Abstract
The International Prognostic Scoring System-Revised (IPSS-R) is one standard for myelodysplastic syndrome (MDS) risk stratification. It divides patients into five categories including an intermediate subset (IPSS-R int-risk). Outcomes and clinical interventions for patients with IPSS-R int-risk are not well defined. We performed an analysis of outcomes of this group of patients. Out of 3167 patients, a total of 298 were identified with IPSS-R int-risk MDS and retrospectively analyzed to assess characteristics affecting outcomes. Cox proportional hazard models for overall survival (OS) were performed to identify statistically significant clinical factors that influence survival. Age of 66 years or greater, peripheral blood blasts of 2% or more, and history of red blood cell (RBC) transfusion were significantly associated with inferior survival. Based on these features, MDS patients with IPSS-R int-risk were classified into two prognostic risk groups for analysis, an int-favorable group and an int-adverse group, and had significantly divergent outcomes. Sequential prognostication was validated using two independent datasets comprising over 700 IPSS-R int-risk patients. The difference in median survival between int-favorable and int-adverse patients was 3.7 years in the test cohort, and 1.8 and 2.0 years in the two validation cohorts. These results confirm significantly variable outcomes of patients with IPSS-R int-risk and need for different prognostic systems.
Keywords: Myelodysplastic syndromes, Outcomes research
Introduction
Research into the clinical and biological underpinnings of myelodysplastic syndromes (MDS) is advancing significantly as tools for analysis are becoming more widely available and as centralized repositories produce data encompassing thousands of patients.1,2 Such tools are meant to help stratify patients for treatment goals, including better quality of life, decreased transfusion dependency, decreased transformation to AML, and ultimately improved overall survival. Among several classification systems, the MDS Revised International Prognostic Scoring System (IPSS-R) has become one of the gold standard models for risk stratification and prognostication for patients with MDS.3,4 It distinguishes patients based on key clinical characteristics and divides them into 5 well-defined risk groups: very low, low, intermediate, high, and very high. IPSS-R scores the disease based on marrow blast percentage, cytogenetics, hemoglobin levels, absolute neutrophil count (ANC), and platelet count.
In the original IPSS scoring system5 patients were frequently considered in binary fashion, as either lower-risk or higher-risk MDS. For IPSS-R, very low-risk and low-risk patients may be considered for more conservative and lower risk strategies, whereas high-risk and very high-risk patients are often treated more assertively. The fifth group of IPSS-R classified patients, whose Kaplan-Meier survival bisects survival of low-risk and high-risk groups, is the intermediate-risk (int-risk) category. These patients present a more challenging clinical dilemma, due to risk uncertainty. While as a group they have an intermediary survival, it is possible that they actually have a more widely variable disease course and outcome. This degree of variance may lead to uncertainty in clinical management, and suggests the group may represent patients that are more challenging to classify, rather than simply those with a mid-range prognosis.15–17 Identification of more favorable and less favorable risk subgroups within the intermediate-risk category would better guide therapeutic decisions and clarify priority treatment goals and help in the design of clinical trials. While patients in the intermediate category have a distinctly less favorable prognosis than those in lower-risk categories (median survival of 3 years vs. 5.3 and 8.8 years for good and very good, respectively)3, a more refined risk classification system for this particular group is warranted. We retrospectively studied a large cohort of patients exclusively with IPSS-R int-risk MDS to identify whether particular characteristics indicated whether a patient had a more or less favorable prognosis. We assessed patient outcomes based on three identified influential factors, and our results indicate that prognosis of this subset of patients is variable with potential implications on their treatment and clinical trial design.
Methods
Patients
A retrospective patient-centered analysis was conducted at the University of Texas, MD Anderson Cancer Center on patients diagnosed with primary MDS between 2000 and 2015. All patients were scored according to the IPSS-R criteria introduced in 2012 prior to analysis.3 Availability of complete data was mandatory for patient selection. Patients having therapy-related MDS disease or chronic myelomonocytic leukemia were excluded. After applying these inclusion criteria, the analytic cohort consisted 298 patients identified as IPSS-R int-risk. All patients provided informed written consent.
