Abstract
Compared to most ophthalmic technologies, adaptive optics, or AO, is relatively young. The first working systems were presented in 1997 and, owing in part to its complexity, the development of AO systems has been relatively slow. Nevertheless, AO for vision science is coming of age and the scope of applications continues to increase. Applications of AO can be broadly split along two lines; for retinal imaging and for testing visual function. This review will focus on the applications of adaptive optics for testing visual function. Since this represents only a subset of the field of AO for ophthalmoscopy, it is possible to cite virtually every paper that has been published in the field to date. As such, this is a comprehensive review whose intent is to get all readers up to speed on the state of the art. More importantly, perhaps, this review will focus on the types of science that can be accomplished with AO with a view to future applications. The reference list alone is informative, since the reader will quickly discover that the community that is using AO for vision science is rather small. Looking at the dates for the cited papers, the reader will also discover that the field is rapidly expanding.
Introduction
The formalization of the concept that vision begins with the formation of an image on the retina set the earliest foundations for understanding the basis of reduced retinal image quality and reduced visual performance. In the early 17th century, Johannes Kepler was the first to accurately describe the optics and corrections of myopia and hyperopia. 200 years later, Thomas Young (1801) measured his own astigmatism, even pointing out that it can be corrected with a tilted lens. George Biddel Airy (1827) devised a cylindrical lens correction for astigmatism. With the correction of defocus and astigmatism (termed second-order aberrations), the optical aberrations that generate retinal blur were largely corrected for the majority of the population. In fact, complaints about the quality of one’s best-corrected vision are rare and often indicate a retinal rather than an optical origin. Therefore, it is not surprising that few efforts were made over the subsequent 200 years to correct the eye’s optics beyond that level. Rather, the effort was directed to devising more comfortable, convenient and cosmetic solutions to refractive errors such as bifocals, contact lenses, progressive addition lenses, and various refractive surgeries including LASIK. The early efforts to produce sharper retinal images were generally developed to probe retinal function. The generation of high-contrast interference fringes, for example, produced high-contrast spatial frequencies on the retina far exceeding anything achievable with conventional viewing methods (Arnulf & Dupuy, 1960; Westheimer, 1960; Campbell & Green, 1965). Even today, interference fringes remain the most effective technique for delivering high contrast images to the retina, albeit with a limited repertoire of stimulus options (Liang & Westheimer, 1993).
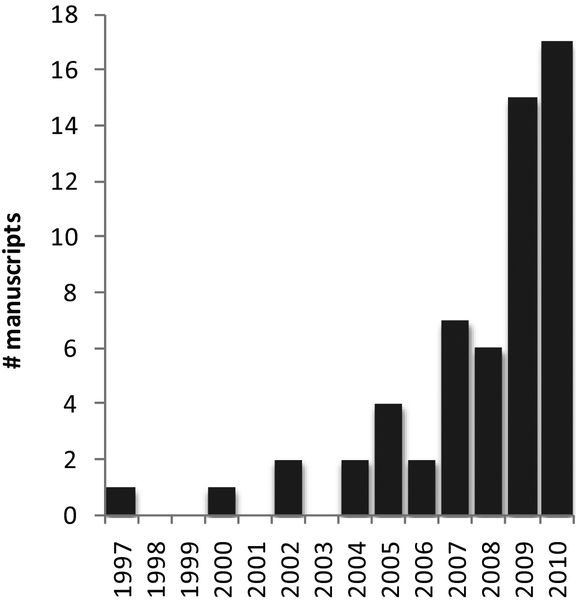
The early studies of aberrations were largely for academic purposes. Smirnov (1962), for example, was the first to record the full wave aberration of the eye. The data collection involved a psychophysical procedure (ie the measurement relied on subject observations and responses), and processing the data, which would take fractions of a second with today’s computer processing speed, took many hours. Nevertheless, the basic principles, which involved the measurement of the slope of the light rays at an array of locations across the pupil aperture, were no different than most modern methods. Smirnov is also credited with recognizing that aberrations do not represent a loss of optical information, just a reconfiguring of it, and that it would be possible to recover the information with advanced optical methods (which were not available at the time). It wasn’t until the use of Shack-Hartmann wavefront sensing for the eye, which provided quick and accurate measures of the eye aberrations, that the notion of correcting the eye’s aberration in any practical way was possible. These foundations were set by Dr. Josef Bille at Heidelberg where he and his student Junzhong Liang developed the first Shack-Hartman wavefront sensor for the eye (Liang, Grimm, Goelz, & Bille, 1994). Bille’s group also made an early attempt at adaptive optics but, at that time, neither the wavefront sensor nor the wavefront corrector was fully developed (Dreher, Bille, & Weinreb, 1989). The full implementation of adaptive optics for the eye was not realized until David Williams led an effort in his lab at the University of Rochester. With a small, but superb, optical team comprised of Donald Miller and Junzhong Liang, they built the world’s first adaptive optics system for the eye (Liang & Williams, 1997; Liang, Williams, & Miller, 1997). Although the primary motivation of the world’s first AO ophthalmoscope might have been for imaging, the seminal 1997 paper also reported the first benefits of high-order aberration correction for vision (Liang et al., 1997). Since that time AO technology has advanced tremendously with real-time AO correction (Fernandez, Iglesias, & Artal, 2001), integration of AO into other imaging modalities (Roorda, Romero-Borja, Donnelly, Queener, Hebert, & Campbell, 2002; Hermann, Fernandez, Unterhuber, Sattmann, Fercher, Drexler, Prieto, & Artal, 2004) and improved optical design (Gomez-Vieyra, Dubra, Malacara-Hernandez, & Williams, 2009). By comparison, the development of new systems and applications for testing vision has been quite slow, but the number of systems and the scope of applications is growing exponentially as can be seen in Fig 1.
Figure 1:
The number of peer-reviewed manuscripts, by year, that have employed adaptive optics to measure some aspect of visual function. For a complete listing of the papers represented in this plot, refer to the bibliography in the appendix.
Since there are already a host of excellent publications describing how an AO system works with some even giving guidance on how to develop your own AO system (Porter, 2006; Miller & Roorda, 2009; Hampson, 2008), only the basic concepts will be described here. An AO system comprises a wavefront sensor, which records the ocular wave aberrations, and a wavefront corrector, which controls, or corrects, for them. An AO controller, usually a computer, interprets the wavefront sensor image and computes the appropriate wavefront corrector drive signals. Most AO systems operate in closed-loop, which means the wavefront sensor is placed so that it can measure the effectiveness of the wavefront correction. As illustrated on Fig 2, the wavefront sensor will record a flat wavefront if the mirror is shaped correctly. The schematic shown on Fig. 2 includes both imaging and stimulus delivery. This schematic is general and applies to both conventional and scanning laser systems – the difference being the relay optics, which are not shown. A property of the system that is worth noting here is that the same correction used to correct light emerging from the eye can be used to correct the light going into the eye. The figure shows how the beam entering the eye is, in effect, pre-aberrated to be equal and opposite to that of the eye so that, upon refraction, the wavefront takes a pure spherical shape.
Figure 2:
Basic layout of an AO system for imaging and vision testing.
Before Adaptive Optics
Before a review on what AO offers for vision applications, it is useful categorize the types of questions that were asked about the limits of human vision prior to AO. These fall into two broad categories, retinal (or neural) and optical. The problem has been that the study of one was confounded by the other and vice versa. Clever methods were developed to handle the confounding factors and, in the years prior to AO, a clearer picture of the optical and retinal limits to vision had already started to emerge.
A useful tool to bypass the optical limits and probe retinal function directly was through the generation of interference fringes which are high contrast, variable frequency sinusoidal gratings formed directly on the retinal surface. Interference between two mutually coherent retinal illumination patches projected through two small apertures in the pupil gives rise to a sinusoidally varying intensity pattern, or interference fringes, on the retina. The small pupil effectively minimizes any optical distortion in the interference fringes and makes the contrast of the pattern immune to ocular aberrations including defocus and astigmatism. Increasing the separation between the apertures increases the spatial frequency, with the maximum frequency being limited by the pupil diameter. The advantage of the method is that the fringes have essentially 100% contrast. Ironically, the challenge has been in how to lower the contrast of the fringes in order to perform threshold tests (Williams, 1985). The retinal contrasts achieved via interference rival anything that can be reached in conventional viewing. However, aside from some innovative modifications (Liang & Westheimer, 1993), the method is primarily limited to projection of sinusoidal gratings over large fields. Nevertheless, the simplicity of the patterns was very effective, especially considering that there was an emerging interest in linear systems theory for human vision during the same time (Campbell & Robson, 1968; Geisler & Banks, 2010). Simply put, the visual system could be thought of as a series of linear filters which, when multiplied together, would generate the final transfer function relating objects in the world to how they are perceived. An excellent example of this approach can be found in Campbell and Green ( 1965) who, in order to measure the degradation of the eye’s optics, measured the eye’s contrast sensitivity function (CSF) with and without the optical limits. The CSF is a measure of the eye’s ability to detect contrast, and is generally plotted as the inverse of the contrast threshold required for detection of the presence of a sinusoidal fringe pattern as a function of its spatial frequency. The shape of the CSF is governed by optics, retinal and neural factors. Campbell and Green measured the CSF including optical limits using conventional methods, and measured the CSF without optical limits with interference fringes formed on the retina. To get the optical degradation, they quantified the contrast losses imposed by the optics in the following way:
What they actually computed was the modulation transfer function, or MTF, of the eye. The MTF is a measure of the ratio of object contrast to image contrast as a function of spatial frequency. Their paper showed the classical results of how the MTF changes with pupil size, and their result was very similar to what is plotted in Fig 4.
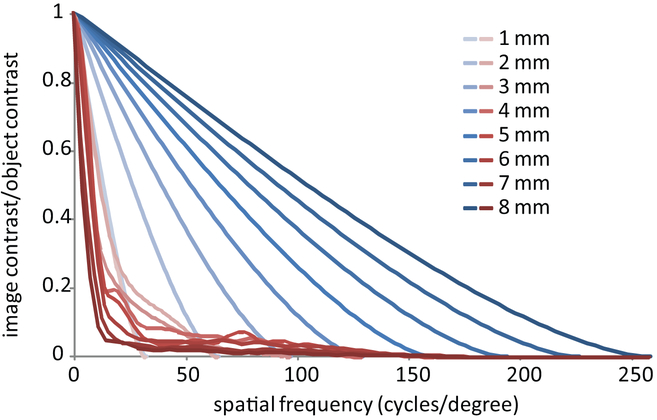
Figure 4:
The Modulation transfer function (MTF) for a diffraction-limited eye (blue lines) and a typical eye (red lines) for pupil sizes ranging from 1 to 8 mm. Aside from the MTFs for the 1 mm pupil, which overlap, the trends with increasing pupil size are in the opposite directions. The contrast reaching the retina increases with pupil size for a diffraction-limited eye but decreases for a typical eye. These MTFs are computed after removal of astigmatism but with an amount of defocus that optimizes the Strehl ratio for each pupil size.
Aside from interference fringes, tools to study the retinal and neural limits to vision on a cellular level were limited to isolating single classes of S-cones (Williams, MacLeod, & Hayhoe, 1981) and projecting small flashes with intensities set low enough to maximize the probability that only a single cone would be involved in the detection (Krauskopf, 1964). Of course, a multitude of other studies has been done on the retinal limits to vision, but they did not rely on precise spatial control of the retinal image.
The other branch of studying limits to vision was to develop a better understanding of the optics irrespective of the retina. These involved measures of the optics with various levels of sophistication (Jackson, 1888; Brudzewski, 1900; Ames & Proctor, 1921; Tscherning, 1924; Pi, 1925; Stine, 1930; Koomen, Tousey, & Scolnik, 1949; Koomen, Scolnik, & Tousey, 1956; Ivanoff, 1956; Smirnov, 1962; Jenkins, 1963; Berny & Slansky, 1969; Howland & Howland, 1977; Campbell, Harrison, & Simonet, 1990; Artal, Santamaria, & Bescos, 1988), but no effort was ever made to correct for them until Shack-Hartmann methods were introduced and adaptive optics was used. Prior to AO, the only method to improve image quality beyond correction of defocus and astigmatism was through pre-filtering which, in its simplest form, involves increasing the contrast of higher frequencies in the object that are suppressed by optical blur (Peli, Goldstein, Young, Trempe, & Buzney, 1991). But if the aberrations are characterized well enough, then an object can be pre-filtered in a way to generate a retinal image that appears as though it were free from any blur from aberrations (Artal, 1990). However, the cost is that there is an overall loss of contrast of the retinal image as well as amplification of noise. Moreover, practical applications of pre-filtering are limited to 2D images and not actual scenes.
