Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, biomarkers, cell cycle arrest, oliguria
Objectives:
Decreased urine output and/or increased serum creatinine may herald the development of acute kidney injury or reflect normal physiology. In this secondary analysis of the Sapphire study, we examined biomarkers of cell cycle arrest in the settings of oliguria and/or azotemia to improve risk assessment when used with conventional indices in predicting severe acute kidney injury (Kidney Disease: Improving Global Outcomes 3 defined by the need for renal replacement therapy or changes in urine output, serum creatinine or both) or death.
Design:
Prospective, international, Sapphire study.
Setting:
Academic Medical Center.
Patients:
Patients without acute kidney injury Kidney Disease: Improving Global Outcomes stage 2 or 3.
Interventions:
None.
Measurements and Main Results:
The primary endpoint being development of severe acute kidney injury or death within 1 week. Secondary analysis examined the relationship between tissue inhibitor of metalloproteinases-2 ([TIMP-2]) and insulin growth factor binding protein 7 ([IGFBP7]) and 9-month death or dialysis conditioned on progression to stage 2–3 acute kidney injury within 1 week. Seventy-nine patients reached the primary endpoint and were more likely to be surgical, with higher nonrenal Acute Physiology and Chronic Health Evaluation III scores and more chronic kidney disease. Stage 1 urine output, serum creatinine, and urinary [TIMP-2]•[IGFBP7] greater than 2.0 were all predictive of progression to the primary endpoint independent from nonrenal Acute Physiology and Chronic Health Evaluation III score. Combinations of predictors increased the hazard ratios considerably (from 2.17 to 4.14 to 10.05, respectively). In the presence of acute kidney injury (stage 1), [TIMP-2]•[IGFBP7] greater than 2.0 leads to an increased risk of death or dialysis at 9 months even in the absence of progression of acute kidney injury (stage 2–3) within 7 days.
Conclusions:
Cell cycle arrest biomarkers, TIMP-2 and IGFBP7, improve risk stratification for severe outcomes in patients with stage 1 acute kidney injury by urine output, serum creatinine or both, with risk increasing with each acute kidney injury indicator. Longer term outcomes demonstrate that the associated risks of a [TIMP-2]•[IGFBP7] greater than 2.0 is equivalent to acute kidney injury progression even where no progression from stage 1 acute kidney injury is observed.
Acute kidney injury (AKI) was first described over a decade ago in order to address limitations in identifying and categorizing individuals deemed to have acute renal failure (1, 2). The prior lack of consensus hindered comparative studies and therefore fundamental questions such as outcomes and the impact of potential interventions were not easily addressed. The current description of AKI has allowed for more precision especially in the critically ill (3, 4). AKI is defined and staged in terms of an increase in serum creatinine (SCr) and/or a reduction in urine output (UO) (5). Using this definition, the development of AKI is associated with an increase in both morbidity and mortality together with an increase in the length of hospital stay and costs (6–8). However, not all stages of AKI are clearly associated with death or the need for dialysis after controlling for baseline covariates. Furthermore, stage 1 AKI, while a useful trigger for clinical action, is not a hard clinical endpoint in the way stage 2 and especially, stage 3 are (9, 10).
Additionally, despite its utility for epidemiology, trial endpoints, and prognosis, the current definition of early stage AKI has limitations. Although a decrease in UO can be an early sign of AKI, it is neither sensitive nor specific. Changes in SCr are more specific but also lack sensitivity and may take up to 48 hours to manifest (11–13). Attempts to improve sensitivity by using increases in SCr greater than 0.3 mg/dL over 48 hours may diminish specificity especially in patients with a baseline SCr greater than 1.5 mg/dL (14). Thus, existing tools are insufficient for determining which patients will progress to stage 3 AKI.
