Abstract
Interventions using methods such as cognitive training and aerobic exercise have shown potential to enhance cognitive abilities. However, there is often pronounced individual variability in the magnitude of these gains. Here, we propose that brain network modularity, a measure of brain sub-network segregation, is a unifying biomarker of intervention-related plasticity. We present work from multiple independent studies demonstrating that individual differences in baseline brain modularity predict gains in cognitive control functions across several populations and interventions, spanning healthy adults to patients with clinical deficits and cognitive training to aerobic exercise. We believe that this predictive framework provides a foundation for developing targeted, personalized interventions to improve cognition
Keywords: Brain injury, Cognitive control, Cognitive training, Executive function, Functional connectivity, Graph theory
Neural Mechanisms of Individual Differences in Cognitive Plasticity
There is a rapidly growing interest to develop interventions that enhance aspects of cognition. A large body of this research has recently focused on computer-based cognitive training (e.g., [1], Box 1), although other interventions, such as aerobic exercise and brain stimulation (e.g., transcranial magnetic stimulation), have also been explored as a means to enhance cognitive abilities. Some of this work has suggested that training can improve cognitive control (see Glossary) abilities, such as attention, working memory, and multi-tasking (e.g., [2–4]; for reviews see [1,5,6]), although it is important to note that the effects of training interventions on enhancing cognitive functions has been mixed (Box 1). Nevertheless, cognitive control is fundamental for goal-directed behavior and, importantly, is linked to scholastic success in children [7,8] and the capacity for independent living in older adults [9]. Further, cognitive control deficits are common in a variety of clinical populations, such as patients with traumatic brain injury (TBI) and attention-deficit disorder. Thus, identifying interventions that can successfully strengthen cognitive control abilities in a wide variety of populations is of critical importance.
Box 1: Training approaches to enhance cognitive control.
Cognitive control functions, such as attention and working memory, comprise a set of neural processes that underlie successful goal-directed behavior. As such, there have been increasing efforts to develop noninvasive interventions to improve cognitive control functions, particularly in the form of ‘cognitive training’. In this approach, individuals are trained on one or more cognitive functions over a period of time, with the aim to improve this and related behaviors (termed ‘near-transfer’) as well as untrained behaviors (termed ‘far-transfer’).
Cognitive training interventions can take on the form of group-based therapies where participants are trained on particular goals or strategies that they practice over time (e.g., [26,30]), or through computerized training where participants are trained on tasks or games that tap particular cognitive functions (e.g., [2,4,68]). Importantly, both forms of training have been shown to be effective in clinical populations and healthy young and older adults. For example, group-based attention regulation training (i.e., strategies to reduce distractibility with mindfulness-based attention techniques) improved cognitive control abilities in patients with traumatic brain injury [26]. Further, training on computer-based video games that tapped aspects of working memory and reasoning improved measures of divided attention in healthy young adults [4] and multitasking training on a custom-designed video game, NeuroRacer, improved behavioral and neural measures of cognitive control in healthy older adults [2].
It is important to note that the results of training studies to improve cognition have been mixed [69], especially regarding the extent to which ‘far-transfer’ has been observed and those that have utilized computerized training approaches (e.g., [70,71]). Flowever, recent meta-analyses have suggested that training moderately improves cognitive abilities, for example in healthy older adults [27–29]. Further, ‘near-transfer’ may be achievable with some training interventions [72,73]. Since it is likely that not all training approaches will be effective at enhancing cognitive control, various intervention parameters that may influence training efficacy, such as adaptivity, dosage, and subject expectancy and motivation, should be considered [1]. Further, targeting subject’s abilities using real-time personalization during training has shown promise to enhance capacity for transfer [1,2,74]. Finally, the heterogeneity of training outcomes across individuals underscores the importance of identifying biomarkers of training-related cognitive plasticity to ultimately develop personalized interventions.
Training-related improvements in cognitive control suggest that these abilities are not immutable, as interventions can drive cognitive plasticity to ultimately enhance cognition. Here, we define ‘cognitive plasticity’ as an individual’s capacity to change and adapt cognitive performance [10]. Although numerous interventions have generally shown promise, there is frequently variability in the magnitude of gains across individuals. In addition, there is also variability in the effectiveness of training across types of interventions. It is therefore crucial to understand the mechanisms that support effective interventions and, further, to understand why certain interventions work for some individuals and not others [11]. Moreover, given the cost and time investments required for training interventions, as well as the limited resources for rehabilitation in our health care system, identifying reliable biomarkers that could predict which individuals have the greatest likelihood of responding to an intervention would help clinicians make more informed decisions before beginning training. Despite this, a common neural mechanism that can explain intervention-related plasticity across a variety of interventions and populations remains relatively under-explored.
Here, we propose that a key organizing principle of brain networks—modularity—is a unifying biomarker of plasticity associated with interventions designed to enhance cognition. A modularity metric can be derived from brain imaging data using a mathematical approach called graph theory (Box 2). This metric can be used to quantify the extent to which individual brain sub-networks, or modules, are segregated from other modules in the whole-brain network, where networks with high modularity have many connections within modules and sparser connections between modules [12,13].
Box 2: Brain networks and modularity: a brief methodological framework.
