Abstract
Introduction of hypothesis
Little information is available regarding the imaging characteristics that assist in differentiating responders from non-responders. We hypothesized that patients with higher pretreatment tumor volume (PTV) would have lower response rates and shorter overall survival (OS).
Methods
Data from patients who received at least one dose of program death-1 (PD-1) inhibitors before August 31, 2016 were captured from our institution’s pharmacy database. The primary objective was to determine the association of PTV with best response, evaluated utilizing RECIST v1.1 criteria. Secondary objectives were estimation of progression-free survival (PFS) and OS. PTV was measured using the Philips Intellispace Multi-Modality Tumor Tracking application.
Results
116 non-small cell lung cancer (NSCLC) patients were evaluated. 66% patients had adenocarcinoma, 28% had squamous cell carcinoma and 5% had poorly differentiated NSCLC. Median PTV was 53.7 cm3 (95% CI: 13.3–107.9). Only one individual had no metastases and the remainder had M1 disease; 38% M1a, 10% M1b, 51% M1c. Most (79%) were previously treated. There were no complete responses; among those followed for at least 6 weeks, 26% had a partial response, 39% stable disease and 34% PD; 4% had no recorded response. There were no strong associations of PTV with any of the demographic or clinical characteristics. There was no association between PTV and OS (HR 1.2, P=0.26) or PFS (HR 1.1, P=0.47). Liver metastasis was associated with shorter survival (HR=2.8, P=0.05).
Conclusion
PTV in NSCLC did not prove to be a predictor of response to PD-1 inhibitors but having liver metastasis was associated with significantly shorter survival.
Keywords: non-small cell lung cancer, tumor volume, tumor burden, checkpoint inhibitors
Plain language summary
Little information is available regarding the imaging characteristics that may assist in differentiating patients with non-small cell lung cancer who respond to immunotherapy and those who do not.
We hypothesized that the more tumor volume the patient had, the lower their response to immunotherapy would be.
Contrary to our hypothesis, tumor volume in non-small cell lung cancer did not prove to be a predictor of response to immunotherapy.
However, cancer spread to the liver which was known prior to treatment was associated with significantly shorter survival.
We believe that the concept and methodology of this study may be valuable to other researchers/clinicians who are involved in the treatment of non-small cell lung cancer.
Introduction
Program death-1 (PD-1) inhibitors are anti-cancer treatments that aim to reinstate anti-cancer immune-mediated cytotoxicity in individuals with cancer. Although this class of therapy offers hope for many patients with advanced stage cancer, little information is available regarding the imaging characteristics that assist in differentiating responders from non-responders. Several studies have shown that higher pretreatment metabolic tumor volume was associated with worse prognosis in non-small cell lung cancer (NSCLC) patients treated with definitive chemoradiotherapy.1,2 However, data on the utility of pretreatment tumor volume (PTV) in assessing response to immunotherapy are lacking.
In patients with metastatic renal cell carcinoma, prior nephrectomy has been associated with improved survival and treatment response to systemic therapy, including targeted therapy and immunotherapy like interferon-α (INF-α).3–6 Similarly, Huang et al reported that in patients with metastatic melanoma, clinical failure to pembrolizumab was not solely due to an inability to induce immune reinvigoration, but rather resulted from an imbalance between T-cell reinvigoration and actual tumor burden.7 Therefore, we hypothesized that NSCLC patients with higher PTV would have lower response rates (RRs) and shorter overall survival (OS) independent of other variables such as age and category of metastasis (i.e., M1a, M1b, M1c).
Materials and methods
Data from patients who received at least one dose of PD-1 inhibitors before August 31, 2016 were captured from our institution’s pharmacy database. Of note, August 3, 2011 was the first date a patient had PD-1 inhibitor administered on trial, which provided us approximately 5 years of treatment data. For patients on trial, the institution’s clinical trial database was used to find all studies involving PD-1 inhibitors. The database was searched using the commercially available name or the drug identification number assigned by the manufacturer. The pharmacy dispensing database was used to validate the first dispense date of drug. For commercially available PD-1 inhibitors, the database was reviewed to identify order data and then nursing records were used to validate administration of drug on that date. The primary objective was to determine the association of PTV with best response, evaluated utilizing RECIST v1.1 criteria. Secondary objectives were estimation of progression-free survival (PFS) and OS. Tumor PD-L1 status was not assessed. This study was reviewed and approved by the Wayne State University’s Institutional Review Board (approval # 062616M1E).