We have previously reported an international, prospective, observational investigation (the Sapphire study) of tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin growth factor binding protein 7 (IGFBP7), both markers of G1 cell cycle arrest (15). These markers appear to increase rapidly after cell stress, even before injury occurs (16, 17). We have validated two [TIMP-2]•[IGFBP7] cut-offs for risk assessment for development of stage 2–3 AKI (18–20) and have reported an association between [TIMP-2]•[IGFBP7] at ICU admission and the composite endpoint of all-cause mortality and/or the receipt of renal replacement therapy at 9 months (21).
The clinical assay for urinary [TIMP-2]•[IGFBP7] became available in Europe in 2012 and the United States in 2014. Clinicians, therefore, currently use [TIMP-2]•[IGFBP7] in conjunction with UO and SCr in assessing the risk for stage 2–3 AKI (22, 23). In order to determine whether urinary [TIMP-2]•[IGFBP7] provides additional information to stage 1 UO and SCr for development of stage 3 AKI, we analyzed data from patients without stage 2–3 AKI at enrollment and compared each criterion along with interactions. Severe AKI (stage 3), dialysis, or death were chosen as endpoints as the prediction of these outcomes as a function of [TIMP-2]•[IGFBP7], and the other biomarkers would enable measures to be undertaken to mitigate the risk of progression. We examined the risk of developing stage 3 AKI, including the need for renal replacement therapy (RRT), or dying over the first week as well as the composite of dialysis or death over the course of the first 9 months following enrollment.
MATERIALS AND METHODS
We conducted a secondary analysis of the Sapphire study, an international, multicenter study of critically ill adults enrolled within 24 hours of ICU admission and evaluated biomarkers for AKI risk assessment (15). We analyzed [TIMP-2]•[IGFBP7], SCr, and UO in patients who did not have AKI stage 2 or 3 at the time of sample collection. The primary outcome was the composite of the development of AKI stage 3, dialysis, or death within 1 week of enrollment. We also examined the 9-month composite outcome of dialysis or death for patients stratified by [TIMP-2]•[IGFBP7], the presence of stage 1 AKI, and progression of AKI (stage 2 or greater) within 1 week.
Urine samples for measurement of [TIMP-2]•[IGFBP7] were collected at enrolment and at 12-hour intervals up to 30 hours after enrollment. The average number of sampling time points per subject was 2.7. Urinary [TIMP-2]•[IGFBP7] was measured in units of (ng/mL)2/1,000 using the NephroCheck Test (Astute Medical, San Diego, CA) (24). AKI status by UO and SCr were determined within ±12 hours relative to the time of [TIMP-2]•[IGFBP7] sample collection according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria (5).
Cox regression analyses were performed to assess the association of AKI status and [TIMP-2]•[IGFBP7] with the primary outcome. AKI status by UO and SCr and [TIMP-2]•[IGFBP7] (dichotomized around either the 0.3 or 2.0 (ng/mL)2/1,000 validated cut-off) (18, 20) were included in the regression models as time-varying covariates. We performed an additional analysis including Acute Physiology and Chronic Health Evaluation (APACHE) III score dichotomized around the median value in the models. Another Cox regression analysis was performed for the association of the number of positive risk factors (AKI status by UO and SCr and [TIMP-2]•[IGFBP7]) with the outcome. Kaplan-Meier curves extended for time-varying covariates were plotted to illustrate these associations (25).
To assess the association of AKI status and [TIMP-2]•[IGFBP7] with the outcome of 9-month dialysis or death, a categorical variable was constructed by whether the maximum [TIMP-2]•[IGFBP7] within 30 hours of enrollment was greater than 2.0 (ng/mL)2/1,000, whether stage 1 AKI was present within ±12 hours of [TIMP-2]•[IGFBP7], and whether stage 2–3 AKI was reached in a week. Cox regression was then performed with this categorical variable as a time-independent variable. Subjects who died or had dialysis in a week were excluded from this analysis. Kaplan-Meier curves were generated to illustrate this association.
