial cell and dissociate in the cytoplasm (200–202). In 2006, lactic acid was documented to be the active antimicrobial molecule of lactobacilli against Salmonella enterica serovar Typhimurium (201, 203, 204). However, lactic acid has also been shown to permeabilize the Gram-negative outer membrane of pathogens like Escherichia coli O157:H7, P. aeruginosa, and S. Typhimurium by utilizing a fluorescent-probe uptake assay and sensitization to bacteriolysis (200). Furthermore, in spent culture supernatant of lactobacilli, lactic acid was shown to play a crucial role in the antibacterial activity against M. catarrhalis (197). This makes it a promising molecule to inhibit Gram-negative URT pathogens like M. catarrhalis and H. influenzae. However, lactic acid is not the only active molecule which can be produced by lactobacilli (205). Species- and strain-specific bacteriocins are produced by many lactobacilli: several Lactobacillus acidophilus strains, for example, produce lactacin (206–208), and many Lactobacillus plantarum strains produce plantaricin (209, 210). By the formation of pores or inhibition of cell wall synthesis, bacteriocins exert their antimicrobial action against (often closely related) bacteria. In addition, seven heat-stable antibacterial peptides active against the enteroaggregative E. coli strain EAEC042, S. Typhimurium, and S. aureus were isolated from L. rhamnosus GG supernatant (211). The genome sequence of L. rhamnosus GG revealed bacteriocin-related genes, which suggests possible production of these antimicrobial peptides (187). However, as far as we know, no bacteriocin of lactobacilli has yet been demonstrated to have antimicrobial activity against OM pathogens, although S. pneumoniae is sensitive to nisin, a bacteriocin produced by Lactococcus lactis (212). Furthermore, lactobacilli like L. rhamnosus GG and L. rhamnosus GR-1 contain lectin-like proteins which are shown to inhibit and/or structurally disrupt pathogenic biofilms (190, 213), while a dairy drink containing L. casei Shirota has been reported to reduce biofilm formation on voice protheses (214). Pericone et al. (215) observed the bactericidal effect of H2O2, produced by S. pneumoniae, against its coinhabitants of the URT, such as H. influenzae and M. catarrhalis, suggesting this mechanism of action might also be mediated in the URT; however, little evidence is yet available. In other human body niches, such as in the vagina of healthy women, H2O2 production by lactobacilli was also proposed as an important antimicrobial mechanism (216). However, since the molecule is highly unstable, this mechanism is quite controversial (217).
Another way of looking at production of antimicrobial molecules is the production of molecules that interact with cell-cell communication of pathogens. Quorum sensing, a system of stimuli and responses correlated to population density, might modulate pathogen infection success by coupling gene expression of immune-alarming virulence factors only to high densities (218). The luxS gene is responsible for the production of autoinducer-2 (AI-2), an important interspecies quorum-sensing molecule, in both Gram-negative and Gram-positive bacteria (218). Most lactobacilli contain this gene and secrete AI-2 (e.g., L. rhamnosus GG [219]), as do the OM pathogens S. pneumoniae (220) and H. influenzae (221). AI-2 is an important factor in biofilm formation by S. pneumoniae and H. influenzae (220, 221). Furthermore, a mutation in the luxS gene causes reductions in virulence and persistence in a murine model of nasopharyngeal carriage of S. pneumoniae, while a luxS mutation increases the virulence of H. influenzae in a chinchilla model (222–224). In contrast to these pathogens, M. catarrhalis cannot produce AI-2 itself, as it does not contain the luxS gene. However, its biofilm formation is promoted by the production of AI-2 by H. influenzae (225). Disrupting AI-2 transport, antagonizing its signaling, inhibiting AI-2 production, or quenching AI-2 would thus be possible strategies to interfere with the interspecies communication in OM infections. However, since the AI-2 synthase LuxS also interferes with general cell metabolism (226), the role of quorum sensing in pathogen exclusion is difficult to investigate.
Enhancement of the nasopharyngeal epithelial barrier.
Another documented probiotic mechanism for the GIT is enhancement of the epithelial barrier function, as reviewed recently by Bron et al. (Table 4) (227). Although barrier defects are coupled to many URT diseases, such as OM (228), barrier enhancement by probiotics has not yet been explored in detail for the URT niche (229). And yet, it is possible to translate possible mechanisms for barrier enhancement, such as enhancement of tight junction functioning, from the GIT to the URT. In an in vivo study, L. plantarum WCFS1 was shown to induce changes in the intestinal epithelial tight junctions, which was demonstrated by an increased presence of zonula occludens-1 and occludin, two tight junction proteins (230). In addition, two soluble proteins produced by L. rhamnosus GG, Msp1/p75 and Msp2/p40, were demonstrated to protect the tight junctions in Caco-2 cell monolayers from hydrogen peroxide-induced disruption (231). Furthermore, these proteins also prevented TNF-induced apoptosis of epithelial cells in cultured cells and ex vivo colon organ culture models (232). In addition to their preventive function, both p75 and p40 have been shown to have potential to repair the intestinal barrier (232, 233), which is of interest for URT therapy (234) but should be further substantiated for nasal and respiratory epithelial cells. Also, symbiont-generated lactate has been shown to support intestinal epithelial cell regeneration (235).
Another epithelial barrier function-promoting mechanism is the induction of antimicrobial peptides like defensins, which protect mucous membranes against invading microorganisms (199, 236). The mechanism of the antimicrobial activity of defensins is multiple: the construction of pores in the membrane of pathogens is the most important one, but they can also inhibit bacterial toxins, such as pneumolysin of S. pneumoniae (237). On the other hand, defensins can influence the immune system to produce proinflammatory cytokines and chemokines. Of the human β-defensins (HBDs), HBD-2 is the most potent antimicrobial peptide (238–240). HBD-2-mediated killing of some strains of S. pneumoniae, H. influenzae, and M. catarrhalis has been reported at low concentrations (241) and can be induced by probiotics (242). In addition to HBD-2, human α-defensins 1 to 4, which are expressed by neutrophil granules, are important in the phagocytosis-mediated killing of bacteria. H. influenzae is especially sensitive to this kind of defensins (243).
Furthermore, human epithelial cells can produce other antimicrobial proteins, such as lysozymes, cathelicidins, C-type lectins, and ribonucleases, which often attack cell wall structures and/or the bacterial membrane. Lysozyme degrades the peptidoglycan of the bacterial cell wall and can kill S. pneumoniae synergistically with HBD-2 (244). Cathelicidins, such as the above-mentioned LL-37, are cationic antimicrobial peptides that also trigger the host’s immune system. In a chinchilla model, a cathelicidin was observed to be able to kill the NTHi strain 86028-NP and M. catarrhalis 1857; however, S. pneumoniae serotype 14 seemed to be less sensitive (245). L. rhamnosus GG can upregulate cathelicidin-related antimicrobial peptides (CRAMPs) in mice (246), but little is known about similar effects in humans. Other examples are the induction of mucus and the induction of cytoprotective molecules (reviewed in Madsen [236]).
Enhancement of the (systemic) immune system.
Besides the stimulation of the production of antimicrobial molecules of the host, the application of probiotics can also modulate host immune responses, both innate and adaptive immunity (Table 4) (247). Probiotic bacteria can, for instance, modulate the maturation of dendritic cells (DCs) toward an anti-inflammatory IL-10 profile. The protein SlpA of L. acidophilus NCFM, a surface-layer-associated protein (SLAP), has been shown to fulfill this immunostimulating role through interaction with DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN) (Fig. 4) (248). In addition, the stimulatory role of probiotics on regulatory T-cell activity is well explored and seems an important probiotic mechanism in controlling overt inflammatory conditions, although there exist large strain differences for this capacity (249, 250). In asthmatic mice, oral administration of L. rhamnosus GG (109 CFU every second day for 8 consecutive weeks) has been shown to suppress allergen-induced proliferative responses associated with an induction of T-regulatory cells in both mesenteric and peribronchial lymph nodes (250). In addition, experiments from our laboratory with nasal administration of L. rhamnosus GG to mice resulted in the prevention of allergic asthma. Mice which received the probiotic for 8 days showed a decrease in lung IL-13 and IL-5 levels, together with a decrease in bronchoalveolar lavage eosinophil counts and airway reactivity (251). These results point to the potential of nasally administered probiotics to prevent inflammatory responses in the host.
Furthermore, probiotics can stimulate increased mucosal immunoglobulin A (IgA) levels and allergen-specific B- and T-cell responses, which can especially influence allergic diseases, e.g., in the URT (reviewed in Toh et al. and Martens et al. [229, 252]). Guglielmetti et al. (195) explored the immunomodulatory effect of the probiotic strain L. helveticus MIMLh5 in FaDu hypopharyngeal carcinoma cells. L. helveticus MIMLh5 could reduce the induction of IL-6, IL-8, and TNF-α, while it enhanced the expression of the heat shock protein coding gene hsp70 (195). Of note, in mice, intranasal administration of L. rhamnosus GG for 3 days increased the cytotoxic activity of pulmonary natural killer (NK) cells after infection with influenza virus H1N1. This probiotic strain was also shown to increase the secretion of IL-1β and TNF-α, which resulted in better survival of the mice after 15 days (253).
The exact molecules by which probiotics can exert these immunomodulatory effects are only fragmentarily known. Moreover, the research into these molecules has primarily focused on the GIT environment. Immune priming molecules include microbe-associated molecular patterns (MAMPs), such as LTA and EPS, that can interact with pattern recognition receptors, such as TLRs (Fig. 4) (247). For example, in intestinal cell and monocytic models, pili of the model probiotic L. rhamnosus GG, for which several URT benefits have also been mentioned above (173, 187, 196, 197, 219, 231, 232, 250), were observed to have an anti-inflammatory effect on the cells. Increased exposure to the pili, obtained via the use of an EPS mutant of L. rhamnosus GG, decreased the IL-8 mRNA induction by 2 times compared to the level in the wild type (254, 255). Similar observations were recently made with a focus on allergy: nasal administration of L. rhamnosus GG in mice decreased the allergy-related inflammation in a more pronounced way than nasal administration of L. rhamnosus GR-1 (251). A key difference between these two strains is the absence of SpaCBA pili in L. rhamnosus GR-1 (256). These results suggest that proper adhesion of the probiotic bacteria to the epithelial cells plays a pivotal role in their immunomodulatory effect.