What are the Limits/Benefits of Wavefront Control?
Before one takes the time, effort and expense to build an adaptive optics system for controlling the retinal stimulus, it is imperative to be aware of what the typical eye’s optical quality is and what can be potentially achieved. Fortunately, physical optical principles can be used to generate accurate estimates of retinal contrast, resolution, PSF size and these techniques will be used here.
First, the image of a point source (called the point spread function or PSF) is never a point. As such, no retinal image ever provides a perfect representation of the object. Even if the eye has no optical imperfections, diffraction still causes the light to spread. This is the reason why an eye that is free from aberrations is still called diffraction-limited. This spread distorts and reduces the contrast of the object, primarily affecting the transfer of high spatial frequencies, or fine details, from the object to the image. Figs 3 and 4 show the two most common ways that the performance of an optical system ir represented: the PSF, which is the most direct representation of blur, and the corresponding MTF, which quantifies the contrast ratio as a function of spatial frequency that remains after an image is blurred by that PSF. One immediately sees how extensive the losses in the human eye are, particularly in the MTF representation.
Figure 3:
PSFs for a diffraction-limited eye (left column) and for a typical eye (right column). These PSFs are computed after removal of astigmatism but include an amount of defocus that optimizes the Strehl ratio for each pupil size. The Strehl ratio is the ratio of peak height of the actual PSF compared to the diffraction-limited case for the same pupil and has been shown to provide good correlations with perceived image quality (Marsack, Thibos, & Applegate, 2004). These PSFs have been normalized so that the structure in the aberrated PSF is visible. The 8 mm pupil actually has a Strehl ratio of 0.0077, and if properly scaled would have a peak value of 0.77 % compared to its diffraction-limited counterpart (left column). This means that small, dim objects, like stars, will be less detectable owing to their lower peak intensity. Note also that the structure within the aberrated PSF has a graininess (or speckle) that is consistent with the size of the diffraction-limited PSF.
Despite the extent of the blur caused by aberrations and diffraction, the non-optimal performance does not seem to pose a major handicap for vision. In fact, most people are not aware of the limits imposed by their aberrations. This is mainly because getting along in our world does not depend on seeing much detail beyond 15 cycles per degree. To put that into context, the three horizontal lines on a letter E of size 20/40 (twice the size of a 20/20 letter) comprise a section of a square wave grating of 15 cycles per degree. 20/40 text is the typical spatial frequency of newspaper text. Moreover, foveal cone sampling imposes a limit of about 60 cycles per degree and so any contrast above that spatial frequency has little value.
Applications of AO for Vision Testing
As is customary with any new technology, the first vision experiments performed with an AO system were not so ambitious, satisfied to simply explore the new space offered by aberration correction. These early experiments showed convincingly that correction of high order aberrations resulted in improved visual performance. Liang et al (1997), in the first AO paper reported dramatic improvements in contrast sensitivity showing, for example, significant contrast sensitivity at 55 cycles per degree, a spatial frequency that was undetectable prior the AO correction. Subsequent studies have continued to confirm the contrast sensitivity and the visual acuity benefits offered by aberration correction (Yoon & Williams, 2002; Artal, Manzanera, Piers, & Weeber, 2010; Li, Xiong, Li, Wang, Dai, Xue, Zhao, Jiang, Zhang, & He, 2009). Even everyday tasks such as face recognition were shown to improve after aberration correction (Sawides, Gambra, Pascual, Dorronsoro, & Marcos, 2010) as well as modest improvements in visual performance in the periphery, where optics are not generally expected be the limiting factor for spatial resolution (Lundstrom, Manzanera, Prieto, Ayala, Gorceix, Gustafsson, Unsbo, & Artal, 2007). The clear improvements shown in the early publications generated a lot of excitement, particularly in the refractive surgery field, since lasers developed for refractive surgery (LASIK and PRK) already had the capability to generate custom corneal ablation profiles that could potentially correct the eye to the diffraction limit. Computing and delivering the appropriate corneal laser ablation pattern from a wavefront measurement was technically possible and so the prospects of correcting the eye’s aberration seemed relatively straightforward. But this turned out to be too simplistic. Not only had it proven difficult to predict the cornea’s response to a laser ablation, but the actual benefits of aberration-correction, if achieved, were beginning to be questioned. AO played an important role in developing a better understanding of the practical benefits as well as the trade-offs of aberration correction. Enthusiasm was dampened first by the reminder that the eye suffers from more than monochromatic aberrations. In fact, chromatic aberrations (the change in refractive power as a function of wavelength) become more apparent after monochromatic aberration correction (Yoon & Williams, 2002) to the extent that it has been suggested that monochromatic aberrations may protect the eye against chromatic aberration (McLellan, Marcos, Prieto, & Burns, 2002). Enthusiasm was further dampened by a collection of other factors. For example, the largest physiological pupils, which stand to benefit the most from aberration correction, are only realized in dim light conditions at which retinal factors limit the benefit (Dalimier & Dainty, 2008; Dalimier, Dainty, & Barbur, 2008; Marcos, Sawides, Gambra, & Dorronsoro, 2008). A potential adverse consequence of aberration correction is the increased possibility of aliasing. At the fovea, the cone spacing is about 0.5 minutes of arc which, from a simplistic sampling point of view, provides a maximum sampling frequency of 60 cycles per degree. Fig. 4 shows that well-corrected optics will transfer frequencies well beyond this cutoff. These higher frequencies can masquerade as something different, or be perceived as an ‘alias’ of the actual pattern. Using high contrast interference fringes, Williams (Williams, 1985) showed that aliasing is in fact perceived and, owing to the size of the cone aperture, can persist for spatial frequencies as high as 200 cycles per degree. Our optical imperfections effectively mitigate against this aliasing for foveal vision. Other factors such as limits imposed by adaptation, potential adverse affects on accommodation and compromises to the depth of field will be discussed in the next sections.
How Much Can Vision Improve?
Although AO-correction provided an immediate benefit to vision, the question remained as to whether the visual system was equipped to take full advantage of the new, unprecedented spatial detail delivered to the retina, and also to what extent the retina could sample the corrected retinal image. After all, if the brain had never been presented with high contrast images at any point during its lifetime, then why would it have developed the neural machinery to process them? This is not an unfamiliar concept, since children who are deprived of a sharp retinal image during critical periods of their development are prone to developing amblyopia (defined as a visual deficit that cannot be improved by sharpening the retinal image). Perhaps past experience sets a limit beyond which no further optical correction can improve visual performance, at least in the short term.
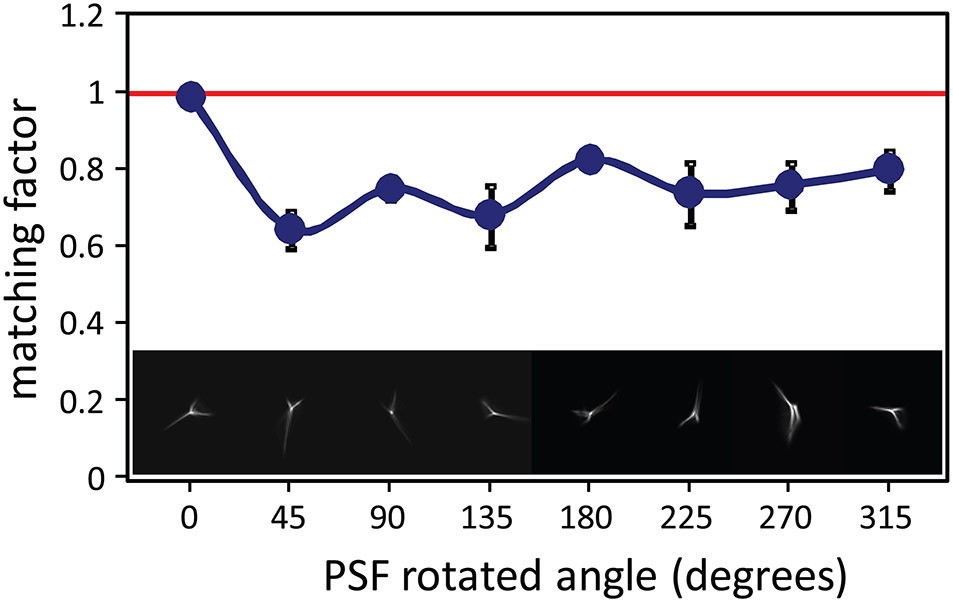
An important paper that set the stage for most of the recent work in this area was by Artal et al (2004). They showed that the eye perceives an image that is blurred by their native PSF as sharper than one blurred by a rotated version of the same PSF, even though the magnitude of the blur is the same and the same spatial frequency content is present in both images. To do this experiment, they used an AO system not to correct the eye’s aberration, but rather to manipulate them in such a way to rotate the eye’s native PSF. Example PSFs are shown on Fig. 5. Subjects were asked to view a scene and compare the scene blurred by their native PSF to a similar scene blurred by their rotated PSF. In order to make a subjective blur match, the subjects had to reduce the magnitude of the aberrations in the rotated PSF condition to match that of the native PSF condition. Fig 5 shows that the aberrations that generate an unfamiliar PSF need to be reduced by over 20% to make the blur appear similar to one generated by the native PSF. They concluded that eye somehow adapts to the blur generated by its own PSF.
Figure 5:
Blur matching as a function of PSF orientation from Artal et al (2004). The lower images illustrate the range of PSF orientations that were tested in the experiment with the leftmost PSF being its native orientation. The matching factor (y-axis) is the amount that that magnitude of the aberrations had to be changed from the initial magnitude to generate a degree of blur that appeared equivalent to the blur produced by the subject’s native PSF orientation. All rotated PSFs appeared blurrier than the native PSF and the subject had to reduce their magnitude to make a subjective blur match.
The adaptation to one’s aberrations not only results in an apparent reduction in perceived blur, but extends to improvements in visual performance. Studies have suggested that people develop strategies to identify letters blurred by their own optics and that these strategies fail when they have someone else’s optics. Mon-Williams et al. (1998), in experiments that did not rely on AO, showed that myopic eyes are better at visual acuity tasks in the presence of blur than emmetropic eyes. To investigate whether this effect extends to higher-order aberrations, Sabesan and Yoon (2010) studied vision and adaptation in eyes with keratoconus, a disease of the cornea where local thinning gives rise to an irregular shape and severe aberrations. They used an AO system with a high-stroke deformable mirror to bestow normal eyes with the aberrations of a keratoconic eye and found that they performed much worse on an acuity task than the eye with the keratoconus. These results begged the question; to what extent does adaptation to our own aberrations limit the effectiveness of an aberration correction? Does past visual experience set a visual performance limit?
To provide a framework for further discussion, we will adopt the terms defined by Sabesan and Yoon, 2009 (2009) who define “neural compensation” as an improvement in visual performance (acuity, contrast sensitivity) or a reduction in perceived blur achieved after adaptation to a blurred retinal image and define “neural insensitivity” as less-than-optimal visual performance when presented with a diffraction-limited retinal image or an image blurred by an unfamiliar PSF.
