Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, child, continuous renal replacement therapy, hospital mortality, severe sepsis
Objectives:
Continuous renal replacement therapy becomes available utilization for pediatric critically ill, but the impact of mortality rate in severe sepsis remains no consistent conclusion. The aim of the study is to assess the effect of continuous renal replacement therapy in pediatric patients with severe sepsis and the impact this therapy may have on their mortality.
Design:
Propensity score-matched cohort study analyzing data prospectively collected by the PICUs over 2 years (2016–2018).
Setting:
Four PICUs of tertiary university children’s hospital in China.
Patients:
The consecutive patients with severe sepsis admitted to study PICUs were enrolled from July 2016 to June 2018.
Interventions:
The patients were divided into the continuous renal replacement therapy group and the conventional (noncontinuous renal replacement therapy) group.
Measurements and Main Results:
A total of 324 patients with severe sepsis were enrolled. The hospital mortality rate was 35.6% (64/180) in the continuous renal replacement therapy group and 47.9% (69/144) in the noncontinuous renal replacement therapy group. After propensity score adjustment, the hospital mortality rate was 21.3% (29/136) in the continuous renal replacement therapy group and 32.4% (44/136) in the noncontinuous renal replacement therapy group. In subgroup analysis, the relative risk of dying was 0.447 (95% CI, 0.208–0.961) only in patients complicated by acute respiratory distress syndrome (p = 0.037), but not in patients with shock, acute kidney injury, acute liver dysfunction, encephalopathy, and fluid overload greater than 10%. The mean duration of continuous renal replacement therapy was 45 hours (26–83 hr) with an ultrafiltration rate of 50 mL/kg/hr. The level of interleukin-6 was decreased, and the percent of natural killer cells (%) was improved in the continuous renal replacement therapy group compared with the noncontinuous renal replacement therapy group. Furthermore, continuous renal replacement therapy was an independently significant risk factor for hospital mortality in pediatric patients with severe sepsis, and the interval between continuous renal replacement therapy initiation and PICU admission was an independent risk factor for hospital mortality in patients receiving continuous renal replacement therapy.
Conclusions:
Continuous renal replacement therapy with an ultrafiltration rate of 50 mL/kg/hr decreases hospital mortality rate in pediatric severe sepsis, especially in patients with acute respiratory distress syndrome.
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection (1). Severe sepsis remains a burdensome health problem in infantile and children population. A worldwide study in 128 PICUs across 26 countries indicated that the hospital mortality rate was 25% in severe sepsis, and 17% of survivors had new moderate or worse disability at hospital discharge (2). Sepsis-induced acute kidney injury (AKI) comprises up to half of critical illness–associated AKI in adults (3, 4). The occurrence rate of AKI was reported 21% in pediatric severe sepsis, and septic AKI plays an independent risk factor for death and new disability (2, 5). Therefore, management of severe sepsis is still a most challenging issue in the PICU.
Accumulated evidences have strongly demonstrated that AKI and fluid overload (FO) were associated with high mortality in severe sepsis (5–7). Surviving Sepsis Campaign 2016 (1) suggests using continuous renal replacement therapy (CRRT) in patients with sepsis and AKI (weak recommendation, moderate quality of evidence), facilitating management of fluid balance in hemodynamically unstable septic patients (weak recommendation, very low quality of evidence). The benefits of CRRT in sepsis include kidney replacement, management of FO, clearance of toxic substances, alleviating of inflammatory reaction, or some combinational actions (6–9). Mortality ranges from 39% to 59% in children with sepsis receiving CRRT, likely reflecting severity of illness of patients election for CRRT application (10). However, well-designed clinical study regarding the role of CRRT in pediatric sepsis is limited in the PICU.
In the present study, we conducted a prospective multicenter cohort study to evaluate the effect of CRRT on the hospital mortality in pediatric severe sepsis.
MATERIALS AND METHODS
Study Design
We set up an observational multicenter prospective cohort of PICU patients with severe sepsis. The study was approved by the ethics committee of Children’s Hospital affiliated to Shanghai Jiao Tong University (Approval number: 2016R011-E02).
