Supplemental Digital Content is available in the text
Keywords: inflammatory bowel diseases, personalized medicine, prediction, risk stratification, treat-to-target
ABSTRACT
Objectives:
Treatment targets in inflammatory bowel disease (IBD) move away from controlling symptoms towards complete recovery of the intestinal mucosa. Currently, the most frequently used noninvasive surrogate marker of mucosal healing is a faecal calprotectin concentration in the target range. This study tested if there was a relation between time-to-reach target calprotectin and first flare.
Methods:
We prospectively included new-onset IBD patients ages 17 and younger in a cloud-based registry (FastForwardCare) and followed them for at least 52 weeks. They were treated according to Dutch national guidelines that advocate a step-up approach. Time-to-reach target was defined as the first calprotectin measurement below 250 μg/g after the start of induction therapy. Time-to-first flare was the time from the first calprotectin measurement below 250 μg/g until reappearance of symptoms with calprotectin values above 250 μg/g.
Results:
We included 76 patients (luminal Crohn disease [CD] 43); ulcerative colitis [UC] 33). Median age at diagnosis was, respectively 14.5 and 14.1 years. Median time-to-reach target calprotectin was 37 weeks in CD and 11 weeks in UC patients (Log-rank test, P = 0.001). Once the calprotectin target was reached, time-to-first flare was significantly longer in CD than in UC patients (Log-rank test, P = 0.001). CD patients with time-to-reach target calprotectin ≤12 weeks after conventional induction therapy (ie, exclusive enteral nutrition or steroids) had a more favorable disease course in the first year than those with time-to-reach target calprotectin >12 weeks (Log-rank test, P = 0.057). In UC patients, time-to-reach target calprotectin ≤12 weeks is not associated with a favorable disease course in the first year.
Conclusions:
The findings of this prospective registry suggest that a quick response to conventional therapy predicts a favorable disease course in new-onset paediatric CD, but not in UC. The concept “time-to-reach target calprotectin level” rationalizes the indefinite term “response to treatment” and is well suited for studying treatment effectiveness in real-world practices.
What Is Known/What Is New
What Is Known
Currently, the most frequently used surrogate for mucosal healing is a faecal calprotectin concentration in the target range.
Faecal calprotectin levels below 250 μg/g correlate well with mucosal healing.
What Is New
A short time-to-reach the calprotectin target on conventional induction therapy, that is, within 12 weeks, is predictive for favorable disease course in patients with new-onset Crohn disease, but not in patients with ulcerative colitis.
Our findings suggest that Crohn disease patients who fail to reach target calprotectin levels within 12 weeks after exclusive enteral nutrition or steroid therapy may be entitled to step-up to anti-Tumor Necrosis Factor agents.
Inflammatory bowel diseases (IBD), including Crohn disease (CD) and ulcerative colitis (UC), develop during childhood or adolescence in approximately 10% of patients (1). Paediatric-onset IBD is associated with more extensive disease, higher disease activity, and a more complicated course than adult-onset IBD (2,3). The focus of modern-day IBD management is moving away from controlling symptoms (or clinical remission) towards reaching and maintaining complete recovery of the intestinal mucosa, constituting both endoscopic and histologic healing (mucosal healing, MH) (4). MH is associated with better patient outcomes and is said to reduce the risk of end-organ damage (5). MH is ideally evaluated by endoscopy with biopsies, but repeated endoscopic procedures are poorly tolerated. Currently, the most frequently used noninvasive surrogate marker of MH is faecal calprotectin (FC). FC levels below 250 μg/g correlate well with MH, FC levels rise quickly above this target threshold in case of disease flare and FC levels rapidly fall with successful treatment (6).
In the past decade, the approach to treating children with luminal CD and UC has been classically described as “step up,” whereas only few exceptional conditions at diagnosis warranted early and continuing use of anti-tumour necrosis factor (TNF) agents, including patients with perianal fistulizing disease, growth failure, or extra-intestinal manifestations (7). Although we acknowledge the importance of recognizing patients who are most at risk of developing a complicated disease course at diagnosis, we argue that risk stratification should not stop there. The identification of high- and low-risk patients for complicated disease should probably continue after diagnosis and extend into the first year of treatment.
For that purpose, we prospectively followed newly diagnosed children with luminal CD or UC who were initially treated with conventional induction therapy and evaluated whether there was a relation between time-to-reach target calprotectin and first flare.