Evidence of neural insensitivity was provided by Chen et al. (2007) who showed that an image that appears the sharpest is not necessarily the one that is generated with a full AO correction. In their experiments, subjects selected an image that was blurred by some remaining aberration as the sharpest image, rather than the diffraction-limited image. Rossi et al (2007) showed results which suggested that performance on a visual acuity task depends on past visual experience. Low myopes, who are more likely to live in a chronically blurred environment, but also have no other adverse ocular complications, performed worse after aberration correction than did emmetropes. In a more extreme case, Sabesan and Yoon (2009) used a high stroke deformable mirror to show that corrected keratoconic eyes perform much worse than emmetropes despite an equally good aberration correction. But are these hard limits? Likely not. Levi (2005) has shown that training can improve visual performance in adults with ambyopia, and it is conventional wisdom in the optometry profession to coach patients on the benefits of adaptation to new spectacles, particularly ones that correct large amounts of astigmatism (Adams, Banks, & van Ee, 2001). It is also reported that the full benefits of wavefront guided LASIK surgeries with excellent optical outcomes are generally not reached until months after the surgery is complete (Pesudovs, 2005). Rossi and Roorda (2010a) showed that even emmetropes, who performed exceptionally well immediately after AO correction (better than 20/10 acuity on average) improved after one week of training for one hour per day. Perhaps myopes and eyes with keratoconus and other conditions (Rocha, Vabre, Chateau, & Krueger, 2010) will take more time to adapt to a sharp retinal image if it is provided, but it seems possible that all eyes could eventually reach a similar performance limit, especially those who had sharp vision during their critical period for vision development. For eyes with keratoconus, who stand the most to gain, the potential of using scleral contact lenses to provide comfortable and effective optical corrections for these eyes should tell us about long term adaptation (Romero-Rangel, Stavrou, Cotter, Rosenthal, Baltatzis, & Foster, 2000).
Role of AO to Study Accommodation
Vision systems that use AO offer a new set of tools to aid in our understanding of accommodation, a process whereby the eye alters the power of its lens to allow it to focus on objects over a range of distances. Questions still remain on what properties in the retinal images reaching one and/or both eyes drive accommodation, and how altering these stimulus properties might affect accommodation. Research in this area not only sheds light on the basic mechanisms of accommodation, but is becoming increasingly relevant with the surge in interest in 3D display technology and the prospect of restoring accommodation in presbyopes (see next section). Table 1 lists known factors that offer signals that indicate the direction that the eye would need to accommodate to focus on an object.
Table 1:
Cues that Provide Signals as to the Sign of Defocus (adapted from (Wolfe J.M., Kluender, Levi, Bartoshuk, Herz, Klatzky, & Lederman, 2006).
| Binocular cues |
|
| Monocular cues |
indicates signals that are not instantaneous but that are derived over time
Although cues from monochromatic aberrations exist, whether the eye uses them to aid accommodation on an object remains undetermined. Until we know this, we can only speculate on how accommodation might be helped or hindered with an aberration correction. Considering the directional signals generated from monochromatic aberrations it has been shown that typical eye aberrations will generate PSF patterns that differ on either side of focus (Wilson, Decker, & Roorda, 2002). These are easily distinguishable, even for complex scenes, and therefore provide a potential cue to for the eye to indicate the sign of defocus. Given this fact, removing aberration cues that consequently remove cues to the sign of defocus could confuse the accommodative system. Additionally, removing or manipulating the aberrations will alter how the image and its contrast will change through focus. If the accommodation system uses the rate of change of image contrast with focus as a feedback signal to drive accommodation (Charman & Jennings, 1976), then how might a more rapid contrast loss with defocus in an AO-corrected eye affect accommodation? On one hand, it could provide a stronger signal and make accommodation more precise. On the other hand, a similar accommodative reaction to an altered stimulus could cause accommodative instability (i.e. result in a poorly controlled feedback system).
Definitive tests of the role of ocular aberrations on accommodation remain to be done. To date, experiments have been mostly limited to the examination of static accommodation responses with fixed corrections, and results in the literature are mixed. Fernandez and Artal (2005) found that removing odd-order aberrations reduced the response time of accommodation, although the removal of odd-order aberrations was a curious choice since odd-order aberrations do not provide a signal to the sign of defocus (Wilson et al., 2002). Chen et al (2006) found mixed results in the eye’s ability to accommodate with and without aberrations corrected and under no circumstance did they find that accommodation ever improved with an AO correction. Chin et al. (2009a) found no effect of aberrations in the eye’s ability to accommodate to a stimulus whose accommodative demand varied sinusoidally, but it is not clear why they should have seen any differences, given the predictability of the stimulus. Gambra et al (2009) were less interested in whether aberrations provided a directional signal, but rather looked at the effects of different aberrations on the accuracy of the static accommodative response to a stimulus. Importantly, the subjects used their physiologic pupil throughout the task, allowing it to dilate or constrict depending on the accommodative conditions. Different aberration conditions produced systematic and different responses. Most notably, the aberration-free conditions resulted in slightly reduced accommodative lag for near objects. Also, accommodative lag was quite small for eyes with negative spherical aberration, an aberration for which a construction in pupil size gives an additional anterior shift in the focal plane (see Fig. 6). The accommodative fluctuations were smallest for the natural aberration condition, perhaps indicating some confusion arising from the unfamiliar aberrations.
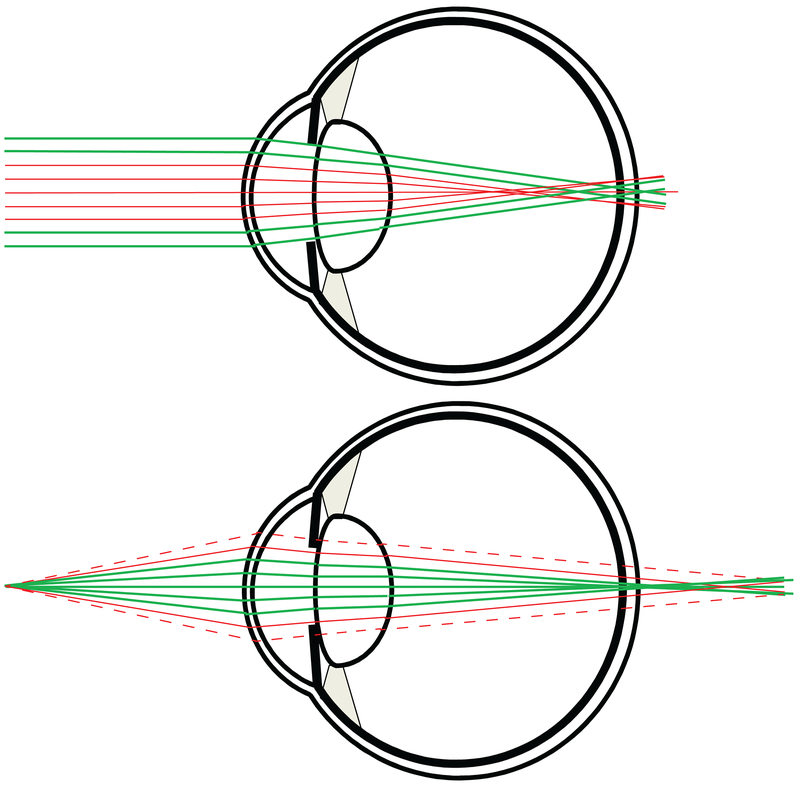
Figure. 6:
This illustration offers the basic rationale for using aberrations, or multifocality, to extend the eye’s depth of field. In both cases, the eye has the same amount of negative spherical aberration. Upper figure: Light from a distance object is refracted by the optics, and the marginal rays (bold and colored green) focus on the retina, while the paraxial rays focus in front. Lower figure: For a near object, the paraxial rays focus on the retina and the marginal rays focus beyond the retina. Constriction of the pupil, which is generally associated with convergence on near objects further augments the effect by blocking some of the non-focusing rays. The vignette rays are shown as dashed in the lower figure. Although the pupil constriction mechanism may be effective for near work, keep in mind that the same pupil constriction would also render the eye myopic when the pupil constricts in bright lights.
Perhaps the most informative paper was by Chin et al (2009b) where, along with a step change in the vergence of the accommodative stimulus, they introduced either (i) no new aberrations, (ii) removed the aberrations, or (iii) reversed the aberrations from their native state. In effect, they generated directional cues that were consistent, nonexistent, or inconsistent with the change in defocus. The defocus steps with the inconsistent cues caused the greatest number of opposite accommodation responses, more initial accommodation errors and longer latencies.
With due appreciation to the efforts already made, the published results have not really shed much new light on the role of monochromatic aberrations for accommodation. The phenomenon is complex - accommodation and disaccommodation themselves are not symmetric processes and the dynamics of each are quite different. Accommodation in the absence of binocular cues and shifts in gaze direction (Schor, Lott, Pope, & Graham, 1999) do not really mimic normal conditions; nor do measures of accommodation under conditions where the pupil size is fixed. What is needed is an AO system that has the frequency response to stay ahead of the accommodation dynamics, and that can limit the accommodative cues well enough so that an eye, when presented with an ambiguous defocused stimulus, makes its initial response in a random direction. Once that starting point is established, the responses under manipulated optical conditions will be much more informative. If such a condition cannot be found, then we need to look for additional cues or physiological mechanisms, beyond the ones we already know, that can indicate the sign of defocus.
Presbyopia
Of course, for many of us, the ability to accommodate is a thing of the past. By the mid-forties presbyopia, the age-related loss in the focusing ability of the crystalline lens, has taken hold. Promising new accommodating intraocular lens designs are on the horizon, but there is currently no cure. At present, the only recourse is to relieve the effects of presbyopia, which can be done in a variety of ways including reading glasses, multifocal spectacles (bifocals, trifocals, progressive addition lenses), monovision (where one eye is given a near correction and the other eye is given distance vision), and pupil size control (Seyeddain, Riha, Hohensinn, Nix, Dexl, & Grabner, 2010). An emerging option has been to generate multifocality within the optical zone of a single lens. This is done by actually adding aberrations to the optical system which, in effect, makes different parts of the optical system focus at different distances (see Fig. 6). Bifocal, multifocal and continuous optical corrections are available today as contact lenses or intra-ocular lenses and are even being implemented in laser refractive surgery. There is a cost to overall contrast however, since for every part of the optical system that provides a sharp image, there is another part that produces an out-of-focus image of the same object. Acceptance of these lens designs are varied, which could be due to individual preferences, or due to the variable optical interactions between the eye aberrations and those of the correction (Martin & Roorda, 2003; Legras, Benard, & Rouger, 2010).
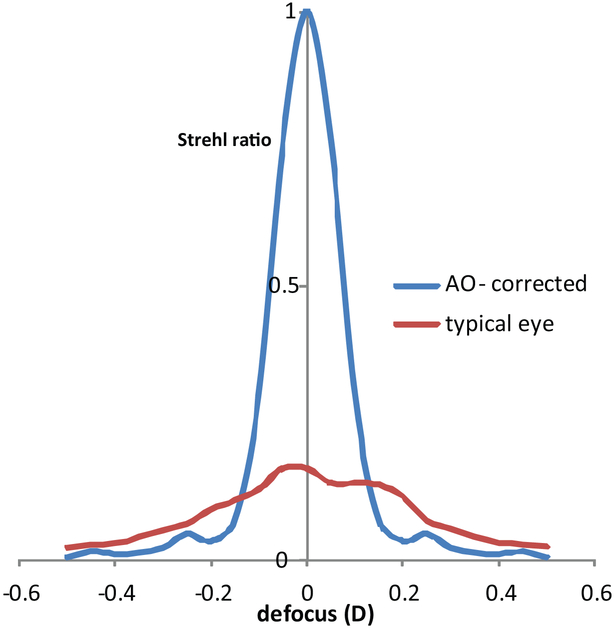
AO can play an important role in this interim effort to relieve presbyopia. If adding aberrations is an effective mechanism to relive presbyopia, then does reducing aberrations make the effects of presbyopia worse? The answer is yes! Fig. 7 shows how the loss in image quality with focus is more precipitous when aberrations are corrected than when they are not.
Figure. 7:
Plot of the Strehl ratio as a function of defocus for an AO-corrected eye and for a typical eye (corrected for astigmatism). Strehl ratio is the ratio of the peak height of the PSF for the actual eye to the peak height of the PSF of a diffraction-limited eye with the same pupil size. Strehl ratios range from 0 to 1 where the higher number indicates a sharper image. The pupil size was 5 mm for both cases. At best focus, the image quality in the aberration-free eye far exceeds that of the typical eye, but image quality drops quickly with defocus. By the time the defocus is +/− 0.15 D or more, the aberration-correction actually results in worse image quality than if it were uncorrected. This illustrates how aberrations can serve to increase the depth of focus of the eye. It also illustrates the cost of correcting ones aberrations.