Other molecules, such as teichoic acids (TAs) and EPS, also show immunomodulatory characteristics in several cell models: LTAs of several Lactobacillus strains can bind TLR2 and activate proinflammatory cytokine release (257–260), while EPS molecules of both L. casei Shirota and L. plantarum seemed to be more immunosuppressive in the gut (261, 262). TLR9, present on endosomes, can, on the other hand, be triggered by unmethylated cytosine-guanine (CpG)-containing DNA. Such CpG-rich DNA is carried by many Lactobacillus species (263) and can thus stimulate Th1 responses, leading to cell-mediated immunity. In addition to MAMPs, the secretion of probiotic effector molecules can influence the host’s immune system as well. Both in in vitro (cell) models and in mice, the production of the cell envelope-associated protease PrtP, also called lactocepin, by Lactobacillus paracasei was shown to selectively degrade proinflammatory cytokines (264). A large genome comparison highlighted the presence of this kind of proteases in many Lactobacillus strains, suggesting that it is an overarching feature among several strains (265). Other Lactobacillus metabolites produced genus wide, such as lactic acid and acetic acid, also exhibit immunostimulatory effects. In macrophages and neutrophils, several short-chain fatty acids (SCFAs; i.e., sodium butyrate, sodium phenylbutyrate, sodium phenylacetate, acetate, propionate, and butyrate, which could be directly produced by lactobacilli or result from cross-feeding with other bacteria) were shown to inhibit IL-6 and TNF-α but stimulate IL-10 production (266–269). Both L. acidophilus and Lactobacillus johnsonii La1, for instance, have been shown to stimulate the production of SCFAs like acetate, butyrate, and/or (iso-)valerate in humans and rats, respectively, after oral administration (270, 271). In addition, lactate is also metabolized to butyrate by lactate-utilizing, butyrate-producing bacteria like Eubacterium hallii and Anaerostipes caccae under the anaerobic conditions of the gut, where it can have additional beneficial functions (272). However, whether these SCFAs are also produced and have a beneficial function in the more oxygen-rich environment of the URT is at present unknown.
Vaccines such as the PCV variants or the 10-valent pneumococcal H. influenzae protein D conjugate vaccine (PHiD-CV10) have already shown a decrease in the incidence of OM (273, 274). For these preventive strategies to reduce OM episodes, probiotic bacteria can also be included as vaccine adjuvants. Up to this point, we have mainly discussed the direct immunomodulating effects of living probiotic bacteria. However, probiotics and their MAMPs are also investigated for their potential to ameliorate humoral responses to vaccines when applied as an adjuvant stimulating PRRs. Several studies have demonstrated, for example, that administration of the model probiotic L. rhamnosus GG before and/or after vaccination can increase the specific antibody production in the human host, leading to enhanced protection rates (275–278). Induction of a higher concentration of such pathogen-specific antibodies may help to inhibit the pathogen’s adhesion to the host’s epithelial cells, as observed for S. pneumoniae (279). In particular, the PCV vaccines where capsule polysaccharides of S. pneumoniae are used, which are less immunogenic than proteins, could benefit from extra immunostimulation via an adjuvant. However, although the URT mucosal immune system is an interesting route for, e.g., vaccination, modulation of immune responses through microbial/probiotic modulations at the URT (for example via nasal application) is currently underexplored.
CONCLUSION
OM is a leading cause of health care visits in children (15), and thanks to major advances in DNA-based bacterial community analyses, our knowledge about the bacterial composition of this disease is steadily increasing. The Human Microbiome Project has already made major efforts for standardization of microbiome-focused studies so that biologically relevant variation in the microbiome composition can be systematically studied. However, due to the use of different sequencing approaches and biological sample material, it is still difficult to define a healthy core microbiome and compare different studies. More uniform sampling protocols and downstream analyses, including algorithms, are required to compare each step adequately, since small details in sample handling can cause large differences in the outcome and interpretation, as reviewed by Vandeputte et al. (38) for gut samples.
Nevertheless, NGS approaches have now substantiated a key role for the classical OM pathogens S. pneumoniae, H. influenzae, and M. catarrhalis in samples from diseased children. In addition, other potential pathogens, such as Turicella and Alloiococcus, are gaining attention (Table 5). However, there is currently little knowledge about their virulence factors and pathogenic impact on the human immune system and barrier function. More knowledge, individually and in biofilms, is thus necessary to target specific causal activities of pathogens in the different disease states.
TABLE 5.
Major conclusions and future research points
| Category | Discussion |
|---|---|
| Major conclusions | Otitis media (OM) is a leading cause of antibiotic prescription in childhood, yet antibiotic use should be limited as much as possible |
| The main bacterial pathogens contributing to OM are S. pneumoniae, H. influenzae, and M. catarrhalis, but microbiome studies of OM also suggest a pathogenic role for other taxa, such as Turicella and Alloiococcus | |
| Microbiome studies in healthy children indicate a protective role against OM for lactic acid bacteria, such as Dolosigranulum, Lactobacillus, and Lactococcus | |
| Microbiome studies give information about the relative abundances of potential pathogens or protective bacteria, but other criteria need to be considered as well, such as those described in (translated probiotic) Koch’s postulates | |
| Both clinical studies and molecular mechanisms suggest that probiotics have potential to reduce OM episodes and the related antibiotic use | |
| Future research points | Microbiome information at the strain level could give more information on strain-specific virulence or protection capacities of specific bacteria |
| Molecular mechanistic studies on the potential of probiotics against OM pathogens and their adaptation to the targeted niches are necessary to select for the most optimal probiotics | |
| Clinical studies in humans will further substantiate the therapeutic effectiveness of such selected probiotics and their influence on the local microbiome | |
In addition, several of the NGS studies reviewed above have correlated the presence of certain bacteria in the nasopharynx of infants with a healthier status in later childhood. The lactic acid genus Dolosigranulum, for example, is increasingly suggested to be a protective bacterial taxon (41, 59, 280). The underlying mechanisms of the potential protective roles of these bacteria are not yet understood. Furthermore, to substantiate the probiotic potential of specific taxa or strains, the importance of validation experiments in which cause-and-effect relationships between the bacteria administered and improved biomarkers for health are demonstrated (see translated “probiotic postulates”) cannot be underestimated. Moreover, targeting the microbiome with health-promoting bacteria, such as probiotics, will gain more interest in the future, as the awareness of the negative consequences of antibiotics is rising. Promising clinical studies with several LAB taxa and strains highlight the potential of probiotic bacteria to reduce the disease burden of OM (Table 5). Further clinical substantiation and strain selection of the most optimal health-promoting bacteria against OM will benefit from more molecular insights into both pathogenic and probiotic mechanisms of action, as exemplified above.
ACKNOWLEDGMENTS
We thank the Lebeer Laboratory of Applied Microbiology and Biotechnology (UAntwerp) and the ENT department of the Antwerp University Hospital for their contributions to the manuscript.
We also thank the funding agencies IWT, FWO, Vlaio, and UAntwerp for their financial contributions to our research. This research was funded by DOC PRO FFB130135 (UAntwerp), IWT-SBO (Vlaio) ProCure IWT 150052, IOF/SBO FF/130233 (UAntwerp), IOF-POC FFI 170288 (UAntwerp), FWO KaN 6522, an FWO travel grant for a short stay abroad (K228515N), and an FWO personal grant to Ilke De Boeck (1S17916N).
Biographies

Marianne F. L. van den Broek, Ph.D., completed her studies of Bioscience Engineering with a focus on cell and gene technology at the Catholic University of Leuven in 2012. In 2013, Dr. Ir. van den Broek started work on a Ph.D. at the University of Antwerp in the laboratory of Prof. Dr. Ir. Sarah Lebeer, researching the in vitro and in vivo probiotic potential of Lactobacillus spp. for otitis media, which she successfully finalized in February 2018. At the moment, she is working as a postdoctoral research scientist in this laboratory, with a focus on the potential of several probiotics for upper respiratory tract diseases and the impact on the local microbiome.

Ilke De Boeck completed her bachelor’s degree in bioscience engineering with a focus on cell and gene technology at the University of Antwerp in 2012 and graduated from her master’s degree program in bioscience engineering, cell and gene technology, at the Catholic University of Leuven in 2014. In 2014, Ir. De Boeck started her Ph.D. at the University of Antwerp in the laboratory of Prof. Dr. Ir. Sarah Lebeer with copromotor Prof. Dr. Olivier Vanderveken, researching the potential of probiotics for chronic rhinosinusitis. She is focusing on the characterization of the upper respiratory tract (URT) microbiome in adults using Illumina MiSeq sequencing and the cultivation of lactic acid bacteria from the URT to explore their potential as URT probiotics.

Filip Kiekens, Pharm.D., completed his studies in pharmaceutical sciences at the University of Ghent. In 2000, Prof. Dr. Kiekens finalized his Ph.D. in the Laboratory of Pharmaceutical Technology at the same university. After obtaining a Ph.D., he worked for 12 years in pharmaceutical product research and development (multinational and small and medium enterprises [SME]). He has headed an academic research group in pharmaceutical technology for the last 5 years, with specific interest in applying pharmaceutical technological processes to a wide field of applications.

An Boudewyns, M.D., completed her studies in Medicine at the University of Antwerp, Belgium. Prof. Dr. Boudewyns obtained the degree of doctor in medical sciences at the University of Antwerp, Belgium, in 1999 and has been a Guest Lecturer at the Faculty of Medicine, Translational Neurosciences, at the University of Antwerp, Belgium, since October 2002. She is a coauthor of 103 peer-reviewed publications. She has been a staff member at the Department of Otorhinolaryngology, Head and Neck Surgery, of the Antwerp University Hospital since 2001 and has a subspecialty in pediatric ear, nose, and throat (ENT) surgery. As a pediatric ENT surgeon, she has a major interest in upper airway problems and infections in children, and she has been working in the area of the upper airway microbiome since 2016.

Olivier M. Vanderveken, M.D., is a full-time ear, nose, and throat (ENT), head, and neck surgeon at the Antwerp University Hospital in Belgium, where he holds an appointment as Chair of the department. Prof. Dr. Vanderveken holds a position as Professor at the Faculty of Medicine of the University of Antwerp. Prof. Dr. Vanderveken received his medical degree from the University of Antwerp in 2001. He completed his residency at the Antwerp University Hospitals. In 2007, he obtained a Ph.D. in medical sciences. His research is in the area of sleep-disordered breathing and the microbiota of the upper respiratory tract. Prof. Dr. Vanderveken has coauthored several professional scientific publications in high-impact peer-reviewed journals. Prof. Dr. Vanderveken has been the recipient of several national and international scientific awards. He holds a Senior Clinical Investigator Fellowship at the Research Foundation Flanders (FWO) in Belgium that supports him in pursuing a full-fledged career in translational research.