METHODS
Study Design and Setting
This was a noninterventional, observational study in which patient data were prospectively entered in an online registry, from diagnosis onwards. We used the cloud-based FastForwardCare (FFC) platform, which is especially designed to capture patients’ records in an easy and comprehensive way (https://www.fastforwardcare.nl/). FFC is a health network in which care providers, researchers, patients and families learn together to improve the care for children with IBD. With effect from January 2015 the University Medical Center Groningen (UMCG) has started the collection of patient data. More IBD treatment centers will be invited to join this health network in the coming years. The FFC platform has options for writing medical summaries, creation of medical letters and building a research database without the drawback of double data entry. Care providers can trace back database information to their individual patients; other users only have access to anonymized data.
Health checks and data collection were done at structured time points, but not at fixed intervals. We used phenotype-specific clinical composite scores (Paediatric Ulcerative Colitis Activity Index [PUCAI] (8) and the shortened Paediatric Crohn Disease Activity Index [shPCDAI]) (9,10). Stool samples were sent to the department of laboratory sciences of the UMCG and were analysed with fCAL ELISA (BÜHLMANN Laboratories AG, Schönenbuch, Switzerland). The results of both the symptom score and the calprotectin stool test cumulated in a relapse risk stratification (Flarometer score) that has been described previously (11,12). In brief, a patient who was in the low-risk stratum (symptom score below 10 and calprotectin below 250 μg/g) was reassured and advised to retest in 3 months. A symptomatic patient with a calprotectin concentration above 250 μg/g was considered to have active disease and required immediate adjustment of therapy and retesting after 1 month. In the intermediate-risk stratum, a test interval of 1 month was advised before progressing to a treatment decision (Fig. 1).
FIGURE 1.
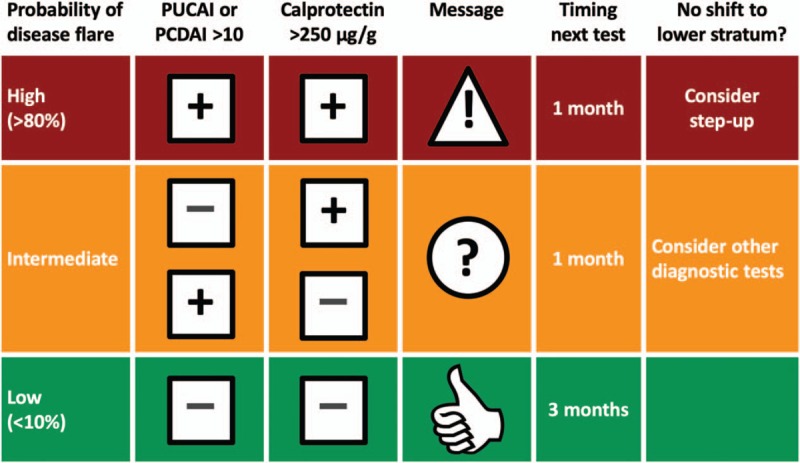
Flarometer strategy. Algorithm with advice on treatment and the timing of re-measurement. Reproduced with permission from Heida et al (11).
The ethical committee of the UMCG reviewed the study protocol and waived the need for informed consent because of the anonymous and noninterventional approach of the registry.
Participants
We used the clinical data of patients up to the age of 17 years who were newly diagnosed with luminal CD or UC between January 2015 and July 2017. National treatment guidelines provided uniformity in treatment among physicians (13). Patients with active luminal CD were treated with steroids (to a maximum of 40 mg/day) and gradual dose tapering, or with exclusive enteral nutrition (EEN) for 6 weeks. Patients with active UC were treated with steroids and aminosalicylate dose escalation. Maintenance therapy was started at the same time as induction therapy and in CD, this included standardized doses of mercaptopurine (1–1.5 mg · kg−1 · day−1), azathioprine (2–2.5 mg · kg−1 · day−1) or methotrexate (15 mg · m−2 · week−1), while in UC this included aminosalicylate monotherapy (60-80 mg · kg−1 · day−1) or combination therapy with azathioprine. Antitumor necrosis factor (TNF) therapy was indicated after failure of conventional therapy.