AO can be used to quantify the extent to which reduced depth of focus that arises with corrected aberrations is a problem for vision (Guo et al., 2008; Legras et al., 2010; Piers et al., 2004). Second, induction of aberrations with AO in combination with vision testing can be used to discover aberration types and magnitudes that prove to be the most beneficial for depth of field at minimal cost to the best vision performance (Werner, Elliott, Choi, & Doble, 2009). After all, considering that our optics are already far from perfect (see Fig 3&4), it seems that we can already tolerate a lot of aberration. Given this built-in tolerance, it ought to be possible to manipulate these aberrations in a sensible way to increase an eye’s depth of focus at little or no cost to the sharpness of its vision. AO systems turn out to be a cost-effective approach to discover the optimal aberration profiles that will achieve this goal, since only the successful profiles would ever be implemented into an actual product. For example, Piers et al, used an AO system to test the cost vs. benefit of removing spherical aberration on visual performance. The study concluded that removing spherical aberration benefits retinal image contrast with little cost to the eye’s depth of focus (Piers et al., 2004; Piers, Manzanera, Prieto, Gorceix, & Artal, 2007). These and related studies examining the practical benefits of spherical aberration correction in the presence of scatter and with or without chromatic aberration (Perez, Manzanera, & Artal, 2009; Artal et al., 2010) led to the development of a very popular intra-ocular lens design, the Tecnis (Abbot Medical Optics, Santa Ana, California) which was designed with an amount of negative spherical aberration that cancels the average positive spherical aberration that is present in the age-group that is likely to be receiving these implants.
Clinically deployable AO systems also have the potential to show the patient the range of optical correction options before they decide on the solution they want. More on this in the technology section……
Technology
As the technology develops, it seems inevitable that AO systems will find their way into routine clinical use. This may be in the form of AO ophthalmoscopes (which are not discussed in this review) but may also be in the form of advanced AO phoropters. An AO phoropter could perform autorefraction based on the wavefront (Cheng, Bradley, Hong, & Thibos, 2003; Guirao & Williams, 2003; Campbell, 2004; Watson & Ahumada, Jr., 2008) as well as expedite the subjective refraction procedure. Additionally, the eye doctor could use it to allow the patient to preview and help guide their decision when selecting from the myriad of options available to relieve presbyopia. An early commercial effort has already been made in this direction (crx1™ Adaptive Optics Visual Simulator, Imagine Eyes, France), but perhaps the most exciting new development comes from Pablo Artal’s group who recently unveiled the first binocular AO vision simulator. The binocular aspect is important because all practical vision tests, vision tasks, and vision corrections employ both eyes and so it is sensible that a practical system should take that into consideration. A typical AO system is usually designed for a single eye and so making a system binocular would normally double the complexity, cost and size. Artal, however, has developed a very practical solution by using a liquid crystal spatial light modulator wavefront corrector (LCOS) that can, on a single device, provide independent corrections to both eyes simultaeneously (Fernandez, Prieto, & Artal, 2010; Fernandez, Prieto, & Artal, 2009). Moreover, the LCOS can correct and generate both low and high order corrections as well as simulate discontinuous optical designs, like diffraction or multifocal lenses (Manzanera, Prieto, Ayala, Lindacher, & Artal, 2007). Although the LCOS design has its limitations (reduced performance with broadband light, reliance on polarization (Doble & Miller, 2006)) it represents a viable path for more widespread use of AO technology for clinical applications. These and other technical achievements are expanding the scope of how AO can be used and increasing the control over our experiments.
Simultaneous Imaging and Stimulus Delivery
AO should be considered as only one part of a system for testing visual function. AO can control the optical properties of whatever stimulus is presented, but controlling other factors, such as axial and lateral placement of the stimulus requires more advanced systems. These comprise a class of systems that are capable of retinal imaging as well as stimulus delivery – the image providing or guiding the where, and the AO providing the what. Some early experiments clarified the usefulness of this capability. In one experiment, the preferred locus of fixation was determined on the retina and compared with the point of maximum cone photoreceptor density (Putnam, Hofer, Doble, Chen, Carroll, & Williams, 2005). They found that the eye did not necessarily use the position of highest cone density as the preferred retinal locus. In another experiment, the color appearance of a sequence of small, AO-corrected spots presented at random locations was recorded and psychophysical results were compared with the known arrangement and proportion of the three cone classes in the vicinity of the test (Hofer, Singer, & Williams, 2005). Finally, detection of small spots was used to infer the functional consequence of ‘holes’ in the cone mosaic of a patients with a unique mutation that lead to functional loss of one spectral class of cone (Makous, Carroll, Wolfing, Lin, Christie, & Williams, 2006). While these showed the strength of the combined measures of structure and function, they did not fully control the stimulus and at best could only determine where it landed after the fact.
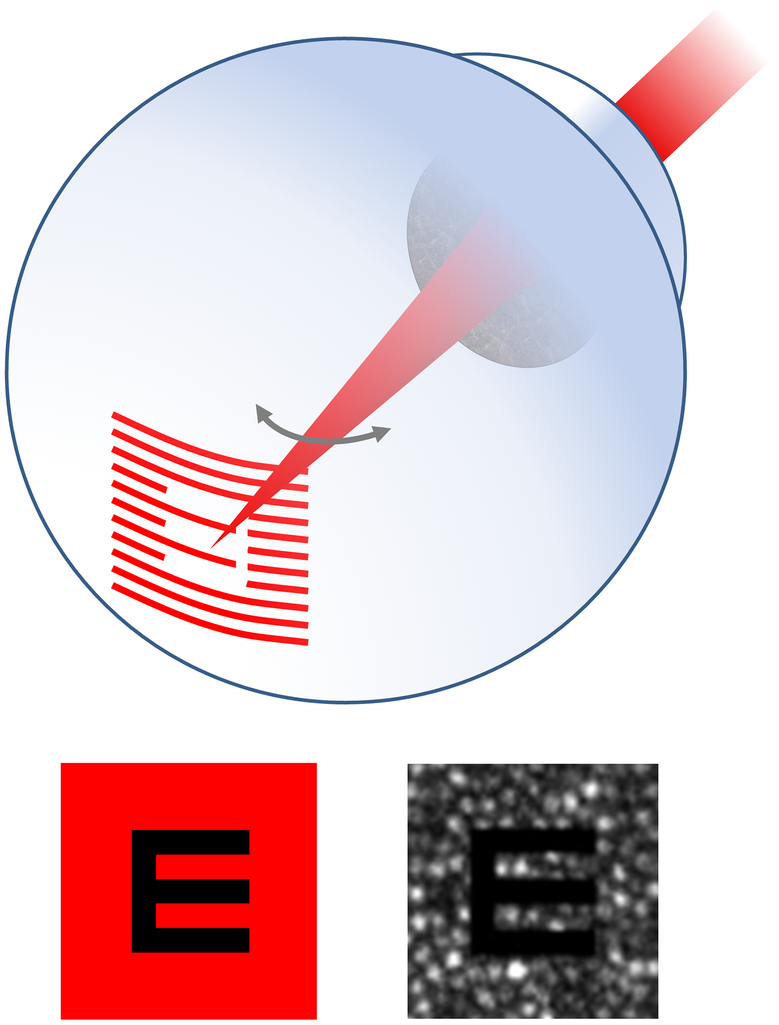
More recently, our group has shown that a scanning laser ophthalmoscope modality with adaptive optics (AOSLO) is an attractive choice for more complete control of the stimulus and its placement. To form an image in an AOSLO, a detector records the scattered light from a small focused spot on the retina as it scans in a raster pattern across a region of interest (typically a 1- 5 degree square field). AO ensures that the focused spot is as small as possible, which improves the contrast and resolution over conventional SLOs. The presentation of a retinal stimulus involves modulating the scanning laser during the scan, much in the same way that electrons are steered and modulated to produce the image on a CRT television monitor (Poonja, Patel, Henry, & Roorda, 2005). Fig. 8 illustrates the basic principles behind this method. Using this method, the stimulus gets encoded directly onto the image and so the operator has an unambiguous and real-time record of where the stimulus has landed. Moreover, multiple lasers can be coaligned with the imaging laser to deliver stimuli of any wavelength. Another feature of the AOSLO system is that it can be used as a high-frequency eye tracker (Stevenson & Roorda, 2005; Vogel, Arathorn, Roorda, & Parker, 2006; Stevenson, Roorda, & Kumar, 2010). Owing to the scanning method, eye motions generate unique distortions in each recorded frame, much like the distortions observed whenever you move a page while it is being imaged on a flat bed scanner. In effect, these distortions are a record of the eye motion and, if decoded fast enough, can provide a real-time record of eye motion. The frequency of eye tracking is not constrained by the frame rate of the AOSLO system (currently 30Hz), but rather depends on how well the distortions can be recovered from each frame. The resolution of AOSLO enables cone-level eye tracking at frequencies over 1 kHz. Combining the high frequency eye tracking with the ability to deliver stimuli has enabled us to deliver AO-corrected stimuli to the retina to targeted retinal locations with an accuracy of a single cone photoreceptor(Arathorn, Yang, Vogel, Zhang, Tiruveedhula, & Roorda, 2007; Yang, Arathorn, Tiruveedhula, Vogel, & Roorda, 2010).
Figure 8:
In the AOSLO, the stimulus is printed directly onto the retina by modulating the scanning laser, line-by-line, as it scans a raster pattern on the retina. The upper cartoon shows a situation where the scanning beam is been modulated to project a letter ‘E’ on the retina. Lower left: The image that the subject sees. Lower right: An actual single frame image from a video showing what the operator sees. The modulated letter is directly encoded onto the retinal image, in this case showing the mosaic of cones. In this way, the operator can ensure that the stimulus is focused accurately on the cones and can also determine exactly which cones have interacted with the stimulus during its presentation.
AOSLO systems provide both structural and functional information and facilitate a new class of experiments. The fact that such systems are in the hands of only a few labs owes more to the complexity than to the scientific potential of these instruments. In time, however, these systems should become more widespread and find new applications.
Learning About Vision at the Scale of Single Photoreceptors
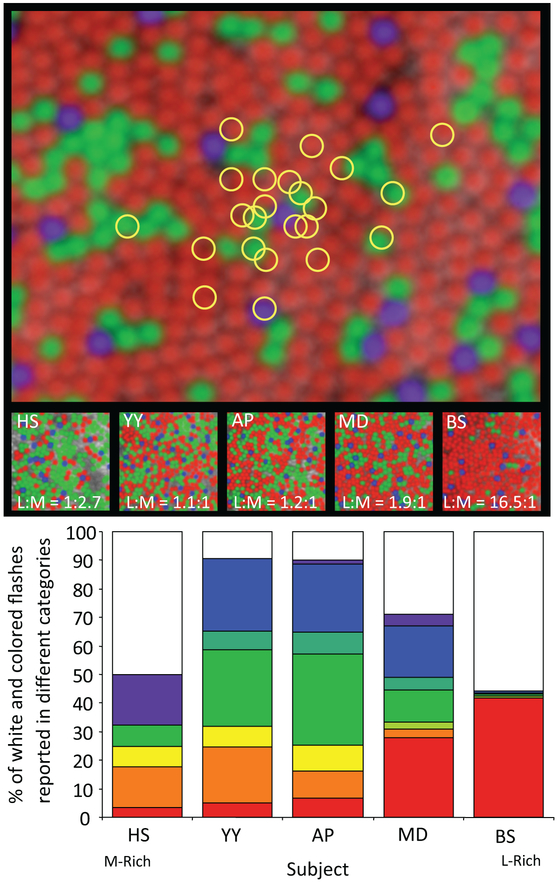
Having microscopic control of the retinal stimulus allows researchers to explore the functional properties of single cells in the same way that electrophysiologists have done using electrodes for decades. Responses to single cell stimulation can help to build a better picture of how the entire system works. Hofer et al (2005) were the first to do this when they explored the color perceptions elicited with single cone stimulation. The basic concepts and main results of their experiment are shown in Fig. 9. They discovered that the color appearance of light stimulating a single cone was much more complex than some of the simpler theories would have suggested. Instead of eliciting three classes of response generated from the stimulation of the three cone classes, they found that subjects demanded as many as 7 color categories. Analysis of the responses suggested that the color appearance generated by a single cone is more a function of how it is situated with respect to other cones rather than by its spectral subtype (Brainard, Williams, & Hofer, 2008). Cones that are in a position to provide strong chromatic cues generate colored percepts, whereas cones that are not in a good position to do so generate achromatic, or white percepts. Given the random arrangement of the three cone classes in the retina (Roorda & Williams, 1999; Hofer, Carroll, Neitz, Neitz, & Williams, 2005), it is sensible that the visual system would develop in this way to best handle the dual role that retina has in conveying both spatial and color vision.