Covariates and outcome measures
The primary outcome was overall survival (OS), defined as the time from the date of diagnosis to the date of death due to any cause. The following characteristics were collected: demographic details such as age, sex and race; IPSS-R score (as well as IPSS score); laboratory investigations including hemoglobin level, serum ferritin level, white blood cell (WBC) count, platelet count, ANC, percentage of peripheral blood (PB) and bone marrow (BM) blasts at diagnosis; and the number of cytopenias defined as ANC <1.8×109/L, hemoglobin <10 g/L, and platelet count <100×10.6 Analysis was performed for the presence of known gene mutations, including FLT3-D835, FLT3-ITD, and in IDH1, IDH2, JAK2, KIT, NPM1, TET2, TP53, NOTCH1, CEBPA, DNMT3A and RAS.7 Cytogenetic group and scoring was defined per the IPSS-R model.3,8 Additional variables including Eastern Cooperative Oncology Group (ECOG) performance status9 at date of diagnosis, duration of MDS prior to progression to AML, prior malignancy, transfusion history for red blood cells (RBC) and platelets, and type of treatment received were also recorded. The response criteria were defined according to the International Working Group (IWG) criteria for MDS.10 Presence of co-morbidities was excluded. The study was complied within the research ethics guidelines of MD Anderson Cancer Center, the MDS Clinical Research Consortium, and institutions contributing validation data, and was in accordance with the ethical standards of the Declaration of Helsinki.
Statistical methods
Summary statistics were used to describe the overall study population. Kaplan-Meier (KM) product limit method11 was used to estimate the KM plot and the median overall survival. Log-rank tests were used to compare survival differences between groups. Univariate Cox proportional hazards regression12 was used to evaluate association between each covariate and overall survival (OS). Classification and regression tree (CART) analysis was used to find the best cut-off points to differentiate OS for some of the continuous variables, including age, PB blast percentage, ferritin, and β2-microglobulin levels. Because outcome is overall survival (and not binary), the CART analysis was accordingly applied using the Martingale residuals of a Cox model. Therefore, CART analysis for failure time data used the Martingale residuals of a Cox model to approximate chi-square values for all possible cut points. A multivariate Cox proportional hazards model was then fitted for OS by including all statistically significant covariates from univariate Cox models. A backward stepwise selection was performed using a threshold of 0.05 for covariates to stay in the final model.13–15 For transfusion requirement analysis, RBC and platelets were analyzed separately. Patients who received transfusions and had length of follow up greater than 30 days were included. Units per patient year were calculated by dividing total number of units by total number of patient years. RBC and platelet transfusion rates for quarterly and semi-annual lengths of follow-up were calculated, and 95% CI’s were calculated using R. Incidence rates were compared using a two-sided t-test in R. Additional methods for analysis of transfusion data is described in supplemental information. Bootstrap method was used for internal validation of the stratification score.16 For each bootstrap data set, differences in median and 1–5-year survival rates were calculated between the two risk groups of patients (score 0–1 vs. score 2–4) using Kaplan–Meier estimation. 95% confidence intervals of these differences between the two subgroups were determined by 1000 bootstrap resamples. Statistical analysis was performed using Stata/SE version 14.1 statistical software (Stata Corp. LP, College Station, TX).
External validation
The proposed scoring system was tested for validation using external cohorts from the Cleveland Clinic, Ohio, USA (external validation cohort 1) and Moffitt Cancer Center, Florida, USA (external validation cohort 2). One variable, “history of any RBC transfusion”, in our proposed score was unavailable for external validation cohort 1 data set. Instead two similar, related variables: “history of RBC transfusion in the 8 weeks prior to presentation” and “HGB<8 as indicator for transfusion need”, were used in the external validation procedure for external validation cohort 1. RBC transfusion history was considered positive if (1) history of RBC transfusion in 8 weeks prior to presentation was positive; or (2) indicator of HGB was <8. Differences in median and 1–5-year survival rates were calculated between the two risk groups of patients (score 0–1 vs. score 2–4) using Kaplan–Meier estimation.