Data Collection
At diagnosis, patient demographics, disease characteristics, and detailed information on diagnostic work-up (including baseline laboratory values, endoscopic examination, histological features, and small bowel imaging) were recorded online in the FFC registry. Stool specimen were collected at home and sent to the hospital laboratory before each face-to-face patient-doctor encounter, as previously described (14). During health checks, all of the following variables were entered in the FFC registry: present treatment, clinical signs and symptoms, follow-up of laboratory values and faecal calprotectin, PUCAI or shPCDAI, a final conclusion (remission, clinical improvement, imminent relapse, relapse), and any treatment adjustments.
Definitions and Outcome Measures
IBD was diagnosed according to the Revised Porto criteria (15) and disease phenotype, location, and behavior were described according to the Paris classification (16). Faecal calprotectin concentrations below 250 μg/g were considered as a proxy for mucosal healing (17–20). Time-to-reach target was defined as the first calprotectin measurement below 250 μg/g after the start of induction therapy (Figure, Supplemental Digital Content 1). Time-to-first flare, which was our primary outcome measure, was defined as the time from the first calprotectin measurement below 250 μg/g until reappearance of symptoms with calprotectin values above 250 μg/g (ie, having a flarometer score in the high-risk stratum). Growth delay was defined as height-for-age less than or equal to −2 SD.
Statistical Analyses
This was an exploratory study, with neither formal hypothesis testing nor sample size calculation. Data analyses were performed using IBM SPSS version 23.0 for Apple Mac (IBM Corporation, Armonk, NY). Baseline demographics and disease characteristics were evaluated using descriptive statistics, including median and interquartile ranges (IQR: 25th percentile, 75th percentile) for continuous variables. For discrete variables, we calculated the 95% confidence interval in OpenEpi, Version 3. Time-to-event data were analysed by Kaplan-Meier and log-rank tests. P values <0.05 were considered statistically significant. Graphs were constructed with GraphPad Prism version 7 for MacBook (GraphPad Software, San Diego, CA). Patients with fistulizing CD were excluded from the survival analysis.
RESULTS
Patient Demographics
A total of 76 newly diagnosed children with IBD were identified between January 2015 to July 2017. Fifty-seven percentage (43/76) had luminal CD and 43% (33/76) had UC. Table 1 shows the patient characteristics. Patients included in the FFC registry were diagnosed at a median age of 14.5 years (IQR 11.9–16.3) and 14.1 years (IQR 12–15.8), for CD and UC, respectively. Fifty-three percentage (23/43) of CD patients was female, compared with 49% (16/33) of UC patients. Growth delay at diagnosis was reported in 17% (7/41) of CD patients and in 6% (2/32) of UC patients. Median follow-up of patients was 67 weeks (IQR 54–86).
TABLE 1.
Patient characteristics at diagnosis
| CD (n = 43) | UC (n = 33) | |
| Demographic features | ||
| Median age in years (IQR) | 14.5 (11.9–16.3) | 14.1 (12–15.8) |
| Female sex | 54% | 49% |
| Family history of IBD | ||
| First degree relatives | 6% (2–17) | 10% (4–26) |
| Second degree relatives | 18% (10–31) | 15% (7–31) |
| IBD-related comorbidity | ||
| Joint inflammation | 20% (11–34) | 14% (5–31) |
| Eye manifestations | 4% (1–14) | - |
| Skin manifestations | 4% (1–14) | - |
| Growth delay* | 17% (9–31) | 6% (2–20) |
| Laboratory markers | ||
| CRP, >10 mg/L | 49% (35–63) | 24% (13–41) |
| ESR, >20 mm/h | 61% (46–74) | 39% (25–56) |
| Hb, <−2SD (sex and age adjusted†) | 49% (35–63) | 42% (27–59) |
| Stool Calprotectin, >250 μg/g | 100% (93–100) | 97% (85–99) |
| Disease location (according to Paris Classification) | ||
| L1: Distal 1/3 ileum | 19% (10–33) | |
| L2: colonic | 42% (28–57) | |
| L3: ileocolonic | 44% (30–59) | |
| L4a: upper disease proximal to ligament of Treitz* | 5% (1–15) | |
| L4b: upper disease distal to ligament of Treitz and proximal to distal 1/3 ileum* | 23% (13–38) | |
| L4ab: upper disease above and below the ligament of Treitz | 37% (24–52) | |
| E1: ulcerative proctitis | 24% (13–41) | |
| E2: left-sided UC (distal to splenic flexure) | 9% (3–24) | |
| E3: extensive (distal to hepatic flexure) | – | |
| E4: pancolitis (proximal to hepatic flexure) | 67% (50–80) | |
| Disease behavior‡ | ||
| B1: nonstricturing, nonpenetrating | 84% (70–92) | |
| B2: structuring | 7% (2-19 | |
| B3: penetrating | 5% (1–15) | |
| B2B3: both penetrating and stricturing disease | 5% (1–15) | |
| p: perianal disease | 35% (22–50) | |
Values are percentages (95% confidence interval) unless otherwise stated.