Figure 9:
The experiment of Hofer et al (2005) involves delivery of AO-corrected stimulus to a cone mosaics whose short-, middle- and long-wavelength sensitive (SML) cone locations are known. The upper image simulates the random landing points of repeated stimulations that arise from natural fixation instability. . For each stimulus, the subject is asked to judge the color of what they saw. The bar chart shows that the five subjects, whose SML cone ratios varied 45-fold (see middle row for images and L:M cone ratios), saw a wide variety of color responses. (adapted from Hofer et al (2005))
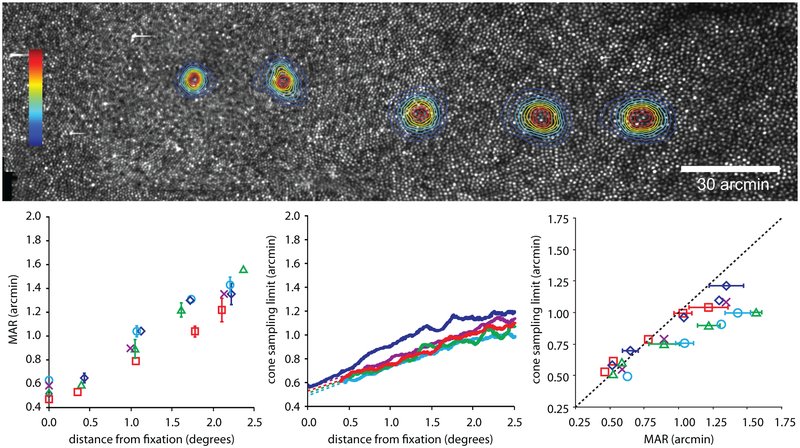
To address questions about retinal limits to spatial vision, advanced AO systems provide for the first time, the ability to measure spatial vision limits and correlate them directly to the sampling frequency of the retinal mosaic. Rossi and Roorda ( 2010b) used an AOSLO to measure visual acuity as a function of eccentricity. At each tested location they carefully measured the cone sampling density and confirmed that, at the foveal center, the acuity matched the sampling limits of the cone mosaic quite well but, almost immediately outside, the functional receptive fields were larger than single cones, suggesting that ganglion cells pool information from more than one cone at smaller eccentricities than previously thought (Fig. 10). They might not have come to that conclusion if it were not for a direct and unambiguous measure of the spatial vision and exact cone spacing and density at each tested location. Other investigators measured similar acuity limits as a function of eccentricity but, because they relied on histological records of cone spacing from different individuals, they came to a different conclusion (see supplemental material in Rossi and Roorda (2010b).
Figure 10:
The experiment of Rossi and Roorda (2010b) involved relating visual acuity measures to cone spacing at and near the fovea. The upper image shows five tested locations in a single eye from the fovea to about 2.5 degrees eccentricity. The lower chart plots cone row spacing against the minimum angle of resolution. If the cone spacing imposed the limit to resolution at all locations, then the slope of the results would have been 1. The slope was actually 0.63, indicating that outside of the foveal center the receptive fields are, on average, larger than single cones.
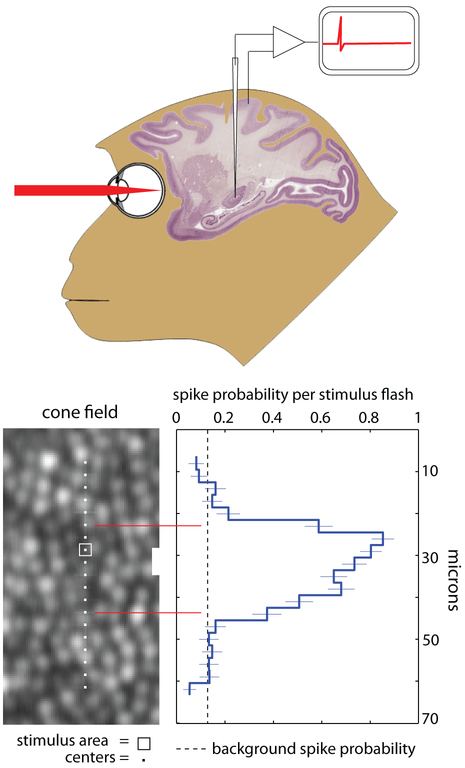
To drill deeper into questions about receptive fields, Sincich et al. (2009) combined all the tracking and light delivery features of the AOSLO and brought them to the electrophysiology lab, where they measured LGN neurons while tracking and optically stimulating individual cones. Simply put, what AO offers to electrophysiologists is unprecedented control of the retinal stimulus. In fact, the experiments of Sincich et al. represented the first time where single cells had been stimulated optically while single neurons were recorded in a living animal (see Fig. 11). With the exception of the delivery of interference fringes to the retina in one ambitious electrophysiology experiment (McMahon, Lankheet, Lennie, & Williams, 2000), the visual stimulation in electrophysiology experiments have generally been limited to presentations on monitors or tangent screens. Because of the limitations of these stimuli caused by eye movements (which persist even under anesthesia and neuromuscular blockage) and by optical blur (which is difficult to monitor), detailed studies of receptive field properties are necessarily more accurate when performed in the periphery. The fovea, which is the most interesting region to study, has largely been avoided. The new control offered by advanced AO systems enables the study of this important retinal location. Of course, recordings can be made in any visually responsive brain area, not just the LGN, and microelectrodes are just one of many possible physiological recording techniques that can be used with AO. With the exception of fMRI, no neural recording method is likely to interfere with AO performance.
Figure 11:
The experiments of Sincich et al. (2009) involved the optical stimulation of targeted cones in the monkey along with simultaneous recording of neural activity in the lateral geniculate nucleus. Example results are shown on the center and right panels of the figure. The image in the central panel indicates the exact locations stimulated with a square spot of light and the plot shows the spike probability per stimulus flash. The sharpness of the edges of the receptor field gave assurance that they were measuring single cone contributions. This is a cross-section of the parvocellular ON-center field at an eccentricity of 3.7 degrees.
Clinical Applications
Combined imaging and delivery applications can also have a large impact in the clinical arena. Although advanced AO systems with tracking, stimulus delivery and imaging may not be used routinely in the clinic anytime soon, these systems offer a useful tool for making important structure-function relationships. As imaging technologies for monitoring and understanding eye disease on a cellular scale continue to mature, the desire to use them to provide diagnoses, assessments of visual function or outcome measures for clinical trials will increase (Talcott, Ratnam, Sundquist, Lucero, Lujan, Tao, Porco, Roorda, & Duncan, 2011). Their acceptance, however, may be hampered until convincing evidence of the functional consequences of the observed structural changes is provided. At present, conventional clinical measures of retinal function do not have an accuracy or precision that is commensurate with the structural measures achieved with AO. So, the onus is on AO systems to make these measurements themselves. An early and very elegant example of relating structure to function was by Makous et al (2006) (Incidentally, this is also a good example of what can happen when we place our AO tools into the hands of experienced psychophysicists). Carroll et al. (2004) had just discovered a rare type of color blindness that led to a unique phenotype where it appeared that an entire class of cones was missing from the mosaic. Rather than reflecting the light as cones normally do, they left black holes in the mosaic. To test whether these ‘holes’ were indeed microscotomas, Makous et al. devised a test to efficiently measure the frequency of seeing of small spots delivered to the retina. Their experimental system was not able to perform targeted stimulus delivery at the time and so they relied on statistical responses but, with their results and a carefully developed model, they were able to confirm that these were indeed cone-sized microscotomas. Future experiments with tracking a targeted stimulus delivery will allow researchers to make these measures more efficiently.
Advanced tests of the functional consequences of abnormal retinal structure will continue to inform us on how to interpret structural changes in the retina. As a result, structural measures from AO ophthalmoscopes will find increasing use and value for clinical applications.
Discussion
It is often the case that an advance in technology is the catalyst that draws a new class of researcher into a field, and the development of AO for the eye is no exception. AO has opened the door to new level of vision correction, approximately 200 years since the last major advance, which was the correction of astigmatism. As AO is driving a paradigm shift in how we do ophthalmoscopy, so too is AO driving a paradigm shift in how we do vision science. In ophthalmoscopy, the single-cell resolution turns the ophthalmoscope into a microscope, which facilitates the translation of a host of innovative tools from the micropscopist’s toolbox into the field of ophthalmoscopy. Similarly, the unprecedented control of the retinal stimulus allows us to carefully probe vision on a scale that was previously only possible in excised retina. Where probing vision on a cellular scale was mainly the domain of the in vitro physiologist, AO enables the translation of some of these methods to the living eye.
Now, as we move forward, I must capitalize on this opportunity to offer some guidance on how we should proceed in this field. Some are based on my 15 years of experience with AO systems, and others are due to the fact that I’ve had to review an ever-increasing number of papers on the topic.
With the maturation of wavefront sensors and AO systems, our ability to measure the eye’s aberrations has become very accurate. Ironically, defocus, the first aberration ever characterized, remains the most difficult aberration to control. In fact, defocus falls into a whole different class of aberration. First, wavefront sensor estimates of defocus are problematic since we are never sure of the source of the reflection from the retina when we interpret wavefront sensor images (Lopez-Gil and Artal, 1997). If the wavefront we recorded were coming from deeper layers than the photoreceptor aperture, for example, then we would incorrectly interpret the eye as being myopic. Furthermore, defocus of the wavefront relative to the retina can be changed by manipulating three factors: the eye’s optics, the eye’s axial length and by the object position, whereas other aberrations are governed only by the eye’s optics. Finally, in the presence of aberrations, it is still not known exactly where the optimal position of the retina should be in the focused beam. That is, what metric needs to be optimized to produce the preferred image quality (Cheng, Bradley, & Thibos, 2004)? Considering this, researchers need to keep in mind that, after AO correction, the perceived retinal image quality will be much more vulnerable to defocus (see Fig. 7) and the demands for getting an accurate correction are much higher than before correction. An AO-correction combined with a small refractive error will give rise to worse performance than if some aberrations were present, and this could lead to erroneous conclusions about whatever phenomena is being explored.
It is no longer sufficient for AO researchers to compare visual performance (visual acuity, contrast sensitivity etc.) with and without an AO correction measured from their single system. The values should be compared to the visual performance that can be achieved on a similar task outside of the AO system. There may be image degradations inherent to the AO system that are common to both conditions and these should be eliminated or at least understood so that they can be part of the interpretation of the results. There are too many reports in the current literature of visual acuity levels that are clearly less than optimal. Be aware that on a typical visual acuity task, we can expect defocus- and astigmatism-corrected subjects to achieve 20/15 or better. (20/15 is equivalent to a decimal acuity of 1.33, a minimal angle of resolution (MAR) of 0.75, and a - logMAR of 0.125).
When reporting on the quality of an AO correction, do not report Strehl ratio unless it is actually measured. Reporting the root mean square (RMS) of the residual aberrations is dangerous enough. Fortunately, the Optical Society of America sponsored a task force to develop standard for reporting of aberrations and researchers have generally adopted the guidelines (Thibos, Applegate, Schwiegerling, Webb, & VSIA Standards Taskforce Members, 2000). But when it comes to reporting AO performance, it is easy for these numbers to be misleading. Keep in mind that the fewer terms one uses to do a wavefront fit, the better the RMS and the higher the Strehl ratio will appear to be. These are good numbers to use internally, but let’s stop pretending that they reflect absolute performance.