All survival analyses were performed with the “survival” package in R (26). The proportional hazard assumption was checked with the “cox.zph” function. Overall Goodness-of-fit of Cox models was assessed by the Grønnesby and Borgan Test using the “gof” function in R package “survMisc” (27). All analyses were performed with R Version 3.4.4 (27, 28). Two-sided p values less than 0.05 were considered statistically significant. The Sapphire study was approved by investigational review boards/ethics committees of each of the participating sites. All subjects (or authorized representatives) provided written informed consent.
RESULTS
Patient Characteristics
Figure S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/E738; legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E742) shows the consort diagram with 661 patients analyzed from the Sapphire cohort of which 79 (10.9%) reached the primary endpoint (death or stage 3 AKI including RRT, over the first week)—50 patients died, and 41 patients had stage 3 AKI, of which 26 received RRT. Baseline patient characteristics are shown in Supplementary Table 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/E739). Patients who developed stage 3 AKI or died had a higher rate of chronic kidney disease were more likely to be surgical and had higher nonrenal APACHE III scores.
Risk of Death or Stage 3 AKI According to Enrollment UO, SCr or [TIMP2]•[IGFBP7]
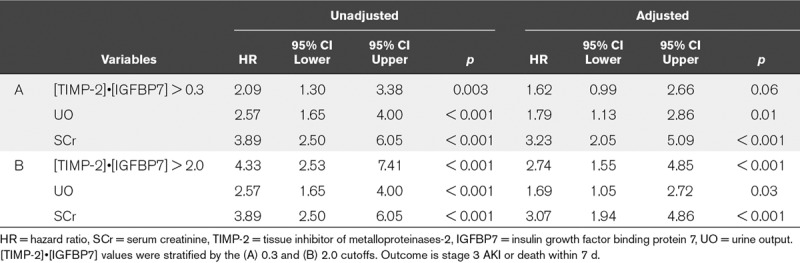
Table 1 shows hazard ratios (HRs) for [TIMP-2]•[IGFBP7], stage 1 UO or SCr for death, or AKI stage 3 within 7 days. HRs were stratified for the two established cut-offs for [TIMP-2]•[IGFBP7] (20). Unadjusted HRs were significant for all three variables, after adjustment [TIMP-2]•[IGFBP7] greater than 0.3 was no longer significant. A [TIMP-2]•[IGFBP7] greater than 2 showed an increased adjusted HR (HR, 2.74; 95% CI, 1.55–4.85) and p value of less than 0.001 with minimal observed decreases in the HRs of UO and SCr (Table 1). Stage 1 SCr showed a similar adjusted HR (3.07; 95% CI, 1.94–4.86) and p value of less than 0.001, and stage 1 UO showed a slightly lower adjusted HR (1.69; 95% CI, 1.05–2.72; p = 0.03). We performed an additional analysis including the nonrenal APACHE III score. [TIMP-2]•[IGFBP7] greater than 2.0 remained a strong predictive factor (HR, 2.49; 95% CI, 1.41–4.42; p < 0.002) in the multivariable model with nonrenal APACHE III score. The negative predictive value of [TIMP-2]•[IGFBP7] in predicting primary endpoint in stage 1 AKI patients was 0.83 (95% CI, 0.78–0.88) for the 2.0 cut-off.
TABLE 1.
Hazard Ratios for tissue inhibitor of metalloproteinases-2 and [IGFBP7], Urine Output, and Serum Creatinine Unadjusted and Adjusted for the Other Variables
Effects of Predictor Variable Combinations
A single predictor variable was defined by combining AKI status by SCr, AKI status by UO, and [TIMP-2]•[IGFBP7]. The reference group being the [TIMP-2]•[IGFBP7] less than or equal to at each cut-off (0.3 or 2.0) as well as the absence of AKI by SCr and UO: “1” positive indicates AKI defined by only one of SCr, UO, or [TIMP-2]•[IGFBP7] greater than 0.3 or 2.0; “2” positive indicates exactly two of the conditions are met; and “3” positive indicates that all three conditions are met (Table 2). Adding each of the single predictor variables, [TIMP-2]•[IGFBP7] greater than 0.3, UO, SCr, resulted in an incremental HR starting from 2.17 for one variable to 10.05 if all three predictor variables were positive (Table 2). HRs were further increased when the 2.0 cut-off for [TIMP-2]•[IGFBP7] was applied with an HR of 16.54 observed where all three variables were positive. This was also reflected in the respective Kaplan-Meier curves for Stage 3 AKI or death by the number of positive variables (Fig. S2, Supplemental Digital Content 3, http://links.lww.com/CCM/E740; legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E742).