*Growth delay was defined as height versus age ≤−2 SD.
†We used age- and sex-adjusted cut points for low haemoglobin blood levels (Hb). Cut-offs were 7.1 g/L for boys and girls younger than 13 years, 8.1 g/L for boys 13 to 17 years, and 7.4 g/L for girls between 13 and 17 years (36).
‡Magnetic resonance enterography was performed in 10 of 43 CD patients. The reported proportion of patients with penetrating or structuring disease may be an underestimation.
Diagnostic Work-up
Abnormal Laboratory Tests at Diagnosis
Blood examination at diagnosis showed abnormal values of C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR) in 67% (29/43) of CD patients and in 45% (15/33) of UC patients. Forty-eight percentage (21/43) of CD patients and 42% (14/33) of UC patients presented with anaemia. All but 1 patient had faecal calprotectin levels out of the normal range (>250 μg/g). One UC patient with continuous inflammation from rectum to sigmoid had a calprotectin level of 220 μg/g at diagnosis. This patient was excluded from Kaplan-Meier analysis for having reached the event of interest at baseline.
Endoscopic and Histological Features at Diagnosis
Disease location and behavior are shown in Table 1. Five patients, who were ultimately diagnosed with UC, had histologic features of focally enhanced gastritis. Small bowel imaging by magnetic resonance enterography (MRE) was performed in 10 patients with indefinite colonic inflammation to distinguish CD from UC. Abnormalities suggestive for CD were seen in 6 patients, whereas the remaining 4 patients with normal MRE findings were initially diagnosed as IBD-unclassified. Follow-up colonoscopies confirmed a diagnosis of CD in 3 patients and UC in 1 patient.
Initial Induction and Maintenance Therapy
Figure, Supplemental Digital Content 2 shows the flow of patients through the first year postdiagnosis. Forty-nine percentage (21/43) of patients diagnosed with luminal CD were initially treated with EEN to induce remission and 51% (22/43) with steroids. Seventy percentage (30/43) of CD patients reached the treatment target during the observation period (whether or not after switch or step-up) and were included in the analysis of the primary outcome measure (time-to-first flare).
Eighty-four percentage (27/32) of UC patients were induced with oral steroids in combination with aminosalicylates, whereas 13% (4/32) of patients, in particular, those with ulcerative proctitis, were induced with topical aminosalicylates. One patient with UC was initially misdiagnosed as luminal CD and put on EEN. The final diagnosis was adjusted after colonoscopy was repeated. Ultimately 94% (30/32) of UC patients reached the treatment target during the observation period (whether or not after switch or step-up) and were included in the analysis of the primary outcome measure.
First choice immunomodulator for CD patients was azathioprine in 93 percentage (40/43) of cases. Eighty-four percentage (27/32) of the UC patients were on oral aminosalicylate maintenance therapy, in 85% (23/27) of cases in combination with azathioprine.
Disease Course in the First Year Postdiagnosis
Time-to-reach Target Calprotectin Levels (<250 μg/g)
Figure 2 shows the time-to-reach target calprotectin levels (<250 μg/g) in the study population in the first year postdiagnosis. Nineteen percentage (8/43) of patients with luminal CD reached the target within 12 weeks after diagnosis. Another 42% (18/43) reached the target between 12 and 52 weeks postdiagnosis, for which 14 patients required a step-up to anti-TNFs. Forty percentage (17/43) had not reached the treatment target after 52 weeks of follow-up. Another 4 patients with CD reached the calprotectin target concentration after 52 weeks of follow-up.
FIGURE 2.
