Abstract
Purpose
To characterize clinical outcomes following Kahook Dual Blade® (KDB)-assisted goniosynechialysis and excisional goniotomy combined with phacoemulsification in eyes with angle-closure glaucoma and cataract.
Setting
Two clinical practices.
Methods
In this retrospective analysis of existing health records, data were collected from 42 eyes of 24 subjects from preoperative, operative, and postoperative encounters through 12 months of follow-up. Outcomes included changes in mean IOP, IOP-lowering medications, and logMAR best-corrected visual acuity (BCVA), as well as the proportions of patients achieving IOP reductions ≥20%, IOP ≤18 mmHg, and a reduction of ≥1 medication.
Results
Preoperative, mean (standard error) IOP was 25.5 (0.7) mmHg and at Month 12 was reduced by 12.3 (0.73) mmHg (−47.2%; p<0.0001). The mean number of IOP-lowering medications used was 2.3 (0.1) preoperatively and was reduced at Month 12 by 2.2 (0.12) (−91.7%; p<0.0001). At Month 12, 92.9% of eyes achieved IOP ≤18 mmHg, 100% achieved IOP reduction of ≥20%, 95.2% required ≥1 fewer medications for IOP control, and 85.7% (36/42) were medication-free. Mean LogMAR BCVA improved from 0.547 (0.06) at baseline to 0.159 (0.07) at Month 12.
Conclusion
KDB-assisted goniosynechialysis and excisional goniotomy at the time of phacoemulsification safely provide significant reductions in both IOP and IOP-lowering medication burden in eyes with angle-closure glaucoma, while simultaneously improving visual acuity.
Keywords: glaucoma, angle closure, synechialysis, goniotomy, MIGS
Introduction
Closure of the iridocorneal angle of the anterior chamber represents a common and often devastating form of glaucoma. The condition exists on a spectrum beginning with the primary angle-closure suspect (characterized by anatomically narrow angles but normal IOP and no glaucomatous optic neuropathy), which can progress to primary angle closure (with elevated IOP and the formation of peripheral anterior synechiae [PAS] but still no neuropathy) and ultimately to primary angle-closure glaucoma (with elevated IOP and resulting optic neuropathy).
The optimal management of primary angle-closure suspects and primary angle closure has been clarified by a series of recent clinical trials. The ZAP trial demonstrated a low rate of progression from suspect to angle closure in an urban Chinese population and thus a little benefit to prophylactic iridotomy.1 The EAGLE study revealed that eyes in acute angle-closure fare better with primary lens extraction (even if the lens is clear) than with iridotomy with or without medications.2
The management of primary angle-closure glaucoma, however, remains less well delineated. Common approaches include filtration surgery (such as trabeculectomy or tube-shunt implantation) or cataract surgery with or without goniosynechialysis, in which PAS are lysed to mechanically open the angle and bare the TM with the goal of improving outflow and lowering IOP.3 This approach assumes that the bared TM is functionally intact once aqueous humor can reach it, which is typically not the case, as chronic synechialization, inflammation, and/or proliferation of iris or fibrous tissue in the region of the TM often render it dysfunctional and impervious to aqueous passage.3,4 In fact, persistent elevated IOP is not uncommon following goniosynechialysis.4
A comprehensive approach to primary angle-closure glaucoma would address the pupillary block component by removing the cataractous lens, would address the angle synechialization with goniosynechialysis, and would address the residual TM dysfunction by creating an opening or a bypass in the damaged TM to allow aqueous egress into Schlemm’s canal. We have developed a combined procedure consisting of cataract extraction, goniosynechialysis to mechanically open the angle and bare the TM, and excisional gonioscopy using the Kahook Dual Blade® (KDB, New World Medical, Inc, Rancho Cucamonga, CA, USA) to functionally open the angle and facilitate aqueous humor outflow bypassing the impaired TM to reach the distal outflow channels.