Graph theory is a mathematical approach to examine network properties, where networks can be represented as graphs (e.g., a mathematical object) comprised of individual units (nodes) and the relationships between them (edges). Large-scale brain networks are comprised of individual brain regions (nodes) and the connections between them (edges). Nodes can be defined anatomically (e.g., the AAL atlas [36]) or functionally, such as through task-based activation maps (e.g., brain regions that are more or less active when comparing different task conditions) or connectivity patterns that define region boundaries (e.g., the Power atlas [37]) (Figure IA). Similarly, edges can be defined anatomically or functionally, such as through white matter structural connectivity (derived from diffusion imaging) or correlations of activity between brain regions obtained over time (e.g. a time series derived from methods such as fMRI, electroencephalography, or magnetoencephalography), respectively (Figures IB). In human studies, large-scale brain networks are often quantified from task-free ‘resting-state’ fMRI (rs-fMRI) data. For functional connections, the time series from every possible pair of brain regions is correlated to form the network edges for the entire brain atlas. The resulting correlation matrix (Figure IC) can then be thresholded and binarized to form unweighted edges (e.g., connections equal 0 or 1 based on a specific network density or correlation value) or the correlation value can be retained to form weighted edges (e.g., connections equal their original correlation value).
After a graph has been defined, various network properties can be quantified. One particularly important organization principle is modularity—the extent to which a network can be segregated into distinct sub-networks, or modules [75] (Figure ID). Modules are groups of nodes that can be identified in a data-driven manner by using various community detection algorithms or by applying previously identified sub-networks (see [13] for a more in-depth discussion of methodological considerations related to examining network modularity). Highly modular networks have many connections within modules and fewer connections between modules. In addition to quantifying modularity of the entire network, the roles of individual nodes, or brain regions, can also be quantified based on their within- and between-module connections. For example, nodes with a high within-module degree score have many connections to other nodes in their own module and nodes with a high participation coefficient have connections distributed across many network modules [76,77].
Importantly, previous work has demonstrated evidence for modular brain network organization across a variety of organisms, including C. elegans [78], Drosophila [79], mouse [80], rat [81], cat [82], macaque [82], and human [83] brains. Further, recent evidence suggests that the diversity of connector hubs (i.e., brain regions with a high participation coefficient) maintains modular organization and supports task performance [84].
A Network Perspective for Studying Cognitive Plasticity
Noting heterogeneity of intervention-related outcomes across individuals, previous studies have attempted to identify neural biomarkers of one’s potential for successful learning. This work has demonstrated that certain types of brain measurements such as regional brain volume (e.g., striatal volume) or brain activity (e.g., frontal alpha power) are related to complex skill learning [14–17] and can also predict improvements in untrained cognitive control abilities [14]. Importantly, however, these types of predictors are not consistent across studies, demonstrating a need for neural biomarkers of outcomes that generalize across subject populations and interventions. Further, these previously identified biomarkers were largely limited to measuring structure or function within isolated brain regions, rather identifying than a biomarker of network-level interactions. We propose that the latter will lead to a more reliable biomarker of intervention-related cognitive plasticity.
As expected, there is significant individual variability in functional brain networks. This uniqueness can be captured with novel brain imaging analytic methods to identify individual participants based on their network connectivity patterns [18,19]. There are increasing efforts to use such prediction frameworks to understand individual differences in cognition as well as to predict future behavioral outcomes [20]. Thus, we believe that a whole-brain network perspective is critical to identifying a common biomarker of intervention-related cognitive plasticity. Focusing on brain networks is likely to be particularly informative, as many interventions aim to enhance complex cognitive processes, such as cognitive control. Such functions cannot be localized to individual brain regions or pairs of brain regions, but rather are thought to rely on the communication across widely distributed brain networks [21,22].
In this Opinion, we argue that brain modularity is a network-level biomarker of potential for intervention success and, more broadly, underlying cognitive plasticity. We base our argument on prior work with several different interventions and populations, showing that individuals with higher brain network modularity at baseline exhibit greater intervention-related cognitive gains [23–25] (Figure 1, Key Figure). After describing these empirical studies, we review other empirical and theoretical work that relates modular network organization and plasticity, forming the basis for potential mechanisms underlying the relationship between modularity and intervention-induced cognitive gains.
Figure 1 (Key Figure). Brain network modularity predicts training-related cognitive gains.

(A) Brain modularity calculated at baseline is predictive of intervention-related cognitive gains across several interventions and populations. Specifically, individuals with higher brain network modularity have larger cognitive gains than those with lower brain network modularity. Toy brain networks are comprised of nodes (circles) and edges that connect them (lines). Nodes are colored according to their module membership; within-module connections are colored to match that of nodes in their own module, while between-module connections are colored black. The network with higher modularity (right side of graph) has many within-module connections and fewer between-module connections, while the network with lower modularity (left side of graph) has fewer within-module connections and many between-module connections. (B) Baseline brain modularity predicted cognitive control gains (on a composite of tasks) in TBI patients who participated in group-based attention training, but not those who participated in a control education intervention (adapted with permission from Wolters Kluwer Health, Inc.: [23]). (C) Baseline brain modularity predicted cognitive control gains (on the Test of Strategic Learning [31]) in healthy older adults who participated in group-based reasoning training, but not those who were in a no-contact control group (adapted from [24]). (D) Baseline brain modularity predicted cognitive control gains (on a composite of tasks) in healthy older adults who participated in exercise training, but not those who participated in a control dance intervention (after controlling for age, in-scanner motion, and baseline cognitive control functioning; adapted from [25]). It is important to note that the range of measured brain modularity values varied across studies due to differences in graph theoretical methodological decisions, such as the number of network nodes and edges and choice of atlas. Nevertheless, the relationship between baseline modularity and intervention-related gains was robust to varying analytic methods.