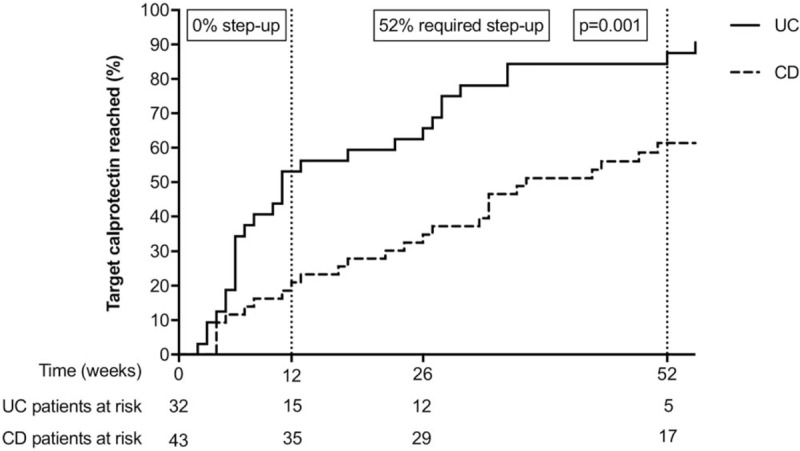
Kaplan-Meier plot demonstrating time-to-reach target calprotectin (<250 μg/g) in the first year postdiagnosis. Patients with ulcerative colitis (solid line) and Crohn disease (dotted line) were treated according to the Dutch step-up approach. One patient with UC had a faecal calprotectin value of 220 μg/g at diagnosis was excluded from the Kaplan-Meier analysis for having reached the event of interest at baseline.
Among patients with UC, 53% (17/32) reached the target within 12 weeks after diagnosis. Another 31% (10/32) reached the target between 12 and 52 weeks postdiagnosis, for which 1 patient needed step-up to anti-TNFs. Thirteen percentage (5/32) had not reached the treatment target at the end of 52 weeks’ follow-up. Another 3 UC patients reached the calprotectin target concentration after 52 weeks of follow-up.
A significant larger proportion of patients with UC reached the calprotectin target in the first year compared with patients with CD, and also at a faster pace. The median time-to-reach target calprotectin levels was, respectively, 11 and 37 weeks (Log-rank test, P = 0.001). The slopes for UC and CD patients in Figure 2 indicate that the difference in time-to-reach target calprotectin is in the first 12 weeks postdiagnosis. Thereafter, both curves follow a parallel track. The patients that ultimately reached the calprotectin target served as the study population to evaluate time-to-first flare.
Time-to-first Flare After Reaching the Calprotectin Target
Figure 3 shows the time-to-first flare after reaching the calprotectin target. Patients with CD who had reached the treatment target remained in remission for a significantly longer time than patients with UC (Log-rank test, P = 0.001). Figure 4 shows that new-onset CD patients who reached the target calprotectin level within 12 weeks were more likely to have a favorable disease course, than CD patients who reached the target calprotectin after 12 weeks (Log-rank test, P = 0.057). In UC patients there was no association between the pace of reaching the target calprotectin and favorable disease course (Log-rank test, P = 0.547).
FIGURE 3.
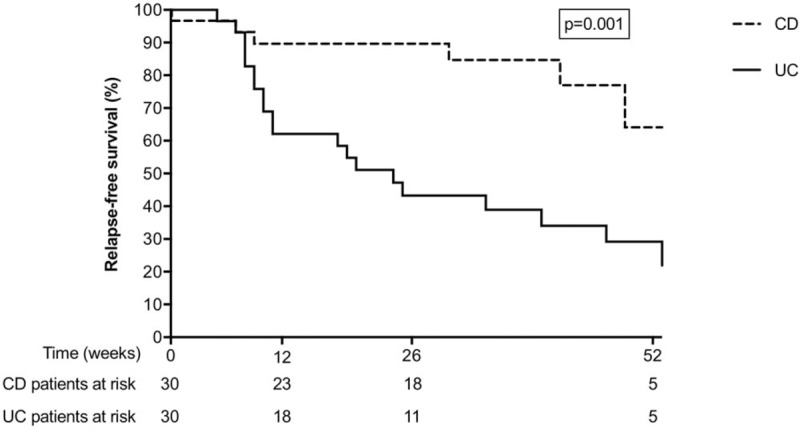
Kaplan-Meier plot demonstrating time-to-first relapse, defined as the time from the first calprotectin measurement below 250 μg/g until reintroduction of induction therapy (whether or not step-up). Patients with ulcerative colitis (solid line) are compared with patients with Crohn disease (dotted line).