TABLE 2.
Hazard ratios for Stage 3 Acute Kidney Injury or Death by Number of Positive Variables
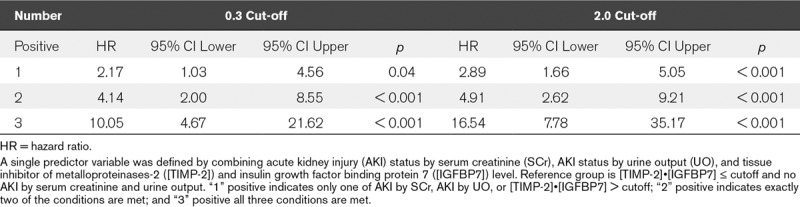
Figure 1 highlights the predictive ability of different combinations of SCr and [TIMP-2]•[IGFBP7] at both cut-offs. There was a clear increase in the probability of reaching the endpoint when both [TIMP-2]•[IGFBP7] and SCr were positive. Applying the 2.0 cut-off of [TIMP-2]•[IGFBP7], when SCr is positive at stage 1, there is an almost three-fold increase in the probability for reaching the endpoint compared with when SCr is at stage 1 without a positive biomarker. Even when using the lower cut-off of 0.3, the probability of meeting the endpoint was increased compared with SCr alone over the first 48 hours, but by 7 days the difference was more modest—about a third higher.
Figure 1.
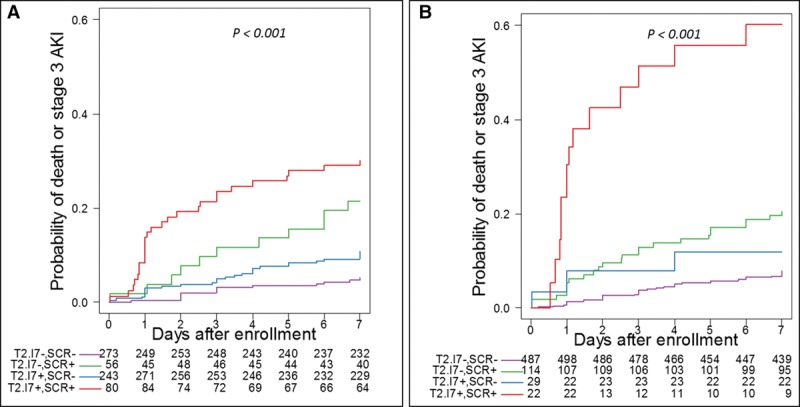
Kaplan-Meier curves for Stage 3 acute kidney injury (AKI) or death by AKI status by serum creatinine (SCr) and tissue inhibitor of metalloproteinases-2 ([TIMP-2]) and insulin growth factor binding protein 7 ([IGFBP7]) level relative to the 0.3 (A) and 2.0 (B) cutoffs. Patients are grouped as [TIMP-2]•[IGFBP7] negative and no AKI by SCr (purple), [TIMP-2]•[IGFBP7] negative and AKI by SCr (green), [TIMP-2]•[IGFBP7] positive and no AKI by SCr (blue), [TIMP-2]•[IGFBP7] positive and AKI by SCr (red). The values below the plots indicate number at risk. Long-rank p < 0.001 for both panels.