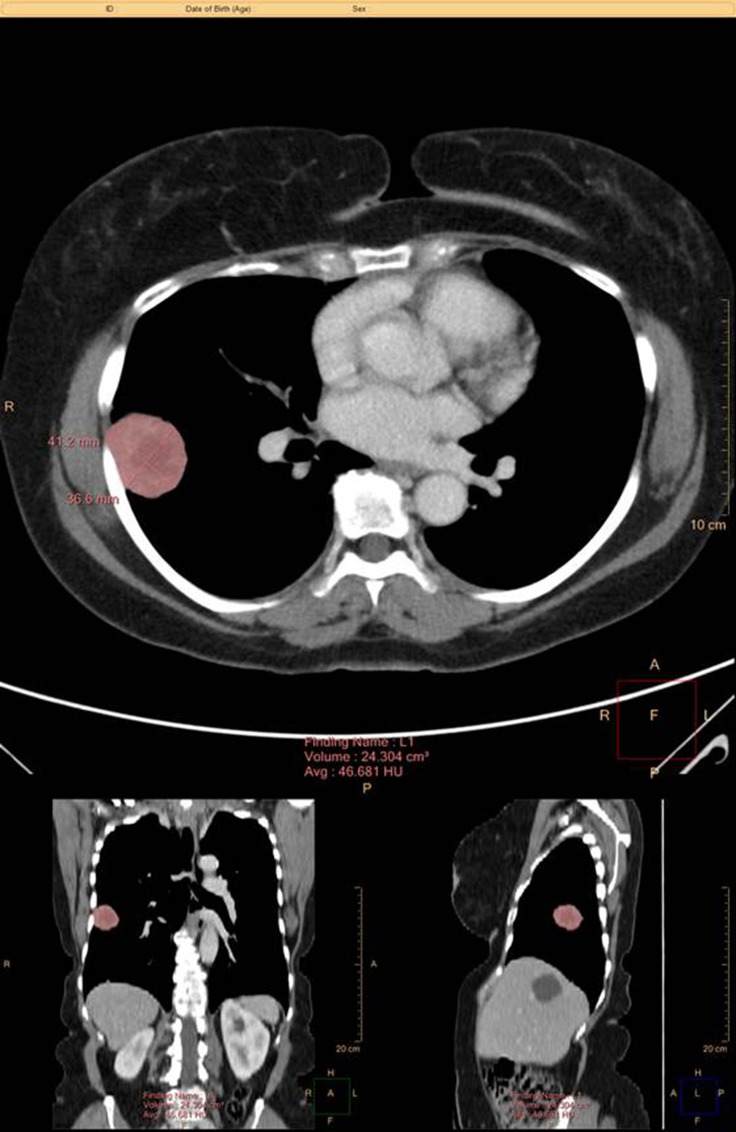
PTV was measured using the Philips Intellispace Multi-Modality Tumor Tracking application (Figure 1). The sum of PTV of all measurable lesions, as defined by any soft tissue mass at or >2 cm in largest diameter and any lymph nodes at or >1.5 cm in shortest diameter, were calculated based on this software and were reported in cubic centimeters. Any soft tissue masses <2 cm in largest diameter were documented as unmeasurable soft tissue mass and categorized in a binary fashion; yes when such lesions were present and no when they were not.
Figure 1.
Example PTV measurement using the Philips Intellispace Multi-Modality Tumor Tracking (MMTT) application.
PTV was found to have an asymmetric frequency distribution which was remedied by a natural loc (ln) transformation. Kendall’s tau-b was used to assess the association between best clinical response and PTV. Univariable proportional hazards were used to assess associations with OS. Multivariable proportional hazard models were not estimated because there were only 47 deaths among the 133 individuals in the dataset.
Statistical consideration
Because the shape of the distribution of total volume was not symmetric, the non-parametric Kruskal–Wallis test was used to compare tumor volume between groups defined by nominal variables such as sex and primary histology. Spearman’s rank correlation was used to estimate the association between tumor volume and ordered measures such as metastatic category and age. Analyses of “best response” and “hospitalization within 6 weeks” were restricted to individuals with at least 6 weeks of follow-up. Univariable proportional hazard models were used to estimate the association of each of the demographic and clinical characteristics with PFS and OS. Caution is urged in the interpretation of reported p-values because no correction for multiplicity was applied.
Results
116 NSCLC patients with at least one site of measurable disease were evaluated. Entry was restricted to individuals who had received at least one dose of a PD-1 or PD-L1 inhibitor prior to the data cut-off of August 31, 2017. Median age was 63 (IQR: 57–70); 67 (58%) were male. 66% patients had adenocarcinoma, 28% had squamous cell carcinoma and 5% had poorly differentiated NSCLC (Table 1). More than half (56%) received Nivolumab with the remainder receiving Pembrolizumab either alone (18%) or in combination (26%). 45 (39%) were treated on a clinical trial. Only one individual had no metastases and the remainder had M1 disease; 38% M1a, 10% M1b, 51% M1c. Most (79%) were previously treated. There were no complete responses; among those followed for at least 6 weeks, 26% had a partial response (PR), 39% stable disease (SD) and 34% PD; 4% had no recorded response. Among those followed for at least 6 weeks, 24% were hospitalized within 6 weeks of their first dose. Overall, median total tumor volume was 53.7 cm3 (95% CI 13.3, 107.9). There were 52 deaths and 90 PFS events among the participants. Median follow-up time for those alive at last follow-up was 27 months (95% CI: 21, 31).