Results
Patient characteristics
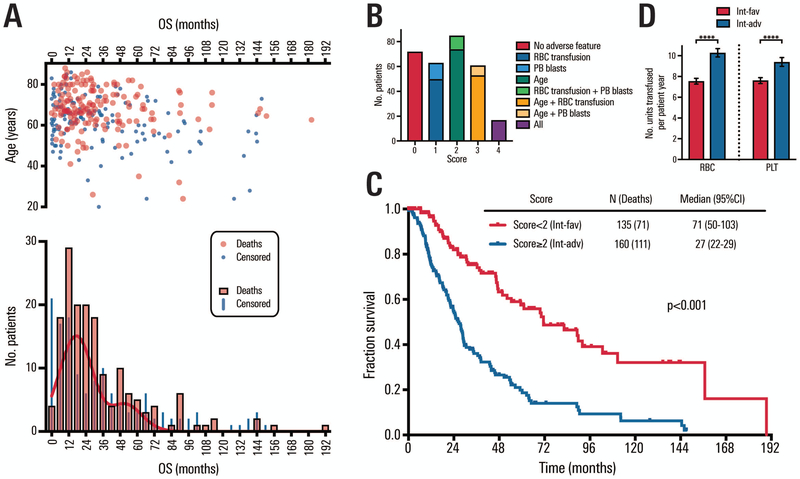
A total of 298 patients with int-risk MDS according to IPSS-R scoring were included in the analysis. Two-hundred and eighteen (73.2%) patients were older than 60 years. Most of the patients had an ECOG performance status score of 0 or 1 (n=282; 95.6%). A total of 141 patients (47.4%) had been transfused with either RBC or platelets (n=33; 11.1% had received both) at the time of diagnosis. Table 1 shows the demographic and baseline clinical characteristics of the patient cohort. The cytogenetic scoring (IPSS-R) was very good/good for the majority of patients (n=225; 75.5%); intermediate for 57 (19.1%), and poor/very poor for 16 (5.4%) patients. Mutation testing was limited for most leukemia-associated genes and is shown in supplemental information (Table S1). The median follow-up time was 23.7 months (range: 0–189.4). Table 1 also outlines the treatment history and response of the patients. A total of 40 patients (13.4%) progressed to AML, and median time to progression was 17.5 months (range, 1–104 months). Individual survival times were evaluated for the entire cohort of patients. Heterogeneous outcomes were noted (Figure 1A, top panel), and histogram analysis demonstrated a shouldered bimodal distribution of outcomes based on deaths among IPSS-R int-risk MDS patients (Figure 1A, bottom panel).
Table 1.
Characteristics including demographics, clinical parameters, and treatment/response for test cohort (n=298) with intermediate-risk myelodysplastic syndrome (MDS).
| Clinical Variable | N (%) |
|---|---|
| Age, years | |
| < 60 | 80 (26.8) |
| 60 – 70 | 112 (37.6) |
| > 70 | 106 (35.6) |
| Gender | |
| Female | 81 (27.2) |
| Male | 217 (72.8) |
| Race | |
| Other | 49 (16.4) |
| White | 249 (83.6) |
| Hemoglobin, g/dL | |
| Median (range) | 9.9 (5.9–16) |
| Platelet Count, × 109/L | |
| Median (range) | 91.0 (2–820) |
| ANC , × 109/L | |
| Median (range) | 1.2 (0–23.1) |
| WBC Count, × 109/L | |
| Median (range) | 2.8 (0.6–26.9) |
| Neutrophil, % | |
| Median (range) | 43.1 (0.6–92.3) |
| Peripheral Blood Blast, % | |
| Median (range) | 0.0 (0–23) |
| Bone Marrow Blast, % | |
| Median (range) | 5.5 (0–27) |
| Ferritin, ng/mL | |
| Median (range) | 339.0 (11–14325) |
| LDH, U/L | |
| Median (range) | 535.0 (191–4161) |
| β2-microglobulin, μg/mL | |
| Median (range) | 2.7 (0.9–21.4) |
| Blood Transfusion Dependency | |
| No | 157 (52.7) |
| Platelet | 10 (3.4) |
| Red Blood Cell | 98 (32.9) |
| Red Blood Cell + Platelet | 33 (11.1) |
| Cytogenetic Change | |
| −5/5q−/−Y/abn 11q | 31 (10.4) |
| 7/7q− | 10 (3.4) |
| 20q−/+8/Misc | 65 (21.8) |
| Normal diploid | 192 (64.4) |
| Treatment Variable | N (%) |
| Treatment History | |
| Bone marrow transplant | 13 (4.4) |
| Chemotherapy | 39 (13.1) |
| Hypomethylating agent/else | 65 (21.8) |
| Single agent/investigational | 48 (16.1) |
| No treatment | 133 (44.6) |
| Response Outcomes | |
| Complete response | 49 (16.4) |
| Complete response without platelet recovery | 11 (3.7) |
| Partial response | 3 (1.0) |
| Hematological improvement | 20 (6.7) |
| No response | 63 (21.1) |
| NE | 3 (1.0) |
| Unknown | 149 (50%) |
| Vital Status | |
| Alive | 133 (44.6) |
| Deceased | 165 (55.4) |
Total N=298 except where indicated. Abbreviations: ANC, absolute neutrophil count; WBC, white blood cell; LDH, lactate dehydrogenase; abn, abnormal; Misc, miscellaneous.
Figure 1. Heterogeneity and divergent outcomes among IPSS-R intermediate-risk patients.