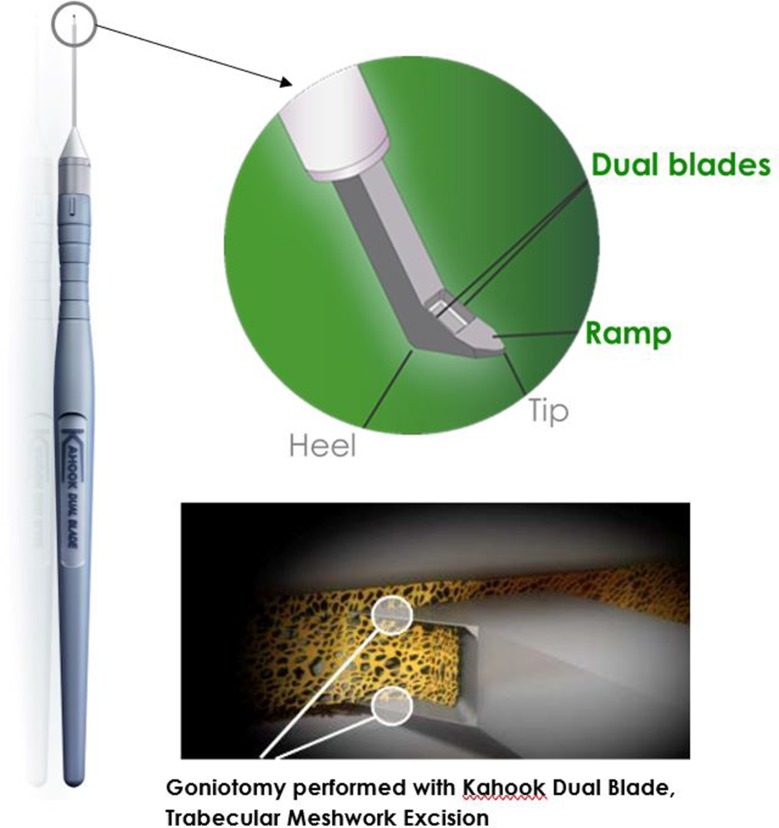
The KDB is a single-use surgical instrument with two blades on either side of a ramp, designed to engage and excise a strip of TM as the instrument is advanced through the iridocorneal angle of the eye (Figure 1). In eyes with open-angle glaucoma, excisional goniotomy with the KDB has been shown to lower IOP 24–36% and reduce the medication burden by 37–63% through 6–12 months of follow-up when performed as a standalone procedure or in combination with cataract surgery.5–9 Little is known regarding the role of excisional goniotomy with the KDB in angle-closure glaucoma. We recently described the 6-month clinical results of this combined surgical approach.10 Recognizing that angle-closure glaucoma is a chronic condition, we herein report the results of this combined procedure through 12 months of follow-up.
Figure 1.
The Kahook Dual Blade. Its tip features a ramp with two parallel blades to engage and excise a strip of trabecular meshwork as it is advanced along the circumference of the iridocorneal angle.
Methods
This was a retrospective interventional case series. Analysis was conducted on a de-identified data set derived from the health records of adult subjects with angle-closure glaucoma and visually significant cataract undergoing combined phacoemulsification with intraocular lens (IOL) implantation, goniosynechialysis, and goniotomy using the KDB. The data set contained no Health Information Portability and Accounting Act–specified Protected Health Information, and the analysis was conducted under an ethics committee’s waiver of consent (Mayo Clinic IRB, Protocol # 18-000006).