Sarah Lebeer is an Associate Professor in Microbiology and Biotechnology at the Bioscience Engineering Department of the University of Antwerp (Belgium). Prof. Dr. Ir. Lebeer obtained her Ph.D. in Bioscience Engineering from the KU Leuven (Belgium) in 2008 after receiving a Ph.D. scholarship from the Research Foundation Flanders (FWO). She specialized in bacterial genetics and functional characterization of probiotics, their cell wall molecules, and associated immunological effects. From 2008 to 2011, she was a postdoctoral research scientist studying the mucosal immunology of probiotics, including a shift of her research interests from the gut to the urogenital tract. When she started her own laboratory as tenure track professor at the University of Antwerp in 2011, she further extended her research interests on probiotics and the human microbiome toward the upper respiratory tract. For example, she coordinates a large project on defining the future of probiotics for respiratory tract diseases (www.procureproject.be).
REFERENCES
- 1.Man WH, de Steenhuijsen Piters WAA, Bogaert D. 2017. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhooge I, Desloovere C, Boudewyns A, Van Kempen M, Dachy JP. 2005. Management of otitis media with effusion in children. B-ENT 1(Suppl 1):3–13. [PubMed] [Google Scholar]
- 3.Schilder AGM, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. 2016. Otitis media. Nat Rev Dis Primers 2:16063. doi: 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosenfeld RM, Sevilla XD, Schwartz RH, Thomas PA, Tunkel DE. 2013. The diagnosis and management of acute otitis media. Pediatrics 131:e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 5.Flynn T, Möller C, Jönsson R, Lohmander A. 2009. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int J Pediatr Otorhinolaryngol 73:1441–1446. doi: 10.1016/j.ijporl.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Maris M, Wojciechowski M, Van de Heyning P, Boudewyns A. 2014. A cross-sectional analysis of otitis media with effusion in children with Down syndrome. Eur J Pediatr 173:1319–1325. doi: 10.1007/s00431-014-2323-5. [DOI] [PubMed] [Google Scholar]
- 7.Buzatto GP, Tamashiro E, Proenca-Modena JL, Saturno TH, Prates MC, Gagliardi TB, Carenzi LR, Massuda ET, Hyppolito MA, Valera FCP, Arruda E, Anselmo-Lima WT. 2017. The pathogens profile in children with otitis media with effusion and adenoid hypertrophy. PLoS One 12:e0171049. doi: 10.1371/journal.pone.0171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld RM, Bluestone CD. 2003. Clinical efficacy of surgical therapy, p 227–239. In Rosenfeld RM, Bluestone CD (ed), Evidence-based otitis media, 2nd ed B. C. Decker, Hamilton, Ontario, Canada. [Google Scholar]
- 9.Bluestone CD. 2003. Definitions, terminology, and classification, p 120–135. In Rosenfeld RM, Bluestone CD (ed), Evidence-based otitis media, 2nd ed B. C. Decker, Hamilton, Ontario, Canada. [Google Scholar]
- 10.Boudewyns A, Antunes J, Bernheim N, Claes J, De Dooy J, De Leenheer E, De Roeck K, Hellings P, de Varebeke SJ, Jorissen M, Ketelslagers K, Lemkens N, Lemkens P, Leupe P, Malfroot A, Maris M, Michiels E, Van Crombrugge L, Vandenplas Y, Verhulst S, Eloy P, Watelet JB. 2012. Specific medical and surgical treatment for chronic inflammatory diseases in children. B-ENT 8(Suppl 19):135–166. [PubMed] [Google Scholar]
- 11.Verhoeff M, Van Der Veen EL, Rovers MM, Sanders EAM, Schilder A. 2006. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol 70:1–12. doi: 10.1016/j.ijporl.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. 2004. Otitis media. Lancet 363:465–473. doi: 10.1016/S0140-6736(04)15495-0. [DOI] [PubMed] [Google Scholar]
- 13.Chonmaitree T, Trujillo R, Jennings K, Alvarez-Fernandez P, Patel JA, Loeffelholz MJ, Nokso-Koivisto J, Matalon R, Pyles RB, Miller AL, McCormick DP. 2016. Acute otitis media and other complications of viral respiratory infection. Pediatrics 137:e20153555. doi: 10.1542/peds.2015-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielhuis GA, Heuvelmans-Heinen EW, Rach GH, Van Den Broek P. 1989. Environmental risk factors for otitis media with effusion in preschool children. Scand J Prim Health Care 7:33–38. doi: 10.3109/02813438909103668. [DOI] [PubMed] [Google Scholar]
- 15.Pettigrew MM, Laufer AS, Gent JF, Kong Y, Fennie KP, Metlay JP. 2012. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol 78:6262–6270. doi: 10.1128/AEM.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akkerman AE, Kuyvenhoven MM, van der Wouden JC, Verheij T. 2005. Analysis of under- and overprescribing of antibiotics in acute otitis media in general practice. J Antimicrob Chemother 56:569–574. doi: 10.1093/jac/dki257. [DOI] [PubMed] [Google Scholar]
- 17.Froom J, Culpepper L, Green LA, de Melker RA, Grob P, Heeren T, van Balen F. 2001. A cross-national study of acute otitis media: risk factors, severity, and treatment at initial visit. Report from the International Primary Care Network (IPCN) and the Ambulatory Sentinel Practice Network (ASPN). J Am Board Fam Pract 14:406–417. [PubMed] [Google Scholar]
- 18.Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. 2015. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015:CD000219. doi: 10.1002/14651858.CD000219.pub4. [DOI] [PubMed] [Google Scholar]
- 19.Venekamp RP, Burton MJ, van Dongen TM, van der Heijden GJ, van Zon A, Schilder AG. 2016. Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev 2016:CD009163. doi: 10.1002/14651858.CD009163.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, Fan J, Henrickson KJ. 2008. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis 46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman CA, Doyle WJ, Skoner DP, Post JC, Alper CM, Seroky JT, Anderson K, Preston RA, Hayden FG, Fireman P, Ehrlich GD. 1995. Influenza A virus-induced acute otitis media. J Infect Dis 172:1348–1351. doi: 10.1093/infdis/172.5.1348. [DOI] [PubMed] [Google Scholar]
- 22.Bakaletz LO. 2010. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol 87:213–222. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revai K, Mamidi D, Chonmaitree T. 2008. Association of nasopharyngeal bacterial colonization during upper respiratory tract infection and the development of acute otitis media. Clin Infect Dis 46:e34–e37. doi: 10.1086/525856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur R, Morris M, Pichichero ME. 2017. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 140:20170181. doi: 10.1542/peds.2017-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel JA, Nguyen DT, Revai K, Chonmaitree T. 2007. Role of respiratory syncytial virus in acute otitis media: implications for vaccine development. Vaccine 25:1683–1689. doi: 10.1016/j.vaccine.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nokso-Koivisto J, Räty R, Blomqvist S, Kleemola M, Syrjänen R, Pitkäranta A, Kilpi T, Hovi T. 2004. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol 72:241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel JA, Nair S, Revai K, Grady J, Chonmaitree T. 2009. Nasopharyngeal acute phase cytokines in viral upper respiratory infection: impact on acute otitis media in children. Pediatr Infect Dis J 28:1002–1007. doi: 10.1097/INF.0b013e3181aa5b13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. 2010. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol 42:450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. 2006. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willner D, Haynes MR, Furlan M, Hanson N, Kirby B, Lim YW, Rainey PB, Schmieder R, Youle M, Conrad D, Rohwer F. 2012. Case studies of the spatial heterogeneity of DNA viruses in the cystic fibrosis lung. Am J Respir Cell Mol Biol 46:127–131. doi: 10.1165/rcmb.2011-0253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willner D, Furlan M, Haynes M, Schmieder R, Angly FE, Silva J, Tammadoni S, Nosrat B, Conrad D, Rohwer F. 2009. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One 4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Yang F, Ren L, Xiong Z, Wu Z, Dong J, Sun L, Zhang T, Hu Y, Du J, Wang J, Jin Q. 2011. Unbiased parallel detection of viral pathogens in clinical samples by use of a metagenomic approach. J Clin Microbiol 49:3463–3469. doi: 10.1128/JCM.00273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lysholm F, Wetterbom A, Lindau C, Darban H, Bjerkner A, Fahlander K, Lindberg AM, Persson B, Allander T, Andersson B. 2012. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS One 7:e30875. doi: 10.1371/journal.pone.0030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhu N, Li Y, Lu R, Wang H, Liu G, Zou X, Xie Z, Tan W. 2016. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin Microbiol Infect 22:458.e1–458.e9. doi: 10.1016/j.cmi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoll J, Rahamat-Langendoen J, Ahout I, de Jonge MI, Jans J, Huijnen MA, Ferwerda G, Warris A, Melchers WJ. 2015. Direct multiplexed whole genome sequencing of respiratory tract samples reveals full viral genomic information. J Clin Virol 66:6–11. doi: 10.1016/j.jcv.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokulich NA, Mills DA. 2013. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol 79:2519–2526. doi: 10.1128/AEM.03870-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngo CC, Massa HM, Thornton RB, Cripps AW. 2016. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One 11:e0150949. doi: 10.1371/journal.pone.0150949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandeputte D, Tito RY, Vanleeuwen R, Falony G, Raes J. 2017. Practical considerations for large-scale gut microbiome studies. FEMS Microbiol Rev 41(Supp_1):S154–S167. doi: 10.1093/femsre/fux027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Steenhuijsen Piters WAA, Sanders EAM, Bogaert D. 2015. The role of the local microbial ecosystem in respiratory health and disease. Philos Trans R Soc 370:20140294. doi: 10.1098/rstb.2014.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch A, Levin E, Van Houten MA, Hasrat R, Kalkman G, Biesbroek G, De Steenhuijsen Piters WAA, De Groot P-K, Pernet P, Keijser BJF, Sanders EAM, Bogaert D. 2016. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 9:336–345. doi: 10.1016/j.ebiom.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, Bogaert D. 2014. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 42.Biesbroek G, Bosch A, Wang X, Keijser BJF, Veenhoven RH, Sanders EAM, Bogaert D. 2014. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med 190:298–308. doi: 10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 43.Santee CA, Nagalingam NA, Faruqi AA, DeMuri GP, Gern JE, Wald ER, Lynch SV. 2016. Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome 4:34. doi: 10.1186/s40168-016-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earl JP, de Vries SPW, Ahmed A, Powell E, Schultz MP, Hermans PWM, Hill DJ, Zhou Z, Constantinidou CI, Hu FZ, Bootsma HJ, Ehrlich GD. 2016. Comparative genomic analyses of the Moraxella catarrhalis serosensitive and seroresistant lineages demonstrate their independent evolution. Genome Biol Evol 8:955–974. doi: 10.1093/gbe/evw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earl JP, Adappa ND, Krol J, Bhat AS, Balashov S, Ehrlich RL, Palmer JN, Workman AD, Blasetti M, Hammond J, Cohen NA, Ehrlich GD, Mell JC. 2018. Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes. bioRxiv doi: 10.1101/338731. [DOI] [PMC free article] [PubMed]