Brain Modularity Predicts Plasticity Across Populations and Interventions
Modularity is an important feature of brain networks. Moreover, both theoretical and empirical work demonstrates that modular organization in large-scale brain networks is beneficial for behavior. Taken together, we hypothesize that a more modular network architecture confers cognitive plasticity during interventions aimed to enhance cognitive abilities, such as cognitive control. In support of this hypothesis, we present results from three studies demonstrating that individuals with higher brain network modularity at baseline (i.e., pre-intervention) exhibit larger gains in cognitive control abilities (Figure 1A). Critically, each of these studies showed that brain modularity was predictive of training outcomes beyond individual differences in baseline cognition, demonstrating that brain modularity adds unique predictive information regarding future intervention-related gains in cognition.
Patients with Traumatic Brain Injury (TBI) Undergoing Cognitive Training
Initial work examining the relationship between baseline brain modularity and intervention gains was conducted in patients with TBI [23]. Here, patients with chronic TBI who exhibited impairments in cognitive control abilities were enrolled in a 5-week goal-oriented attention self-regulation intervention. The intervention incorporated group-based training, individual sessions with trainers, and at-home practice with a focus on mindfulness attention regulation and practical application of these skills in daily life. Participants were randomized to this intervention or an active control brain health education intervention. Compared to the control intervention, performance on a composite measure of cognitive control (e.g., letter number sequencing, response inhibition and switching, fluency) improved after the cognitive intervention [26]. To examine the relationship between baseline brain modularity and training-related gains, modularity was quantified from a 5-minute eyes-open ‘resting-state’ fMRI (rs-fMRI) scan. Remarkably, patients with higher modularity at baseline exhibited greater training-related cognitive gains (Figure 1B) [23]. Importantly, there was no relationship between baseline modularity and cognitive changes in the education intervention group. Further, the relationship between modularity and training-related gains could not be explained by individual differences in cognitive abilities prior to training, suggesting that modularity was a unique contributor to predicting training gains.
Healthy Individuals Undergoing Cognitive Training
In a second study, the relationship between brain modularity and training-related cognitive gains was examined in healthy older individuals [24]. While TBI patients typically exhibit cognitive control deficits, healthy older adults can also experience difficulties in these functions. A large body of recent work suggests that interventions can improve cognitive control abilities in older adults with moderate effect sizes [27–29]. In one example, a previous study demonstrated that training improved cognitive control abilities in a group of older adults compared to a no-contact control group [30]. Here, older adults (aged 56-71 years old) with non-clinical cognitive control difficulties were enrolled in 12-week gist reasoning training (‘Strategic Memory and Reasoning Training’; SMART) that incorporated training in small groups and at-home practice. Compared to no-contact controls, individuals in the SMART group improved on measures of gist reasoning (Test of Strategic Learning; TOSL [31]) and concept abstraction (WAIS-III Similarities [32]). To examine the relationship between baseline brain modularity and training-related gains, modularity was quantified from a 4-minute rs-fMRI scan. As in TBI patients who completed a different type of cognitive training [23], older adults with higher brain modularity at baseline exhibited greater SMART-related improvements on the TOSL (Figure 1C) [24]. Importantly, there was no relationship between baseline brain modularity and SMART or TOSL changes in the control group. Further, the relationship between baseline brain modularity and SMART-related TOSL gains was not related to age, in-scanner motion, or baseline cognitive abilities.
While the findings in TBI patients focused on whole-brain modularity measures, there is evidence that aging has more pronounced effects on sub-networks mediating ‘associative’ functions, such as the fronto-parietal control network, compared to sub-networks mediating sensory-motor functions, such as the visual network [33]. In a secondary analysis, it was demonstrated that association cortex modularity, but not sensory-motor cortex modularity, was related to TOSL gains in the SMART group, suggesting that modularity of association networks may be more informative in predicting training-related gains in older adults.
Healthy Individuals Undergoing Exercise Training
Although a large focus of recent efforts to improve cognitive functioning has utilized group-based or computerized cognitive training, exercise interventions have also shown positive effects on brain function and behavior, particularly in older adults [34,35]. In a final study, the association between brain modularity and cognitive improvements associated with exercise training was examined [25]. Here, healthy, low active, older adults (aged 60-80 years old) were enrolled in a 6-month physical exercise intervention led by trained exercise specialists. Participants were randomized to one of four groups: (1) participants in the Walk group walked within their target heart rate during training, (2) participants in the Walk+ group walked in the same target heart rate and were provided a daily beta-alanine supplement, (3) participants in the SSS group performed exercises focused on stretching, strengthening, and stability, and (4) participants in the Dance group were instructed on social dance sequences. Compared to the Dance group, individuals in the Walk, Walk+, and SSS groups improved on a composite measure of cognitive control (e.g., spatial working memory, task switching) and cardiorespiratory fitness. To examine the relationship between baseline brain modularity and training-related gains, modularity was quantified from a 6-minute eyes-closed rs-fMRI scan. Similar to previous work, higher baseline modularity was related to greater exercise-related cognitive control gains specifically in the groups that exhibited intervention-related cognitive improvements (i.e., Walk, Walk+, and SSS; Figure 1D) [25]. In the Walk and Walk+ groups, this relationship was stronger in individuals with lower baseline cognitive control abilities. Importantly, there was no relationship between brain modularity and cognitive changes in the Dance control group, indicating that this predictive relationship was selective to groups that showed training-related cognitive and cardiorespiratory fitness gains. Further, the relationship between baseline modularity and cognitive gains was not related to individual differences in age, in-scanner motion, years of education, or brain volume.