A: Variability and distribution of IPSS-R int-risk MDS patients. Top panel shows distribution of survival times vs. age. Bottom panel shows histogram of overall survival (bin width=6 months). Black curve shows non-linear fit of histogram of non-censored survival times using two Gaussian curves. B: Distribution of patients by sequentially stratification, based on the sum of three classification parameters: age (0 or 2 points), PB blasts (0 or 1 point), and RBC transfusion history (0 or 1 point). Each numerical score is subdivided to indicate how patients achieved specified scores. C: Overall survival among patients with IPSS-R intermediate-risk MDS according to two prognostic groups sequentially stratified as described, according to age, PB blasts, and transfusion history. D: Transfused units of red blood cells and platelets per patient year among int-favorable and int-adverse patients who required transfusions after diagnosis of MDS (p=<0.0001 for both red blood cells and platelets).
Univariate and multivariate survival analyses
Association between overall survival and patient characteristics was assessed by univariate and multivariate analysis (Table S2). In univariate analysis, significant statistical association was found between overall survival and the following covariates: hemoglobin (p=0.003), neutrophil percentage (p=0.007), performance status (p=0.047), red blood cell (RBC) transfusion dependency (p<0.001), age (p<0.001), peripheral blast percentage (p<0.001), ferritin (p<0.001), and β2-microglobulin levels (p<0.001).
Ferritin levels ≥1222 ng/mL were associated with inferior survival (median survival = 22 months vs. 32 months for patients with ferritin <1222 ng/mL), however this was not included in the multivariate analysis since ferritin levels were unavailable for 61% of patients. Full mutation testing was not available for a majority of patients, and when mutations were investigated for patients stratified by OS, no significant correlations were discovered (data not shown). Due to incomplete data and few numbers of patients with most mutations, mutation status was not included in the univariate or multivariate analysis. Fitting results of the full and reduced multivariate Cox models are shown in Table S2. Based on the reduced model, age ≥ 66 years (<0.001), peripheral blood blast percentage ≥ 2% (p=0.009) and RBC transfusion dependency (p=0.003) were each independently and significantly associated with a less favorable survival.
Sequential stratification based on age, PB blasts, and RBC transfusion
We proposed an optimal dichotomous stratification based on the findings from the reduced Cox proportional hazards multivariate model using the using the values assigned to each patient. The covariates included in the multivariate model were factors identified as significant by backward elimination method analysis as described.15 The calculated risk value was defined by each coefficient multipliers to weight relative influence of dichotomized covariates on risk, and was described for each patient by the equation:
where each of the covariates (Age ≥ 66 years, PB blast ≥ 2%, and RBC transfusion history) is equal to 1 if present and equal to 0 if absent. The median risk value among all patients was 0.89 and was considered the cut-off for additional analysis. To further simplify patient assessment, each coefficient was divided by 0.51 (smallest coefficient in the reduced multivariate model) and rounded to the nearest integer, providing a simple 4-point system by which risk could be assessed. Table 2 shows the coefficients and hazard ratios with their weighted points.
Table 2.
Prognostic score for patients with intermediate risk myelodysplastic syndrome (0–4 score).
| Variable | Coefficient | HR (95% CI) | p | Score | No. Patients |
|---|---|---|---|---|---|
| * Age ≥ 66 years | |||||
| No | 0 | 143 | |||
| Yes | 0.87 | 2.43 (1.74–3.38) | <0.001 | 2 | 152 |
| * Peripheral blood blasts ≥ 2% | |||||
| No | 0 | 247 | |||
| Yes | 0.52 | 1.69 (1.14–2.50) | 0.009 | 1 | 48 |
| Red blood cell transfusion | |||||
| No | 0 | 199 | |||
| Yes | 0.51 | 1.66 (1.18–2.32) | 0.003 | 1 | 96 |
Score were obtained by dividing each coefficient by 0.51 (smallest coefficient) and rounding each results to the nearest integer and changing the results to the absolute value.
Based on CART. CART = Classification and Regression Tree analysis. CART analysis for failure time data uses the martingale residuals of a Cox model to calculate (approximate) chi-square values for all possible cut point on all the CART covariates. Coefficient, HR, and p are given for reduced model using backward elimination methods as described. Complete univariate and multivariate analysis are included in supplemental Table S
Divergent risk based on sequential stratification
Intermediate-risk MDS patients were stratified as determined by adding points from three individual clinical characteristics: patients with a score of 0–1 were considered favorable (int-favorable) and patients with a score of 2–4 were considered less favorable (int-adverse). There were 72 patients with a score of 0, 63 with a score of 1 (50 from RBC transfusion history and 13 from PB blast), 85 with a score of 2 (74 from age and 11 from RBC transfusion history + PB blast), 61 with a score of 3 (53 from age + RBC transfusion history and 8 from age + PB blast), and 17 with a score of 4 (Figure 1B). Overall survival was significantly different between the two risk groups, with median survival of approximately 5.9 and 2.3 years for int-favorable and int-adverse, respectively (Figure 1C, P <0.001).