Conclusion
At 14 years since the first seminal paper in the field, adaptive optics systems for vision testing applications are still in their adolescence. Like adolescents, AO systems tend to be a bit awkward and clunky, but the physical tools are essentially in place. Although AO systems are already being used to make significant advances in vision science, there is a lot of room for maturation.
In the next ten years, we should expect to see different classes of AO systems for vision science emerge. Low cost systems with specific uses will likely start coming available for routine clinical use, such as the AO phoropter. At the same time, it should not be too long before any researcher with appropriate funding will be able to purchase a robust, user-friendly AO system with advanced imaging and visual testing capabilities. At present, few advanced instruments exist outside of the labs that have developed them. But history has proven that the factors that will increase accessibility to the technology - reduced cost and a more robust, user-friendly design with user-friendly control - will happen with time.
Acknowledgements
The author thanks a number of people who provided constructive reviews on the paper: Martin Banks, Lawrence Sincich, David Williams, Pablo Artal, Heidi Hofer and Ethan Rossi. Funding support for much of the work in Roorda’s lab comes from an NIH Bioengineering Research Partnership NIH EY014375.
Appendix A.
AO for Vision Science Bibliography by topic
This is a list of all the peer-reviewed papers that the author could find where adaptive optics was employed to test some aspect of vision. They are listed chronologically within each topic area.
Visual Benefit of Correcting Aberrations of Visual Consequences of Blur
- Liang J, Williams DR, and Miller D(1997). Supernormal vision and high-resolution retinal imaging through adaptive optics. J.Opt.Soc.Am.A, 14(11)2884–2892 [DOI] [PubMed] [Google Scholar]
- Williams D, Yoon GY, Porter J, Guirao A, Hofer H, and Cox I(2000). Visual benefit of correcting higher order aberrations of the eye. J.Refract.Surg., 16(5)S554–S559 [DOI] [PubMed] [Google Scholar]
- Yoon GY and Williams DR(2002). Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J.Opt.Soc.Am.A, 19(2)266–275 [DOI] [PubMed] [Google Scholar]
- Lundstrom L, Manzanera S, Prieto PM, Ayala DB, Gorceix N, Gustafsson J, Unsbo P, and Artal P(2007). Effect of optical correction and remaining aberrations on peripheral resolution acuity in the human eye. Opt.Express, 15(20)12654–12661 [DOI] [PubMed] [Google Scholar]
- Rocha KM, Vabre L, Harms F, Chateau N, and Krueger RR(2007). Effects of Zernike wavefront aberrations on visual acuity measured using electromagnetic adaptive optics technology. J.Refract.Surg., 23(9)953–959 [DOI] [PubMed] [Google Scholar]
- Dalimier E, Dainty C, and Barbur JL(2008). Effects of higher-order aberrations on contrast acuity as a function of light level. Journal of Modern Optics, 55(4-5)791–803 [Google Scholar]
- Dalimier E and Dainty C(2008). Use of a customized vision model to analyze the effects of higher-order ocular aberrations and neural filtering on contrast threshold performance. J.Opt.Soc.Am.A Opt.Image Sci.Vis., 25(8)2078–2087 [DOI] [PubMed] [Google Scholar]
- Marcos S, Sawides L, Gambra E, and Dorronsoro C(2008). Influence of adaptive-optics ocular aberration correction on visual acuity at different luminances and contrast polarities. J.Vis., 8(13)1–12 [DOI] [PubMed] [Google Scholar]
- Atchison DA, Guo H, Charman WN, and Fisher SW(2009). Blur limits for defocus, astigmatism and trefoil. Vision Res, 49(19)2393–2403 [DOI] [PubMed] [Google Scholar]
- Atchison DA, Guo H, and Fisher SW(2009). Limits of spherical blur determined with an adaptive optics mirror. Ophthalmic Physiol Opt, 29(3)300–311 [DOI] [PubMed] [Google Scholar]
- Elliott SL, Choi SS, Doble N, Hardy JL, Evans JW, and Werner JS(2009). Role of high-order aberrations in senescent changes in spatial vision. J.Vis., 9(2)24–16 tested CSF with age [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiong Y, Wang N, Li S, Dai Y, Xue L, Zhao H, Jiang W, and Zhang Y(2009). Effects of spherical aberration on visual acuity at different contrasts. J.Cataract Refract.Surg., 35(8)1389–1395 [DOI] [PubMed] [Google Scholar]
- Li S, Xiong Y, Li J, Wang N, Dai Y, Xue L, Zhao H, Jiang W, Zhang Y, and He JC(2009). Effects of monochromatic aberration on visual acuity using adaptive optics. Optometry and Vision Science, 86(7)868–874 [DOI] [PubMed] [Google Scholar]
- Perez GM, Manzanera S, and Artal P(2009). Impact of scattering and spherical aberration in contrast sensitivity. J.Vis., 9(3)19–10 [DOI] [PubMed] [Google Scholar]
- Rouger H, Benard Y, and Legras R(2009). Effect of Monochromatic Induced Aberrations on Visual Performance Measured by Adaptive Optics Technology. J.Refract.Surg, 1–10 [DOI] [PubMed] [Google Scholar]
- Artal P, Manzanera S, Piers P, and Weeber H(2010). Visual effect of the combined correction of spherical and longitudinal chromatic aberrations. Opt.Express, 18(2)1637–1648 [DOI] [PubMed] [Google Scholar]
- Guo H and Atchison DA(2010). Subjective blur limits for cylinder. Optometry and Vision Science, 87(8)E549–E559 [DOI] [PubMed] [Google Scholar]
- Gupta P, Guo H, Atchison DA, and Zele AJ(2010). Effect of optical aberrations on the color appearance of small defocused lights. J.Opt.Soc.Am.A Opt.Image Sci.Vis., 27(5)960–967 [DOI] [PubMed] [Google Scholar]
- Rocha KM, Vabre L, Chateau N, and Krueger RR(2010). Enhanced visual acuity and image perception following correction of highly aberrated eyes using an adaptive optics visual simulator. J.Refract.Surg., 26(1)52–56 [DOI] [PubMed] [Google Scholar]
- Sawides L, Gambra E, Pascual D, Dorronsoro C, and Marcos S(2010). Visual performance with real-life tasks under adaptive-optics ocular aberration correction. J.Vis., 10(5)19. [DOI] [PubMed] [Google Scholar]
- de Gracia P, Dorronsoro C, Gambra E, Marin G, Hernandez M, and Marcos S(2010). Combining coma with astigmatism can improve retinal image over astigmatism alone. Vision Res, 50(19)2008–2014 [DOI] [PubMed] [Google Scholar]
New technology for AO Vision Testing
- Fernandez EJ, Manzanera S, Piers P, and Artal P(2002). Adaptive optics visual simulator. J.Refract.Surg., 18(5)S634–S638 [DOI] [PubMed] [Google Scholar]
- Poonja S, Patel S, Henry L, and Roorda A(2005). Dynamic visual stimulus presentation in an adaptive optics scanning laser ophthalmoscope. J.Refract.Surg., 21(5)S575–S580 [DOI] [PubMed] [Google Scholar]
- Manzanera S, Prieto PM, Ayala DB, Lindacher JM, and Artal P(2007). Liquid crystal Adaptive Optics Visual Simulator: Application to testing and design of ophthalmic optical elements. Opt.Express, 15(24)16177–16188 [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Prieto PM, and Artal P(2009). Binocular adaptive optics visual simulator. Opt.Lett, 34(17)2628–2630 [DOI] [PubMed] [Google Scholar]
- Canovas C, Prieto PM, Manzanera S, Mira A, and Artal P(2010). Hybrid adaptive-optics visual simulator. Opt.Lett, 35(2)196–198 [DOI] [PubMed] [Google Scholar]
- Yang Q, Arathorn DW, Tiruveedhula P, Vogel CR, and Roorda A(2010). Design of an integrated hardware interface for AOSLO image capture and cone-targeted stimulus delivery. Opt.Express, 18(17)17841–17858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Neural Adaptation and Neural Insensitivity
- Artal P, Chen L, Fernandez EJ, Singer B, Manzanera S, and Williams DR(2004). Neural compensation for the eye's optical aberrations. J.Vis., 4(4)281–287 [DOI] [PubMed] [Google Scholar]
- Chen L, Artal P, Gutierrez D, and Williams DR(2007). Neural compensation for the best aberration correction. J.Vis., 7(10)9. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Weiser P, Tarrant J, and Roorda A(2007). Visual performance in emmetropia and low myopia after correction of high-order aberrations. J.Vis., 7(8)1–14 [DOI] [PubMed] [Google Scholar]
- Sabesan R, Jeong TM, Carvalho L, Cox IG, Williams DR, and Yoon G(2007). Vision improvement by correcting higher-order aberrations with customized soft contact lenses in keratoconic eyes. Opt.Lett, 32(8)1000–1002 [DOI] [PubMed] [Google Scholar]
- Sabesan R and Yoon G(2009). Visual performance after correcting higher order aberrations in keratoconic eyes. J.Vis., 9(5)6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IJ, Elliott SL, Pallikaris A, Werner JS, Choi S, and Tahir HJ(2010). The oblique effect has an optical component: orientation-specific contrast thresholds after correction of high-order aberrations. J.Vis., 10(11)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesan R and Yoon G(2010). Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol.Vis.Sci, 51(7)3835–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA and Roorda A(2010). Is visual resolution after adaptive optics correction susceptible to perceptual learning? J.Vis., 10(12)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, Marcos S, Ravikumar S, Thibos L, Bradley A, and Webster M(2010). Adaptation to astigmatic blur. J.Vis., 10(12)22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evaluation of Refraction Aids for Presbyopia
- Piers PA, Fernandez EJ, Manzanera S, Norrby S, and Artal P(2004). Adaptive optics simulation of intraocular lenses with modified spherical aberration. Invest Ophthalmol.Vis.Sci, 45(12)4601–4610 [DOI] [PubMed] [Google Scholar]
- Piers PA, Manzanera S, Prieto PM, Gorceix N, and Artal P(2007). Use of adaptive optics to determine the optimal ocular spherical aberration. J.Cataract Refract.Surg., 33(10)1721–1726 [DOI] [PubMed] [Google Scholar]
- Guo H, Atchison DA, and Birt BJ(2008). Changes in through-focus spatial visual performance with adaptive optics correction of monochromatic aberrations. Vision Res, 48(17)1804–1811 [DOI] [PubMed] [Google Scholar]
- Rocha KM, Vabre L, Chateau N, and Krueger RR(2009). Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J.Cataract Refract.Surg., 35(11)1885–1892 [DOI] [PubMed] [Google Scholar]
- Werner JS, Elliott SL, Choi SS, and Doble N(2009). Spherical aberration yielding optimum visual performance: evaluation of intraocular lenses using adaptive optics simulation. J.Cataract Refract.Surg., 35(7)1229–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legras R, Benard Y, and Rouger H(2010). Through-focus visual performance measurements and predictions with multifocal contact lenses. Vision Res, 50(12)1185–1193 [DOI] [PubMed] [Google Scholar]
AO For Testing Mechanisms of Accommodation
- Fernandez EJ and Artal P(2005). Study on the effects of monochromatic aberrations in the accommodation response by using adaptive optics. J.Opt.Soc.Am.A Opt.Image Sci.Vis., 22(9)1732–1738 [DOI] [PubMed] [Google Scholar]
- Chen L, Kruger PB, Hofer H, Singer B, and Williams DR(2006). Accommodation with higher-order monochromatic aberrations corrected with adaptive optics. J.Opt.Soc.Am.A Opt.Image Sci.Vis., 23(1)1–8 [DOI] [PubMed] [Google Scholar]
- Hampson KM(2008). Adaptive optics and vision. Journal of Modern Optics, 55(21)3425–3467 [Google Scholar]
- Chin SS, Hampson KM, and Mallen EA(2009). Role of ocular aberrations in dynamic accommodation control. Clin.Exp.Optom., 92(3)227–237 [DOI] [PubMed] [Google Scholar]