Table 1.
Demographic and clinical characteristics
| N=116 | |
|---|---|
| Sex | |
| Male | 67 (58%) |
| Female | 49 (42%) |
| Age | |
| Median (IQR) | 63 (57, 70) |
| Primary | |
| Lung adeno | 77 (66%) |
| Lung squam | 33 (28%) |
| Lung poorly diff | 6 (5%) |
| Mets category | |
| M0 | 1 (1%) |
| M1a | 44 (38%) |
| M1b | 12 (10%) |
| M1c | 59 (51%) |
| Number of lines of previous tx | |
| 0 | 24 (21%) |
| 1 | 64 (55%) |
| 2 | 23 (20%) |
| 3 | 2 (2%) |
| 4 | 2 (2%) |
| 5 | 1 (1%) |
| Agent | |
| Pembro | 21 (18%) |
| Pembro plus chemo | 15 (13%) |
| Pembro plus investigational | 7 (6%) |
| Pembro plus ipi | 8 (7%) |
| Nivo | 65 (56%) |
| Protocol | |
| No | 71 (61%) |
| Yes | 45 (39%) |
| Best responsea | |
| PR | 26 (26%) |
| SD | 39 (39%) |
| PD | 34 (34%) |
| Not recorded | 4 (4%) |
| Reason for discontinuation | |
| Progression | 37 (32%) |
| PD-1 Adverse events | 16 (14%) |
| PS Decline, hospital admit, hospice | 43 (37%) |
| Completed therapy | 11 (10%) |
| Unknown, lost | 6 (5%) |
| Long vacation | 1 (1%) |
| No response | 2 (2%) |
| Hospitalization within 6 weeks of first dosea | |
| No | 78 (76%) |
| Yes | 25 (24%) |
| Total volume, median (IQR) | |
| Median (IQR) | 53.7 (13.3, 107.9) |
| Lung volume | |
| Median (IQR) | 15.1 (0.0, 65.4) |
| Unmeasurable lung | |
| No | 27 (23.3%) |
| Yes | 89 (76.7%) |
| LN volume | |
| Median (IQR) | 5.1 (0.0, 18.1) |
| Unmeasurable LN | |
| No | 43 (37.1%) |
| Yes | 73 (62.9%) |
| Liver volume | |
| Median (IQR) | 0.0 (0.0, 0.0) |
| Unmeasurable liver | |
| No | 93 (80%) |
| Yes | 23 (20%) |
| Soft tissue volume | |
| Median (IQR) | 0.0 (0.0, 0.0) |
| Unmeasurable soft tissue | |
| No | 85 (73%) |
| Yes | 31 (27%) |
Notes: aFor those followed for at least weeks.
Abbreviations: SD, stable disease; PD-1, program death-1; PR, partial response; LN, lymph node; PS, performance status; PD, progression of disease.
There were no strong associations of total volume with any of the demographic or clinical characteristics (Table 2). In addition, tumor volume was not associated with PFS (HR per 100 cm3=1.1,95% CI 0.9, 1.3, P=0.47) (Table 3) or OS (HR per 100 cm3=1.2 95% CI: 0.9, 1.5, P=0.26) (Table 4). Liver metastasis was associated with shorter survival (HR=2.8, 95% CI: 1, 7.9, P=0.05).
Table 2.