RBC transfusion rate was significantly lower in int-favorable patients than in int-adverse patients (IRR: 0.73; 95% CI: 0.69–0.77; p<0.0001, Figure 1D). Int-favorable patients (N=94), had a RBC transfusion rate of 7.54 units/patient-year (95% CI: 7.27–7.82), while int-adverse patients (N=101) were transfused RBC at a rate of 10.27 units/patient-year (95% CI: 9.88–10.68). Platelet transfusion rate was also significantly lower in int-favorable patients compared to int-adverse (IRR: 0.81; 95% CI: 0.76–0.86; p<0.0001, Figure 1D). Platelet transfusion rate was 7.61 units/patient-year (95% CI: 7.32–7.89 in the int-favorable group (N=87) vs. 9.38 units/patient-year (95% CI: 8.97–9.82) in the int-adverse risk group (N=81).
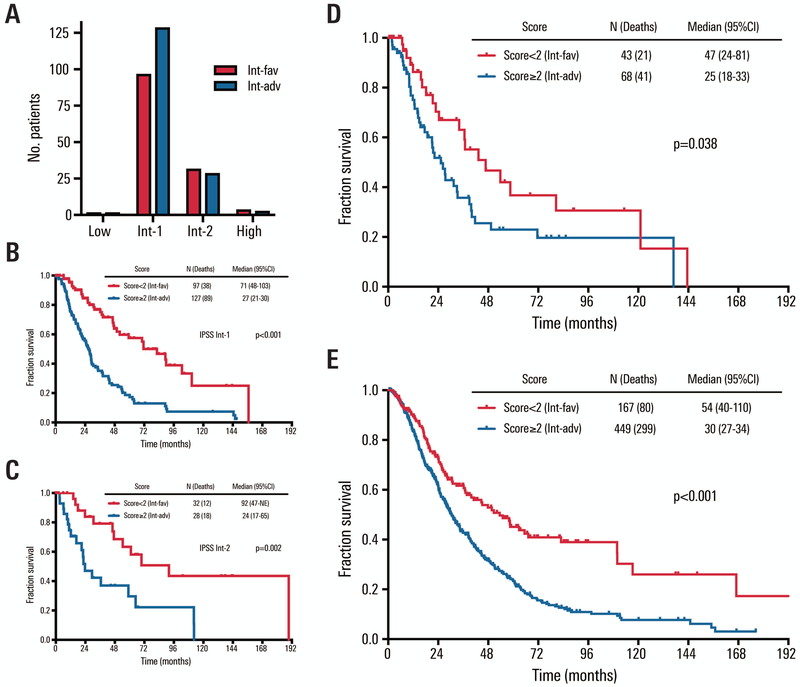
We compared the survival outcomes between the sequentially stratified risk groups when these patients were classified according to IPSS (Figure 2A–C). Sequential stratification was significantly prognostic for the two IPSS intermediate groups (p<0.001 and p=0.002 for intermediate-1 and intermediate-2 risk, respectively), suggesting the importance of these three clinical characteristics in prognosis among IPSS intermediate-risk patients also. Limited numbers of IPSS-R intermediate-risk patients were IPSS low-risk (n=4) or IPSS high-risk (n=7) (Figure S1).
Figure 2. IPSS classification of test cohort and survival of external validation cohorts.
A: Distribution of intermediate-favorable (int-fav) and intermediate-adverse (int-adv) test cohort groups when classified by IPSS. Survival for the two sequentially stratified prognostic groups using proposed risk score described. B: Survival based on sequential score for test cohort patients classified as IPSS intermediate-1 (int-1) risk. C: Survival based on sequential score for test cohort patients classified as IPSS intermediate-2 (int-2) risk. D: Survival of external validation cohort 1, IPSS-R int-risk patients from outside institution (n=111), classified by sequential scoring as int-favorable vs. int-adverse. E: Survival of external validation cohort 2, IPSS-R int-risk patients from outside institution (n=616), classified by sequential scoring as int-favorable vs. int-adverse.
The influence of age ≥ 66 years was strongest in our sequential stratification. Age-adjusted IPSS-R (IPSS-RA)3 was considered in our test cohort, and this classification also sequentially stratified patients based on the additional consideration of age according to IPSS-RA (p<0.001, see supplemental Figure S2A). Finally, in order to assess the influence of PB blasts and RBC transfusion requirement regardless of age, the original reduced model was analyzed without age as a factor. The difference in overall survival remained strongly significant (p=0.003, Figure S2B), indicating these two factors are influential among int-risk patients independent of age.