Where stage 1 UO is positive (Fig. 2A), [TIMP-2]•[IGFBP7] applied at the lower cut-off approximately doubled the probability of reaching the endpoint. Conversely, when UO is normal, [TIMP-2]•[IGFBP7] applied at the lower cut-off did not affect risk. However, [TIMP-2]•[IGFBP7] values greater than 2.0 discriminated for both UO positive and negative patients (Fig. 2B). Finally, we examined the effect of [TIMP-2]•[IGFBP7] for various combinations of positive or negative UO and SCr (Fig. S3, Supplemental Digital Content 4, http://links.lww.com/CCM/E741; legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E742). Here, we can observe that [TIMP-2]•[IGFBP7] further discriminated even when both SCr and UO criteria were positive (red curves) but did not discriminate (at either cutoff) when both SCr and UO criteria were negative (purple curves).
Figure 2.
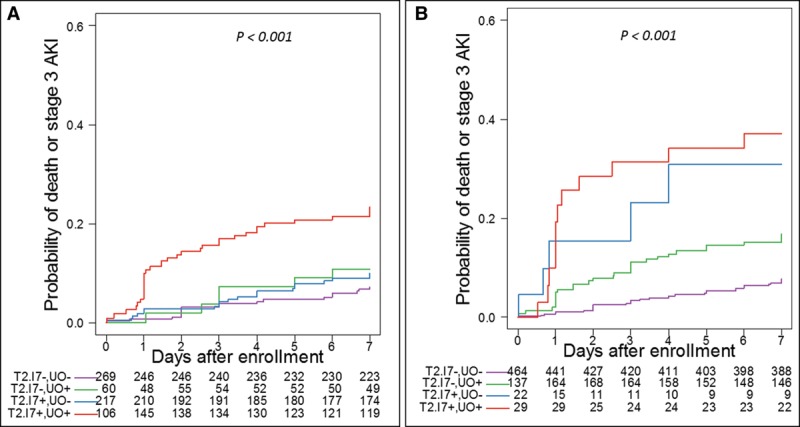
Kaplan-Meier curves for Stage 3 acute kidney injury (AKI) or death by AKI status by urine output (UO) and tissue inhibitor of metalloproteinases-2 ([TIMP-2]) and insulin growth factor binding protein 7 ([IGFBP7]) level relative to the 0.3 (A) and 2.0 (B) cutoffs. Patients are grouped as [TIMP-2]•[IGFBP7] negative and no AKI by UO (purple), [TIMP-2]•[IGFBP7] negative and AKI by UO (green), [TIMP-2]•[IGFBP7] positive and no AKI by UO (blue), [TIMP-2]•[IGFBP7] positive and AKI by UO (red). The values below the plots indicate number at risk. Long-rank p < 0.001 for both panels.
Biomarker Elevations and Long-Term Risk
Patients may not always manifest kidney injury by changes in functional biomarkers (SCr and UO), and this has been termed “subclinical” AKI (29). The relationship between [TIMP-2]•[IGFBP7] greater than 2.0 with or without evidence of AKI and 9-month death or dialysis conditional on development/progression of AKI in the first week is shown in Figure 3. In patients without clinical evidence of AKI (stage 1) and no progression, [TIMP-2]•[IGFBP7] did not discriminate the risk of death or dialysis at 9 months. However, in the presence of AKI, [TIMP-2]•[IGFBP7] greater than 2.0 led to a roughly two-fold increased risk of death or dialysis even in the absence of progression of AKI within the first week with a [TIMP-2]•[IGFBP7] greater than 2.0 essentially equivalent to the progression of AKI.
Figure 3.