Robustness of Brain Modularity as a Biomarker of Plasticity
Together, the findings of these three empirical studies suggest that brain network modularity assessed during task-free, ‘resting-state’ fMRI may be a biomarker of potential for training-related cognitive control gains that generalizes across a variety of populations and interventions (Figure 1). First, the modularity-gain relationship was observed in several different populations that exhibited intervention-related gains, on average, ranging from patients with clinical deficits to healthy individuals. Importantly, this relationship was found across individuals with a range of educational backgrounds. Second, the modularity-gain relationship was observed in several different interventions that improved functioning, ranging from group-based cognitive therapies to physical fitness interventions. Third, this relationship was found for a variety of outcome measures that were unique to each study, but all tapped aspects of cognitive control functioning.
The modularity-gain relationship also appears to be robust to methodological choices in terms of data collection and analysis. This relationship was observed in datasets with varying rs-fMRI scan length and parameters, including eyes-open or eyes-closed rest. Importantly, several steps were also taken demonstrate that the relationship between modularity and cognitive gains was not related to in-scanner head motion. Further, the modularity-gain relationship generalized across graph theoretical analysis methods, including node selection (e.g., AAL [36] or Power [37] atlases), edge selection (e.g., thresholding and binarization or retention of edge weights [33]), and methods for modularity optimization (e.g., simulated annealing [38], spectral clustering [39], or imposing pre-defined modules). Given that some of these methodological choices (e.g., node and edge number) can affect the range of measured modularity values, it is difficult to meaningfully compare the magnitude of the modularity metric between studies and, instead, should be compared within studies where the same analytic methods are used. Nonetheless, the correlation between baseline brain modularity and intervention-related gains was replicated in several independent studies that used somewhat different graph theoretical methods (Figure 1).
Interestingly, a similar robustness to in-scanner motion, scan sequence, and atlas selection has been found for other functional connectome prediction studies [19]. However, it should be noted that some of these choices may result in lower prediction accuracy (i.e., prediction of individual participants based on connectivity patterns), such as the use of anatomically-based atlases with large network nodes or insufficient processing to account for motion artifacts [19] [40]. Additionally, some work has suggested that performing a task in the scanner may increase the detectability of inter-individual variability and reduce subject motion [41]. Future work could examine the relationship between modularity quantified during a cognitive task and intervention-related cognitive gains.
Additional Work Supporting a Brain Modularity-Plasticity Relationship
Results from the independent studies described above demonstrate that baseline brain network modularity is predictive of cognitive control gains in multiple populations after cognitive and exercise training. Broadly, these results suggest that modularity indexes cognitive and underlying neural plasticity. Notably, brain modularity has also recently been shown to be predictive of task learning rates in early stages of working memory training [42]. In further support of modularity as a biomarker of intervention plasticity, a metric of baseline default and visual network modularity also predicts reductions in symptom severity after cognitive behavioral therapy in patients with obsessive-compulsive disorder (OCD) [43] and default network segregation predicts clinical treatment outcomes in patients with schizophrenia [44].
Moreover, brain modularity metrics also predict outcomes associated with forms of plasticity that are not directly intervention-related, such as recovery from injury. In one study, modularity measured from resting EEG data predicted future behavioral outcomes in patients with disorders of consciousness [45]. Specifically, patients with higher modularity had more positive outcomes (i.e., recovered consciousness) approximately one year later. In a second study, rs-fMRI connectivity within brain sub-networks predicted future cognitive decline in cognitively normal older adults [46]. Specifically, older adults with higher baseline connectivity within ‘associative’ networks had the least cognitive decline approximately three years later.
Collectively, these additional studies demonstrate that brain modularity is also predictive of other clinically-based outcomes, such as OCD severity and recovery of consciousness. Further, they suggest that brain modularity can be related to forms of plasticity even in the absence of an active intervention, such as recovery from brain injury and cognitive decline from normal aging.
Potential Mechanisms Underlying the Relationship between Brain Modularity and Plasticity
There is a large body of empirical and computational work demonstrating the advantages of a modular network organization. While recent reviews have described the behavioral relevance of modular network organization in detail (e.g., [47]), the specific focus of this Opinion is to propose network-level mechanisms relating brain modularity to cognitive plasticity that may, in turn, explain how interventions lead to cognitive benefits.
Empirical Work
Human studies have shown that modular brain networks are beneficial for cognitive performance. First, higher network modularity quantified from task-free ‘resting’ functional connectivity data is related to better working memory capacity [48] and episodic memory [33] across individuals. Further, greater modularity of sensorimotor networks is related to future motor skill learning [49] and functional networks become more segregated over the course of practice and learning [50]. Second, modular network organization is disrupted in both healthy older individuals and patients with cognitive control deficits [33,51–56]. Finally, lesions to brain regions important for maintaining modular organization [57] lead to more extensive cognitive deficits [58]. Network modularity also increases over the first weeks to months during stroke recovery and, importantly, is associated with recovery of complex cognitive functions, such as attention and memory [59]. Collectively, these empirical studies in humans suggest that trait-like features of brain modularity predict behavioral performance, namely that a more modular intrinsic network organization measured in ‘resting’ states is advantageous for behavior.