Subjects were included in the analysis if they were aged 18 years or older and diagnosed with both visually significant cataract and ACG with uncontrolled IOP on medical therapy and had undergone combined phacoemulsification with IOL implantation, goniosynechialysis, and excisional goniotomy using the KDB. For this analysis, angle-closure glaucoma was defined as elevated IOP, classic glaucomatous optic nerve, and visual field changes, and at least 3 clock hours of PAS in the anterior chamber angle. The procedure consisted of a standard phacoemulsification and IOL implantation procedure, after which the pupil was pharmacologically constricted. Ophthalmic viscosurgical device (OVD) was injected into the anterior chamber to dissect the iris from the angle to the greatest extent possible. The KDB was then inserted through the surgical incision and, under direct gonioscopy, its pointed tip was utilized to engage peripheral iris anterior to the center of each PAS. Gentle centripetal force was applied within the iris plane toward the pupillary center to disrupt the peripheral adhesions and expose the TM for goniotomy. The KDB was then employed to perform goniotomy as described previously.5 Briefly, under direct gonioscopy, the instrument’s tip was guided to the nasal angle, inserted through the TM into Schlemm’s canal, and advanced for several clock hours, excising a narrow strip of TM. A second pass in the opposite direction removed several additional clock hours of TM. The strip was then removed from the eye either with the KDB or forceps. The OVD was then irrigated from the anterior chamber. Postoperative care included prednisolone acetate 1% instilled 4–6 times per day tapered over 6–8 weeks, pilocarpine 1–2% 4 times per day for 4–6 weeks, and a broad-spectrum antibiotic (typically a 4th-generation fluoroquinolone) 4 times per day for a week.
Distance visual acuity was measured using the Snellen acuity chart at the 20-foot equivalent distance. IOP was measured using Goldmann applanation tonometry. Detailed anterior segment examination was conducted with a slit-lamp, and dark room indentation gonioscopy was performed with the Zeiss four mirror lens. Fundus examination including optic disc and periphery were documented using 78D lens and 20D lens, respectively.
Baseline data included age, gender, severity of glaucoma, Snellen best-corrected visual acuity (BCVA), Goldmann IOP, and the IOP-lowering medication regimen (fixed combinations were counted as 2 medications). Postoperatively, BCVA, IOP, and IOP-lowering medication use was recorded 1 day, 1 week, and 1, 3, 6, 9, and 12 months postoperatively. Medication use in the immediate postoperative period (1 day and 1 week) was not analyzed as it was highly variable between patients and not indicative of long-term outcomes. Intraoperative and postoperative complications were noted.
The primary statistical goal of this analysis was to characterize the long-term (12-month) reduction from baseline in both IOP and IOP-lowering medication use following the combined procedure. Additional exploratory endpoints included IOP reduction of ≥20% from baseline, the proportion of eyes with IOP ≤18 mmHg, and the proportion of eyes requiring ≥1 fewer medication, all assessed at each postoperative time point. Arithmetic means and changes from baseline are presented ± SE. Changes from baseline in both absolute and percent IOP and medication reductions, as well as the significance of these changes, were assessed using standard nested mixed regression modeling with both fixed and random effects to account for fellow-eye correlation given that some subjects contributed data from both eyes (utilizing an autoregressive first-order correlation matrix that assumes that values are more strongly correlated between fellow eyes and in shorter-interval follow-ups) and Bonferroni’s correction to address multiplicity. As no specific hypothesis testing was planned, formal power and sample size calculations were not performed.
Results
The cohort of 42 eyes of 24 subjects has been previously described (Table 1).10 Briefly, the mean (SE) age of subjects was 66.5 (2.4) years, the majority (54.2%) were female, and most eyes had moderate (31.0%) or severe (61.9%) angle-closure glaucoma in the worse eye, most of which were treated with 2 (47.6%) or 3 (42.9%) IOP-lowering medications. All eyes of all subjects were examined at 12 months postoperatively.
Table 1.