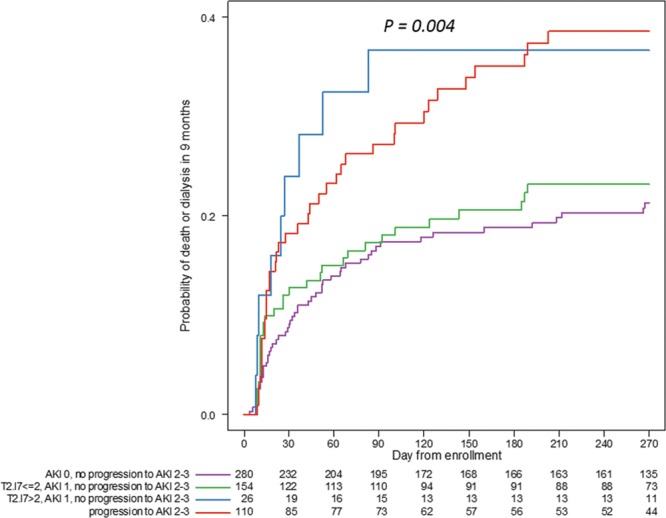
Kaplan-Meier curves for death or dialysis within 9 mo among patients who were alive and dialysis-free at 7 d after enrolment. Patients who did not progress to stage 2–3 AKI within 7 d from enrollment were divided into three categories based on the maximum tissue inhibitor of metalloproteinases-2 ([TIMP-2]) and insulin growth factor binding protein 7 ([IGFBP7]) value within 30 hr of enrollment and the maximum AKI stage within 12 hr of [TIMP-2]•[IGFBP7] as (1) no AKI, (2) stage 1 AKI and [TIMP-2]•[IGFBP7] less than or equal to 2.0, and (3) stage 1 AKI and [TIMP-2]•[IGFBP7] greater than 2.0. Patients who progressed to stage 2–3 AKI within 7 d were included as a single category. Long-rank p = 0.004.
DISCUSSION
Our current understanding of AKI dictates that risk assessment is ongoing—not only the risk for developing AKI but also the risk for progression in patients with earlier stages of AKI. This is especially true since most patients developing stage 1 AKI will reverse in the first week (30). Although risk prediction models do exist, they are often limited to specific cohorts and are often poorly generalizable (31–33). Therefore, methods to better assess risk for AKI progression are needed. Our results confirm that patients who develop stage 1 AKI are at significant risk for progression to stage 3 AKI—a level of severity unequivocally associated with adverse outcomes. Our results also indicate that urinary [TIMP-2]•[IGFBP7] greater than 2.0 alone poses a risk for subsequent stage 3 AKI or death, similar to stage 1 AKI by either UO or SCr alone. Future staging systems for AKI might well consider this relationship.
Furthermore, our results clearly demonstrate that combining biomarkers with UO or SCr enables substantially enhanced risk prediction by way of a composite risk assessment. This combined approach may well be of direct clinical relevance as previous studies in patients undergoing cardiac surgery demonstrate that SCr alone does not predict AKI progression (34). A previous study has shown that the combination of UO and SCr criteria conveys a substantially increased risk of death over either criterion alone (35). Our results, in this investigation, extend this finding further by demonstrating that when all three variables are positive (stage 1 UO, stage 1 SCr, and biomarkers), the HR for stage 3 AKI or death is 10.05 when using the 0.3 cut-off for [TIMP-2]•[IGFBP7] and 16.54 when using the greater than 2.0 cut-off (Table 2). These represent very large increases in the HR from 4.14 and 4.91 when only two variables are positive. Whether this increase in HR may be considered clinically relevant will depend heavily on the patient’s baseline risk. If, for example, baseline risk is 0.5%, then 1.5–2% might not be considered very different from 5% to 8% because the absolute risk increase is rather small. However, if the baseline is 5%, then the absolute risk increases from 15–20% to 50–80% and likely will be relevant for clinical risk assessment.
In this context, the question is raised whether this may be of clinical relevance because of changing treatment regimen for these patients. Whereas it must be conceded that biomarker-guided management of AKI has not become standard of care in many centers, there are two prospective randomized controlled trials that indicate a clinical benefit when choosing such an approach in specific patients. The prevAKI study showed a reduced rate of AKI including stage 2 and 3, by applying a certain bundle of interventions comprising hemodynamic optimization, avoidance of nephrotoxins and of hyperglycemia in biomarker positive cardiac surgery patients (36). Similar results were obtained in the Biomarker-guided intervention to prevent Acute Kidney injury (BigPAK) study, which included biomarker-positive general surgery patients and used an approach concentrating on optimizing fluid management and nephrology consultation (37).