Finally, although recent work has described state-like changes in modular network organization (e.g., during task performance) in depth (e.g., [47,60]), one particularly relevant study found that individuals with higher general intelligence have smaller changes in connectivity between a task-free ‘resting-state’ and task performance, suggesting that these individuals have a more ‘optimal’ intrinsic network organization that supports more efficient changes in patterns of connectivity during task performance [61]. During cognitive training, we propose that individuals with higher network modularity may also be in a more “optimal” network state that requires less network reconfiguration to achieve the “optimal” network state necessary for a successful response to a training intervention. Although our hypotheses and review of previous studies emphasize trait-level differences in brain modularity, we believe that leveraging both state- and trait-based approaches in future work will be informative for identifying biomarkers as well as understanding network-level mechanisms underlying cognitive plasticity.
Theoretical Work
Computational modeling studies provide a mechanistic framework for the empirical findings described above, as well as demonstrate evolutionary benefits of having a modular network architecture. In particular, network modularity may offer information encapsulation that, in turn, allows for faster processing [62]. Further, previous work has shown that modular networks spontaneously evolve to solve varying task goals and, additionally, networks that are more modular have greater adaptability in changing environments [63]. Similarly, other work has shown that modular networks are better at learning new skills without forgetting old ones [64]. In particular, a problem in the field of artificial intelligence referred to as catastrophic forgetting (i.e., forgetting previously acquired skills after learning new ones) was reduced in modular neural networks. It is proposed that modular networks aid new learning because processing occurs selectively in a module dedicated to learning a new task [64]. Modular biological networks may have also evolved to reduce network wiring costs [65]. Specifically, this may allow modular networks of sufficient size, similar to the scale of many biological networks, to be more efficient and faster at problem solving (for example, identifying a subset of specific patterns from a larger set [66]).
Taken together, this computational work suggests that modular networks are more adaptable and are better at learning and problem solving. These advantages of modular networks have been attributed to the compartmentalization of connections offered by a modular organization [13]. Here, networks with many connections concentrated within modules can more independently adapt to changing external demands [67] and, further, are not as affected if other modules are modified or damaged. Relatedly, modular networks are thought to act more independently from the rest of the network because they have relatively few between-module connections, which leads to increased flexibility.
Considering the empirical and computational evidence for advantages of a modular network organization, we propose possible network-level mechanisms underlying the relationship between brain modularity and intervention-induced cognitive gains. Specifically, we hypothesize that higher network modularity represents a brain network trait that allows for greater plasticity during interventions. As the empirical work suggests, robust trait-like differences in modularity exist across individuals that are related to better behavioral performance in a variety of cognitive domains, such as working memory and episodic memory. Further, as the computational work suggests, modular networks show greater adaptability in changing environments and are better at learning and problem solving.
In the context of cognitive training interventions, we propose that individuals with more modular brains are better at adapting to changing training demands and faster at solving novel tasks that must be learned during training. In this way, given the relationship between brain modularity and cognitive performance, individuals with a more modular brain are likely better at learning training paradigms [49]. This proposal is supported by empirical work linking modularity to early learning rates during training [42], in which individuals with more modular brains exhibited faster learning of the training paradigm than those with less modular brains. Thus, we propose that during the course of cognitive training, individuals with higher brain network modularity have a greater capacity to train more and at higher levels of difficulty sooner than individuals with lower brain network modularity, leading to larger intervention-induced cognitive improvements. Future studies can directly test these hypotheses.
Concluding Remarks and Future Directions
Based on recent studies, we propose that assessments of brain network modularity serve as a key biomarker that predicts intervention-related cognitive plasticity across individuals engaging in various forms of training. Moreover, modularity may also be a valuable metric for understanding recovery from brain injury and progression of brain pathology. We believe that this level of analysis (i.e., examining large-scale brain network organization) is critical for capturing information that is not as well explained by behavior or the activity and structure of individual brain regions. For complex behaviors like cognitive control, examining the interactions within and between brain sub-networks is likely to be informative. The extant literature provides evidence for the significance of modular brain network organization in shaping both current and future behavioral outcomes. Further, it broadly points to modularity as an important index of underlying plasticity and, as such, offers potential mechanisms for individual differences in intervention success. Finally, we anticipate that this framework can be used prospectively to guide personalized interventions that improve outcomes across individuals. Future studies should examine whether the relationship between modularity and cognitive plasticity extends to other populations, such as learning in children and adolescents (see Outstanding Questions). Importantly, these future lines of work will help generate testable hypotheses for underlying neurobiological mechanisms of brain modularity as a biomarker of plasticity.
Outstanding Questions Box.
Is brain modularity also predictive of underlying neural changes that occur with interventions? Intervention studies also frequently report concurrent changes in brain structure and function, but the relationship between brain modularity and training-related neural changes remains unexplored.
Is brain modularity related to intervention-related outcomes, learning, and academic achievement in children and adolescents? Variability in cognitive control abilities is related to scholastic success in children and adolescents, but the relationship between modularity and these abilities is not well understood.
Is brain modularity predictive of brain stimulation-based cognitive improvements or intervention-related gains in other cognitive domains? For example, brain stimulation (e.g., transcranial magnetic stimulation) is another promising method to improve cognitive control. Further, interventions have also shown potential to improve other aspects of complex behavior (e.g., mnemonic training to improve memory). Future research should examine the relationship between brain modularity, other interventions that enhance cognition, and other cognitive domains.