- 47.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ton-That H, Schneewind O. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol Microbiol 50:1429–1438. doi: 10.1046/j.1365-2958.2003.03782.x. [DOI] [PubMed] [Google Scholar]
- 49.Tarr PE, Stock F, Cooke RH, Fedorko DP, Lucey DR. 2003. Multidrug-resistant Corynebacterium striatum pneumonia in a heart transplant recipient. Transpl Infect Dis 5:53–58. doi: 10.1034/j.1399-3062.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 50.Ridaura VK, Bouladoux N, Claesen J, Chen YE, Byrd AL, Constantinides MG, Merrill ED, Tamoutounour S, Fischbach MA, Belkaid Y. 2018. Contextual control of skin immunity and inflammation by Corynebacterium. J Exp Med 215:785–799. doi: 10.1084/jem.20171079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Boeck I, Wittouck S, Wuyts S, Oerlemans EFM, van den Broek MFL, Vandenheuvel D, Vanderveken O, Lebeer S. 2017. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front Microbiol 8:2372. doi: 10.3389/fmicb.2017.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, Bochkov YA, Grindle K, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. 2015. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu CM, Price LB, Hungate BA, Abraham AG, Larsen LA, Christensen K, Stegger M, Skov R, Andersen PS. 2015. Staphylococcus aureus and the ecology of the nasal microbiome. Sci Adv 1:e1400216. doi: 10.1126/sciadv.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos-Cortez RLP, Chiong CM, Frank DN, Ryan AF, Giese APJ, Bootpetch Roberts T, Daly KA, Steritz MJ, Szeremeta W, Pedro M, Pine H, Yarza TKL, Scholes MA, Llanes EdV, Yousaf S, Friedman N, Tantoco MLC, Wine TM, Labra PJ, Benoit J, Ruiz AG, de la Cruz RAR, Greenlee C, Yousaf A, Cardwell J, Nonato RMA, Ray D, Ong KMC, So E, Robertson CE, Dinwiddie J, Lagrana-Villagracia SM, Gubbels SP, Shaikh RS, Cass SP, Einarsdottir E, Lee NR, Schwartz DA, Gloria-Cruz TLI, Bamshad MJ, Yang IV, Kere J, Abes GT, Prager JD, Riazuddin S, Chan AL, Yoon PJ, Nickerson DA, Cutiongco-de la Paz EM, Streubel SO, Reyes-Quintos MRT, Jenkins HA, et al. . 2018. FUT2 variants confer susceptibility to familial otitis media. Am J Hum Genet 103:679–690. doi: 10.1016/j.ajhg.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mika M, Mack I, Korten I, Qi W, Aebi S, Frey U, Latzin P, Hilty M. 2015. Dynamics of the nasal microbiota in infancy: a prospective cohort study. J Allergy Clin Immunol 135:905–912.e11. doi: 10.1016/j.jaci.2014.12.1909. [DOI] [PubMed] [Google Scholar]
- 56.Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, Bruin J, Montijn R, Bonten M, Sanders E. 2011. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One 6:e17035. doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenberg D, Givon-Lavi N, Broides A, Blancovich I, Peled N, Dagan R. 2006. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin Infect Dis 42:897–903. doi: 10.1086/500935. [DOI] [PubMed] [Google Scholar]
- 58.Man WH, van Dongen TMA, Venekamp RP, Pluimakers VG, Chu M, van Houten MA, Sanders EAM, Schilder AGM, Bogaert D. 2019. Respiratory microbiota predicts clinical disease course of acute otorrhea in children with tympanostomy tubes. Pediatr Infect Dis J 38:e116–e125. doi: 10.1097/INF.0000000000002215. [DOI] [PubMed] [Google Scholar]
- 59.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. 2011. Microbial communities of the upper respiratory tract and otitis media in children. mBio 2:e00245-10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hilty M, Qi W, Brugger SD, Frei L, Agyeman P, Frey PM, Aebi S, Mühlemann K. 2012. Nasopharyngeal microbiota in infants with acute otitis media. J Infect Dis 205:1048–1055. doi: 10.1093/infdis/jis024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sillanpää S, Kramna L, Oikarinen S, Sipilä M, Rautiainen M, Aittoniemi J, Laranne J, Hyöty H, Cinek O. 2017. Next-generation sequencing combined with specific PCR assays to determine the bacterial 16S rRNA gene profiles of middle ear fluid collected from children with acute otitis media. mSphere 2:e00006-17. doi: 10.1128/mSphere.00006-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez-Garces JL, Alhambra A, Alos JI, Barrera B, García G. 2004. Acute and chronic otitis media and Turicella otitidis: a controversial association. Clin Microbiol Infect 10:854–857. doi: 10.1111/j.1198-743X.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- 63.Ashhurst-Smith C, Hall ST, Walker P, Stuart J, Hansbro PM, Blackwell CC. 2007. Isolation of Alloiococcus otitidis from Indigenous and non-Indigenous Australian children with chronic otitis media with effusion. FEMS Immunol Med Microbiol 51:163–170. doi: 10.1111/j.1574-695X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 64.Lappan R, Imbrogno K, Sikazwe C, Anderson D, Mok D, Coates H, Vijayasekaran S, Bumbak P, Blyth CC, Jamieson SE, Peacock CS. 2018. A microbiome case-control study of recurrent acute otitis media identified potentially protective bacterial genera. BMC Microbiol 18:13. doi: 10.1186/s12866-018-1154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu CM, Cosetti MK, Aziz M, Buchhagen JL, Contente-Cuomo TL, Price LB, Keim PS, Lalwani AK. 2011. The otologic microbiome: a study of the bacterial microbiota in a pediatric patient with chronic serous otitis media using 16S rRNA gene-based pyrosequencing. Arch Otolaryngol Head Neck Surg 137:664–668. doi: 10.1001/archoto.2011.116. [DOI] [PubMed] [Google Scholar]
- 66.Jervis-Bardy J, Rogers GB, Morris PS, Smith-Vaughan HC, Nosworthy E, Leong LEX, Smith RJ, Weyrich LS, De Haan J, Carney AS, Leach AJ, O’Leary S, Marsh RL. 2015. The microbiome of otitis media with effusion in Indigenous Australian children. Int J Pediatr Otorhinolaryngol 79:1548–1555. doi: 10.1016/j.ijporl.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Chan CL, Wabnitz D, Bardy JJ, Bassiouni A, Wormald P-J, Vreugde S, Psaltis AJ. 2016. The microbiome of otitis media with effusion. Laryngoscope 126:2844–2851. doi: 10.1002/lary.26128. [DOI] [PubMed] [Google Scholar]
- 68.Chan CL, Wabnitz D, Bassiouni A, Wormald P-J, Vreugde S, Psaltis AJ. 2017. Identification of the bacterial reservoirs for the middle ear using phylogenic analysis. JAMA Otolaryngol Head Neck Surg 143:155. doi: 10.1001/jamaoto.2016.3105. [DOI] [PubMed] [Google Scholar]
- 69.Principi N, Marchisio P, Rosazza C, Sciarrabba CS, Esposito S. 2017. Acute otitis media with spontaneous tympanic membrane perforation. Eur J Clin Microbiol Infect Dis 36:11–18. doi: 10.1007/s10096-016-2783-9. [DOI] [PubMed] [Google Scholar]
- 70.Johnston J, Hoggard M, Biswas K, Astudillo‐García C, Radcliff FJ, Mahadevan M, Douglas RG. 2019. Pathogen reservoir hypothesis investigated by analyses of the adenotonsillar and middle ear microbiota. Int J Pediatr Otorhinolaryngol 118:103–109. doi: 10.1016/j.ijporl.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 71.Krueger A, Val S, Pérez-Losada M, Panchapakesan K, Devaney J, Duah V, DeMasson C, Poley M, Rose M, Preciado D. 2017. The relationship of the middle ear effusion microbiome to secretory mucin production in pediatric patients with chronic otitis media. Pediatr Infect Dis J 36:635–640. doi: 10.1097/INF.0000000000001493. [DOI] [PubMed] [Google Scholar]
- 72.Neeff M, Biswas K, Hoggard M, Taylor MW, Douglas R. 2016. Molecular microbiological profile of chronic suppurative otitis media. J Clin Microbiol 54:2538–2546. doi: 10.1128/JCM.01068-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falkow S. 1988. Molecular Koch’s postulates applied to microbial pathogenicity. Clin Infect Dis 10(Suppl 2):S274–S276. doi: 10.1093/cid/10.Supplement_2.S274. [DOI] [PubMed] [Google Scholar]
- 74.Fredricks DN, Relman DA. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev 9:18–33. doi: 10.1128/CMR.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 76.O’Toole PW, Marchesi JR, Hill C. 2017. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol 2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 77.van der Poll T, Opal SM. 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 78.Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 79.de Vries SPW, Bootsma HJ, Hays JP, Hermans P. 2009. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol Mol Biol Rev 73:389–406. doi: 10.1128/MMBR.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duell BL, Su YC, Riesbeck K. 2016. Host-pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen. FEBS Lett 590:3840–3853. doi: 10.1002/1873-3468.12351. [DOI] [PubMed] [Google Scholar]
- 81.Rose MC, Voynow JA. 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 82.Komatsu K, Jono H, Lim JH, Imasato A, Xu H, Kai H, Yan C, Li JD. 2008. Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phosphatase-1-dependent inhibition of p38 MAPK. Biochem Biophys Res Commun 377:763–768. doi: 10.1016/j.bbrc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 83.Jedrzejas MJ. 2001. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev 65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziane H, Manageiro V, Ferreira E, Moura IB, Bektache S, Tazir M, Caniça M. 2016. Serotypes and antibiotic susceptibility of Streptococcus pneumoniae isolates from invasive pneumococcal disease and asymptomatic carriage in a pre-vaccination period, in Algeria. Front Microbiol 7:803. doi: 10.3389/fmicb.2016.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahrén IL, Janson H, Forsgren A, Riesbeck K. 2001. Protein D expression promotes the adherence and internalization of non-typeable Haemophilus influenzae into human monocytic cells. Microb Pathog 31:151–158. doi: 10.1006/mpat.2001.0456. [DOI] [PubMed] [Google Scholar]
- 86.Val DS. 2015. Basic science concepts in otitis media pathophysiology and immunity: Role of mucins and inflammation, p 53–77. In Preciado D. (ed), Otitis media: state of the art concepts and treatment. Springer, Cham, Switzerland. [Google Scholar]