Demographic and glaucoma severity data of the study cohort
| (N=24 subjects) | ||
|---|---|---|
| Age, yr (mean (SE)) | 66.5 (2.4) | |
| Gender (n (%)) | Female | 13 (54.2) |
| Male | 11 (45.8) | |
| (N=42 eyes) | ||
| Severity (n (%)) | Mild | 3 (7.1) |
| Moderate | 13 (31.0) | |
| Severe | 26 (61.9) | |
| Glaucoma medications (n (%)) | 1 | 4 (9.5) |
| 2 | 20 (47.6) | |
| 3 | 18 (42.9) | |
| Study eye (n (%)) | Right eye | 20 (47.6) |
| Left eye | 22 (52.4) |
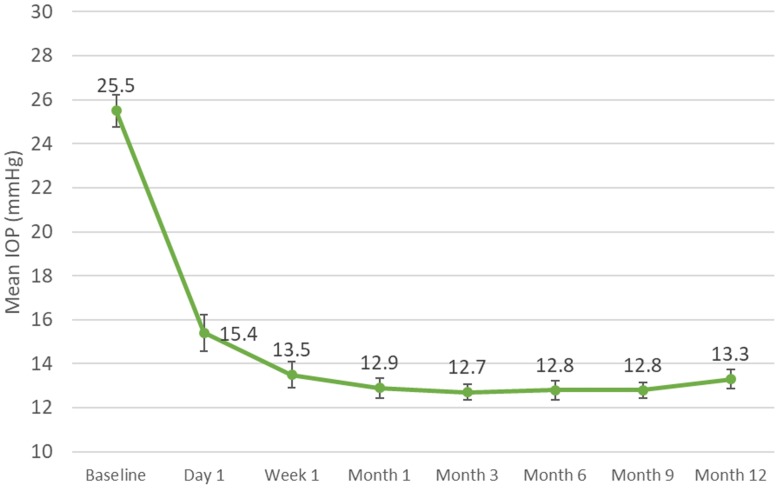
Mean (SE) IOP at baseline was 25.5 (0.7) mmHg (Table 2 and Figure 2). Compared to the IOP control achieved through the first 6 months, mean IOP remained unchanged through an additional 6 months of follow-up. From mean IOP of 12.8 (0.4) mmHg at Month 6, mean IOP at Months 9 and 12 was 12.8 (0.4) and 13.3 (0.4) mmHg (p<0.0001 for both), representing IOP reductions of 49.2% and 47.2%, respectively (compared to 48.8% at Month 6). At Month 12, 92.9% (39/42) of eyes achieved IOP ≤18 mmHg, and 100% (42/42) achieved a minimum IOP reduction of 20% from baseline (Table 3).
Table 2.
Mean IOP, medication and visual acuity data, and changes from baseline, at each study time point
| Baseline | Day 1 | Week 1 | Month 1 | Month 3 | Month 6 | Month 9 | Month 12 | |
|---|---|---|---|---|---|---|---|---|
| N=42 | N=42 | N=42 | N=42 | N=42 | N=42 | N=42 | N=42 | |
| Mean IOP (SE) | 25.5 (0.73) | 15.4 (0.82) | 13.5 (0.61) | 12.9 (0.46) | 12.7 (0.35) | 12.8 (0.43) | 12.8 (0.35) | 13.3 (0.44) |
| Change from baseline | – | −10.1 (0.98) | −12.0 (0.82) | −12.6 (0.69) | −12.8 (0.67) | −12.7 (0.75) | −12.8 (0.66) | −12.3 (0.73) |
| Percent change from baseline | – | −38.4 (3.26) | −45.9 (2.53) | −48.5 (1.76) | −49.5 (1.46) | −48.8 (1.86) | −49.2 (1.48) | −47.2 (1.61) |
| Significance | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean number of IOP-lowering medications (SE) | 2.33 (0.10) | – | – | 0.10 (0.06) | 0.05 (0.03) | 0.14 (0.06) | 0.10 (0.05) | 0.14 (0.06) |
| Change from baseline | – | – | – | −2.24 (0.10) | −2.29 (0.10) | −2.19 (0.12) | −2.23 (0.12) | −2.19 (0.12) |
| Percent change from baseline | – | – | – | −96.8 (1.90) | −98.4 (1.11) | −91.7 (3.65) | −93.3 (3.55) | −91.7 (3.65) |
| Significance | – | – | – | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean logMAR best-corrected visual acuity (SE) | 0.547 (0.06) | 0.405 (0.09) | 0.252 (0.07) | 0.210 (0.07) | 0.183 (0.07) | 0.183 (0.07) | 0.171 (0.07) | 0.159 (0.07) |
| Change from baseline | – | −0.141 (0.06) | −0.295 (0.02) | −0.337 (0.03) | −0.363 (0.03) | −0.364 (0.03) | −0.376 (0.03) | −0.388 (0.03) |
| Percent change from baseline | – | −33.1 (11.68) | −65.0 (4.79) | −73.1 (4.69) | −77.8 (4.03) | −77.6 (4.32) | −79.3 (4.32) | −81.7 (4.45) |
| Significance | – | 0.0072 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Figure 2.