Associations of total volume with demographic and clinical characteristics
| N | Median total vol |
IQR | P-value | |
|---|---|---|---|---|
| Sex | 0.21b | |||
| Male | 67 | 66 | 14, 116 | |
| Female | 49 | 37 | 13, 79 | |
| Age | 0.73c | |||
| ≤59 yrs | 41 | 49 | 15, 99 | |
| 60–69 yrs | 42 | 57 | 11, 111 | |
| ≥70 yrs | 33 | 58 | 15, 103 | |
| Primary | 0.56b | |||
| Lung adeno | 77 | 53 | 13, 105 | |
| Lung squam | 33 | 63 | 28, 111 | |
| Lung poorly diff | 6 | 28 | 10, 72 | |
| Best responsea | 0.61c | |||
| PR | 26 | 43 | 13, 88 | |
| SD | 39 | 37 | 13, 79 | |
| PD | 34 | 60 | 18, 84 | |
| Mets category | 0.07c | |||
| M0/M1a | 45 | 35 | 13, 72 | |
| M1b | 12 | 59 | 22, 116 | |
| M1c | 59 | 56 | 21, 128 | |
| Lines of previous Tx | 0.70a | |||
| 0 | 24 | 35 | 13, 115 | |
| 1 | 64 | 60 | 15, 105 | |
| 2 or more | 28 | 71 | 13, 108 | |
| Agent | 0.29b | |||
| Pembro | 21 | 36 | 11, 70 | |
| Pembro + other | 30 | 43 | 14, 132 | |
| Nivo | 65 | 65 | 18, 116 | |
| On protocol | 0.76b | |||
| No | 71 | 51 | 13, 101 | |
| Yes | 45 | 63 | 15, 123 | |
| Hospitalizations w/i 6 wksa | 0.91b | |||
| No | 78 | 47 | 13, 88 | |
| Yes | 25 | 55 | 15, 80 | |
| Reason discontinuation | 0.14b | |||
| PD, decline, PD-1 AE | 96 | 56 | 15, 113 | |
| Completed therapy | 11 | 36 | 7, 71 | |
| Other | 7 | 193 | 2, 462 | |
| Unmeasurable lung | 0.04b | |||
| No | 27 | 71 | 36, 132 | |
| Yes | 89 | 44 | 13, 92 | |
| Unmeasurable LN | 0.33b | |||
| No | 43 | 36 | 13, 84 | |
| Yes | 73 | 61 | 14, 116 | |
| Unmeasurable liver | 0.64b | |||
| No | 93 | 55 | 13, 103 | |
| Yes | 23 | 37 | 18, 138 | |
| Unmeasurable soft tissue | 0.56b | |||
| No | 85 | 63 | 13, 126 | |
| Yes | 31 | 50 | 20, 79 |
Notes: aFor those followed for at least weeks; bKruskal–Wallis Test; cSpearman’s rank correlation.
Abbreviations: AE, adverse events; SD, stable disease; PD-1, program death-1; PR, partial response; LN, lymph node; PD, progression of disease.
Table 3.
Tumor volume in association with PFS
| HR (95% CI) | P-value | |
|---|---|---|
| Total volume (per 100 cm3) | 1.1 (0.9, 1.3) | 0.47 |
| Sex | 0.35 | |
| Male | 1.0 | |
| Female | 1.2 (0.8, 1.9) | |
| Age | 0.59 | |
| ≤59 yrs | 1.0 | |
| 60–69 yrs | 1.2 (0.7, 1.9) | |
| ≥70 yrs | 0.9 (0.5, 1.5) | |
| Primary | 0.94 | |
| Lung adeno | 1.0 | |
| Lung squam | 1.0 (0.7, 1.6) | |
| Lung poorly diff | 0.9 (0.2, 2.4) | |
| Best Responsea | <0.001 | |
| PR | 1.0 | |
| SD | 4.6 (2.3, 9.4) | |
| PD | 43.9 (17.9, 107.3) | |
| Metastasizes | 0.007 | |
| M0/M1a | 1.0 | |
| M1b | 1.0 (0.5. 2.3) | |
| M1c | 2.0 (1.3, 3.1) | |
| Previous lines of Tx | 0.005 | |
| 0 | 1.0 | |
| 1 | 2.4 (1.4, 4.3) | |
| 2 or more | 2.1 (1.1, 4.2) | |
| Agent | 0.006 | |
| Pembro | 1.0 | |
| Pembro + other | 1.0 (0.5, 2.1) | |
| Nivo | 2.0 (1.1, 3.8) | |
| On protocol | 0.006 | |
| No | 1.0 | |
| Yes | 0.5 (0.3, 0.8) | |
| Hospitalization w/i 6 wka | 0.001 | |
| No | 1.0 | |
| Yes | 2.3 (1.4, 3.9) | |
| Reason for discontinuation | <0.001 | |
| PD, decline, PD-1 AE | 1.0 | |
| Completed Tx | 0.1 ((0.05, 0.4) | |
| Other | 0.4 (0.1, 1.2) | |
| Unmeasurable lung | 0.03 | |
| No | 1.0 | |
| Yes | 1.8 (1.0, 3.0) | |
| Unmeasurable LN | 0.55 | |
| No | 1.0 | |
| Yes | 0.9 (0.6, 1.3) | |
| Unmeasurable Liver | 0.06 | |
| No | 1.0 | |
| Yes | 1.7 (1.0, 2.8) | |
| Unmeasurable soft tissue | 0.41 | |
| No | 1.0 | |
| Yes | 1.2 (0.8, 1.9) | |
| Lung (per 100 cm3) >2 cm | 1.0 (0.8, 1.3) | 0.99 |
| LN (per 100 cm3) >1.5 cm | 0.8 (0.4, 1.7) | 0.59 |
| Liver (per 100 cm3) >2 cm | 2.6 (1.0, 7.0) | 0.11 |
| Soft tissue (per 100 cm3) >2 cm | 1.8 (1.1, 2.9) | 0.02 |
Notes: aFor those followed for at least weeks.
Abbreviations: AE, adverse events; SD, stable disease; PD-1, program death-1; PR, partial response; PFS, progression free survival; LN, lymph node; PD, progression of disease.