- 87.Murphy TF, Bakaletz LO, Smeesters PR. 2009. Microbial interactions in the respiratory tract. Pediatr Infect Dis J 28(10 Suppl):S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- 88.Van Eldere J, Slack MPE, Ladhani S, Cripps AW. 2014. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis 14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 89.Lanie JA, Ng W-L, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME. 2007. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 189:38–51. doi: 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen H, Yoshida H, Yan F, Li W, Xu F, Huang H, Jono H, Li JD. 2008. Synergistic induction of MUC5AC mucin by nontypeable Haemophilus influenzae and Streptococcus pneumoniae. Biochem Biophys Res Commun 365:795–800. doi: 10.1016/j.bbrc.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 91.Kerschner JE, Hong W, Khampang P, Johnston N. 2014. Differential response of gel-forming mucins to pathogenic middle ear bacteria. Int J Pediatr Otorhinolaryngol 78:1368–1373. doi: 10.1016/j.ijporl.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. 2014. Muc5b is required for airway defence. Nature 505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson MK, Geoffroy C, Alouf JE. 1980. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun 27:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirst RA, Kadioglu A, O’Callaghan C, Andrew PW. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol 138:195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marriott HM, Mitchell TJ, Dockrell DH. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med 8:497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 96.Feldman C, Anderson R, Cockeran R, Mitchell T, Cole P, Wilson R. 2002. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir Med 96:580–585. doi: 10.1053/rmed.2002.1316. [DOI] [PubMed] [Google Scholar]
- 97.Post DMB, Ketterer MR, Coffin JE, Reinders LM, Munson RS, Bair T, Murphy TF, Foster ED, Gibson BW, Apicella MA. 2016. Comparative analyses of the lipooligosaccharides from nontypeable Haemophilus influenzae and Haemophilus haemolyticus show differences in sialic acid and phosphorylcholine modifications. Infect Immun 84:765–774. doi: 10.1128/IAI.01185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. 2007. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun 75:325–333. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnston JW, Coussens NP, Allen S, Houtman JCD, Turner KH, Zaleski A, Ramaswamy S, Gibson BW, Apicella MA. 2008. Characterization of the N-acetyl-5-neuraminic acid-binding site of the extracytoplasmic solute receptor (SiaP) of nontypeable Haemophilus influenzae strain 2019. J Biol Chem 283:855–865. doi: 10.1074/jbc.M706603200. [DOI] [PubMed] [Google Scholar]
- 100.Jurcisek J, Greiner L, Watanabe H, Zaleski A, Apicella MA, Bakaletz LO. 2005. Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear. Infect Immun 73:3210–3218. doi: 10.1128/IAI.73.6.3210-3218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect Immun 72:106–113. doi: 10.1128/iai.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, Hultenby K, Taddei AR, Beiter K, Wartha F, von Euler A, Covacci A, Holden DW, Normark S, Rappuoli R, Henriques-Normark B. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci U S A 103:2857–2862. doi: 10.1073/pnas.0511017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danne C, Dramsi S. 2012. Pili of Gram-positive bacteria: roles in host colonization. Res Microbiol 163:645–658. doi: 10.1016/j.resmic.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 104.Bagnoli F, Moschioni M, Donati C, Dimitrovska V, Ferlenghi I, Facciotti C, Muzzi A, Giusti F, Emolo C, Sinisi A, Hilleringmann M, Pansegrau W, Censini S, Rappuoli R, Covacci A, Masignani V, Barocchi MA. 2008. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J Bacteriol 190:5480–5492. doi: 10.1128/JB.00384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Basset A, Herd M, Daly R, Dove SL, Malley R. 2017. The pneumococcal type 1 pilus genes are thermoregulated and are repressed by a member of the Snf2 protein family. J Bacteriol 199:e00078-17. doi: 10.1128/JB.00078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laurenceau R, Péhau-Arnaudet G, Baconnais S, Gault J, Malosse C, Dujeancourt A, Campo N, Chamot-Rooke J, Le Cam E, Claverys J-P, Fronzes R. 2013. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog 9:e1003473. doi: 10.1371/journal.ppat.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muschiol S, Erlendsson S, Aschtgen M-S, Oliveira V, Schmieder P, de Lichtenberg C, Teilum K, Boesen T, Akbey U, Henriques-Normark B. 2017. Structure of the competence pilus major pilin ComGC in Streptococcus pneumoniae. J Biol Chem 292:14134–14146. doi: 10.1074/jbc.M117.787671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luke-Marshall NR, Sauberan SL, Campagnari AA. 2011. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene 477:19–23. doi: 10.1016/j.gene.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 109.Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS, Bakaletz LO. 2012. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol 194:1927–1933. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novotny LA, Bakaletz LO. 2016. Intercellular adhesion molecule 1 serves as a primary cognate receptor for the type IV pilus of nontypeable Haemophilus influenzae. Cell Microbiol 18:1043–1055. doi: 10.1111/cmi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luke NR, Howlett AJ, Shao J, Campagnari AA. 2004. Expression of type IV pili by Moraxella catarrhalis is essential for natural competence and is affected by iron limitation. Infect Immun 72:6262–6270. doi: 10.1128/IAI.72.11.6262-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staunton DE, Merluzzi VJ, Rothlein R, Barton R, Marlin SD, Springer TA. 1989. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 113.Fink DL, Buscher AZ, Green B, Fernsten P, St Geme JW III.. 2003. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell Microbiol 5:175–186. doi: 10.1046/j.1462-5822.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 114.Ahrén IL, Williams DL, Rice PJ, Forsgren A, Riesbeck K. 2001. The importance of a β-glucan receptor in the nonopsonic entry of nontypeable Haemophilus influenzae into human monocytic and epithelial cells. J Infect Dis 184:150–158. doi: 10.1086/322016. [DOI] [PubMed] [Google Scholar]
- 115.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 116.Young NM, Foote SJ, Wakarchuk WW. 2013. Review of phosphocholine substituents on bacterial pathogen glycans: synthesis, structures and interactions with host proteins. Mol Immunol 56:563–573. doi: 10.1016/j.molimm.2013.05.237. [DOI] [PubMed] [Google Scholar]
- 117.Johnson RW, McGillivary G, Denoël P, Poolman J, Bakaletz LO. 2011. Abrogation of nontypeable Haemophilus influenzae protein D function reduces phosphorylcholine decoration, adherence to airway epithelial cells, and fitness in a chinchilla model of otitis media. Vaccine 29:1211–1221. doi: 10.1016/j.vaccine.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 118.Clark SE, Weiser JN. 2013. Microbial modulation of host immunity with the small molecule phosphorylcholine. Infect Immun 81:392–401. doi: 10.1128/IAI.01168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pang B, Winn D, Johnson R, Hong W, West-Barnette S, Kock N, Swords WE. 2008. Lipooligosaccharides containing phosphorylcholine delay pulmonary clearance of nontypeable Haemophilus influenzae. Infect Immun 76:2037–2043. doi: 10.1128/IAI.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nelson AL, Ries J, Bagnoli F, Dahlberg S, Fälker S, Rounioja S, Tschöp J, Morfeldt E, Ferlenghi I, Hilleringmann M, Holden DW, Rappuoli R, Normark S, Barocchi MA, Henriques-Normark B. 2007. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol Microbiol 66:329–340. doi: 10.1111/j.1365-2958.2007.05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosch JW, Mann B, Thornton J, Sublett J, Tuomanen E. 2008. Convergence of regulatory networks on the pilus locus of Streptococcus pneumoniae. Infect Immun 76:3187–3196. doi: 10.1128/IAI.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hallström T, Singh B, Resman F, Blom AM, Mörgelin M, Riesbeck K. 2011. Haemophilus influenzae protein E binds to the extracellular matrix by concurrently interacting with laminin and vitronectin. J Infect Dis 204:1065–1074. doi: 10.1093/infdis/jir459. [DOI] [PubMed] [Google Scholar]
- 123.Singh B, Al-Jubair T, Mörgelin M, Thunnissen MM, Riesbeck K. 2013. The unique structure of haemophilus influenzae protein E reveals multiple binding sites for host factors. Infect Immun 81:801–814. doi: 10.1128/IAI.01111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su YC, Mukherjee O, Singh B, Hallgren O, Westergren-Thorsson G, Hood D, Riesbeck K. 2016. Haemophilus influenzae p4 interacts with extracellular matrix proteins promoting adhesion and serum resistance. J Infect Dis 213:314–323. doi: 10.1093/infdis/jiv374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Voges M, Bachmann V, Kammerer R, Gophna U, Hauck CR. 2010. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol 10:117. doi: 10.1186/1471-2180-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hill DJ, Toleman MA, Evans DJ, Villullas S, Van Alphen L, Virji M. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol Microbiol 39:850–862. doi: 10.1046/j.1365-2958.2001.02233.x. [DOI] [PubMed] [Google Scholar]
- 127.Skotnicka B, Hassmann E. 2008. Proinflammatory and immunoregulatory cytokines in the middle ear effusions. Int J Pediatr Otorhinolaryngol 72:13–17. doi: 10.1016/j.ijporl.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 128.Kerschner JE, Meyer TK, Burrows A. 2004. Chinchilla middle ear epithelial mucin gene expression in response to inflammatory cytokines. Arch Otolaryngol Head Neck Surg 130:1163–1167. doi: 10.1001/archotol.130.10.1163. [DOI] [PubMed] [Google Scholar]
- 129.Leibovitz E, Dagan R, Laver JH, Piglansky L, Raiz S, Abboud MR, Fliss DM, Leiberman A, Barzilai A. 2000. Interleukin 8 in middle ear fluid during acute otitis media: correlation with aetiology and bacterial eradication. Arch Dis Child 82:165–168. doi: 10.1136/adc.82.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Si Y, Zhang ZG, Chen SJ, Zheng YQ, Chen YB, Liu Y, Jiang H, Feng LQ, Huang X. 2014. Attenuated TLRs in middle ear mucosa contributes to susceptibility of chronic suppurative otitis media. Hum Immunol 75:771–776. doi: 10.1016/j.humimm.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 131.Zielnik-Jurkiewicz B, Stankiewicz-Szymczak W. 2016. Pro-inflammatory interleukins in middle ear effusions from atopic and non-atopic children with chronic otitis media with effusion. Eur Arch Otorhinolaryngol 273:1369–1378. doi: 10.1007/s00405-015-3683-9. [DOI] [PubMed] [Google Scholar]
- 132.Lee HY, Chung JH, Lee SK, Byun JY, Kim YI, Yeo SG. 2013. Toll-like receptors, cytokines & nitric oxide synthase in patients with otitis media with effusion. Indian J Med Res 138:523–530. [PMC free article] [PubMed] [Google Scholar]
- 133.Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, N’guessan PD, Lucka L, Riesbeck K, Zimmermann W, Zweigner J, Temmesfeld-Wollbrueck B, Suttorp N, Singer BB. 2008. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol 9:1270–1278. doi: 10.1038/ni.1661. [DOI] [PubMed] [Google Scholar]