Setting and Patients
Patients were recruited in four university tertiary PICUs located in China mainland from July 2016 to June 2018. The clinical examination and data collection for enrolled patients were performed using standardized case report forms. The patients with severe sepsis were diagnosed based on the International Pediatric Sepsis Consensus Conference in 2005 (11) and Surviving Sepsis Campaign International Guidelines in 2012 (12). Sepsis-associated AKI was defined as infectious episode with AKI according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria (13). Sepsis-associated FO was defined as the FO greater than 10% (FO = [CRRT initial weight – PICU admission weight]/PICU admission weight × 100%) (14).
The inclusion criteria were as follows: 1) patients from 29 days to 18 years old; 2) patients with severe sepsis within 7 days; and 3) patients with one or more organ dysfunctions such as AKI, acute respiratory distress syndrome (ARDS), acute liver dysfunction, septic shock, or multiple organ dysfunction syndromes. Patients were excluded if they had the following condition: 1) patients with PICU stay less than 24 hours; 2) patients with primary immune function defect; 3) irreversible neurologic dysfunction; and 4) patients without the informed consent.
Patient’s Treatment
Patients were treated in accordance to current guidelines for treatment of sepsis. Initial management included fluid therapy, antibiotics, vasoactive drugs, nutrition, and other supportive therapy recommended by the International Pediatric Sepsis Consensus Conference in 2005 (11) and Surviving Sepsis Campaign International Guidelines in 2012 (12). The indications for CRRT include sepsis complicated by AKI, FO (> 10%), or hemodynamic instability, electrolyte disturbance, or moderate or severe ARDS (Pao2/Fio2, < 150 mm Hg). Electrolyte disturbance as a indication for CRRT was defined as the intractable electrolyte disturbance with poor response to conventional therapy. Sepsis complicated by moderate or severe ARDS (Pao2/Fio2, < 150 mm Hg) is an indication for CRRT initiation.
According to whether received CRRT, the patients were divided into CRRT group and non-CRRT group. Patients were excluded for CRRT and treated with conventional therapies if they met any of the following criteria: 1) complicated with severe coagulopathy disorder (international normalized ratio, > 3.0; or platelet count, < 10 × 109/L); 2) a history of biofilm or hemofilter allergy; 3) without consent for CRRT; 4) with difficult to set CRRT catheter access; and 5) acute leukemia or malignant tumor with a life expectancy of less than 2 years. In the non-CRRT group, besides of following current guidelines for treatment of sepsis, solution restriction and the application of diuretics should be considered if blood pressure could be stable with vasoactive drugs. All therapeutic decisions were independently made by the attending intensivists according to standard practice in each PICU.
CRRT Procedures
We performed CRRT with an ultrafiltration rate of 50 mL/kg/hr, performed using commercially available pump-driven machines (Prisma or Prismaflex; Gambro, Lund, Sweden) and HF20, M100, M60, or hemofilter of AN69 membrane (Gambro) dependent on body weights. For the patient complicated with AKI, hemofiltration and hemodialysis were performed. The blood flow rate was set on 4–6 mL/kg/min to achieve a filtration fraction of 25–35% in patients with FO. The filter circuit was prewashed with saline containing 5,000–10,000 IU/L unfractionated heparin (UFH). Vascular access was obtained with 6.5F–12F central venous catheters (GamCath; Gambro, Colombes, France) in the right internal jugular or femoral vein, according to patient body weight. Anticoagulation was achieved with UFH adjusted to a target activated partial thromboplastin time (APTT) of 40–55 seconds (1.5- to two-fold of normal value) or activated coagulation time (ACT) of 150–180 seconds. ACT and APTT were detected once per 4–6 hours. The hemofilter was changed every 24 hours, transmembrane pressure greater than 200 mm Hg, or when clotted as described in our previous study (15). The indications, performance, and management for CRRT maintained uniform standards among four PICUs in our study (supplemental data, Supplemental Digital Content 1, http://links.lww.com/CCM/E733).
Clinical Data Collection
Data on demographics, clinical manifestations, comorbidities, illness severity, and outcome were collected prospectively. Underlying conditions on PICU admission were classified following the pediatric complex chronic conditions classification system (16). Illness severity was measured by the Pediatric Risk of Mortality (PRISM) score III (17). All subject records were encoded with a unique identifier that corresponded to the center and respective patient number.