Mean intraocular pressure (IOP) values over time for eyes with angle closure glaucoma undergoing goniosynechialysis and excisional goniotomy with the Kahook Dual Blade along with phacoemulsification (with SE bars).
Table 3.
Pre-specified IOP and medication outcomes at each study time point
| Day 1 (N=42) | Week 1 (N=42) | Month 1 (N=42) | Month 3 (N=42) | Month 6 (N=42) | Month 9 (N=42) | Month 12 (N=42) | |
|---|---|---|---|---|---|---|---|
| Proportion achieving IOP ≤18 mmHg | 31 (73.8) | 37 (88.1) | 41 (97.6) | 42 (100) | 39 (92.9) | 42 (100) | 39 (92.9) |
| Proportion achieving IOP reduction ≥20% compared to baseline | 32 (76.2) | 38 (90.5) | 42 (100) | 42 (100) | 41 (97.6) | 42 (100) | 42 (100) |
| Proportion using ≥1 fewer medication compared to baseline | – | – | 42 (100) | 42 (100) | 40 (95.2) | 40 (95.2) | 40 (95.2) |
| Proportion medication-free | – | – | 39 (92.9) | 40 (95.2) | 36 (85.7) | 38 (90.5) | 36 (85.7) |
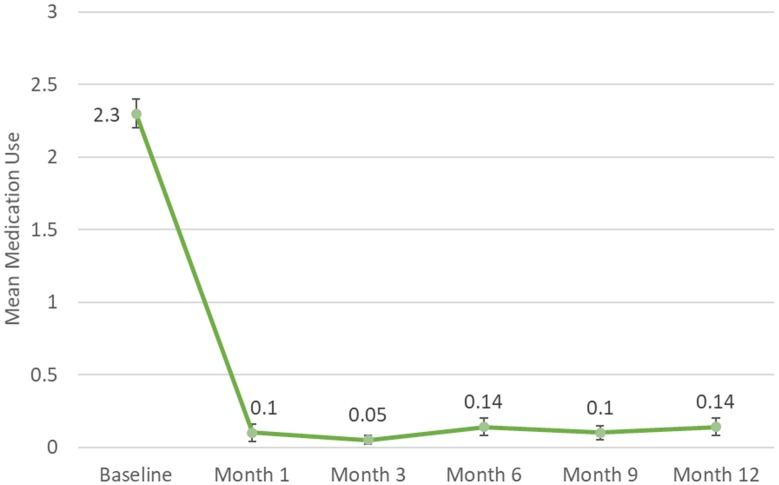
Mean (SE) number of IOP medications at baseline was 2.3 (0.1) (Table 2 and Figure 3). Compared to the number of medications required through the first 6 months, the mean number of medications remained unchanged through an additional 6 months of follow-up. From mean medications of 0.1 (0.1) at Month 6, the number of medications at both Months 9 and 12 was 0.1 (0.1) (p<0.0001), representing medication reductions of 93.3% and 91.7%, respectively (unchanged from 91.7% at Month 6). At Month 12, 95.2% (40/42) of eyes were using at least 1 less medication compared to preoperatively, and 85.7% (36/42) were medication-free (Table 3).
Figure 3.
Mean medication use over time for eyes with angle closure glaucoma undergoing goniosynechialysis and excisional goniotomy with the Kahook Dual Blade along with phacoemulsification (with SE bars).
Mean (SE) BCVA (logMAR) was 0.55 (0.1) at baseline. Compared to BCVA through the first 6 months, mean BCVA remained stable through Month 12. At Months 9 and 12, mean logMAR BCVA was 0.17 (0.1) and 0.16 (0.1) (p<0.0001 for both), respectively. Overall, final postoperative BCVA improved in 40 eyes (95.2%) and was unchanged in the remaining 2 eyes (4.8%); no eye lost any BCVA compared to baseline at Month 12.