- 134.Weidenmaier C, Peschel A. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 135.Gisch N, Kohler T, Ulmer AJ, Müthing J, Pribyl T, Fischer K, Lindner B, Hammerschmidt S, Zähringer U. 2013. Structural reevaluation of Streptococcus pneumoniae lipoteichoic acid and new insights into its immunostimulatory potency. J Biol Chem 288:15654–15667. doi: 10.1074/jbc.M112.446963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Q, Bagrade L, Bernatoniene J, Clarke E, Paton JC, Mitchell TJ, Nunez DA, Finn A. 2007. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis 195:1194–1202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 137.Rabes A, Suttorp N, Opitz B. 2016. Inflammasomes in pneumococcal infection: innate immune sensing and bacterial evasion strategies. Curr Top Microbiol Immunol 397:215–227. doi: 10.1007/978-3-319-41171-2_11. [DOI] [PubMed] [Google Scholar]
- 138.Jalalvand F, Su YC, Mörgelin M, Brant M, Hallgren O, Westergren-Thorsson G, Singh B, Riesbeck K. 2013. Haemophilus influenzae protein F mediates binding to laminin and human pulmonary epithelial cells. J Infect Dis 207:803–813. doi: 10.1093/infdis/jis754. [DOI] [PubMed] [Google Scholar]
- 139.Forsgren A, Brant M, Möllenkvist A, Muyombwe A, Janson H, Woin N, Riesbeck K. 2001. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J Immunol 167:2112–2120. doi: 10.4049/jimmunol.167.4.2112. [DOI] [PubMed] [Google Scholar]
- 140.Clark SE, Snow J, Li J, Zola TA, Weiser JN. 2012. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLoS Pathog 8:e1002521. doi: 10.1371/journal.ppat.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lysenko ES, Gould J, Bals R, Wilson JM, Weiser JN. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun 68:1664–1671. doi: 10.1128/iai.68.3.1664-1671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jurcisek JA, Bakaletz LO. 2007. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Webster P, Wu S, Gomez G, Apicella M, Plaut AG, St Geme JW III.. 2006. Distribution of bacterial proteins in biofilms formed by Non-typeable Haemophilus influenzae. J Histochem Cytochem 54:829–842. doi: 10.1369/jhc.6A6922.2006. [DOI] [PubMed] [Google Scholar]
- 144.West-Barnette S, Rockel A, Swords WE. 2006. Biofilm growth increases phosphorylcholine content and decreases potency of nontypeable Haemophilus influenzae endotoxins. Infect Immun 74:1828–1836. doi: 10.1128/IAI.74.3.1828-1836.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones EA, McGillivary G, Bakaletz LO. 2013. Extracellular DNA within a nontypeable haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun 5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol 25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 147.Clark SE, Eichelberger KR, Weiser JN. 2013. Evasion of killing by human antibody and complement through multiple variations in the surface oligosaccharide of Haemophilus influenzae. Mol Microbiol 88:603–618. doi: 10.1111/mmi.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michel LV, Snyder J, Schmidt R, Milillo J, Grimaldi K, Kalmeta B, Khan MN, Sharma S, Wright LK, Pichichero ME. 2013. Dual orientation of the outer membrane lipoprotein P6 of nontypeable Haemophilus influenzae. J Bacteriol 195:3252–3259. doi: 10.1128/JB.00185-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iannelli F, Chiavolini D, Ricci S, Oggioni MR, Pozzi G. 2004. Pneumococcal surface protein C contributes to sepsis caused by Streptococcus pneumoniae in mice. Infect Immun 72:3077–3080. doi: 10.1128/iai.72.5.3077-3080.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quin LR, Carmicle S, Dave S, Pangburn MK, Evenhuis JP, McDaniel LS. 2005. In vivo binding of complement regulator factor H by Streptococcus pneumoniae. J Infect Dis 192:1996–2003. doi: 10.1086/497605. [DOI] [PubMed] [Google Scholar]
- 151.Nordström T, Blom AM, Forsgren A, Riesbeck K. 2004. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol 173:4598–4606. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- 152.Nordstrom T, Blom AM, Tan TT, Forsgren A, Riesbeck K. 2005. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J Immunol 175:3628–3636. doi: 10.4049/jimmunol.175.6.3628. [DOI] [PubMed] [Google Scholar]
- 153.Attia AS, Ram S, Rice PA, Hansen EJ. 2006. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect Immun 74:1597–1611. doi: 10.1128/IAI.74.3.1597-1611.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosadini CV, Ram S, Akerley BJ. 2014. Outer membrane protein p5 is required for resistance of nontypeable haemophilus influenzae to both the classical and alternative complement pathways. Infect Immun 82:640–649. doi: 10.1128/IAI.01224-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pang B, Swords WE. 2017. Haemophilus parainfluenzae strain ATCC 33392 forms biofilms in vitro and during experimental otitis media infections. Infect Immun 85:e01070-16. doi: 10.1128/IAI.01070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gu X, Keyoumu Y, Long L, Zhang H. 2014. Detection of bacterial biofilms in different types of chronic otitis media. Eur Arch Otorhinolaryngol 271:2877–2883. doi: 10.1007/s00405-013-2766-8. [DOI] [PubMed] [Google Scholar]
- 158.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. 1998. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 279:296. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 159.Post JC. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 160.Ehrlich GD, Veeh R, Wang X, Costerton JW, Hayes JD, Hu FZ, Daigle BJ, Ehrlich MD, Post JC. 2002. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- 161.Wolcott RD, Ehrlich GD. 2008. Biofilms and chronic infections. JAMA 299:2682. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- 162.Schachern PA, Tsuprun V, Cureoglu S, Ferrieri P, Briles DE, Paparella MM, Juhn S. 2009. Virulence of pneumococcal proteins on the inner ear. Arch Otolaryngol Head Neck Surg 135:657–661. doi: 10.1001/archoto.2009.72. [DOI] [PubMed] [Google Scholar]
- 163.Idicula WK, Jurcisek JA, Cass ND, Ali S, Goodman SD, Elmaraghy CA, Jatana KR, Bakaletz LO. 2016. Identification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope 126:1946–1951. doi: 10.1002/lary.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Novotny L, Brockman K, Mokrzan E, Jurcisek J, Bakaletz L. 2019. Biofilm biology and vaccine strategies for otitis media due to nontypeable Haemophilus influenzae. J Pediatr Infect Dis 14:69–77. doi: 10.1055/s-0038-1660818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Broides A, Dagan R, Greenberg D, Givon‐Lavi N, Leibovitz E. 2009. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis 49:1641–1647. doi: 10.1086/647933. [DOI] [PubMed] [Google Scholar]
- 166.Perez AC, Pang B, King LB, Tan L, Murrah KA, Reimche JL, Wren JT, Richardson SH, Ghandi U, Swords WE. 2014. Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathog Dis 70:280–288. doi: 10.1111/2049-632X.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Weimer KED, Juneau RA, Murrah KA, Pang B, Armbruster CE, Richardson SH, Swords WE. 2011. Divergent mechanisms for passive pneumococcal resistance to β-lactam antibiotics in the presence of Haemophilus influenzae. J Infect Dis 203:549–555. doi: 10.1093/infdis/jiq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cope EK, Goldstein-Daruech N, Kofonow JM, Christensen L, McDermott B, Monroy F, Palmer JN, Chiu AG, Shirtliff ME, Cohen NA, Leid JG. 2011. Regulation of virulence gene expression resulting from Streptococcus pneumoniae and nontypeable Haemophilus influenzae interactions in chronic disease. PLoS One 6:e28523. doi: 10.1371/journal.pone.0028523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kumpu M, Swanljung E, Tynkkynen S, Hatakka K, Kekkonen RA, Järvenpää S, Korpela R, Pitkäranta A. 2013. Recovery of probiotic Lactobacillus rhamnosus GG in tonsil tissue after oral administration: randomised, placebo-controlled, double-blind clinical trial. Br J Nutr 109:2240–2246. doi: 10.1017/S0007114512004540. [DOI] [PubMed] [Google Scholar]
- 170.Swanljung E, Tapiovaara L, Lehtoranta L, Mäkivuokko H, Roivainen M, Korpela R, Pitkäranta A. 2015. Lactobacillus rhamnosus GG in adenoid tissue: double-blind, placebo-controlled, randomized clinical trial. Acta Otolaryngol 135:824–830. doi: 10.3109/00016489.2015.1027412. [DOI] [PubMed] [Google Scholar]
- 171.Tapiovaara L, Lehtoranta L, Swanljung E, Mäkivuokko H, Laakso S, Roivainen M, Korpela R, Pitkäranta A. 2014. Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration. Int J Pediatr Otorhinolaryngol 78:1637–1641. doi: 10.1016/j.ijporl.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 172.Broeckx G, Vandenheuvel D, Claes IJJ, Lebeer S, Kiekens F. 2016. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505:303–318. doi: 10.1016/j.ijpharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 173.Segers ME, Lebeer S. 2014. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb Cell Fact 13:S7. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Niittynen L, Pitkäranta A, Korpela R. 2012. Probiotics and otitis media in children. Int J Pediatr Otorhinolaryngol 76:465–470. doi: 10.1016/j.ijporl.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 175.Marom T, Marchisio P, Tamir SO, Torretta S, Gavriel H, Esposito S. 2016. Complementary and alternative medicine treatment options for otitis media. Medicine (Baltimore) 95:e2695. doi: 10.1097/MD.0000000000002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roos K, Hakansson EG, Holm S. 2001. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ 322:210–212. doi: 10.1136/bmj.322.7280.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tano K, Grahn Håkansson E, Holm SE, Hellström S. 2002. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int J Pediatr Otorhinolaryngol 62:17–23. doi: 10.1016/S0165-5876(01)00581-X. [DOI] [PubMed] [Google Scholar]
- 178.Santagati M, Scillato M, Muscaridola N, Metoldo V, La Mantia I, Stefani S. 2015. Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. Eur J Clin Microbiol Infect Dis 34:2075–2080. doi: 10.1007/s10096-015-2454-2. [DOI] [PubMed] [Google Scholar]
- 179.La Mantia I, Varricchio A, Ciprandi G. 2017. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for preventing recurrent acute otitis media in children: a real-life clinical experience. Int J Gen Med 10:171–175. doi: 10.2147/IJGM.S137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Skovbjerg S, Roos K, Holm SE, Grahn Hakansson E, Nowrouzian F, Ivarsson M, Adlerberth I, Wold AE. 2009. Spray bacteriotherapy decreases middle ear fluid in children with secretory otitis media. Arch Dis Child 94:92–98. doi: 10.1136/adc.2008.137414. [DOI] [PubMed] [Google Scholar]
- 181.Marchisio P, Santagati M, Scillato M, Baggi E, Fattizzo M, Rosazza C, Stefani S, Esposito S, Principi N. 2015. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur J Clin Microbiol Infect Dis 34:2377–2383. doi: 10.1007/s10096-015-2491-x. [DOI] [PubMed] [Google Scholar]