Pre-CRRT initiation data collected were comprised of the following: age, gender, primary disease, comorbidity, and indications for CRRT initiation, the interval between CRRT initiation and PICU admission. The biochemical parameters in patients including lactate, the ratio of the Pao2 to the Fio2 (Pao2/Fio2), alanine transaminase (ALT), total bilirubin (TBIL), serum creatinine (sCr), blood urea nitrogen (BUN), inflammatory factors (tumor necrosis factor [TNF]-α and interleukin-6), and the percent of immune cells (natural killer [NK] cells, cluster of differentiation [CD]19+, CD4+, and CD8+) were prospectively collected.
Outcome Measures
The primary outcome was all-cause hospital mortality. Secondary outcomes included the length of PICU stay, 28-day mortality, changes of biochemical indexes, and the factors related to prognosis in patients received CRRT. The 28-day mortality was defined as the survival status after diagnosis with severe sepsis. All data were collected in well-designed case report forms. Monthly telephone conferences, biannual meetings, and clinical protocols including case definitions, data audits, and monitoring ensured uniform procedures among study sites.
Propensity Score Methods
In this prospective cohort study, propensity score matching analysis was performed to reduce the imbalance between the CRRT and non-CRRT groups. All the possible covariables (age, gender, shock, AKI, and FO) were included in the propensity score matching. Propensity scores were calculated using a logistic regression model. A one-to-one nearest neighbor matching algorithm was applied with a caliper of 0.02 and without replacement. The balance of variables in both groups was assessed by the standardized differences.
Statistical Analysis
Continuous variables were summarized as means ± sds for normal distribution data and as median (interquartile range) for abnormal distribution data. All variables were tested for normal distribution by using the Kolmogorov-Smirnov test. Independent-samples t test (for normal distribution data), Mann-Whitney U tests (for abnormal distribution data), or one-way analysis of variance (for repeated measurement data) was used to compare parameters in different groups. Categorical variables were presented as numbers and percentages and compared using chi-square tests. Primary analysis for hospital mortality was performed on the complete cases and the propensity-matched cohort. We performed exploratory subgroup analysis, restricting to patients with different organ dysfunction or receiving CRRT. The multivariate logistic regression analysis was used to access the independent risk factors for mortality of patients with severe sepsis with or without CRRT.
All tests were two sided, and p values that were less than 0.05 were considered to be statistically significant. All statistical analyses were performed with the SPSS version 23.0 statistical software program (SPSS, Chicago, IL).
RESULTS
Baseline Characteristics of Pediatric Patients With Severe Sepsis
Over the 2-year study period, a total of 407 patients were screened and 324 cases were enrolled. Among these patients, the mean age was 23.5 months (9–48 mo) and 182 patients (56.2%) were male. Of 324 patients with severe sepsis, 133 patients died and overall hospital mortality was 41.1%. Among 324 children with severe sepsis, 180 patients (55.6%) received CRRT (CRRT group) and 144 cases (44.4%) management in conventional strategy (non-CRRT group). After propensity score adjustment, there were 136 cases included in each group (Fig. 1).
Figure 1.
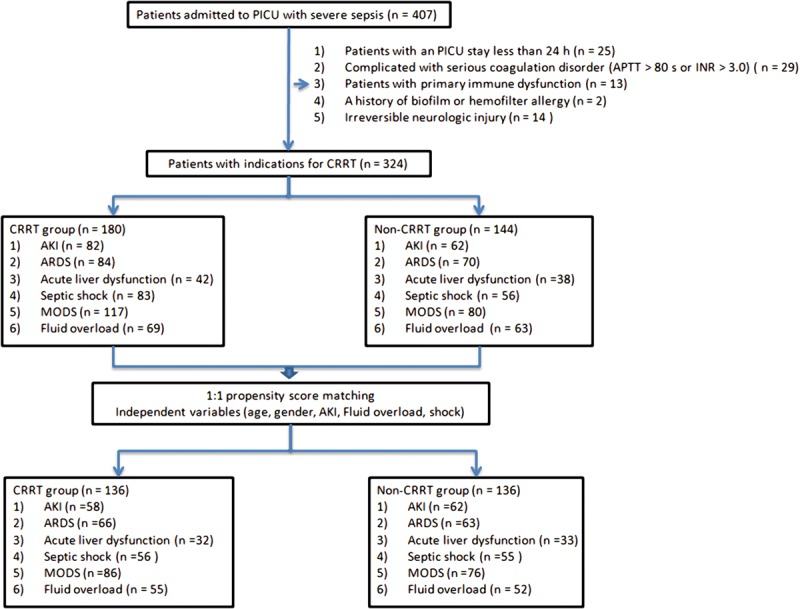
Flowchart of patients with severe sepsis enrolled in this multicenter study and the propensity score methods. AKI = acute kidney injury, APTT = activated partial thromboplastin time, ARDS = acute respiratory distress syndrome, CRRT = continuous renal replacement therapy, INR = international normalized ratio, MODS = multiple organ dysfunction syndrome.