Can brain network biomarkers be used prospectively to develop and guide personalized interventions? Thus far, brain modularity and other biomarkers have been used to predict intervention outcomes retrospectively. A promising line of future work will be to use network biomarkers prospectively to maximize outcomes. For example, can baseline brain modularity measures be used to identify optimal intervention dosage? Further, could brain state be manipulated prior to an intervention (e.g., increase modularity) to enhance an individual’s outcome? Finally, can differential predictors of success be identified to assign individuals to an ‘optimal’ intervention given their baseline brain network organization?
Figure I. Functional brain network analysis pipeline.
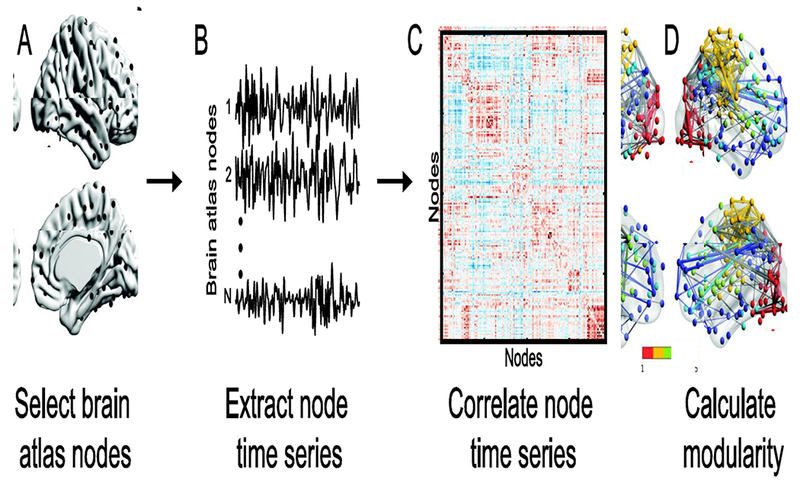
(A) The brain is first parcellated into a set of brain regions to form network nodes (e.g., the Power et al. atlas [37]). (B) For functional network analyses, the time series of each node is then extracted. (C) The time series of every possible pair of brain regions is then correlated to form the network edges. (D) The network is then partitioned into sub-networks or modules. Finally, the modularity of the network can be calculated by comparing within and between module connections. Brain network images in (A) and (D) were visualized with the BrainNet Viewer [85].
Trends Box.
There is a rapidly growing interest to use interventions to enhance cognition. Although some interventions show promise, there is often variability in the magnitude of improvements across individuals.
Graph theory analyses of human neuroimaging data are a useful tool to examine large-scale network properties of the brain. Modularity is an important organizational principle that quantifies the extent to which a network is divided into distinct sub-networks, or modules.
Baseline assessments of brain modularity predict intervention-related cognitive improvements across several different populations and interventions, including patients with traumatic brain injury and healthy individuals participating in cognitive and exercise interventions.
Brain modularity is a unifying biomarker of potential for intervention success and, more broadly, cognitive plasticity.
Acknowledgements
This work was supported by a National Science Foundation SBE Postdoctoral Research Fellowship (Grant 1808384 to CLG), the NIH (Grant NS79698 to MD), and the VA Research Administration (to MD). We would also like to thank Zoe D’Esposito for assistance with figure creation.
Glossary
- Biomarker:
a biological marker that can be used to characterize ongoing normal or abnormal biological processes as well as to predict responses to interventions or treatments.
- Brain network:
a group of neural components and the connections between them. Neural components can scale to represent individual neurons to brain regions. Connections can be anatomical (e.g., white matter tracts) or functional (e.g., time series correlations of brain imaging data).
- Cognitive control:
a set of complex processes important for goal-directed behavior, including aspects of attention and working memory.
- Cognitive plasticity:
the capacity to change and adapt cognitive functioning.
- Functional connectivity:
a method to identify connections between brain regions based on statistical correlations of their activity across time. Functional connectivity is often quantified using task-free ‘resting-state’ functional MRI data.
- Graph theory:
a mathematical approach to examine properties of networks, or graphs (e.g., a mathematical object). Graphs are comprised of individual components (nodes) and the relationships between them (edges).
- Network modularity:
a network property that quantifies the extent to which a network is partitioned into individual sub-networks, or modules. A network with high modularity has many connections within modules and fewer connections between modules.