The combined procedure was safe and well tolerated. No new adverse events were identified between months 6 and 12, and best-corrected visual acuity was not reduced in any eye at Month 12 compared to preoperatively.
Discussion
This longer-term analysis of subjects with angle-closure glaucoma and visually significant cataract demonstrates that the short-term IOP and medication reductions observed at 6 months following combined phacoemulsification/IOL implantation/goniosynechialysis/excisional goniotomy with the KDB persist through 12 months of follow-up. Rapid and sustained IOP reductions of ~50% were achieved while nearly eliminating the need for IOP-lowering medications and improving BCVA in nearly all eyes.
The IOP reductions seen in this study are comparable to results of prior reports of cataract surgery combined with goniosynechialysis performed with devices other than the KDB.11–17 These prior studies, however, have not generally accomplished these IOP reductions while simultaneously reducing medication use by >90% as was seen in the current study. We hypothesize that the addition of the KDB-assisted excisional goniotomy provided the additional efficacy necessary for both IOP and medication reduction in these eyes. The goal of glaucoma therapy is to preserve patients’ quality of life.18,19 This combined procedure improves visual acuity, lowers IOP to levels that would be expected to prevent further visual field loss, and does so in most eyes without the need for medications, while avoiding the long-term risks associated with bleb-based procedures20 and intraocular devices21,22—together these attributes of the procedure are likely to convey substantial quality of life to patients with angle-closure glaucoma.
These results have broad generalizability throughout the world. There are an estimated 20 million people worldwide with angle-closure glaucoma, of whom 87% are Asian, and more than 5 million people are bilaterally blind from angle-closure glaucoma.23 As approximately half of the patients in our study were native Vietnamese, our results should apply to those most affected by angle closure glaucoma. This combined procedure has the potential to reduce global blindness from angle closure glaucoma.
This study’s impact is limited both by its retrospective nature and its size, representing the surgical outcomes of only 2 physicians. The clinical assessments—particularly the IOP measurements—were conducted according to routine clinical standards and not the more robust standards of prospective clinical trials. The consistency of results from 6 to 12 months of follow-up, however, suggests that—despite its relatively short follow-up period—the initial results endure significantly beyond the early postoperative period. Also, as mentioned above, the inclusion of Asian eyes enhances the external validity of findings in the most affected population in the world.
In summary, phacoemulsification combined with KDB-assisted goniosynechialysis and excisional goniotomy safely provides statistically and clinically meaningful reductions in both IOP and the need for IOP-lowering medications in eyes with angle-closure glaucoma, while also improving visual acuity.
Disclosure
The authors acknowledge that New World Medical, Inc, manufacturer of the Kahook Dual Blade, provided support for manuscript preparation. SD reports travel support from New World Medical, Inc., outside the submitted work. GKB reports personal fees from New World Medical, Inc., for biostatistical analysis during the conduct of this and other studies. The authors report no other conflicts of interest in this work.