Table 4.
Tumor volume in association with OS
| HR (95% CI) | P-value | |
|---|---|---|
| Total volume (per 100 cm3) | 1.2 (0.9, 1.5) | 0.26 |
| Sex | 0.24 | |
| Male | 1.0 | |
| Female | 1.4 (0.8, 2.3) | |
| Age | 0.65 | |
| ≤59 yrs | 1.0 | |
| 60–69 yrs | 1.2 (0.6, 2.2) | |
| ≥70 yrs | 0.9 (0.4, 1.7) | |
| Primary | 0.72 | |
| Lung adeno | 1.0 | |
| Lung squam | 0.8 (0.4, 1.5) | |
| Lung poorly diff | 1.2 (0.4, 4.0) | |
| Best responsea | <0.001 | |
| PR | 1.0 | |
| SD | 5.6 (1.6, 19.1) | |
| PD | 17.4 (5.1, 59.3) | |
| Metastasizes | 0.008 | |
| M0/M1a | 1.0 | |
| M1b | 1.0 (0.3, 3.1) | |
| M1c | 2.2 (1.3, 4.4) | |
| Previous lines of Tx | 0.001 | |
| 0 | 1.0 | |
| 1 | 2.3 (1.0, 5.3) | |
| 2 or more | 4.4 (1.9, 10.6) | |
| Agent | 0.19 | |
| Pembro | 1.0 | |
| Pembro + other | 0.9 (0.4, 2.3) | |
| Nivo | 1.6 (0.7, 3.5) | |
| On protocol | 0.10 | |
| No | 1.0 | |
| Yes | 0.6 (0.4, 1.1) | |
| Hospitalization w/i 6 wka | <0.001 | |
| No | 1.0 | |
| Yes | 6.0 (3.2, 11.0) | |
| Reason for discontinuationb | – | |
| PD, decline, PD-1 AE | 1.0 | |
| Completed Tx | – | |
| Other | – | |
| Unmeasurable lung | 0.02 | |
| No | 1.0 | |
| Yes | 2.2 (1.0, 4.7) | |
| Unmeasurable LN | 0.74 | |
| No | 1.0 | |
| Yes | 0.9 (0.5, 1.6) | |
| Unmeasurable liver | 0.09 | |
| No | 1.0 | |
| Yes | 1.7 (0.9, 3.3) | |
| Unmeasurable soft tissue | 0.46 | |
| No | 1.0 | |
| Yes | 1.3 (0.7, 2.3) | |
| Lung (per 100 cm3) 2 cm | 1.1 (0.8, 1.4) | 0.54 |
| LN (per 100 cm3) 1.5 cm | 1.1 (0.5, 2.6) | 0.77 |
| Liver (per 100 cm3) 2 cm | 2.8 (1.0, 7.9) | 0.05 |
| Soft tissue (per 100 cm3) 2 cm | 1.3 (0.5, 3.3) | 0.66 |
Notes: aFor those followed for at least weeks; bKruskal–Wallis Test.
Abbreviations: AE, adverse events; SD, stable disease; PD-1, program death-1; PR, partial response; OS, overall survival; LN, lymph node; PD, progression of disease.
Discussion
Checkpoint inhibitors targeting the PD-1–PDL1 axis have substantially changed the landscape of the treatment of advanced metastatic stage NSCLC. Nivolumab, pembrolizumab, and atezolizumab have demonstrated superiority against docetaxel in the second-line setting for advanced metastatic NSCLC.8–10 A randomized Phase II study11 and subsequently the Phase III Keynote-189 trial12 showed the benefits of combining carboplatin/pemetrexed with pembrolizumab in the first-line setting and this combination has been approved for its use, regardless of PD-L1 expression. In addition, the Phase III KEYNOTE-407 trial showed improved PSF and OS when pembrolizumab was combined with carboplatin and paclitaxel/nabpaclitaxel, compared to chemotherapy alone in previously untreated metastatic squamous NSCLC patients. This was first-line chemotherapy irrespective of PD-L1 level.13 Single-agent pembrolizumab showed superiority compared to first-line chemotherapy in patients with tumors expressing PD-L1 ≥50%. Pembrolizumab has gained FDA approval as a first-line single-agent therapy in this setting.14 Recently, single-agent Pembrolizumab was shown to improve OS even in patients with low PD-L1 expression (PD-L1 TPS level ≥1%) compared to chemotherapy alone in untreated patients with locally advanced or metastatic NSCLC. This led to the FDA approval of pembrolizumab in this subset of patients as well.15
Although this new class of therapy offers hope for many advanced stage cancer patients, little is known on the characteristics of patients who are likely to respond.