- Network modules:
a collection of nodes that form an individual sub-network in a graph, where nodes within a module have many connections to others in their own sub-network and fewer connections to other sub-networks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anguera JA and Gazzaley A (2015) Video games, cognitive exercises, and the enhancement of cognitive abilities. Current Opinion in Behavioral Sciences 4, 160–165 [Google Scholar]
- [2].Anguera JA et al. (2013) Video game training enhances cognitive control in older adults. Nature 501, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buschkuehl M et al. (2014) Neural effects of short-term training on working memory. Cogn Affect Behav Neurosci 14, 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baniqued PL et al. (2014) Cognitive training with casual video games: points to consider. Frontiers in Psychology 4, 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mishra J and Gazzaley A (2014) Harnessing the neuroplastic potential of the human brain & the future of cognitive rehabilitation. Frontiers in Human Neuroscience 8, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Green CS and Bavelier D (2008) Exercising your brain: A review of human brain plasticity and training-induced learning. Psychology and Aging 23, 692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gathercole SE et al. (2003) Working memory assessments at school entry as longitudinal predictors of National Curriculum attainment levels. Educational and Child Psychology 20, 109–122 [Google Scholar]
- [8].Bryck RL and Fisher PA (2012) Training the brain: Practical applications of neuralplasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. American Psychologist 67, 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Willis SL et al. (2006) Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA: the journal of the American Medical Association 296, 2805–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pascual-Leone A et al. (2005) The plastic human brain cortex. Annu. Rev. Neurosci 28, 377–401 [DOI] [PubMed] [Google Scholar]
- [11].Katz B et al. (2018) How to play 20 questions with nature and lose: Reflections on 100 years of brain-training research. Proceedings of the National Academy of Sciences 115, 9897–9904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Newman ME and Girvan M (2004) Finding and evaluating community structure in networks. Phys. Rev. E 69, 026113. [DOI] [PubMed] [Google Scholar]
- [13].Sporns O and Betzel RF (2016) Modular Brain Networks. Annu. Rev. Psychol 67, 613–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mathewson KE et al. (2012) Different slopes for different folks: Alpha and delta EEG power predict subsequent video game learning rate and improvements in cognitive control tasks. Psychophysiol 49, 1558–1570 [DOI] [PubMed] [Google Scholar]
- [15].Vo LTK et al. (2011) Predicting Individuals’ Learning Success from Patterns of Pre-Learning MRI Activity. PLoS ONE 6, e16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Basak C et al. (2011) Regional differences in brain volume predict the acquisition of skill in a complex real-time strategy videogame. Brain and Cognition 76, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Erickson KI et al. (2010) Striatal Volume Predicts Level of Video Game Skill Acquisition. Cerebral Cortex 20, 2522–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Finn ES et al. (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature neuroscience 18, 1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Horien C et al. (2019) The uniqueness of the individual functional connectome In Connectomics, pp. 63–81, Academic Press [Google Scholar]
- [20].Gabrieli JDE et al. (2015) Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron 85, 11–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Medaglia JD et al. (2015) Cognitive Network Neuroscience. Journal of Cognitive Neuroscience 27, 1471–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mesulam M-M (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28, 597–613 [DOI] [PubMed] [Google Scholar]
- [23].Arnemann KL et al. (2015) Functional brain network modularity predicts response to cognitive training after brain injury. Neurology 84, 1568–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gallen CL et al. (2016) Modular Brain Network Organization Predicts Response to Cognitive Training in Older Adults. PLoS ONE 11, e0169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baniqued PL et al. (2017) Brain Network Modularity Predicts Exercise-Related Executive Function Gains in Older Adults. Fronti.Ag.Neurosci 9, 924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Novakovic-Agopian T et al. (2011) Rehabilitation of executive functioning with training in attention regulation applied to individually defined goals: a pilot study bridging theory, assessment, and treatment. Journal of Head Trauma Rehabilitation 26, 325–338 [DOI] [PubMed] [Google Scholar]
- [27].Chiu H-L et al. (2017) The effect of cognitive-based training for the healthy older people: A meta-analysis of randomized controlled trials. PLoS ONE 12, e0176742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lampit A et al. (2014) Computerized Cognitive Training in Cognitively Healthy Older Adults: A Systematic Review and Meta-Analysis of Effect Modifiers. Plos Med 11, e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kelly ME et al. (2014) Ageing Research Reviews. Ageing Research Reviews 15, 28–43 [DOI] [PubMed] [Google Scholar]
- [30].Chapman SB et al. (2015) Neural Mechanisms of Brain Plasticity with Complex Cognitive Training in Healthy Seniors. Cerebral Cortex 25, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vas AK et al. (2011) Higher-Order Reasoning Training Years After Traumatic Brain Injury in Adults. Journal of Head Trauma Rehabilitation 26, 224–239 [DOI] [PubMed] [Google Scholar]
- [32].Wechsler D (1997) WAIS-III, Wechsler adult intelligence scale: Administration and scoring manual, Psychological Corporation. [Google Scholar]
- [33].Chan MY et al. (2014) Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences 111, E4997–E5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Voss MW et al. (2011) Exercise, brain, and cognition across the life span. Journal of Applied Physiology 111, 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Voss MW et al. (2013) Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences 17, 525–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tzourio-Mazoyer N et al. (2002) Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 15, 273–289 [DOI] [PubMed] [Google Scholar]
- [37].Power JD et al. (2011) Functional Network Organization of the Human Brain. Neuron 72, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kirkpatrick S et al. (1983) Optimization by simulated annealing. Science 220, 671–680 [DOI] [PubMed] [Google Scholar]