FIGURE 4.
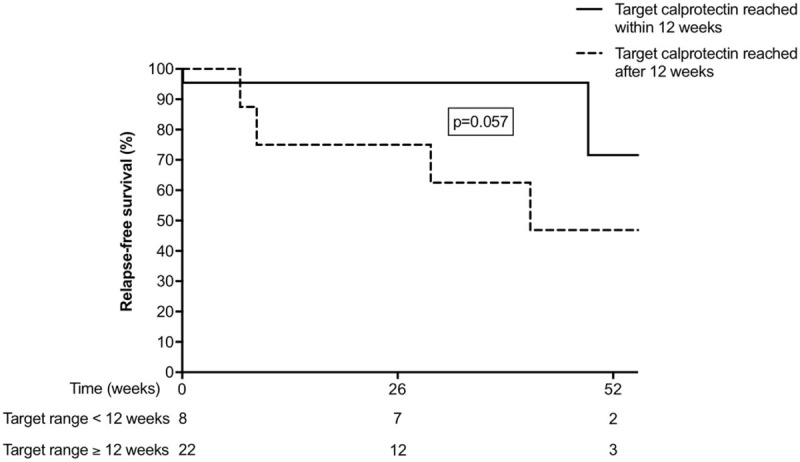
Kaplan-Meier plot demonstrating time-to-first relapse, defined as the time from the first calprotectin measurement below 250 μg/g until reintroduction of induction therapy (whether or not step-up) in 30 patients with Crohn disease. Patients who reached the target calprotectin within 12 weeks (solid line) are compared with patients who reached the target calprotectin beyond 12 weeks (dotted line).
In both CD and UC patients, 1-year outcomes including time-to-reach target calprotectin and time-to-first flare were similar regardless of initial choice of induction therapy, although our study was not powered to address this question (Table, Supplemental Digital Content 3).
Figure, Supplemental Digital Content 4 gives an overview of changes in faecal calprotectin from baseline to the moment of reaching the target range (ΔFC↓) and from the last result in range to the first out-of-range result (ΔFC↑). The median ΔFC↓ was −2095 (interquartile range, IQR −3000 to 1281) μg/g. The median ΔFC↑ was 860 (interquartile range, IQR 557–1922) ug/g. Ninety-five percentage (72/76) of IBD patients in our cohort had FC concentrations over 500 μg/g at baseline. Eighty-eight percent (53/60) of patients had FC concentrations below 200 μg/g when the target range was reached. Similarly, in those who flared during the observation period, FC concentrations were below 200 μg/g shortly before the flare in 92% (23/25) of cases. The first out-of-range measurement was over 500 μg/g in 96% (24/25) of cases.
DISCUSSION
Key Findings
We quantified time-to-reach target calprotectin levels and its relation with first flare in patients with new-onset paediatric IBD. The data were drawn from the FastForwardCare Registry. We showed that patients with UC reached the treatment target (FC <250 μg/g) at a faster pace than patients with CD, but they also experienced a first flare sooner than patients with CD. Additionally, we observed that CD patients with time-to-reach target calprotectin ≤12 weeks after conventional induction therapy (ie, exclusive enteral nutrition or steroids) had a more favorable disease course in the first year than those with time-to-reach target calprotectin >12 weeks. In UC patients, time-to-reach target calprotectin ≤12 weeks was not associated with a favorable disease course in the first year.
Comparison With Existing Literature
Several research groups have identified factors at disease diagnosis that warranted early and ongoing use of anti-TNF agents, including perianal fistulizing disease, presence of strictures or penetrating disease in CD, and extensive disease (pancolitis) or extraintestinal manifestations in UC (21–26).
In addition, we have now shown that the absence of response to conventional induction therapy (with EEN or steroids), rationalized in a failure to reach target calprotectin levels within 12 weeks from initiation, facilitates selection of patients with CD that may require an accelerated step-up therapy. A recently published Pan-European study among patients with new-onset paediatric CD also stressed the prognostic power of calprotectin values at week 12 of induction therapy, and showed that response to induction therapy has more impact than baseline disease severity or inflammation (27).