Treatment effects for int-favorable and int-adverse patients
We assessed the association between the primary treatment received and OS among all patients, and those who were sequentially stratified. Table S3 shows the univariate Cox proportional hazard models for initial treatment effect overall and by sequential stratification. There were no significant differences by treatment among the entire int-risk group, however there were trends for better overall survival among 13 patients who underwent transplant and 65 patients who received a hypomethylating agent (HMA) when compared to patients without treatment (p=0.08 and 0.09, respectively). Univariate assessment was performed in both sequentially stratified risk groups individually. In the int-favorable group, patients treated with a HMA had significantly improved survival compared to those without treatment (p=0.05), however this was not true for int-adverse patients (p=0.73). The use of transplant in the int-adverse was associated with greater risk of death than the other treatment groups, however only two patients in the int-adverse group underwent transplant, making the finding difficult to interpret. A multivariate analysis was also performed (Table S3) to detect whether survival with a particular treatment could be accounted for by using that treatment in int-favorable vs. int-adverse patients. No significant effect was noted outside of treatment with transplant in int-adverse patients, a finding again confounded by the very limited number of patients undergoing transplant. In summary, the treatment analysis most strongly suggested that the use of HMA therapy in int-favorable patients was beneficial.
Validation of sequential stratification
First, an internal validation was performed using bootstrapping method. Differences in median and 1–5-year survival rates between the two risk groups of patients (score 0–1 vs. score 2–4) are shown in Table S4. The lower confidence limits of the 95% confidence intervals of the differences between the two risk groups were all greater than zero, indicating that there were statistically significant differences with respect to median and survival rates between the two groups (score 0–1 vs. score 2–4). The stratification was robust in the classification of patients with IPSS-R intermediate-risk myelodysplastic syndrome by internal validation.
Next we analyzed two additional independent datasets that included 111 and 616 IPSS-R int-risk MDS patients from two other, separate institutions, annotated for age, history of RBC transfusion, and presence of peripheral blood blast >2% at presentation. Summary patient characteristics of the external validation cohorts are provided in Table S5. Each patient was assigned a score based on supplied information (Figure S3), and determined to be int-favorable risk (score <2) or int-adverse risk (score ≥2). For the first dataset (N=111), survival rates among int-favorable and int-adverse patients were significantly different (p=0.038, HR=1.76 [95% CI, 1.03–3.01], Figure 2D) when using history of RBC transfusion within 8 weeks or hemoglobin < 8 g/dL as a surrogate for history of any RBC transfusion, the metric used in test cohort data. The second and larger independent external validation cohort (N=616) also demonstrated significantly different survival for int-favorable vs. int-adverse patients (p<0.001, HR=1.84 [95%CI, 1.44–2.36], Figure 2E). Median survival times were 47 vs. 25 months for external validation cohort 1 and 54 vs. 30 months for external validation cohort 2.
Univariate Cox proportional hazards regression models were used to assess the association between score and OS for the two external validation cohorts. Differences in median and 1–5-year survival rates between the two risk groups of patients (score 0–1 vs. score 2–4) are shown in Table S6. The lower confidence limits of the 95% confidence intervals for the differences between the two risk groups for external cohort 1 were near-zero or positive (ranging from −0.1 to 0.4), indicating excellent when not significant differences for each time point (additional analysis in Tables S7–S9 and Figure S4). All lower confidence limits were ≥ 0 for external cohort 2, demonstrating significant differences for median survival and survival rates among int-favorable and int-adverse patients. In total, independent validation cohort data strongly supported the idea that int-risk MDS patients have heterogenous outcomes and could be reliably sequentially stratified based on common characteristics even when evaluated at different institutions.
Discussion
In this analysis we demonstrate that the outcomes of patients with intermediate risk MDS by IPSS-R is variable. Sequentially stratifying patients based on specific clinical information powerfully demonstrates the heterogeneity of outcomes among int-risk MDS patients. The information has important implications for clinical management and trial design. Using multivariate analysis and backwards elimination method, age < 66 years, peripheral blood blasts < 2% and the absence of RBC transfusion history were found to be strong indicators of more favorable outcome. Survival data from hundreds of patients with heterogeneous outcomes were successfully stratified into int-favorable and int-adverse groups, by taking these straightforward clinical measures into account. The sequential approach also appeared utilitarian for patients classified as int-1 and int-2 according to IPSS and was well-validated using bootstrap methods and in two large independent datasets from additional institutions. The differences in median survival between int-favorable and int-adverse groups were 22 and 24 months for the two validation cohorts.