PD-L1 expression has been one of the most extensively studied biomarkers in NSCLC. However, this marker is far from perfect, and is subject to several limitations Objective responses have been reported in both PD-L1 positive and negative NSCLCs.8,10–12,16,17 For example, the aforementioned Keynote-189 trial reported higher RRs with the pembrolizumab-chemotherapy combination group than in the placebo-chemotherapy combination group across all categories of PD-L1 tumor proportion score, although the greatest between-group difference in patients was seen in the PD-L1 high (50% or greater) group.12
The limitations of PD-L1 testing likely come from multiple variables. PD-L1 expression is thought to be regulated by various mechanisms, including the MAPK and PI3K pathways, multiple transcriptional factors such as HIF1 and STAT3 as well as epigenetic factors.18 In addition, PD-L1 expression can be transient. Intra-patient, as well as intra-tumor heterogeneity in PD-L1 expression is possible.19
Another challenge is the poor uniformity, and PD-L1 positivity thresholds of the various immunohistochemistry testing methods.20 Furthermore, PD-L1 expression alone does not take into account other factors that could influence anti-tumor response such as immune-cell engagement in the tumor micro-environment and the presence of other concurrent suppressive pathways.21 Despite these limitations, PD-L1 expression is one of the few widely available biomarkers that has been most extensively studied for NSCLC at this time. Testing for PD-L1 is considered to be standard of care for patients with newly diagnosed advanced metastatic NSCLC.22
Since mutations are considered to produce neoantigens that could elicit an immune response, somatic mutational burden is another predictive biomarker that is being explored in this setting. Tumors with high rates of somatic mutations such as melanoma, NSCLC, and microsatellite-unstable colorectal cancer have been found to have a higher probability of benefiting from immune checkpoint blockade than those with lower rates of somatic mutations.23,24 In the CheckMate 227 study, Hellmann et al showed that among patients with NSCLC and a high tumor mutational burden (≥10 mutations per megabase determined by the FoundationOne CDx assay), the PFS was significantly longer with first-line nivolumab plus ipilimumab than with chemotherapy, irrespective of their PD-L1 expression level.17
Tumor-infiltrating lymphocytes may also serve as a prognostic biomarker. In a melanoma study assessing 40 patients, a RR of 79% was demonstrated when pre-treatment tumor biopsies had >20% of tumor-infiltrating PD-1 high and CTLA-4 high CD8 T cells, whereas there were no responders in patients with fewer than 20% infiltrating PD-1 high and CTLA-4 high CD8 T cells.25 However, one must be cautious about the use of tumor-infiltrating lymphocytes in clinical practice, because lymphocyte infiltration is also known to appear upon disease progression. Detection techniques and cut-off values have not been standardized.
Furthermore, Tumeh et al investigated whether the narrow repertoire of T cell receptors correlated with response to pembrolizumab in patients with melanoma. Their observations showed that T-cell receptor beta chain usage was more restricted in responders versus those who were showing signs of progression; suggesting that those whose tumor has a low tumor-infiltrating lymphocyte density may still benefit from anti-PD-1 therapy if the tumor-infiltrating lymphocyte population has restricted T-cell receptor clonality specific to the tumor antigen.26
Many studies have evaluated the association between tumor burden and response to therapy. The classic example is metastatic renal cell carcinoma, where prior cytoreductive nephrectomy has been associated with a survival advantage and improved response to systemic therapy, including anti-VEGF treatment and immunotherapy like INF-α.3–6 In a trial by Flanigan et al, nephrectomy prior to INF-α-2b treatment was associated with significantly improved median OS (8.1 vs 11.1 months).3 Similarly, a trial by Mickisch et al, showed improved survival and increased time to progression in patients who underwent nephrectomy prior to INF-α treatment compared to INF-α alone.4 In addition, there have been reports for improved survival in the combination of nephrectomy and IL-2 therapy.27,28
In melanoma studies, patients with high LDH levels had lower RRs to immunotherapy when compared to those without high LDH levels.29,30 While non-specific, as LDH is easily detected in serum during tissue turnover and damage, this has been a classic marker for tumor burden.