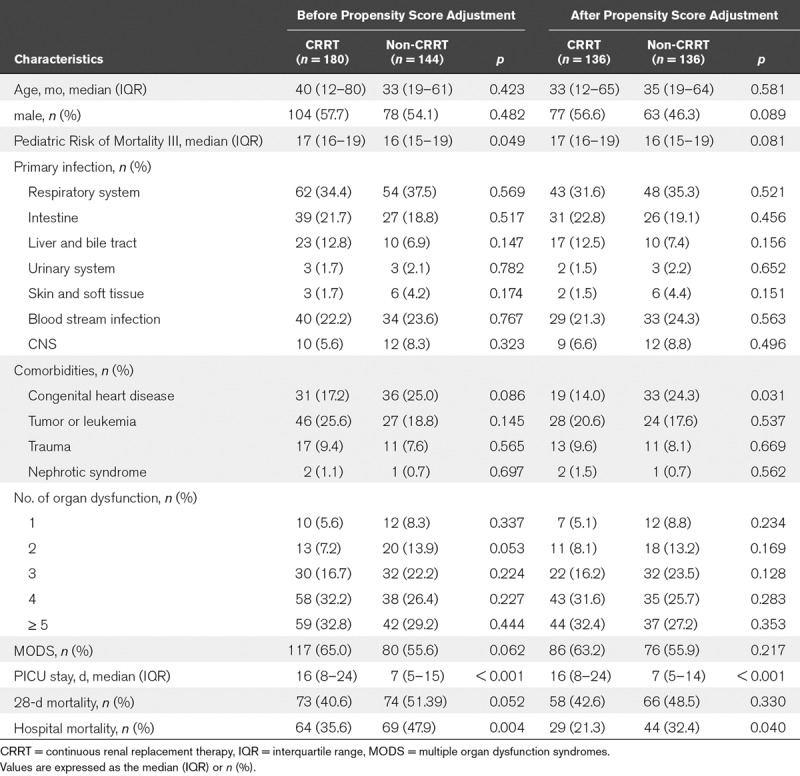
The mainly primary infection was pneumonia in 116 cases (35.8%), followed by blood stream infection in 74 cases (22.8%), intestine in 66 cases (20.4%). The occurrence rate of comorbidities in enrolled patients was 52.8% (171/324) at admission. The most common comorbidity was congenital heart disease (20.7%; n = 67), followed by malignancies (22.5%; n = 73) and trauma (8.6%; n = 28). After propensity score adjustment, there were 136 cases in each group, and there was no significant difference in aspects of age, gender, PRISM III score, and comorbidities expect of congenital heart disease (all p > 0.05). The baseline characteristics of the patients with severe sepsis were presented in Table 1.
TABLE 1.
Demographic and Clinical Characteristics of Pediatric Severe Sepsis Receiving Continuous Renal Replacement Therapy and Without Continuous Renal Replacement Therapy
Hospital Mortality as Primary Outcome in the Propensity-Matched Cohort
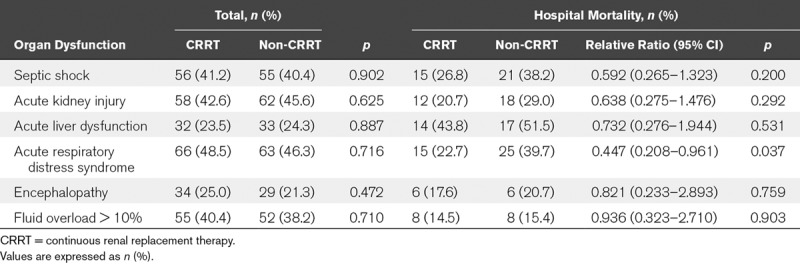
The hospital mortality rate was 21.3% (29/136) in the CRRT group and 32.4% (44/136) in the non-CRRT group, respectively. The duration of CRRT was 45 hours (26–83 hr) in the CRRT group. In subgroup, the hospital mortality rates in ARDS were 22.7% (15/66) in the CRRT group and 39.7% (25/63) in the non-CRRT group (relative risk [RR], 0.447; 95% CI, 0.208–0.961; p = 0.037). The hospital mortality rates in AKI were 20.7% (12/58) in the CRRT group and 29.0% (18/62) in the non-CRRT group (RR, 0.638; 95% CI, 0.275–1.476; p = 0.292). There were decreased tendency without statistic difference between CRRT and non-CRRT group in the subgroups of septic shock (26.8% vs 38.2%; p = 0.200), acute liver dysfunction (43.8% vs 51.5%; p = 0.531), encephalopathy (17.6% vs 20.7%; p = 0.759), or FO greater than 10% (14.5% vs 15.4%; p = 0.903) (Table 2).