- [39].Newman ME (2006) Modularity and community structure in networks. Proceedings of the National Academy of Sciences 103, 8577–8582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen X et al. (2018) Topological analyses of functional connectomics: A crucial role of global signal removal, brain parcellation, and null models. Hum. Brain Mapp 39, 4545–4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Finn ES et al. (2017) Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage 160, 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Iordan AD et al. (2018) Aging and Network Properties: Stability Over Time and Links with Learning during Working Memory Training. Fronti.Ag.Neurosci 9, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reggente N et al. (2018) Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive–compulsive disorder. Proceedings of the National Academy of Sciences 115, 2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Doucet GE et al. (2018) Baseline brain structural and functional predictors of clinical outcome in the early course of schizophrenia. Molecular Psychiatry 380, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chennu S et al. (2017) Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 140, 2120–2132 [DOI] [PubMed] [Google Scholar]
- [46].Buckley RF et al. (2017) Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology 89, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wig GS (2017) Segregated Systems of Human Brain Networks. Trends in Cognitive Sciences 21, 981–996 [DOI] [PubMed] [Google Scholar]
- [48].Stevens AA et al. (2012) Functional Brain Network Modularity Captures Inter- and Intra-Individual Variation in Working Memory Capacity. PLoS ONE 7, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mattar MG et al. (2018) Predicting future learning from baseline network architecture. NeuroImage 172, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bassett DS et al. (2015) Learning-induced autonomy of sensorimotor systems. Nature neuroscience 18, 744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gallen CL et al. (2016) Reconfiguration of brain network architecture to support executive control in aging. Neurobiology of Aging 44, 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Onoda K and Yamaguchi S (2013) Small-worldness and modularity of the resting-state functional brain network decrease with aging. Neuroscience Letters 556, 104–108 [DOI] [PubMed] [Google Scholar]
- [53].Chen ZJ et al. (2011) Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. NeuroImage 56, 235–245 [DOI] [PubMed] [Google Scholar]
- [54].Geerligs L et al. (2014) A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral Cortex 25, 1987–1999 [DOI] [PubMed] [Google Scholar]
- [55].Alexander-Bloch A et al. (2012) The discovery of population differences in network community structure: New methods and applications to brain functional networks in schizophrenia. NeuroImage 59, 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Alexander-Bloch AF et al. (2010) Disrupted Modularity and Local Connectivity of Brain Functional Networks in Childhood-Onset Schizophrenia. Front. Syst. Neurosci 4, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gratton C et al. (2012) Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. Journal of Cognitive Neuroscience 24, 1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Warren DE et al. (2014) Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences 111, 14247–14252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Siegel JS et al. (2018) Re-emergence of modular brain networks in stroke recovery. Cortex 101, 44–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shine JM and Poldrack RA (2018) Principles of dynamic network reconfiguration across diverse brain states. NeuroImage 180, 396–405 [DOI] [PubMed] [Google Scholar]
- [61].Schultz DH and Cole MW (2016) Higher Intelligence Is Associated with Less Task-Related Brain Network Reconfiguration. Journal of Neuroscience 36, 8551–8561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Coltheart M (1999) Modularity and cognition. Trends in Cognitive Sciences 3, 115–120 [DOI] [PubMed] [Google Scholar]
- [63].Kashtan N and Alon U (2005) Spontaneous evolution of modularity and network motifs. Proceedings of the National Academy of Sciences 102, 13773–13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ellefsen KO et al. (2015) Neural Modularity Helps Organisms Evolve to Learn New Skills without Forgetting Old Skills. PLoS Comput Biol 11, e1004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Clune J et al. (2013) The evolutionary origins of modularity. Proc. R. Soc. B 280, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tosh CR and McNally L (2015) The relative efficiency of modular and non-modular networks of different size. Proc. R. Soc. B 282, 20142568–20142568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schlosser G and Wagner GP, eds (2004) Modularity in Development and Evolution, University of Chicago Press [Google Scholar]
- [68].Mishra J et al. (2014) Adaptive Training Diminishes Distractibility in Aging across Species. Neuron 84, 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Simons DJ et al. (2016) Do “Brain-Training” Programs Work? Psychological Science in the Public Interest 17, 103–186 [DOI] [PubMed] [Google Scholar]
- [70].Thompson TW et al. (2013) Failure of Working Memory Training to Enhance Cognition or Intelligence. PLoS ONE 8, e63614–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kable JW et al. (2017) No Effect of Commercial Cognitive Training on Brain Activity, Choice Behavior, or Cognitive Performance. J. Neurosci 37, 7390–7402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Melby-Lervåg M and Hulme C (2013) Is working memory training effective? A meta-analytic review. Developmental Psychology 49, 270–291 [DOI] [PubMed] [Google Scholar]
- [73].Melby-Lervåg M et al. (2016) Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of “Far Transfer.” Perspect Psychol Sci 11, 512–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mishra J and Gazzaley A (2015) Closed-loop cognition: the next frontier arrives. Trends in Cognitive Sciences 19, 242–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Newman ME (2004) Detecting community structure in networks. The European Physical Journal B-Condensed Matter and Complex Systems 38, 321–330 [Google Scholar]
- [76].Guimera R and Amaral LAN (2005) Functional cartography of complex metabolic vnetworks. Nature 433, 895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guimera R et al. (2006) Classes of complex networks defined by role-to-role connectivity profiles. Nat Phys 3, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sohn Y et al. (2011) Topological Cluster Analysis Reveals the Systemic Organization of the Caenorhabditis elegans Connectome. PLoS Comput Biol 7, e1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shih C-T et al. (2015) Connectomics-Based Analysis of Information Flow in the Drosophila Brain. Current Biology 25, 1249–1258 [DOI] [PubMed] [Google Scholar]
- [80].Wang Q et al. (2012) Network Analysis of Corticocortical Connections Reveals Ventral and Dorsal Processing Streams in Mouse Visual Cortex. J. Neurosci 32, 4386–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bota M et al. (2015) Architecture of the cerebral cortical association connectome underlying cognition. Proceedings of the National Academy of Sciences 112, E2093–E2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hilgetag CC et al. (2000) Anatomical connectivity defines the organization of clusters of cortical areas in the macaque and the cat. Philosophical Transactions of the Royal Society of London B: Biological Sciences 355, 91–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hagmann P et al. (2008) Mapping the structural core of human cerebral cortex. Plos Biol 6, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bertolero MA et al. (2018) A mechanistic model of connector hubs, modularity, and cognition. arXiv preprint arXiv, 1803.08109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xia M et al. (2013) BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]