Interestingly, [TIMP-2]•[IGFBP7] provides additional information as to the risk for stage 3 AKI or death for various combinations of UO and SCr (positive for stage 1, either, or both). Accordingly, assessment of kidney stress using urinary [TIMP-2]•[IGFBP7] represents an additional risk assessment tool whose information cannot be supplanted by SCr or UO. In fact, the 7-day risk of developing AKI stage 3 or death approaches 60% when urinary [TIMP-2]•[IGFBP7] is greater than 2.0 and stage 1 AKI is present using both UO and SCr. Furthermore, even in the absence of meeting this endpoint (or even progression to stage 2), [TIMP-2]•[IGFBP7] greater than 2.0, together with stage 1 AKI, significantly increases the risk of death or dialysis at 9 months. Thus, biomarker positive ([TIMP-2]•[IGFBP7] > 2.0) stage 1 AKI can be viewed on a par with stage 2–3 AKI in terms of the severity of the event. From a practicing clinicians perspective, the use of [TIMP-2]•[IGFBP7] in patients with stage 1 AKI would enable the introduction of measures known to reduce the further progression of AKI (36). Conversely, as we have previously shown (21, 33), an elevation of [TIMP-2]•[IGFBP7] in the absence of AKI does not influence 9-month death or dialysis. This result should be interpreted with caution; however, since it has been recently shown that in cardiac surgery patients, an increase in [TIMP-2]•[IGFBP7] confers a risk for the loss of functional renal reserve even in patients without clinical AKI (38) which supports the concept of early identification of subclinical AKI (29).
Our study is not without limitations. As a post hoc analysis of the Sapphire study, there are known risks of bias inherent to post hoc analyses like unrecognized confounding. Although we performed several multivariate analyses to control for confounding, the use of [TIMP-2]•[IGFBP7] levels in combination with SCr and/or UO in risk prediction still needs to be confirmed prospectively.
CONCLUSIONS
We have shown in a heterogeneous cohort of patients that cell cycle arrest biomarkers, TIMP-2 and IGFBP7, can improve risk stratification for severe outcomes (stage 3 AKI or death) even in patients with stage 1 AKI by UO, SCr, or both. We also found that risk for this outcome increases with each indicator of AKI, UO, SCr, and biomarkers. Finally, longer term outcomes (9 months) are also influenced by the elevated biomarkers in stage 1 AKI, even when they do not appear to progress. This analysis reflects the reality of clinical practice where multiple sources of information are integrated into medical decision-making.
Supplementary Material
Footnotes
For full list of Sapphire Investigators, see Appendix 1 (Supplemental Digital Content 6, http://links.lww.com/CCM/E860).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by Astute Medical.
Drs. Joannidis’, Forni’s, Koyner’s and Kellum’s institutions received funding from Astute Medical. Dr. Haase received funding from Astute Medical and lecture fees from Abbott and Alere. Dr. Koyner’s institution received funding from the University of Chicago (for enrolling patients into this prospective observational cohort study), and he received funding from Astute Medical (consulting). Drs. Shi and Chawla received funding from Astute Medical (consultant). Dr. Kellum received support for article research from Astute Medical. Dr. Kashani disclosed that he does not have any potential conflicts of interest.