TABLE 2.
Hospital Mortality in Pediatric Severe Sepsis Complicated by Organ Dysfunction With Continuous Renal Replacement Therapy or Without Continuous Renal Replacement Therapy in the Propensity-Matched Cohort
Changes of Biochemical Parameters in the Propensity-Matched Cohort
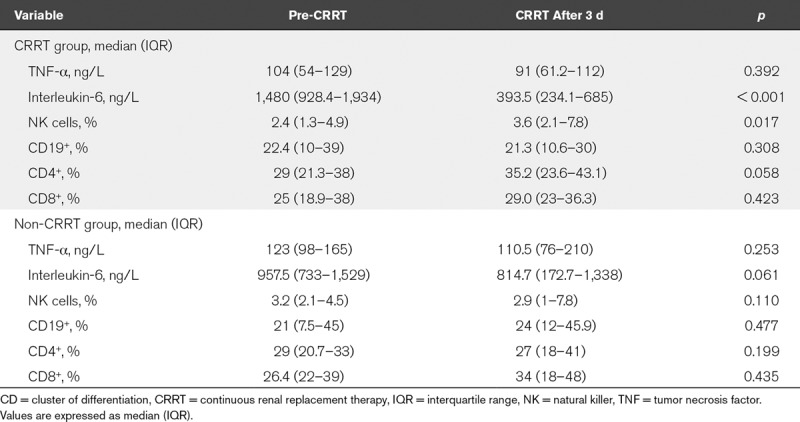
The changes in biochemical parameters for organ functions were showed in Supplemental Figure 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/E734; and legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E737) and Table 3. The plasma lactate levels were reduced gradually from 0 to 72 hours after CRRT (3 mmol/L [2–6.5 mmol/L] vs 2 mmol/L [1–3 mmol/L]; p < 0.001). The value of Pao2/Fio2 was significantly increased at 72 hours after CRRT (156 mm Hg [110–198 mm Hg] vs 166 mm Hg [121–210 mm Hg]; p < 0.001), and the clinical relevance of this effect needs further investigation. In addition, the levels of ALT were decreased after CRRT (p < 0.001), but not TBIL (p = 0.612). The indicators for renal function including sCr and BUN were significantly decreased after CRRT (both p < 0.001) (Supplemental Fig. 1, Supplemental Digital Content 2, http://links.lww.com/CCM/E734; and legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E737). However, there was no significant change after conventional therapy except of serum lactate level (Supplemental Fig. 1, Supplemental Digital Content 2, http://links.lww.com/CCM/E734; and legend, Supplemental Digital Content 5, http://links.lww.com/CCM/E737). Interleukin-6 level was significantly decreased at 72 hours after CRRT (1,480 ng/L [928.4–1,934 ng/L] vs 393.5 ng/L [234.1–685 ng/L]; p < 0.001). However, the levels of TNF-α were no difference before and after CRRT (p = 0.392). In addition, the percent of NK cells (NK%) was significantly increased at 72 hours after CRRT treatment (2.4% [1.3%–4.9%] vs 3.6% [2.1%–7.8%]; p = 0.017) (Table 3). In the non-CRRT group, the levels of interleukin-6 showed a decreased tread after 72 hours treatment without statistical significance (957.5 ng/L [733–1,529 ng/L] vs 814.7 ng/L [172.7–1,338 ng/L]; p = 0.061), and there was no significant change in the level of TNF-α and the percent of NK%, CD19+, CD4+, and CD8+ (all p > 0.05) (Table 3).
TABLE 3.