Tumor volumes, oftentimes derived through calculations of target volumes, have been previously studied in the field of radiation oncology. Techniques to overcome respiratory motion have been an area of focus especially in the treatment planning of lung cancer.31,32 The prognostic value of PTV has also been studied in stage III NSCLC patients undergoing definitive chemoradiotherapy. In this single institutional study of 52 patients, tumor volumes were measured four times during the course of their treatment and found that greater relative tumor volume reduction during treatment correlates with improved disease control and OS.33 Through their analysis of 88 patients, Kuo et al calculated the decrease of tumor volume post-chemotherapy in advanced NSCLC. The authors reported the efficacy of their proposed survival prediction index through tumor volume measurements, but these findings are yet to be validated.34
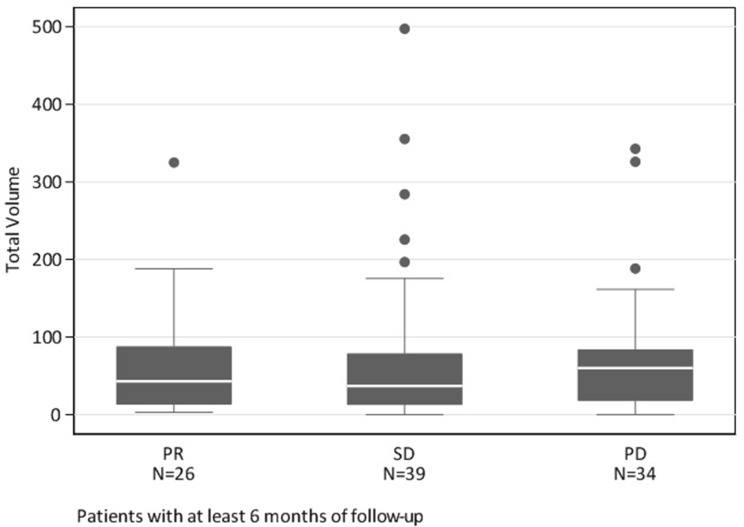
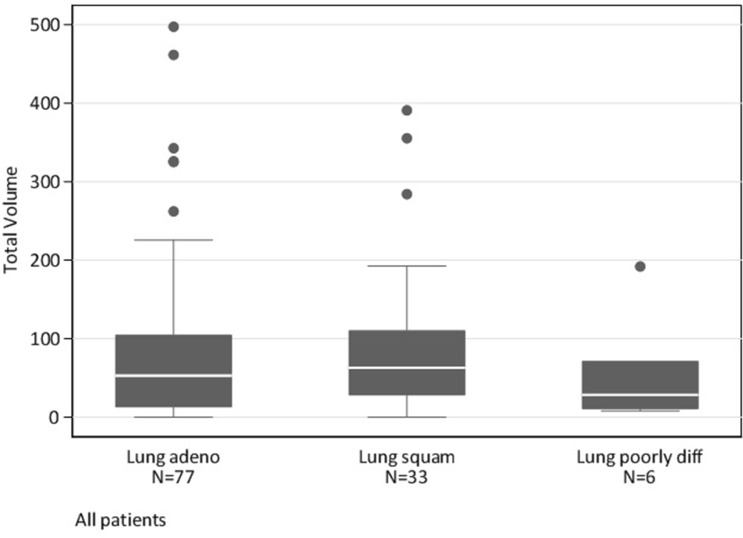
To our knowledge, our study is the first to estimate an association between PTV and best response in NSCLC patients treated with immunotherapy. The association between PTV and best response, considered as an ordered 4-category variable was not strong. Neither were PTV, age, sex, PD-1 agent, protocol status associated with OS (Table 2). Best response, metastasis category, previous lines of therapy, hospitalization within 6 weeks and having liver metastasis >2 cm in size were associated with shorter survival. Contrary to our hypothesis, PTV in NSCLC did not prove to be a strong predictor of response. As demonstrated in Figure 2 although some patients with PR had low PTV, some of those with high PTV did have a response, thereby suggesting the presence of other factors contributing to response. As shown in Figure 3, there were no associations of PTV based on the lung tumor histology.
Figure 2.
Box plots illustrating the distribution of pretreatment tumor volume according to best response.
Figure 3.
Boxplots illustrating the distribution of pretreatment tumor volume according to lung tumor histology.
Interestingly, Huang et al recently reported that the magnitude of reinvigoration of circulating exhausted-phenotype CD8 T cells calibrated to pretreatment tumor burden was associated with clinical response in advanced melanoma patients.7 In this study, tumor burden was defined as the sum of the long axis of all measurable lesions reported on the pretreatment imaging reports. The authors did comment that the tumor burden alone was not a perfect predictor of response to anti-PD-1 therapy and that there were likely other parameters, such as anatomical location of metastases, PD-L1 expression and mutational phenotype, which may add further to resolve the relationship between T-cell reinvigoration and tumor burden.
In our study, liver metastasis prior to treatment was associated with significantly shorter survival. This result is consistent with reports from previous studies with Riihimäki et al showing that mortality was 1.53 fold higher for patients with liver metastasis than for those with brain metastasis (P<0.05).35 Similarly, Tamura et al demonstrated that the mortality was 1.55 fold higher for patients with liver metastasis than for those with other distant metastasis (P<0.001).36 As demonstrated, liver metastases in NSCLC are likely a negative prognostic factor regardless of the use of immunotherapy.
Conclusion
Contrary to our hypothesis, PTV in NSCLC did not prove to be a predictor of response to PD-1 inhibitors but having liver metastasis prior to treatment was associated with significantly shorter survival.
Acknowledgments
The authors wish to thank the patients and the families who were treated at our site. The preliminary results of this manuscript were presented as a poster on 10/17/2017 at IASLC 18th World Lung Cancer Conference at Yokohama, Japan. This study did not receive any funding.