- 182.Mårtensson A, Greiff L, Lamei SS, Lindstedt M, Olofsson TC, Vasquez A, Cervin A. 2016. Effects of a honeybee lactic acid bacterial microbiome on human nasal symptoms, commensals, and biomarkers. Int Forum Allergy Rhinol 6:956–963. doi: 10.1002/alr.21762. [DOI] [PubMed] [Google Scholar]
- 183.Mandal MD, Mandal S. 2011. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1:154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Claes IJ, Vargas García CE, Lebeer S. 2015. Novel opportunities for the exploitation of host–microbiome interactions in the intestine. Curr Opin Biotechnol 32:28–34. doi: 10.1016/j.copbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 185.Lebeer S, Vanderleyden J, De Keersmaecker S. 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deborah S, Prathibha KM. 2014. Measurement of nasal mucociliary clearance. Clin Res Pulmonol 2:1019. [Google Scholar]
- 187.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S, De Keersmaecker SCJ, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjarvi T, Auvinen P, de Vos WM. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci 106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Call EK, Klaenhammer TR. 2013. Relevance and application of sortase and sortase-dependent proteins in lactic acid bacteria. Front Microbiol 4:73. doi: 10.3389/fmicb.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wuyts S, Wittouck S, De Boeck I, Allonsius CN, Pasolli E, Segata N, Lebeer S. 2017. Large-scale phylogenomics of the Lactobacillus casei group highlights taxonomic inconsistencies and reveals novel clade-associated features. mSystems 2:e00061-17. doi: 10.1128/mSystems.00061-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Petrova MI, Lievens E, Verhoeven TLA, Macklaim JM, Gloor G, Schols D, Vanderleyden J, Reid G, Lebeer S. 2016. The lectin-like protein 1 in Lactobacillus rhamnosus GR-1 mediates tissue-specific adherence to vaginal epithelium and inhibits urogenital pathogens. Sci Rep 6:37437. doi: 10.1038/srep37437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Burgain J, Scher J, Lebeer S, Vanderleyden J, Corgneau M, Guerin J, Caillet C, Duval JFL, Francius G, Gaiani C. 2015. Impacts of pH-mediated EPS structure on probiotic bacterial pili-whey proteins interactions. Colloids Surfaces B Biointerfaces 134:332–338. doi: 10.1016/j.colsurfb.2015.06.068. [DOI] [PubMed] [Google Scholar]
- 192.De Rudder C, Calatayud Arroyo M, Lebeer S, Van de Wiele T. 2018. Modelling upper respiratory tract diseases: getting grips on host-microbe interactions in chronic rhinosinusitis using in vitro technologies. Microbiome 6:75. doi: 10.1186/s40168-018-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lafontaine ER, Wall D, Vanlerberg SL, Donabedian H, Sledjeski DD. 2004. Moraxella catarrhalis coaggregates with Streptococcus pyogenes and modulates interactions of S. pyogenes with human epithelial cells. Infect Immun 72:6689–6693. doi: 10.1128/IAI.72.11.6689-6693.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Allonsius CN, van den Broek MFL, De Boeck I, Kiekens S, Oerlemans EFM, Kiekens F, Foubert K, Vandenheuvel D, Cos P, Delputte P, Lebeer S. 2017. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb Biotechnol 10:1753–1763. doi: 10.1111/1751-7915.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Zanoni I, Stuknyte M, Granucci F, Karp M, Mora D. 2010. A dairy bacterium displays in vitro probiotic properties for the pharyngeal mucosa by antagonizing group A streptococci and modulating the immune response. Infect Immun 78:4734–4743. doi: 10.1128/IAI.00559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wong S-S, Quan Toh Z, Dunne EM, Mulholland EK, Tang MLK, Robins-Browne RM, Licciardi PV, Satzke C. 2013. Inhibition of Streptococcus pneumoniae adherence to human epithelial cells in vitro by the probiotic Lactobacillus rhamnosus GG. BMC Res Notes 6:135. doi: 10.1186/1756-0500-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van den Broek MFL, De Boeck I, Claes IJJ, Nizet V, Lebeer S. 2018. Multifactorial inhibition of lactobacilli against the respiratory tract pathogen Moraxella catarrhalis. Benef Microbes 9:429–439. doi: 10.3920/BM2017.0101. [DOI] [PubMed] [Google Scholar]
- 198.Siegel SJ, Weiser JN. 2015. Mechanisms of bacterial colonization of the respiratory tract. Annu Rev Microbiol 69:425–444. doi: 10.1146/annurev-micro-091014-104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Underwood M, Bakaletz L. 2011. Innate immunity and the role of defensins in otitis media. Curr Allergy Asthma Rep 11:499–507. doi: 10.1007/s11882-011-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alakomi H, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 66:2001–2005. doi: 10.1128/aem.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.De Keersmaecker SCJ, Verhoeven TLA, Desair J, Marchal K, Vanderleyden J, Nagy I. 2006. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259:89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 202.Makras L, Triantafyllou V, Fayol-Messaoudi D, Adriany T, Zoumpopoulou G, Tsakalidou E, Servin A, De Vuyst L. 2006. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res Microbiol 157:241–247. doi: 10.1016/j.resmic.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 203.Makras L, De Vuyst L. 2006. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J 16:1049–1057. doi: 10.1016/j.idairyj.2005.09.006. [DOI] [Google Scholar]
- 204.Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. 2006. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol 100:1324–1332. doi: 10.1111/j.1365-2672.2006.02857.x. [DOI] [PubMed] [Google Scholar]
- 205.Marianelli C, Cifani N, Pasquali P. 2010. Evaluation of antimicrobial activity of probiotic bacteria against Salmonella enterica subsp. enterica serovar typhimurium 1344 in a common medium under different environmental conditions. Res Microbiol 161:673–680. doi: 10.1016/j.resmic.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 206.Barefoot SF, Klaenhammer TR. 1983. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol 45:1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Muriana PM, Klaenhammer TR. 1991. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol 57:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tabasco R, García-Cayuela T, Peláez C, Requena T. 2009. Lactobacillus acidophilus La-5 increases lactacin B production when it senses live target bacteria. Int J Food Microbiol 132:109–116. doi: 10.1016/j.ijfoodmicro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 209.Gonzalez B, Arca P, Mayo B, Suarez JE. 1994. Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl Environ Microbiol 60:2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zacharof MP, Lovitt RW. 2012. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia 2:50–56. doi: 10.1016/j.apcbee.2012.06.010. [DOI] [Google Scholar]
- 211.Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A. 2009. Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr 49:23–30. doi: 10.1097/MPG.0b013e3181924d1e. [DOI] [PubMed] [Google Scholar]
- 212.Goldstein BP, Wei J, Greenberg K, Novick R. 1998. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J Antimicrob Chemother 42:277–278. doi: 10.1093/jac/42.2.277. [DOI] [PubMed] [Google Scholar]
- 213.Petrova MI, Imholz NCE, Verhoeven TLA, Balzarini J, Van Damme EJM, Schols D, Vanderleyden J, Lebeer S. 2016. Lectin-like molecules of Lactobacillus rhamnosus GG inhibit pathogenic Escherichia coli and Salmonella biofilm formation. PLoS One 11:e0161337. doi: 10.1371/journal.pone.0161337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schwandt LQ, Van Weissenbruch R, Stokroos I, Van Der Mei HC, Busscher HJ, Albers F. 2004. Prevention of biofilm formation by dairy products and N-acetylcysteine on voice prostheses in an artificial throat. Acta Otolaryngol 124:726–731. doi: 10.1080/00016480410022516. [DOI] [PubMed] [Google Scholar]
- 215.Pericone CD, Overweg K, Hermans PWM, Weiser JN. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 68:3990–3997. doi: 10.1128/IAI.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Servin AL. 2004. Antagonistic activities of Lactobacilli and Bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 217.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. 2015. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol 6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect Immun 68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lebeer S, De Keersmaecker SCJ, Verhoeven TLA, Fadda AA, Marchal K, Vanderleyden J. 2007. Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. J Bacteriol 189:860–871. doi: 10.1128/JB.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vidal JE, Ludewick HP, Kunkel RM, Zähner D, Klugman KP. 2011. The luxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect Immun 79:4050–4060. doi: 10.1128/IAI.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, Byrd MS, Love CF, Kock ND, Swords WE. 2009. LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface. Infect Immun 77:4081–4091. doi: 10.1128/IAI.00320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Joyce EA, Kawale A, Censini S, Kim CC, Covacci A, Falkow S. 2004. LuxS is required for persistent pneumococcal carriage and expression of virulence and biosynthesis genes. Infect Immun 72:2964–2975. doi: 10.1128/iai.72.5.2964-2975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stroeher UH, Paton AW, Ogunniyi AD, Paton JC. 2003. Mutation of luxS of Streptococcus pneumoniae affects virulence in a mouse model. Infect Immun 71:3206–3212. doi: 10.1128/IAI.71.6.3206-3212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Daines DA, Bothwell M, Furrer J, Unrath W, Nelson K, Jarisch J, Melrose N, Greiner L, Apicella M, Smith AL. 2005. Haemophilus influenzae luxS mutants form a biofilm and have increased virulence. Microb Pathog 39:87–96. doi: 10.1016/j.micpath.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 225.Armbruster CE, Hong W, Pang B, Weimer KED, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1:e00102-10. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. 2005. Making “sense” of metabolism: autoinducer-2, LUXS and pathogenic bacteria. Nat Rev Microbiol 3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 227.Bron PA, Kleerebezem M, Brummer R-J, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM. 2017. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yeo N-K, Jang YJ. 2010. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope 120:346–352. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]
- 229.Martens K, Pugin B, De Boeck I, Spacova I, Steelant B, Seys SF, Lebeer S, Hellings PW. 2018. Probiotics for the airways: potential to improve epithelial and immune homeostasis. Allergy 73:1954–1963. doi: 10.1111/all.13495. [DOI] [PubMed] [Google Scholar]