Comparison of Immunology and Cytokines Between the Continuous Renal Replacement Therapy and Non-Continuous Renal Replacement Therapy Groups in the Propensity-Matched Cohort
Application of CRRT and the Interval Between CRRT Initiation and PICU Admission as Independent Factors for Mortality of Pediatric Severe Sepsis in the Propensity-Matched Cohort
The ratio of CRRT in survivors was 78.7% (107/199), which was higher than that in nonsurvivors (21.3%; 29/73) (p = 0.040). The levels of sCr, BUN, Paco2, lactate, ALT, TBIL, TNF-α, interleukin-6, as well as NK%, CD19+, CD4+, and CD8+ cells displayed no difference between nonsurvivors and survivors (all p > 0.05) (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/CCM/E735). In the subgroup of patients receiving CRRT, the interval between CRRT initiation and PICU admission was significantly longer in nonsurvivors than in survivors (23 hr [19–37 hr] vs 14 hr [11–22 hr]; p < 0.001). The basic value of Pao2/Fio2 showed a lower tendency in nonsurvivors than in survivors (121 [99–154] vs 143 [112–202]; p = 0.044) (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/CCM/E735).
Additionally, multivariate logistic analysis indicated that CRRT application was an independently protective factor for hospital mortality in pediatric severe sepsis (odds ratio [OR], 0.567 [0.328–0.978]; p = 0.041). Furthermore, the interval between CRRT initiation and PICU admission was an independently risk factor for hospital morbidity in patients with severe sepsis receiving CRRT (OR, 1.067 [1.029–1.106]; p < 0.001) adjusted by Pao2/Fio2 value (Table 4). Supplemental Worksheet for this study is available online (Supplemental Digital Content 4, http://links.lww.com/CCM/E736).
TABLE 4.
Logistic Analysis of Variables Independently Associated With Hospital Mortality in Pediatric Patients With Severe Sepsis in the Propensity-Matched Cohort

DISCUSSION
In this multicenter, propensity score-matched, observational prospective cohort study, we demonstrated that CRRT decreases the hospital mortality rate in pediatric severe sepsis, especially in sepsis-associated ARDS. In addition, hospital mortality rate showed a reduced tendency without statistic significance in the CRRT group compared with the non-CRRT group in the subgroups of AKI, shock, acute liver dysfunction, or encephalopathy. Furthermore, CRRT application was a protective factor in pediatric severe sepsis, and the interval between CRRT initiation and PICU admission was significantly associated with the prognosis of children with severe sepsis under CRRT.
Various extracorporeal therapies including CRRT had been successfully conducted in critically ill patients (18). Until now, the dose of hemofiltration used in pediatric sepsis has not reached a consensus. In high-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE) study, high-volume hemofiltration (70 mL/kg/hr) did not significantly reduce 28-day mortality compared with standard volume (35 mL/kg/hr) in adult patients with septic shock complicated by AKI (19). Recent study indicated that high dose (80 mL/kg/hr) of continuous venovenous hemodiafiltration did not improve 28-day mortality in patient with sepsis with AKI (20). Based on our previous study (15, 21) and to avoid the difference among four centers, we set the dose of hemofiltration at 50 mL/kg/hr in the present study.
In adult ICU, hospital mortality and 28-day mortality range from 50.4% to 64.5% in patients with septic AKI under CRRT (20, 22), and the survival rate is significantly associated with the timing of CRRT (22). Furthermore, mortality ranges from 39% to 59% in children with sepsis receiving CRRT (10). Until now, the benefits of CRRT are still no consistent conclusion in pediatric sepsis (12, 23). In present study, the hospital mortality rate was significantly decreased in the CRRT group (21.3%) compared with the non-CRRT group (32.4%) in the propensity-matched cohort. More importantly, the hospital mortality rate in sepsis-associated ARDS was significantly decreased, which needs further investigation. Although AKI is a specific indication for CRRT, the hospital mortality rate in septic AKI received CRRT had decreased mortality tendency compared with non-CRRT group without statistic significance, partially due to relatively small sample size.