- Chin SS, Hampson KM, and Mallen EA(2009). Effect of correction of ocular aberration dynamics on the accommodation response to a sinusoidally moving stimulus. Opt.Lett, 34(21)3274–3276 similar to above [DOI] [PubMed] [Google Scholar]
- Gambra E, Sawides L, Dorronsoro C, and Marcos S(2009). Accommodative lag and fluctuations when optical aberrations are manipulated. J.Vis., 9(6)4–15 [DOI] [PubMed] [Google Scholar]
Relationships Between Retinal Structure and Visual Function
- Hofer H, Singer B, and Williams DR(2005). Different sensations from cones with the same pigment. J.Vis., 5(5)444–454 [DOI] [PubMed] [Google Scholar]
- Putnam NM, Hofer H, Doble N, Chen L, Carroll J, and Williams DR(2005). The locus of fixation and the foveal cone mosaic. J.Vis., 5(7)632–639 [DOI] [PubMed] [Google Scholar]
- Makous W, Carroll J, Wolfing JI, Lin J, Christie N, and Williams DR(2006). Retinal microscotomas revealed with adaptive-optics microflashes. Invest Ophthalmol.Vis.Sci, 47(9)4160–4167 [DOI] [PubMed] [Google Scholar]
- Raghunandan A, Frasier J, Poonja S, Roorda A, and Stevenson SB(2008). Psychophysical measurements of referenced and unreferenced motion processing using high-resolution retinal imaging. J.Vis., 8(14)14–11 [DOI] [PubMed] [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, and Roorda A(2009). Resolving single cone inputs to visual receptive fields. Nat.Neurosci, 12(8)967–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalimier E and Dainty C(2010). Role of ocular aberrations in photopic spatial summation in the fovea. Opt.Lett, 35(4)589–591 [DOI] [PubMed] [Google Scholar]
- Li KY, Tiruveedhula P, and Roorda A(2010). Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophthalmol.Vis.Sci, 51(12)6858–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA and Roorda A(2010). The relationship between visual resolution and cone spacing in the human fovea. Nat.Neurosci, 13(2)156–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson Roorda, &Kumar. Eye tracking with the adaptive optics scanning laser ophthalmoscope Spencer SN Proceedings of the 2010 Symposium on Eye-Tracking Research & Applications , 195-198. 2010. New York, NY, USA, Association for Computed Machinery. [Google Scholar]
Testing Binocular Vision
- Fernandez EJ, Prieto PM, and Artal P(2010). Adaptive optics binocular visual simulator to study stereopsis in the presence of aberrations. J.Opt.Soc.Am.A Opt.Image Sci.Vis., 27(11)A48–A55 [DOI] [PubMed] [Google Scholar]
References
- Adams WJ, Banks MS, & van Ee R (2001). Adaptation to three-dimensional distortions in human vision. Nat.Neurosci, 4(11), 1063–1064. [DOI] [PubMed] [Google Scholar]
- Airy GB (1827). On a peculiar Defect in the Eye, and a mode of correcting it. Edinborough Journal of Science, 7, 322–326. [Google Scholar]
- Ames A, & Proctor CA (1921). Dioptrics of the eye. J.Opt.Soc.Am, 5, 22–49. [Google Scholar]
- Arathorn DW, Yang Q, Vogel CR, Zhang Y, Tiruveedhula P, & Roorda A (2007). Retinally Stabilized Cone-Targeted Stimulus Delivery. Opt.Express, 15, 13731–13744. [DOI] [PubMed] [Google Scholar]
- Arnulf MA, & Dupuy MO (1960). La transmission des contrastes par le système optique de l'oeil et les seuils de contrastes retinines. C.R.Acad.Sci.(Paris), 250, 2757–2759. [PubMed] [Google Scholar]
- Artal P (1990). Calculations of two-dimensional foveal retinal images in real eyes. J.Opt.Soc.Am.A, 7, 1374–1381. [DOI] [PubMed] [Google Scholar]
- Artal P, Chen L, Fernandez EJ, Singer B, Manzanera S, & Williams DR (2004). Neural compensation for the eye's optical aberrations. J.Vis, 4(4), 281–287. [DOI] [PubMed] [Google Scholar]
- Artal P, Manzanera S, Piers P, & Weeber H (2010). Visual effect of the combined correction of spherical and longitudinal chromatic aberrations. Opt.Express, 18(2), 1637–1648. [DOI] [PubMed] [Google Scholar]
- Artal P, Santamaria J, & Bescos J (1988). Retrieval of wave aberration of human eyes from actual point-spread-function data. J.Opt.Soc.Am.A, 5, 1201–1206. [DOI] [PubMed] [Google Scholar]
- Berny F, & Slansky S (1969). Wavefront Determination resulting from Foucault Test applied to the Human Eye and Visual Instruments In Home Dickon J (Ed.), Optical Instruments and Techniques. (pp. 375–385). London: Oriel Press. [Google Scholar]
- Brainard DH, Williams DR, & Hofer H (2008). Trichromatic reconstruction from the interleaved cone mosaic: Bayesian model and the color appearance of small spots. J.Vis., 8(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzewski K (1900). Beitrag zur dioptrik des auges. Arch.f.Augenh., 40, 296–333. [Google Scholar]
- Campbell CE (2004). Improving visual function diagnostic metrics with the use of higher-order aberration information from the eye. J.Refract.Surg., 20(5), S495–S503 [DOI] [PubMed] [Google Scholar]
- Campbell FW, & Green DG (1965). Optical and retinal factors affecting visual resolution. J.Physiol, 181, 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, & Robson JG (1968). Application of Fourier analysis to the visibility of gratings. J.Physiol, 197(3), 551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MCW, Harrison EM, & Simonet P (1990). Psychophysical measurement of the blur on the retina due to optical aberrations of the eye. Vision Res, 30, 1587–1602. [DOI] [PubMed] [Google Scholar]
- Carroll J, Neitz M, Hofer H, Neitz J, & Williams DR (2004). Functional photoreceptor loss revealed with adaptive optics: an alternate cause of color blindness. Proc.Natl.Acad.Sci.U.S.A, 101(22), 8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN, & Jennings JAM (1976). The optical quality of the monochromatic retinal image as a function of focus. British Journal of Physiolical Optics, 31(3), 119–134. [PubMed] [Google Scholar]
- Chen L, Artal P, Gutierrez D, & Williams DR (2007). Neural compensation for the best aberration correction. J.Vis., 7(10), 9. [DOI] [PubMed] [Google Scholar]
- Chen L, Kruger PB, Hofer H, Singer B, & Williams DR (2006). Accommodation with higher-order monochromatic aberrations corrected with adaptive optics. J.Opt.Soc.Am.A, 23(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Cheng X, Bradley A, Hong X, & Thibos L (2003). Relationship between refractive error and monochromatic aberrations of the eye. Optometry and Vision Science, 80(1), 43–49. [DOI] [PubMed] [Google Scholar]
- Cheng X, Bradley A, & Thibos LN (2004). Predicting subjective judgment of best focus with objective image quality metrics. J.Vis., 4(4), 310–321. [DOI] [PubMed] [Google Scholar]
- Chin SS, Hampson KM, & Mallen EA (2009a). Effect of correction of ocular aberration dynamics on the accommodation response to a sinusoidally moving stimulus. Opt.Lett, 34(21), 3274–3276. [DOI] [PubMed] [Google Scholar]
- Chin SS, Hampson KM, & Mallen EA (2009b). Role of ocular aberrations in dynamic accommodation control. Clin.Exp.Optom., 92(3), 227–237. [DOI] [PubMed] [Google Scholar]
- Dalimier E, & Dainty C (2008). Use of a customized vision model to analyze the effects of higher-order ocular aberrations and neural filtering on contrast threshold performance. J.Opt.Soc.Am.A, 25(8), 2078–2087. [DOI] [PubMed] [Google Scholar]
- Dalimier E, Dainty C, & Barbur JL (2008). Effects of higher-order aberrations on contrast acuity as a function of light level. Journal of Modern Optics, 55(4–5), 791–803. [Google Scholar]
- Doble N, & Miller DT (2006). Wavefront Correctors for Vision Science In Porter J, Queener H, Lin J, Thorn K, & Awwal A (Eds.), Adaptive Optics for Vision Science. (pp. 83–117). Hoboken: Wiley-Interscience. [Google Scholar]
- Dreher AW, Bille JF, & Weinreb RN (1989). Active optical depth resolution improvement of the laser tomographic scanner. Appl.Optics, 28, 804–808. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, & Artal P (2005). Study on the effects of monochromatic aberrations in the accommodation response by using adaptive optics. J.Opt.Soc.Am.A, 22(9), 1732–1738. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Iglesias I, & Artal P (2001). Closed-loop adaptive optics in the human eye. Opt.Lett, 26(10), 746–748. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Prieto PM, & Artal P (2009). Binocular adaptive optics visual simulator. Opt.Lett, 34(17), 2628–2630. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Prieto PM, & Artal P (2010). Adaptive optics binocular visual simulator to study stereopsis in the presence of aberrations. J.Opt.Soc.Am.A, 27(11), A48–A55 [DOI] [PubMed] [Google Scholar]
- Gambra E, Sawides L, Dorronsoro C, & Marcos S (2009). Accommodative lag and fluctuations when optical aberrations are manipulated. J.Vis., 9(6), 4–15. [DOI] [PubMed] [Google Scholar]
- Geisler WS, & Banks MS (2010). Visual Performance In Enoch JM & Lakshminarayanan V (Eds.), Handbook of Optics, Volume III: Vision and Vision Optics. New York: McGraw Hill. [Google Scholar]
- Gomez-Vieyra A, Dubra A, Malacara-Hernandez D, & Williams DR (2009). First-order design of off-axis reflective ophthalmic adaptive optics systems using afocal telescopes. Opt.Express, 17(21), 18906–18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao A, & Williams DR (2003). A method to predict refractive errors from wave aberration data. Optometry and Vision Science, 80(1), 36–42. [DOI] [PubMed] [Google Scholar]
- Guo H, Atchison DA, & Birt BJ (2008). Changes in through-focus spatial visual performance with adaptive optics correction of monochromatic aberrations. Vision Res, 48(17), 1804–1811. [DOI] [PubMed] [Google Scholar]
- Hampson KM (2008). Adaptive optics and vision. Journal of Modern Optics, 55(21), 3425–3467. [Google Scholar]
- Hermann B, Fernandez EJ, Unterhuber A, Sattmann H, Fercher AF, Drexler W, Prieto PM, & Artal P (2004). Adaptive-optics ultrahigh-resolution optical coherence tomography. Opt.Lett, 29(18), 2142–2144. [DOI] [PubMed] [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, & Williams DR (2005). Organization of the human trichromatic cone mosaic. J.Neurosci, 25(42), 9669–9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, Singer B, & Williams DR (2005). Different sensations from cones with the same pigment. J.Vis., 5(5), 444–454. [DOI] [PubMed] [Google Scholar]
- Howland HC, & Howland B (1977). A subjective method for the measurement of monochromatic aberrations of the eye. J.Opt.Soc.Am, 67, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Ivanoff A (1956). About the spherical aberration of the eye. J.Opt.Soc.Am, 46, 901–903. [DOI] [PubMed] [Google Scholar]
- Jackson E (1888). Symmetrical aberration of the eye. Trans.Amer.Ophth.Soc., 5, 141–149. [PMC free article] [PubMed] [Google Scholar]
- Jenkins TCA (1963). Aberrations of the eye and their effects on vision: Part I. British Journal of Physiolical Optics, 20, 59–91. [PubMed] [Google Scholar]
- Koomen M, Scolnik R, & Tousey R (1956). Spherical aberration of the eye and the choice of axis. J.Opt.Soc.Am, 46, 903–904. [DOI] [PubMed] [Google Scholar]
- Koomen M, Tousey R, & Scolnik R (1949). The spherical aberration of the human eye. Journal of the American Optometric Association, 39, 370–376. [DOI] [PubMed] [Google Scholar]
- Krauskopf J (1964). Color appearance of small stimuli and the spatial distribution of color receptors. J.Opt.Soc.Am, 54, 1171 [Google Scholar]