References
- 1.He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393:1609–1618. doi: 10.1016/S0140-6736(18)32607-2 [DOI] [PubMed] [Google Scholar]
- 2.Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388:1389–1397. doi: 10.1016/S0140-6736(16)30956-4 [DOI] [PubMed] [Google Scholar]
- 3.Lai JS. The role of goniosynechialysis in the management of chronic angle-closure glaucoma. Asia-Pac J Ophthalmol. 2013;2:277–278. doi: 10.1097/APO.0b013e3182a8146b [DOI] [PubMed] [Google Scholar]
- 4.Lai J, Choy BN, Shum JW. Management of primary angle-closure glaucoma. Asia-Pac J Ophthalmol. 2016;5:59–62. doi: 10.1097/APO.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 5.Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43:1197–1201. doi: 10.1016/j.jcrs.2017.06.046 [DOI] [PubMed] [Google Scholar]
- 6.Dorairaj SK, Kahook MY, Williamson BK, Seibold LK, ElMallah MK, Singh IP. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin Ophthalmol. 2018;12:791–797. doi: 10.2147/OPTH.S158403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-month outcomes of goniotomy performed using the kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35:1460–1469. doi: 10.1007/s12325-018-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdahl JP, Gallardo MJ, ElMallah MK, et al. Six-month outcomes of goniotomy performed with the Kahook Dual Blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35:2093–2102. doi: 10.1007/s12325-018-0803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy using the Kahook Dual Blade in severe and refractory glaucoma: 6-month outcomes. J Glaucoma. 2018;27:849–855. doi: 10.1097/IJG.0000000000001019 [DOI] [PubMed] [Google Scholar]
- 10.Dorairaj S, Tam MD. Kahook Dual Blade excisional goniotomy and goniosynechialysis combined with phacoemulsification for angle closure glaucoma: 6-month results. J Glaucoma. 2019. (in press). doi: 10.1097/IJG.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 11.Teekhasaenee C, Ritch R. Combined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucoma. Ophthalmology. 1999;106:669–674. doi: 10.1016/S0161-6420(99)90149-5 [DOI] [PubMed] [Google Scholar]
- 12.Harasymowycz PJ, Papamatheakis DG, Ahmed I, et al. Phacoemulsification and goniosynechialysis in the management of unresponsive primary angle closure. J Glaucoma. 2005;14:186–189. [DOI] [PubMed] [Google Scholar]
- 13.Kameda T, Inoue T, Inatani M, Tanihara H; Japanese Phaco-Goniosynechialysis Multicenter Study G. Long-term efficacy of goniosynechialysis combined with phacoemulsification for primary angle closure. Graefes Arch Clin Exp Ophthalmol. 2013;251:825–830. doi: 10.1007/s00417-012-2091-8 [DOI] [PubMed] [Google Scholar]
- 14.White AJ, Orros JM, Healey PR. Outcomes of combined lens extraction and goniosynechialysis in angle closure. Clin Exp Ophthalmol. 2013;41:746–752. doi: 10.1111/ceo.12121 [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zou YP. Endoscope-assisted goniosynechialysis combined with phacoemulsification and intraocular lens implantation to manage primary angle-closure glaucoma. Int J Ophthalmol. 2013;6:174–177. doi: 10.3980/j.issn.2222-3959.2013.02.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda M, Watanabe M, Ichikawa K. Goniosynechialysis using an ophthalmic endoscope and cataract surgery for primary angle-closure glaucoma. J Glaucoma. 2014;23:174–178. doi: 10.1097/IJG.0b013e31826aaf3b [DOI] [PubMed] [Google Scholar]
- 17.Fakhraie G, Vahedian Z, Moghimi S, Eslami Y, Zarei R, Oskouee JF. Phacoemulsification and goniosynechialysis for the management of refractory acute angle closure. Eur J Ophthalmol. 2012;22:714–718. doi: 10.5301/ejo.5000101 [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Ophthalmology. Primary Open-angle Glaucoma: Preferred Practice Pattern. San Francisco, CA: American Academy of Ophthalmology; 2015. [Google Scholar]
- 19.European Glaucoma Society. Terminology and Guidelines for Glaucoma. 4th ed. Savona, Italy: PubliComm; 2014. [Google Scholar]
- 20.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. doi: 10.1016/j.ajo.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane S. Overview of the results from the 5 yr follow up study of the CyPass microstent. European Society for Cataract and Refractive Surgery Annual Meeting Vienna, Austria; 2018. [Google Scholar]
- 22.US Food and Drug Administration. UPDATE: potential eye damage from Alcon CyPass micro-stent used to treat open-angle glaucoma: FDA safety communication; 2018. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624283.htm. Accessed December14, 2018.
- 23.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- US Food and Drug Administration. UPDATE: potential eye damage from Alcon CyPass micro-stent used to treat open-angle glaucoma: FDA safety communication; 2018. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624283.htm. Accessed December14, 2018.