Faecal Calprotectin Cut-point
The discussion about the best calprotectin cut-point for mucosal healing is on-going, and the debate is blurred by the intended purpose of this stool test. In many articles, the use of a single calprotectin measurement is compared with a reference test (such as endoscopy or MRE) (28), whereas we use calprotectin at every health check (ie, periodically) and now adjust our therapy plans based on the trend in calprotectin results. By analogy with the most frequently used monitoring test in medicine—the measurement of blood pressure—the variability over time makes a single measurement a poor monitoring test. Fecal calprotectin becomes a good monitoring test when therapy adjustment is based on a series of measurements.
Choosing a cut-point that is considerably lower than 250 μg/g used in this project (eg, 150 μg/g) will definitely improve the specificity of the calprotectin test. At the same time, this may be detrimental to the outcome of patients with calprotectin results between 150 and 250 μg/g, who may need to undergo pointless changes of therapy that lead to quick exhaustion of the therapeutic arsenal. Evaluation of the best calprotectin cut-point should, therefore, not be limited to the reporting of receiver operating characteristic (ROC) curves, as is done in many publications (28), but must also include the monitoring strategy, definitions of action thresholds, and the actions to be taken if any of the thresholds is reached.
Limitations
Although there is an imminent danger of over-interpretation of results in an observational study, our results offer insight into the short-term outcomes of new-onset paediatric IBD patients, where double-blind randomized controlled trials are unlikely to occur. The University Medical Center in Groningen was the first center to start capturing data in the FastForwardCare prospective registry. We realize that single-center studies are unlikely to have sufficient power to draw reliable conclusions, especially when evaluating the effect of treatment choices on time-to-event analyses, and we, therefore, aim to encourage more paediatric IBD treatment centers to join the registry in the upcoming years.
Implications for Paediatric Practice and Further Research
Several trials have shown that early use of anti-TNF agents is associated with improved outcomes and fewer complications in adult as well as in paediatric patients with CD (25,29–34). This does not necessarily mean that every new-onset IBD patient will profit from the early institution of anti-TNF induction treatment. A significant proportion of patients with IBD have a relatively favorable disease course, with long time-to-first flare and low risk of complications. We are the first to have shown that a time-to-reach target calprotectin level within 12 weeks is predictive for a favorable disease course in the short run in patients with new-onset CD, but not in patients with UC.
Given that calprotectin is a cytosolic protein, particularly, abundant in neutrophils, these results are not unexpected. Influx of neutrophils in the epithelium or in the intestinal lumen are known to increase the amount of calprotectin in the gut lumen (35). ln UC, the inflammation is confined to the mucosal layer, whereas in CD also the deeper layers may be affected. We postulate that time-to-elimination of neutrophils (in other words time-to-histologic healing) takes longer in CD, and this may be reflected in the longer time-to-reach target calprotectin levels. CD patients with a swift normalization of calprotectin values by induction therapy may have had a less deep type of inflammation, but we were unable to prove this in the present study, because of absence of follow-up biopsies.
CONCLUSIONS
With the use of a prospective registry, we have shown that reaching calprotectin levels <250 μg/g within 12 weeks of the initiation of induction therapy, facilitates recognition of new-onset CD patients with a favorable disease course, but this does not apply to new-onset UC patients. CD patients who fail to reach target calprotectin levels within 12 weeks after conventional induction therapy may be entitled to step-up to anti-TNF agents.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Ghione S, Sarter H, Fumery M, et al. Dramatic increase in incidence of ulcerative colitis and Crohn's disease (1988-2011): a population-based study of French adolescents. Am J Gastroenterol 2018; 113:265–272. [DOI] [PubMed] [Google Scholar]
- 2.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology 2008; 135:1106–1113. [DOI] [PubMed] [Google Scholar]
- 3.Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010; 16:953–961. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol 2014; 12:929.e2–934.e2. [DOI] [PubMed] [Google Scholar]
- 5.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 6.Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis 2016; 22:623–630. [DOI] [PubMed] [Google Scholar]