Treatment options for MDS vary from observation to HSCT. Therapeutic guidelines are divided according to “lower risk” and “higher risk” MDS as established by conventional risk categories.8,17 Treatment for the higher risk group aims to modify the natural disease course with therapy, while a main priority for low-risk MDS is correction of cytopenias if present.4 Patients with int-risk MDS are not limited to lower risk disease treatment options, may have resistance to first-line treatment,18 and may require treatments appropriate for higher risk patients.19 Our analysis demonstrates that patients classified first as int-risk can be further divided into well-differentiated prognostic categories. Further, patients in the int-favorable group who had HMA therapy had superior outcomes compared to other therapies. This was not true in the int-adverse group suggesting that defining features (age, transfusion dependence, and peripheral blasts) and pathobiological features of int-adverse patients are associated with HMA-resistance. RBC and platelet transfusion dependency was significantly greater in int-adverse group of patients compared to int-favorable group, supporting an earlier analysis that found both anemia and thrombocytopenia to be significant risk factors for shorter OS and AML transformation.20
There was shorter median overall survival for older patients, as noted in other MDS studies.8,17,21 A majority of the patients with MDS are older than 55 years. Older patients (especially with int-risk) often present a therapeutic conundrum for clinicians given co-morbid illnesses that may affect the management of MDS. While supportive therapy and quality-of-life improvements may be the current primary goal in managing some such patients, the current study suggests that others may benefit from specific therapeutic interventions guided by upfront sequential stratification. Additionally, as novel treatment options become available and as patients live longer, it is imperative to recognize candidates who would benefit from specific therapeutic approaches.
There was a significant impact of PB blast percentage on survival, with median overall survival of 24 months in patients with ≥2% blast cells compared to 46 months in those with <2% blast cells. While the IPSS-R uses BM blast cell percentage as a variable in its prognostic score, PB blast percentage was found to be an independent prognostic indicator among IPSS-R int-risk patients. Prior evidence also suggests that having a higher blast percentage in PB than in BM is associated with more aggressive MDS22 and AML transformation.23,24
The IPSS and IPSS-R are good tools for predicting survival outcome and their prognostic impact has been validated by many studies.25–27 Both tools may be deficient in how to incorporate transfusion history and dependency. The current analysis noted a significant difference in median overall survival for patients who demonstrated some dependency on RBC transfusion, compared to those who were not dependent (28 months vs. 54 months). Transfusion dependence has also been noted as an independent prognostic factor previously.28,29 Malcovati et al. noted that the development of secondary iron overload in transfusion-dependent patients also significantly affected their survival (p=0.003).29 Although in our study ferritin levels of ≥ 1222 ng/mL were associated with significantly worse survival outcomes, these were not incorporated into the final risk model due to incompletely available data. Other studies have also detected the prognostic importance of ferritin, and whether that is a function of transfusion history or a surrogate marker (for example, for inflammation), is not well understood.29–31 Serum β−2 microglobulin is also a relevant biomarker in predicting survival in MDS as well as risk of AML transformation.30 In our dataset, baseline plasma β−2 microglobulin was not available for every patient and was not considered in the final model. Finally, the influence of prior erythropoietin-stimulating agents and baseline erythropoietin levels was not evaluated, since this data was not captured.
While both karyotype complexity and TET2 mutations have been implicated in accelerating leukemic transformation, no significant impact of mutated TET2 on survival has been reported.32 Complex cytogenetics, on the other hand, have been associated with poor prognosis.8,17,33 Mutation status and cytogenetics were not significant influential factors in the current study, however cytogenetics were pre-selected for int-risk and mutational data was limited, highlighting a shortcoming of this analysis. The prognostic impact of a variety of MDS-associated mutations continues to undergo analysis as more patients have such testing, and its current use may not be available to all clinicians.
The sequential stratification based on patients from a single institution was further validated using bootstrap method, and with external data from two separate, independent institutions. The strength of significance and successful validation suggests that stratifying int-risk MDS patients based on age, peripheral blood blast percentage and RBC transfusion history, is a meaningful way of bifurcating patients with IPSS-R int-risk MDS using readily available clinical factors. Further stratifying int-risk patients will be useful in treatment choice and trial design. Our results indicate that patients with intermediate risk MDS have variable prognosis and that new prognostic systems, likely incorporating genomic data are needed.
Supplementary Material
Acknowledgements
This work was supported by the Cancer Center Support Grant from the NIH/NCI, P30 CA016672, the MDS Clinical Research Consortium funded by the Edwards P. Evans Foundation, and by generous philanthropic contributions to MD Anderson’s Moon Shot Program.