Ethics approval/copyright
This study was reviewed and approved by the Wayne State University’s Institutional Review Board (approval # 062616M1E). The database held only de-identified patient data.
Abbreviations
INF, interferon-α; NSCLC, non-small cell lung cancer; OS, overall survival; PR, partial response; PD-1, program death-1; PFS, progression-free survival, PTV, pretreatment tumor volume; RR, response rate; SD, stable disease.
Author contributions
The first and last authors contributed to the conception, data acquisition and prepared the first draft. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Antoinette Wozniak reports grants, personal fees from Boehringer Ingelheim, personal fees from Astra Zeneca, DSMB from BeyondSpring, DSMB from HUYA Bioscience, personal fees from Takeda, personal fees from Karyopharm Therapeutics, personal fees from Premier Inc, during the conduct of the study. Dr Shirish Gadgeel reports personal fees from Boehringer-Ingelheim, non-financial support from Astra-Zeneca, non-financial support from Novocure, non-financial support from Takeda, non-financial support from Genentech/Roche, non-financial support from Bristol Myers-Squibb, non-financial support from Xcovery, non-financial support from Abbvie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Bazan JG, Duan F, Snyder BS, et al. Metabolic tumor volume predicts overall survival in patients with stage III non-small cell lung cancer treated on ACRIN 6668/RTOG 0235: locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(5):S3–S4. doi: 10.1016/j.ijrobp.2014.08.027 [DOI] [Google Scholar]
- 2.Lee P, Bazan JG, Lavori PW, et al. Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer. 2012;13(1):52–58. doi: 10.1016/j.cllc.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi: 10.1056/NEJMoa003013 [DOI] [PubMed] [Google Scholar]
- 4.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi: 10.1016/s0140-6736(01)06103-7 [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185(1):60–66. doi: 10.1016/j.juro.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Sun M, Meyer CP, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a national cancer data base study. J Clin Oncol. 2016;34(27):3267–3275. doi: 10.1200/JCO.2016.66.7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ration associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 10.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 15.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 16.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–416. doi: 10.1093/annonc/mdv615 [DOI] [PubMed] [Google Scholar]
- 19.Mansfield AS, Murphy SJ, Peikert T, et al. Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res. 2016;22(9):2177–2182. doi: 10.1158/1078-0432.CCR-15-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint Phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311. doi: 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12):e542–e551. doi: 10.1016/S1470-2045(16)30406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology-Non-Small Cell Lung Cancer.Version 7. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed Spetember 02, 2019. [Google Scholar]
- 23.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):3447–3452. doi: 10.1172/JCI87324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin JR, Figlin R, Rauch J, et al. Cytoreductive surgery in the management of metastatic renal cell carcinoma: the UCLA experience. Semin Urol Oncol. 1996;14(4):230–236. [PubMed] [Google Scholar]
- 28.Mani S, Todd MB, Katz K, Poo WJ. Prognostic factors for survival in patients with metastatic renal cancer treated with biological response modifiers. J Urol. 1995;154(1):35–40. [PubMed] [Google Scholar]
- 29.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 30.Long GV, Blank C, Ribas A, et al. Impact of baseline serum lactate dehydrogenase concentration on the efficacy of pembrolizumab and ipilimumab in patients with advanced melanoma: data from KEYNOTE-006. Eur J Cancer. 2017;72:S122–S123. doi: 10.1016/S0959-8049(17)30482-3 [DOI] [Google Scholar]
- 31.Nishibuchi I, Kimura T, Nakashima T, et al. Time-adjusted internal target volume: a novel approach focusing on heterogeneity of tumor motion based on 4-dimensional computed tomography imaging for radiation therapy planning of lung cancer. Int J Radiat Oncol Biol Phys. 2014;89(5):1129–1137. doi: 10.1016/j.ijrobp.2014.04.050 [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Ge H, Cheng S, et al. Evaluation of interfractional variation of the centroid position and volume of internal target volume during stereotactic body radiotherapy of lung cancer using cone-beam computed tomography. J Appl Clin Med Phys. 2016;17(2):461–472. doi: 10.1120/jacmp.v17i2.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald P, Mo X, Barney C, et al. Prognostic value of primary tumor volume changes on kV-CBCT during definitive chemoradiotherapy for stage III non-small cell lung cancer. J Thorac Oncol. 2017;12(12):1779–1787. doi: 10.1016/j.jtho.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 34.Kuo CJ, Ke BH, Wu NY, et al. Prognostic value of tumor volume for patients with advanced lung cancer treated with chemotherapy. Comput Methods Programs Biomed. 2017;144:165–177. doi: 10.1016/j.cmpb.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 35.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 36.Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3(1):217–221. doi: 10.3892/mco.2014.410 [DOI] [PMC free article] [PubMed] [Google Scholar]