- 7.Ruemmele FM, Veres G, Kolho KL, et al. European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014; 8:1179–1207. [DOI] [PubMed] [Google Scholar]
- 8.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007; 133:423–432. [DOI] [PubMed] [Google Scholar]
- 9.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr 1991; 12:439–447. [PubMed] [Google Scholar]
- 10.Kappelman MD, Crandall WV, Colletti RB, et al. Short pediatric Crohn's disease activity index for quality improvement and observational research. Inflamm Bowel Dis 2011; 17:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heida A, Dijkstra A, Muller Kobold A, et al. Efficacy of home telemonitoring versus conventional follow-up: a randomized controlled trial among teenagers with inflammatory bowel disease. J Crohns Colitis 2018; 12:432–441. [DOI] [PubMed] [Google Scholar]
- 12.Dijkstra A, Heida A, van Rheenen PF. Exploring the challenges of implementing a web-based telemonitoring strategy for teenagers with inflammatory bowel disease: empirical case study. J Med Internet Res 2019; 21:e11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13:144–164. [DOI] [PubMed] [Google Scholar]
- 14.Haisma SM, van Rheenen PF, Wagenmakers L, et al. Calprotectin instability may lead to undertreatment in children with IBD. Arch Dis Child 2019; DOI: 10.1136/archdischild-2018-316584. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine YY, Koletzko J, Turner D. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. Zhonghua Er Ke Za Zhi 2016; 54:728–732. [DOI] [PubMed] [Google Scholar]
- 16.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011; 17:1314–1321. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018; 390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen V, Roseth A, Ahmad T, et al. Fecal calprotectin: a reliable predictor of mucosal healing after treatment for active ulcerative colitis. Gastroenterol Res Pract 2017; 2017:2098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jauregui-Amezaga A, Lopez-Ceron M, Aceituno M, et al. Accuracy of advanced endoscopy and fecal calprotectin for prediction of relapse in ulcerative colitis: a prospective study. Inflamm Bowel Dis 2014; 20:1187–1193. [DOI] [PubMed] [Google Scholar]
- 20.Zhulina Y, Cao Y, Amcoff K, et al. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther 2016; 44:495–504. [DOI] [PubMed] [Google Scholar]
- 21.Guizzetti L, Zou G, Khanna R, et al. Development of clinical prediction models for surgery and complications in Crohn's Disease. J Crohns Colitis 2018; 12:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn's disease. Gastroenterology 2006; 130:650–656. [DOI] [PubMed] [Google Scholar]
- 23.Khanna R, Jairath V, Feagan BG. The evolution of treatment paradigms in Crohn's Disease: beyond better drugs. Gastroenterol Clin North Am 2017; 46:661–677. [DOI] [PubMed] [Google Scholar]
- 24.Van Assche G, Dignass A, Panes J, et al. European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4:7–27. [DOI] [PubMed] [Google Scholar]
- 25.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet 2017; 389:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarur AJ, Strobel SG, Deshpande AR, et al. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol 2011; 7:652–659. [PMC free article] [PubMed] [Google Scholar]
- 27.Ziv-Baran T, Hussey S, Sladek M, et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-onset paediatric Crohn's disease. Aliment Pharmacol Ther 2018; 48:1242–1250. [DOI] [PubMed] [Google Scholar]
- 28.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110:802–819. [DOI] [PubMed] [Google Scholar]
- 29.D’Haens G, Baert F, van Assche G, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008; 371:660–667. [DOI] [PubMed] [Google Scholar]
- 30.Walters TD, Kim MO, Denson LA, et al. PRO-KIIDS Research Group. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn's disease. Gastroenterology 2014; 146:383–391. [DOI] [PubMed] [Google Scholar]
- 31.Khanna R, Bressler B, Levesque BG, et al. REACT Study Investigators. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386:1825–1834. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn's disease. J Crohns Colitis 2013; 7:213–221. [DOI] [PubMed] [Google Scholar]
- 33.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010; 362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 34.Jongsma MME, Cozijnsen MA, van Pieterson M, et al. Top-down infliximab superior to step-up in children with moderate-to-severe Crohn's disease - a multicenter randomized controlled trial. J Pediatr Gastroenterol Nutr 2019. 40–41. 68S1. [Google Scholar]
- 35.Magro F, Lopes J, Borralho P, et al. Portuguese IBD Study Group (GEDII). Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut 2018; 68:594–603. [DOI] [PubMed] [Google Scholar]
- 36.Nathan DG, Orkin SH, Lock AT, et al. Nathan and Oski's Hematology of Infancy and Childhood. Philadelphia: Saunders; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