- Legras R, Benard Y, & Rouger H (2010). Through-focus visual performance measurements and predictions with multifocal contact lenses. Vision Res, 50(12), 1185–1193. [DOI] [PubMed] [Google Scholar]
- Levi DM (2005). Perceptual learning in adults with amblyopia: a reevaluation of critical periods in human vision. Dev.Psychobiol., 46(3), 222–232. [DOI] [PubMed] [Google Scholar]
- Li S, Xiong Y, Li J, Wang N, Dai Y, Xue L, Zhao H, Jiang W, Zhang Y, & He JC (2009). Effects of monochromatic aberration on visual acuity using adaptive optics. Optometry and Vision Science, 86(7), 868–874. [DOI] [PubMed] [Google Scholar]
- Liang J, Grimm B, Goelz S, & Bille JF (1994). Objective measurement of wave aberrations of the human eye with use of a Hartmann-Shack wave-front sensor. J.Opt.Soc.Am.A, 11, 1949–1957. [DOI] [PubMed] [Google Scholar]
- Liang J, & Westheimer G (1993). Method for measuring visual resolution at the retinal level. J.Opt.Soc.Am.A, 10(8), 1691–1696. [DOI] [PubMed] [Google Scholar]
- Liang J, & Williams DR (1997). Aberrations and retinal image quality of the normal human eye. J.Opt.Soc.Am.A, 14(11), 2873–2883. [DOI] [PubMed] [Google Scholar]
- Liang J, Williams DR, & Miller D (1997). Supernormal vision and high-resolution retinal imaging through adaptive optics. J.Opt.Soc.Am.A, 14(11), 2884–2892. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil N, & Artal P (1997). Comparison of double-pass estimates of the retinal-image quality obtained with green and near-infrared light. J.Opt.Soc.Am.A, 14(5), 961–971. [DOI] [PubMed] [Google Scholar]
- Lundstrom L, Manzanera S, Prieto PM, Ayala DB, Gorceix N, Gustafsson J, Unsbo P, & Artal P (2007). Effect of optical correction and remaining aberrations on peripheral resolution acuity in the human eye. Opt.Express, 15(20), 12654–12661. [DOI] [PubMed] [Google Scholar]
- Makous W, Carroll J, Wolfing JI, Lin J, Christie N, & Williams DR (2006). Retinal microscotomas revealed with adaptive-optics microflashes. Invest Ophthalmol.Vis.Sci, 47(9), 4160–4167. [DOI] [PubMed] [Google Scholar]
- Manzanera S, Prieto PM, Ayala DB, Lindacher JM, & Artal P (2007). Liquid crystal Adaptive Optics Visual Simulator: Application to testing and design of ophthalmic optical elements. Opt.Express, 15(24), 16177–16188. [DOI] [PubMed] [Google Scholar]
- Marcos S, Sawides L, Gambra E, & Dorronsoro C (2008). Influence of adaptive-optics ocular aberration correction on visual acuity at different luminances and contrast polarities. J.Vis., 8(13), 1–12. [DOI] [PubMed] [Google Scholar]
- Marsack JD, Thibos LN, & Applegate RA (2004). Metrics of optical quality derived from wave aberrations predict visual performance. J.Vis., 4(4), 322–328. [DOI] [PubMed] [Google Scholar]
- Martin JA, & Roorda A (2003). Predicting and assessing visual performance with multizone bifocal contact lenses. Optometry and Vision Science, 80(12), 812–819. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Marcos S, Prieto PM, & Burns SA (2002). Imperfect optics may be the eye's defense against chromatic blur. Nature, 417(6885), 174–176. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Lankheet MJ, Lennie P, & Williams DR (2000). Fine structure of parvocellular receptive fields in the primate fovea revealed by laser interferometry. J.Neurosci, 20(5), 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, & Roorda A (2009). Adaptive Optics in Retinal Microscopy and Vision In Bass M (Ed.), Handbook of Optics Vol III Rochester: Optical Society of America. [Google Scholar]
- Mon-Williams M, Tresilian JR, Strang NC, Kochhar P, & Wann JP (1998). Improving vision: neural compensation for optical defocus. Proc.Biol.Sci, 265(1390), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peli E, Goldstein RB, Young GM, Trempe CL, & Buzney SM (1991). Image enhancement for the visually impaired. Simulations and experimental results. Invest Ophthalmol.Vis.Sci, 32(8), 2337–2350. [PubMed] [Google Scholar]
- Perez GM, Manzanera S, & Artal P (2009). Impact of scattering and spherical aberration in contrast sensitivity. J.Vis., 9(3), 19. [DOI] [PubMed] [Google Scholar]
- Pesudovs K (2005). Involvement of neural adaptation in the recovery of vision after laser refractive surgery. J.Refract.Surg, 21(2), 144–147. [DOI] [PubMed] [Google Scholar]
- Pi HT (1925). Total peripheral aberration of the eye. Trans.Ophth.Soc.U.K., 45, 393–399. [Google Scholar]
- Piers PA, Fernandez EJ, Manzanera S, Norrby S, & Artal P (2004). Adaptive optics simulation of intraocular lenses with modified spherical aberration. Invest Ophthalmol.Vis.Sci, 45(12), 4601–4610. [DOI] [PubMed] [Google Scholar]
- Piers PA, Manzanera S, Prieto PM, Gorceix N, & Artal P (2007). Use of adaptive optics to determine the optimal ocular spherical aberration. J.Cataract Refract.Surg., 33(10), 1721–1726. [DOI] [PubMed] [Google Scholar]
- Poonja S, Patel S, Henry L, & Roorda A (2005). Dynamic visual stimulus presentation in an adaptive optics scanning laser ophthalmoscope. J.Refract.Surg., 21(5), S575–S580 [DOI] [PubMed] [Google Scholar]
- Porter J (2006). Adaptive Optics for Vision Science. Hoboken, NJ: Wiley-Interscience. [Google Scholar]
- Putnam NM, Hofer H, Doble N, Chen L, Carroll J, & Williams DR (2005). The locus of fixation and the foveal cone mosaic. J.Vis., 5(7), 632–639. [DOI] [PubMed] [Google Scholar]
- Rocha KM, Vabre L, Chateau N, & Krueger RR (2010). Enhanced visual acuity and image perception following correction of highly aberrated eyes using an adaptive optics visual simulator. J.Refract.Surg., 26(1), 52–56. [DOI] [PubMed] [Google Scholar]
- Romero-Rangel T, Stavrou P, Cotter J, Rosenthal P, Baltatzis S, & Foster CS (2000). Gas-permeable scleral contact lens therapy in ocular surface disease. American Journal of Ophthalmology, 130(1), 25–32. [DOI] [PubMed] [Google Scholar]
- Roorda A, Romero-Borja F, Donnelly WJ, Queener H, Hebert TJ, & Campbell MCW (2002). Adaptive optics scanning laser ophthalmoscopy. Opt.Express, 10(9), 405–412. [DOI] [PubMed] [Google Scholar]
- Roorda A, & Williams DR (1999). The arrangement of the three cone classes in the living human eye. Nature, 397, 520–522. [DOI] [PubMed] [Google Scholar]
- Rossi EA, & Roorda A (2010a). Is visual resolution after adaptive optics correction susceptible to perceptual learning? J.Vis., 10(12), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, & Roorda A (2010b). The relationship between visual resolution and cone spacing in the human fovea. Nat.Neurosci, 13(2), 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, Weiser P, Tarrant J, & Roorda A (2007). Visual performance in emmetropia and low myopia after correction of high-order aberrations. J.Vis., 7(8), 1–14. [DOI] [PubMed] [Google Scholar]
- Sabesan R, & Yoon G (2009). Visual performance after correcting higher order aberrations in keratoconic eyes. J.Vis., 9(5), 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesan R, & Yoon G (2010). Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol.Vis.Sci, 51(7), 3835–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, Gambra E, Pascual D, Dorronsoro C, & Marcos S (2010). Visual performance with real-life tasks under adaptive-optics ocular aberration correction. J.Vis., 10(5), 19. [DOI] [PubMed] [Google Scholar]
- Schor CM, Lott LA, Pope D, & Graham AD (1999). Saccades reduce latency and increase velocity of ocular accommodation. Vision Res, 39(22), 3769–3795. [DOI] [PubMed] [Google Scholar]
- Seyeddain O, Riha W, Hohensinn M, Nix G, Dexl AK, & Grabner G (2010). Refractive surgical correction of presbyopia with the AcuFocus small aperture corneal inlay: two-year follow-up. J.Refract.Surg., 26(10), 707–715. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, & Roorda A (2009). Resolving single cone inputs to visual receptive fields. Nat.Neurosci, 12(8), 967–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov MS (1962). Measurement of the wave aberration of the human eye. Biophysics, 6, 776–795. [PubMed] [Google Scholar]
- Stevenson SB, & Roorda A (2005). Correcting for miniature eye movements in high resolution scanning laser ophthalmoscopy In Manns F, Soderberg P, & Ho A (Eds.), Ophthalmic Technologies XI. (pp. 145–151). Bellingham, WA: SPIE. [Google Scholar]
- Stevenson SB, Roorda A, & Kumar G Eye tracking with the adaptive optics scanning laser ophthalmoscope In Spencer SN (Ed.), Proceedings of the 2010 Symposium on Eye-Tracking Research & Applications New York, NY, USA: Association for Computed Machinery. [Google Scholar]
- Stine GH (1930). Variations in refraction of the visual and extravisual pupillary zones. American Journal of Ophthalmology, 13, 101–112. [Google Scholar]
- Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Lujan BJ, Tao W, Porco TC, Roorda A, & Duncan JL (2011). Longitudinal Study of Cone Photoreceptors during Retinal Degeneration and in Response to Ciliary Neurotrophic Factor Treatment. Invest Ophthalmol.Vis.Sci, 52(5), 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos LN, Applegate RA, Schwiegerling J, Webb RH, & VSIA Standards Taskforce Members. Standards for reporting the optical aberrations of eyes In Lakshminarayanan V (Ed.), Vision Science and its Applications Trends in Optics and Photonics: 35 Optical Society of America. [Google Scholar]
- Tscherning M (1924). Physiologic Optics. (4 ed.). Philadelphia: Keystone Publishing Co. [Google Scholar]
- Vogel CR, Arathorn DW, Roorda A, & Parker A (2006). Retinal motion estimation and image dewarping in adaptive optics scanning laser ophthalmoscopy. Opt.Express, 14(2), 487–497. [DOI] [PubMed] [Google Scholar]
- Watson AB, & Ahumada AJ Jr. (2008). Predicting visual acuity from wavefront aberrations. J.Vis., 8(4), 17–19. [DOI] [PubMed] [Google Scholar]
- Werner JS, Elliott SL, Choi SS, & Doble N (2009). Spherical aberration yielding optimum visual performance: evaluation of intraocular lenses using adaptive optics simulation. J.Cataract Refract.Surg., 35(7), 1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G (1960). Modulation thresholds for sinusoidal light distributions on the retina. J.Physiol, 152, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (1985). Aliasing in human foveal vision. Vision Res, 25, 195–205. [DOI] [PubMed] [Google Scholar]
- Williams DR, MacLeod DIA, & Hayhoe MM (1981). Punctate sensitivity of the blue-sensitive mechanism. Vision Res, 21, 1357–1375. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Decker KE, & Roorda A (2002). Monochromatic aberrations provide an odd-error cue to focus direction. J.Opt.Soc.Am.A, 19(5), 833–839. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Kluender KR, Levi DM, Bartoshuk LM, Herz RS, Klatzky RL, & Lederman SJ (2006). Sensation and Perception. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- Yang Q, Arathorn DW, Tiruveedhula P, Vogel CR, & Roorda A (2010). Design of an integrated hardware interface for AOSLO image capture and cone-targeted stimulus delivery. Opt.Express, 18(17), 17841–17858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon GY, & Williams DR (2002). Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J.Opt.Soc.Am.A, 19(2), 266–275. [DOI] [PubMed] [Google Scholar]
- Young T (1801). On the mechanism of the eye. Philosophical Transactions of the Royal Society of London, 91, 23–88. [Google Scholar]