Intriguingly, we found that CRRT significantly decreases hospital mortality rate in pediatric ARDS (22.7% in the CRRT group vs 39.7% in the non-CRRT group). In the past decade, there were studies suggesting CRRT as an effective therapy for ARDS treatment in patients associated with sepsis, pancreatitis, pneumonia, etc (24–26). In addition, reports in adults also suggested improvement of oxygenation and survival benefit using CRRT in ARDS, renal transplantation (27), bone morrow transplantation or chemotherapy (28), and allogeneic hematopoietic stem cell transplantation (29). Recently, meta-analysis of adult randomized trials reports that continuous venovenous hemofiltration might be associated with lower mortality in a population of critically ill patients with ARDS or severe sepsis/septic shock. However, the quality of evidence is still insufficient to support a conclusion of benefit (30). Until now, little information about the benefits of CRRT is available in pediatric ARDS. To our knowledge, this is the largest study about CRRT in pediatric septic ARDS in China. All these results suggested that CRRT could be potential therapy for patients with ARDS.
Fluid management is associated with clinical outcome including ventilator-free days and oxygenation, especially in patients with ARDS complicated by FO (31). Nonsurvivors receiving CRRT with higher FO demonstrated less improvement in mechanical ventilation during CRRT (32). In addition, FO impairs gas exchange and reduces lung compliance in ARDS (33). In our study, the Pao2/Fio2 was improved after 72 hours of CRRT, indicating that improved oxygenation is partially contributed by CRRT. Consistently, a previous study reported that Pao2/Fio2 after 24 hours of CRRT was higher in ARDS patients with extrapulmonary etiology than in those with pulmonary etiology (34). Furthermore, early initiation (within 12 hr after ARDS onset) of CRRT significant improves oxygenation and significantly decreased bronchoalveolar lavage fluid after CRRT (26). In our study, we found that the interval between CRRT initiation and PICU admission was independently associated with the outcome of pediatric severe sepsis received CRRT. So, we speculated that the improved oxygenation of CRRT in septic ARDS was partially reduction of FO and bronchoalveolar lavage fluid.
We previously indicated that CRRT significantly reduced the inflammatory factors interleukin-6 in patients with severe sepsis (21) and secondary hemophagocytic lymphohistiocytosis (15). Furthermore, the percentage of NK cells at day 1 was associated with mortality in patients with severe sepsis (35). We speculated that the decreased interleukin-6 level and increased NK% contributed to the impact of CRRT in severe sepsis, which needs pay attention to confirm in the future.
Our study has several limitations. First, the observational design precludes accurate conclusions due to lacking of randomization for CRRT. Second, we only enrolled the patients from the four PICUs located in China mainland. This may reduce generalization of our results. Third, for safety, we exclude patients with severe coagulopathy. Fourth, we did not systematically monitor the extravascular lung water (EVLW) in children with severe ARDS because EVLW monitoring is difficult to perform in pediatric population. It may impact the observed outcomes in ARDS received CRRT. Fifth, we only performed the CRRT with the ultrafiltration dose of 50 mL/kg/hr. The effects of different ultrafiltration dose of CRRT in pediatric sepsis need further investigation.
CONCLUSIONS
In our prospective, propensity score-matched, multicenter cohort study, we demonstrated that CRRT therapy decreases the hospital mortality in pediatric severe sepsis, especially in patients with severe sepsis complicated by ARDS. CRRT application is protective for pediatric patients with severe sepsis. Of pediatric patients supported by CRRT, the interval between CRRT initiation and PICU admission is significantly correlated with the prognosis.
Supplementary Material
Footnotes
Drs. Miao and Shi contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by grants from the Science and Technology Commission of Shanghai Municipality (16411970300,18411951000), funded by Clinical Multicenter Study supported by Clinical Research Center of Shanghai Jiao Tong University School of Medicine (DLY201618, 20171928), New Advanced Technology Project at the Shanghai City Hospital Development Center (SHDC12014116).