REFERENCES
- 1.Bellomo R, Ronco C, Kellum JA. Acute renal failure[mdash]definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care 2004; 8:R204–R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum J, Ronco C. Acute renal failure: Time for consensus. Intensive Care Med 2001; 27:1685–1688 [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Kellum JA. Acute kidney injury: Epidemiology and diagnostic criteria. Curr Opin Crit Care 2006; 12:531–537 [DOI] [PubMed] [Google Scholar]
- 4.Petäjä L, Vaara S, Liuhanen S, et al. Acute kidney injury after cardiac surgery by complete KDIGO criteria predicts increased mortality. J Cardiothorac Vasc Anesth 2017; 31:827–836 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Nagaraja HN, Nadasdy T, et al. A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int 2012; 81:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clec/’h C, Gonzalez F, Lautrette A. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: A competing risks analysis. Critical care 2011; 15:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the acute kidney injury network criteria. Crit Care Med 2011; 39:2659–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchino S, Bellomo R, Bagshaw SM, et al. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 2010; 25:1833–1839 [DOI] [PubMed] [Google Scholar]
- 9.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 2015; 41:1411–1423 [DOI] [PubMed] [Google Scholar]
- 10.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 2009; 35:1692–1702 [DOI] [PubMed] [Google Scholar]
- 11.Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest 2012; 141:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelbaum T, Lee J, Scott DJ, et al. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med 2013; 39:414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 2009; 20:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Fernandez H, Shashaty MG, et al. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol 2015; 10:1723–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarbock A, Schmidt C, Van Aken H, et al. ; RenalRIPC Investigators: Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. JAMA 2015; 313:2133–2141 [DOI] [PubMed] [Google Scholar]
- 17.Emlet DR, Pastor-Soler N, Marciszyn A, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: Differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 2017; 312:F284–F296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014; 189:932–939 [DOI] [PubMed] [Google Scholar]
- 19.Chindarkar NS, Chawla LS, Straseski JA, et al. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]·[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta 2016; 452:32–37 [DOI] [PubMed] [Google Scholar]
- 20.Hoste EA, McCullough PA, Kashani K, et al. ; Sapphire Investigators: Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014; 29:2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyner JL, Shaw AD, Chawla LS, et al. ; Sapphire Investigators: Tissue Inhibitor Metalloproteinase-2 (TIMP-2)⋅IGF-Binding Protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 2015; 26:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellum JA, Chawla LS. Cell-cycle arrest and acute kidney injury: The light and the dark sides. Nephrol Dial Transplant 2016; 31:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayan A, Faubel S, Askenazi DJ, et al. ; American Society of Nephrology Acute Kidney Injury Advisory Group: Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 2016; 68:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uettwiller-Geiger DL, Vijayendran R, Kellum JA, et al. Analytical characteristics of a biomarker-based risk assessment test for acute kidney injury (AKI). Clin Chim Acta 2016; 455:93–98 [DOI] [PubMed] [Google Scholar]
- 25.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 2005; 59:301–307 [Google Scholar]
- 26.Therneau T.A Package for Survival Analysis in S. version 2.38, 2015. Available at: http://CRAN.R-project.org/package=survival. Accessed January 11, 2017.
- 27.Dardis C.survMisc: Miscellaneous Functions for Survival Data. R package version 0.5.5, 2018. Available at: https://CRAN.R-project.org/package=survMisc Accessed July 5. 2018.
- 28.R Core Team: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2018Vienna, Austria. [Google Scholar]
- 29.Haase M, Kellum JA, Ronco C. Subclinical AKI–an emerging syndrome with important consequences. Nat Rev Nephrol 2012; 8:735–739 [DOI] [PubMed] [Google Scholar]
- 30.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demirjian S, Schold JD, Navia J, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis 2012; 59:382–389 [DOI] [PubMed] [Google Scholar]
- 32.Forni LG, Dawes T, Sinclair H, et al. Identifying the patient at risk of acute kidney injury: A predictive scoring system for the development of acute kidney injury in acute medical patients. Nephron Clin Pract 2013; 123:143–150 [DOI] [PubMed] [Google Scholar]
- 33.Koyner JL, Adhikari R, Edelson DP, et al. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol 2016; 11:1935–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyner JL, Garg AX, Coca SG, et al. ; TRIBE-AKI Consortium: Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 2012; 23:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 2015; 26:2231–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med 2017; 43:1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gocze I, Jauch D, Gotz M, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: The Prospective Randomized BigpAK Study. Ann Surg 2017; 267:1013–1020 [DOI] [PubMed] [Google Scholar]
- 38.Husain-Syed F, Ferrari F, Sharma A, et al. Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant 2019; 34:308–317 [DOI] [PubMed] [Google Scholar]