- 230.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJM, Wells JM. 2010. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 231.Seth A, Yan F, Polk DB, Rao RK. 2008. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 294:G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. 2007. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM, Wilson KT, Polk DB. 2011. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest 121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. 2016. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology 54:195–205. doi: 10.4193/Rhin15.376. [DOI] [PubMed] [Google Scholar]
- 235.Lee Y-S, Kim T-Y, Kim Y, Lee S-H, Kim S, Kang SW, Yang J-Y, Baek I-J, Sung YH, Park Y-Y, Hwang SW, O E, Kim KS, Liu S, Kamada N, Gao N, Kweon M-N. 2018. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24:833–846.e6. doi: 10.1016/j.chom.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 236.Madsen KL. 2012. Enhancement of epithelial barrier function by probiotics. J Epithel Biol Pharmacol 5:55–59. doi: 10.2174/1875044301205010055. [DOI] [Google Scholar]
- 237.Lehrer RI, Jung G, Ruchala P, Wang W, Micewicz ED, Waring AJ, Gillespie EJ, Bradley KA, Ratner AJ, Rest RF, Lu W. 2009. Human β-defensins inhibit hemolysis mediated by cholesterol-dependent cytolysins. Infect Immun 77:4028–4040. doi: 10.1128/IAI.00232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schröder JM, Bevins CL, Fellermann K, Stange EF. 2004. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal β-defensin expression. Gut 53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Delcour J, Ferain T, Deghorain M, Palumbo E, Hols P. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie van Leeuwenhoek 76:159–184. doi: 10.1023/A:1002089722581. [DOI] [PubMed] [Google Scholar]
- 240.Macho Fernandez E, Fernandez EM, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, Grangette C. 2011. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 241.Maxson S, Yamauchi T. 1996. Acute otitis media. Pediatr Rev 17:191–195. doi: 10.1542/pir.17-6-191. [DOI] [PubMed] [Google Scholar]
- 242.Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. 2008. Probiotic lactobacilli and VSL#3 induce enterocyte β-defensin 2. Clin Exp Immunol 151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bishop-Hurley SL, Schmidt FJ, Erwin AL, Smith AL. 2005. Peptides selected for binding to a virulent strain of Haemophilus influenzae by phage display are bactericidal. Antimicrob Agents Chemother 49:2972–2978. doi: 10.1128/AAC.49.7.2972-2978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee H-Y, Andalibi A, Webster P, Moon S-K, Teufert K, Kang S-H, Li J-D, Nagura M, Ganz T, Lim DJ. 2004. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McGillivary G, Ray WC, Bevins CL, Munson RS, Bakaletz LO. 2007. A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media. Mol Immunol 44:2446–2458. doi: 10.1016/j.molimm.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bu H-F, Wang X, Zhu Y-Q, Williams RY, Hsueh W, Zheng X, Rozenfeld RA, Zuo X-L, Tan X-D. 2006. Lysozyme-modified probiotic components protect rats against polymicrobial sepsis: role of macrophages and cathelicidin-related innate immunity. J Immunol 177:8767–8776. doi: 10.4049/jimmunol.177.12.8767. [DOI] [PubMed] [Google Scholar]
- 247.Lebeer S, Vanderleyden J, De Keersmaecker S. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 248.Konstantinov SR, Smidt H, de Vos WM, Bruijns SCM, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci U S A 105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. 2009. Probiotics and immunity. J Gastroenterol 44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 250.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. 2007. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy 37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 251.Spacova I, Petrova MI, Fremau A, Pollaris L, Vanoirbeek J, Ceuppens JL, Seys S, Lebeer S. 2019. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy 74:100–1. doi: 10.1111/all.13502. [DOI] [PubMed] [Google Scholar]
- 252.Toh ZQ, Anzela A, Tang MLK, Licciardi PV. 2012. Probiotic therapy as a novel approach for allergic disease. Front Pharmacol 3:171. doi: 10.3389/fphar.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, Yausi H. 2010. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol 50:597–602. doi: 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- 254.Lebeer S, Claes I, Tytgat HLP, Verhoeven TLA, Marien E, von Ossowski I, Reunanen J, Palva A, de Vos WM, De Keersmaecker SCJ, Vanderleyden J. 2012. Functional analysis of lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78:185–193. doi: 10.1128/AEM.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vargas García CE, Petrova M, Claes IJJ, De Boeck I, Verhoeven TLA, Dilissen E, von Ossowski I, Palva A, Bullens DM, Vanderleyden J, Lebeer S. 2015. Piliation of Lactobacillus rhamnosus GG promotes adhesion, phagocytosis, and cytokine modulation in macrophages. Appl Environ Microbiol 81:2050–2062. doi: 10.1128/AEM.03949-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Petrova MI, Macklaim JM, Wuyts S, Verhoeven T, Vanderleyden J, Gloor G, Lebeer S, Reid G. 2018. Comparative genomic and phenotypic analysis of the vaginal probiotic Lactobacillus rhamnosus GR-1. Front Microbiol 9:1278. doi: 10.3389/fmicb.2018.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A 102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Claes IJJ, De Keersmaecker SCJ, Vanderleyden J, Lebeer S. 2011. Lessons from probiotic-host interaction studies in murine models of experimental colitis. Mol Nutr Food Res 55:1441–1453. doi: 10.1002/mnfr.201100139. [DOI] [PubMed] [Google Scholar]
- 259.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 10:259–266. doi: 10.1128/cdli.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O'Flaherty S, Barrett T, Klaenhammer TR. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A 108(Suppl 1):4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yasuda E, Serata M, Sako T. 2008. Suppressive effect on activation of macrophages by Lactobacillus casei strain shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl Environ Microbiol 74:4746–4755. doi: 10.1128/AEM.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Remus DM, van Kranenburg R, van Swam II, Taverne N, Bongers RS, Wels M, Wells JM, Bron PA, Kleerebezem M. 2012. Impact of 4 Lactobacillus plantarum capsular polysaccharide clusters on surface glycan composition and host cell signaling. Microb Cell Fact 11:149. doi: 10.1186/1475-2859-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kant R, de Vos WM, Palva A, Satokari R. 2014. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J Med Microbiol 63:293–308. doi: 10.1099/jmm.0.064220-0. [DOI] [PubMed] [Google Scholar]
- 264.von Schillde M-A, Hörmannsperger G, Weiher M, Alpert C-A, Hahne H, Bäuerl C, Van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G, Kuster B, Haller D. 2012. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11:387–396. doi: 10.1016/j.chom.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 265.Sun Z, Harris HMB, McCann A, Guo C, Argimón S, Zhang W, Yang X, Jeffery IB, Cooney JC, Kagawa TF, Liu W, Song Y, Salvetti E, Wrobel A, Rasinkangas P, Parkhill J, Rea MC, O’Sullivan O, Ritari J, Douillard FP, Paul Ross R, Yang R, Briner AE, Felis GE, de Vos WM, Barrangou R, Klaenhammer TR, Caufield PW, Cui Y, Zhang H, O’Toole PW. 2015. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun 6:8322. doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vinolo MAR, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. 2011. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 267.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Di Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Park J-S, Lee E-J, Lee J-C, Kim W-K, Kim H-S. 2007. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: involvement of NF-κB and ERK signaling pathways. Int Immunopharmacol 7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 270.Mountzouris KC, Kotzampassi K, Tsirtsikos P, Kapoutzis K, Fegeros K. 2009. Effects of Lactobacillus acidophilus on gut microflora metabolic biomarkers in fed and fasted rats. Clin Nutr 28:318–324. doi: 10.1016/j.clnu.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 271.Yamano T, Iino H, Takada M, Blum S, Rochat F, Fukushima Y. 2006. Improvement of the human intestinal flora by ingestion of the probiotic strain Lactobacillus johnsonii La1. Br J Nutr 95:303–312. doi: 10.1079/BJN20051507. [DOI] [PubMed] [Google Scholar]
- 272.Louis P, Flint HJ. 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 273.Lewnard JA, Givon-Lavi N, Tähtinen PA, Dagan R. 2018. Pneumococcal phenotype and interaction with nontypeable Haemophilus influenzae as determinants of otitis media progression. Infect Immun 86:e00727-17. doi: 10.1128/IAI.00727-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Eythorsson E, Hrafnkelsson B, Erlendsdóttir H, Gudmundsson SA, Kristinsson KG, Haraldsson Á. 2018. Decreased acute otitis media with treatment failure after introduction of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine. Pediatr Infect Dis J 37:361–366. doi: 10.1097/INF.0000000000001870. [DOI] [PubMed] [Google Scholar]
- 275.de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. 2005. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr 44:406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- 276.Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. 1995. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine. Vaccine 13:310–312. doi: 10.1016/0264-410X(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 277.Davidson L, Fiorino A-M, Snydman D, Hibberd P. 2011. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr 65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Boyle RJ, Ismail IH, Kivivuori S, Licciardi PV, Robins-Browne RM, Mah L-J, Axelrad C, Moore S, Donath S, Carlin JB, Lahtinen SJ, Tang M. 2011. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 66:509–516. doi: 10.1111/j.1398-9995.2010.02507.x. [DOI] [PubMed] [Google Scholar]
- 279.Amerighi F, Valeri M, Donnarumma D, Maccari S, Moschioni M, Taddei A, Lapazio L, Pansegrau W, Buccato S, De Angelis G, Ruggiero P, Masignani V, Soriani M, Pezzicoli A. 2016. Identification of a monoclonal antibody against pneumococcal pilus 1 ancillary protein impairing bacterial adhesion to human epithelial cells. J Infect Dis 213:516–522. doi: 10.1093/infdis/jiv461. [DOI] [PubMed] [Google Scholar]
- 280.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. 2018. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol 49:207–216. doi: 10.1016/j.copbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 282.Chonmaitree T, Jennings K, Golovko G, Khanipov K, Pimenova M, Patel JA, McCormick DP, Loeffelholz MJ, Fofanov Y. 2017. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One 12:e0180630. doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]