Drs. Wang and Zhang received funding from Clinical Multicenter Study supported by Clinical Research Center of Shanghai Jiao Tong University School of Medicine (DLY201618, 20171928). Dr. Zhang received funding from Science and Technology Commission of Shanghai Municipality (16411970300); Science and Technology Commission of Shanghai Municipality (18411951000). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45:486–552 [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee: Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care 2008; 12:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. ; VANISH Investigators: Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH randomized clinical trial. JAMA 2016; 316:509–518 [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. ; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network: Acute kidney injury in pediatric severe sepsis: An independent risk factor for death and new disability. Crit Care Med 2016; 44:2241–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr Opin Crit Care 2014; 20:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming GM, Sahay R, Zappitelli M, et al. The Incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: A multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med 2016; 17:1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: A retrospective analysis. Crit Care Med 2004; 32:1771–1776 [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Cass A, Cole L, et al. ; RENAL Replacement Therapy Study Investigators: Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361:1627–1638 [DOI] [PubMed] [Google Scholar]
- 10.Davis AL, Carcillo JA, Aneja RK, et al. American college of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017; 45:1061–1093 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 2–8 [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup: Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637 [DOI] [PubMed] [Google Scholar]
- 13.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 2015; 10:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein SL, Currier H, Graf Cd, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001; 107:1309–1312 [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Zhang YC, Kang YL, et al. High-volume hemofiltration in critically Ill patients with secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome: A prospective study in the PICU. Pediatr Crit Care Med 2016; 17:e437–e443 [DOI] [PubMed] [Google Scholar]
- 16.Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack MM, Ruttimann UE, Getson PR. Pediatric Risk of Mortality (PRISM) score. Crit Care Med 1988; 16:1110–1116 [DOI] [PubMed] [Google Scholar]
- 18.Villa G, Neri M, Bellomo R, et al. ; Nomenclature Standardization Initiative (NSI) Alliance: Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: Practical applications. Crit Care 2016; 20:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joannes-Boyau O, Honoré PM, Perez P, et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med 2013; 39:1535–1546 [DOI] [PubMed] [Google Scholar]
- 20.Park JT, Lee H, Kee YK, et al. ; HICORES Investigators: High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: A randomized controlled trial. Am J Kidney Dis 2016; 68:599–608 [DOI] [PubMed] [Google Scholar]
- 21.Miao H, Wang F, Xiong X, et al. Clinical benefits of high-volume hemofiltration in critically Ill pediatric patients with severe sepsis: A retrospective cohort study. Blood Purif 2018; 45:18–27 [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Fernández X, Sabater-Riera J, Sileanu FE, et al. Clinical variables associated with poor outcome from sepsis-associated acute kidney injury and the relationship with timing of initiation of renal replacement therapy. J Crit Care 2017; 40:154–160 [DOI] [PubMed] [Google Scholar]
- 23.Fortenberry JD, Paden ML, Goldstein SL. Acute kidney injury in children: An update on diagnosis and treatment. Pediatr Clin North Am 2013; 60:669–688 [DOI] [PubMed] [Google Scholar]
- 24.Matsuda K, Moriguchi T, Oda S, et al. Efficacy of continuous hemodiafiltration with a cytokine-adsorbing hemofilter in the treatment of acute respiratory distress syndrome. Contrib Nephrol 2010; 166:83–92 [DOI] [PubMed] [Google Scholar]
- 25.Cui HX, Xu JY, Li MQ. Efficacy of continuous renal replacement therapy in the treatment of severe acute pancreatitis associated acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci 2014; 18:2523–2526 [PubMed] [Google Scholar]
- 26.Han F, Sun R, Ni Y, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. Am J Med Sci 2015; 349:199–205 [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Liu ZH, Chen J, et al. An aggressive systematic strategy for acute respiratory distress syndrome caused by severe pneumonia after renal transplantation. Transpl Int 2006; 19:110–116 [DOI] [PubMed] [Google Scholar]
- 28.DiCarlo JV, Alexander SR, Agarwal R, et al. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol 2003; 25:801–805 [DOI] [PubMed] [Google Scholar]
- 29.Elbahlawan L, West NK, Avent Y, et al. Impact of continuous renal replacement therapy on oxygenation in children with acute lung injury after allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 2010; 55:540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putzu A, Fang MX, Boscolo Berto M, et al. Blood purification with continuous veno-venous hemofiltration in patients with sepsis or ARDS: A systematic review and meta-analysis. Minerva Anestesiol 2017; 83:867–877 [DOI] [PubMed] [Google Scholar]
- 31.Ingelse SA, Wösten-van Asperen RM, Lemson J, et al. Pediatric acute respiratory distress syndrome: Fluid management in the PICU. Front Pediatr 2016; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005; 67:653–658 [DOI] [PubMed] [Google Scholar]
- 33.Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann Intensive Care 2015; 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W, Hong J, Zeng Q, et al. Improvement of oxygenation in severe acute respiratory distress syndrome with high-volume continuous veno-venous hemofiltration. Glob Pediatr Health 2016; 3:2333794X16645699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andaluz-Ojeda D, Iglesias V, Bobillo F, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care 2011; 15:R243. [DOI] [PMC free article] [PubMed] [Google Scholar]