Footnotes
Conflict of Interest Disclosures
The authors have no conflicts to report.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Kouides PA, Bennett JM. Understanding the Myelodysplastic Syndromes. Oncologist. 1997;2(6):389–401. [PubMed] [Google Scholar]
- 3.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2017;15(1):60–87. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 6.Sanz GF, Sanz MA. Vallespí T, Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients. Blood. 1989;74(1):395–408. [PubMed] [Google Scholar]
- 7.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30(8):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–3674. [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 12.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34(2):187.-+. [Google Scholar]
- 13.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkler D, Plischke M, Leffondre K, Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PLoS One. 2014;9(11):e113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge ; New York, NY, USA: Cambridge University Press; 1997. [Google Scholar]
- 17.Valcarcel D, Sanz G, Ortega M, et al. Use of newer prognostic indices for patients with myelodysplastic syndromes in the low and intermediate-1 risk categories: a population-based study. Lancet Haematol. 2015;2(6):e260–266. [DOI] [PubMed] [Google Scholar]
- 18.Kelaidi C, Park S, Sapena R, et al. Long-term outcome of anemic lower-risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis-stimulating agents. Leukemia. 2013;27(6):1283–1290. [DOI] [PubMed] [Google Scholar]
- 19.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120(7):1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J, Shao ZH, Liu H, et al. Transformation of myelodysplastic syndromes into acute myeloid leukemias. Chinese Medical Journal. 2004;117(7):963–967. [PubMed] [Google Scholar]
- 21.Sanz GF, Sanz MA, Greenberg PL. Prognostic factors and scoring systems in myelodysplastic syndromes. Haematologica. 1998;83(4):358–368. [PubMed] [Google Scholar]
- 22.Amin HM, Yang Y, Shen Y, et al. Having a higher blast percentage in circulation than bone marrow: clinical implications in myelodysplastic syndrome and acute lymphoid and myeloid leukemias. Leukemia. 2005;19(9):1567–1572. [DOI] [PubMed] [Google Scholar]
- 23.Vehmeyer K, Haase D, Alves F. Increased peripheral stem cell pool in MDS: an indication of disease progression? Leuk Res. 2001;25(11):955–959. [DOI] [PubMed] [Google Scholar]
- 24.Berer A, Jager E, Sagaster V, et al. Circulating myeloid colony-forming cells predict survival in myelodysplastic syndromes. Ann Hematol. 2003;82(5):271–277. [DOI] [PubMed] [Google Scholar]
- 25.Voso MT, Fenu S, Latagliata R, et al. Revised International Prognostic Scoring System (IPSS) Predicts Survival and Leukemic Evolution of Myelodysplastic Syndromes Significantly Better Than IPSS and WHO Prognostic Scoring System: Validation by the Gruppo Romano Mielodisplasie Italian Regional Database. Journal of Clinical Oncology. 2013;31(21):2671.-+. [DOI] [PubMed] [Google Scholar]
- 26.Neukirchen J, Lauseker M, Blum S, et al. Validation of the revised international prognostic scoring system (IPSS-R) in patients with myelodysplastic syndrome: a multicenter study. Leuk Res. 2014;38(1):57–64. [DOI] [PubMed] [Google Scholar]
- 27.van Spronsen MF, Ossenkoppele GJ, Holman R, van de Loosdrecht AA. Improved risk stratification by the integration of the revised international prognostic scoring system with the myelodysplastic syndromes comorbidity index. Eur J Cancer. 2014;50(18):3198–3205. [DOI] [PubMed] [Google Scholar]
- 28.Cazzola M, Malcovati L. Myelodysplastic syndromes - Coping with ineffective hematopoiesis. New England Journal of Medicine. 2005;352(6):536–538. [DOI] [PubMed] [Google Scholar]
- 29.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–7603. [DOI] [PubMed] [Google Scholar]
- 30.Gatto S, Ball G, Onida F, Kantarjian HM, Estey EH, Beran M. Contribution of beta-2 microglobulin levels to the prognostic stratification of survival in patients with myelodysplastic syndrome (MDS). Blood. 2003;102(5):1622–1625. [DOI] [PubMed] [Google Scholar]
- 31.Neumann F, Gattermann N, Barthelmes HU, Haas R, Germing U. Levels of beta 2 microglobulin have a prognostic relevance for patients with myelodysplastic syndrome with regard to survival and the risk of transformation into acute myelogenous leukemia. Leukemia Research. 2009;33(2):232–236. [DOI] [PubMed] [Google Scholar]
- 32.Lin TL, Nagata Y, Kao HW, et al. Clonal leukemic evolution in myelodysplastic syndromes with TET2 and IDH1/2 mutations. Haematologica. 2014;99(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morel P, Hebbar M, Lai JL, et al. Cytogenetic Analysis Has Strong Independent Prognostic Value in De-Novo Myelodysplastic Syndromes and Can Be Incorporated in a New Scoring System - a Report on 408 Cases. Leukemia. 1993;7(9):1315–1323. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.