Abstract
Mental disorders are the predominant chronic diseases of youth, with substantial lifespan morbidity and mortality. A wealth of evidence demonstrates that the neurodevelopmental roots of common mental health problems are present in early childhood. Unfortunately, this has not been translated to systematic strategies for improving population level mental health at this most malleable neurodevelopmental period. We lay out a translational Mental Health, Earlier roadmap as a key future direction for prevention of mental disorder. This paradigm shift aims to reduce population attributable risk of mental disorder emanating from early life, by preventing, attenuating or delaying onset/course of chronic psychopathology via the promotion of self-regulation in early childhood within large scale healthcare delivery systems. The Earlier Pillar rests on a “science of when to worry” that (a) optimizes clinical assessment methods for characterizing probabilistic clinical risk beginning in infancy via deliberate incorporation of neurodevelopmental heterogeneity; and (b) universal primary care based screening targeting patterns of dysregulated irritability as a robust transdiagnostic marker of vulnerability to lifespan mental health problems. The core of the Healthier Pillar is provision of low intensity selective intervention promoting self-regulation for young children with developmentally atypical patterns of irritability within an implementation science framework in pediatric primary care to ensure highest population impact and sustainability. These Mental Health, Earlier strategies hold much promise for transforming clinical outlooks and ensuring young children’s mental health and wellbeing in a manner that reverberates throughout the lifespan.
Mental disorders are the predominant chronic diseases of youth (7 – 29% of the population), accounting for greater lifespan morbidity and mortality than physical diseases (Insel, 2009; Merikangas et al., 2010; Pennap et al., 2018). Mental health and disorder are neurodevelopmental in nature, with the seeds of vulnerability and resilience planted in early life (Mittal & Wakschlag, 2017; Pine & Fox, 2015). Thus, the unfolding clinical sequence of mental disorder flows from vulnerability to prodromal symptoms to frank disorder with clinically recognizable symptoms.
Prevention offers a compelling opportunity to reduce the public health burden of mental disorder by mitigating risks and promoting mental health before disorders are present (Casey, Oliveri, & Insel, 2014; Uddin & Karlsgodt, 2018). For the common preventable mental disorders of childhood, this points to prevention in early childhood. Neurodevelopmental risk factors are evident as early as infancy, and common clinical syndromes are identifiable by preschool age (Dougherty et al., 2015). Promotion of early childhood mental health, particularly reductions in dysregulation may have a profound impact on overall health, disease, and human capital across the lifespan. Our framework centers on irritability, a robust indicator of dysregulation and transdiagnostic mental health risk marker, identifiable as early as the first year of life. For example, preventing early childhood dysregulation results in lifespan improvements in health, and costs savings of 13% per year in service utilization (Knudsen, Heckman, Cameron, & Shonkoff, 2006).
Considering these disparate bodies of work together underscores the tremendous promise for ameliorating the significant population attributable risk for common, preventable emotional and behavioral disorders of childhood (e.g., depression, disruptive behavior) emanating from early dysregulation during the period of greatest neurodevelopmental plasticity. Regrettably, this science base has not translated into concomitant investments in comprehensive, population-level early-life prevention of mental disorders and its impact on healthcare cost containment (Boat, 2015; Shonkoff, 2003). The key objective of this paper is to set an agenda for closing this science-to-application gap and driving transformative change.
We propose a Mental Health, Earlier roadmap as a central future direction for mental health prevention by harnessing transdisciplinary strengths from developmental, clinical, population, and implementation science to catalyze a long overdue paradigm shift in the mental health field. Pillar I: “Earlier” calls for identification of mental health risk as soon as possible in early childhood using reliable clinical risk thresholds, which capture those children at increased risk for mental health problems in childhood and beyond. This rests on a “science of when to worry,” grounded in an integrative and theoretically and empirically derived developmental specification framework (Wakschlag, Perlman, et al., 2018; Wakschlag, Tolan, & Leventhal, 2010). Earlier requires a shift from a categorically-based diagnostic process to one that is transdiagnostic, dimensional, probabilistic, and grounded in normal-versus-abnormal differentiation of neurodevelopment. Pillar II: “Healthier” draws on the foundation of the “Earlier” pillar to drive a shift from the current reactive, treatment-focused, clinic-based approach to a pre-emptive and population-based early-life mental health selective prevention program. To actualize this, we propose to capitalize on the centrality and effectiveness of pediatric primary care medical homes (PCMH) to enact a population-based strategy for mental health promotion, drawing on advances in health information technology, prevention science, and implementation science (Medical Home Initiatives Advisory Committee, 2002). These strategic priorities will drive actualization of the pivot needed to achieve Mental Health, Earlier. We provide the empirical evidence undergirding this approach and specify the critical steps towards realization.
Earlier: Integrating Developmental and Clinical Science towards Earlier Identification
Ironically, one of the most significant obstacles to scalable clinical and population health applications in early life has been human development itself. The complexities of development continue to be viewed, scientifically and clinically, as a major barrier to early mental health prevention. These “challenging” developmental features include the rapid pace of developmental changes and skill acquisition (measured in weeks and months, not years), and extensive normative variation (including transient self-correcting perturbations). These challenges are further amplified by the non-developmental nature of current nosologic systems. Traditional mental health syndromes are conceptualized as downward extensions of adult or adolescent phenomenology. Further, there is substantial overlap of normative misbehavior and many clinical symptoms (Wakschlag, Leventhal, & Thomas, 2007). The predominant response to such challenges is clinical approaches that erase developmental “noise” and favor entrenched “they’ll grow out of it” myths (Dirks, De Los Reyes, Briggs-Gowan, Cella, & Wakschlag, 2012; Luby, 2012).
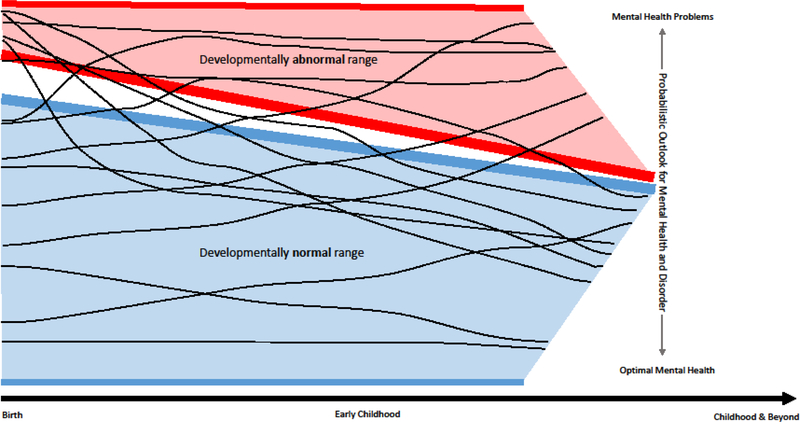
Central to the Earlier pillar is the notion of embracing, rather than erasing, developmental variation to achieve earlier, accurate identification of mental health risk. Figure 1 provides a heuristic illustration of typical and atypical patterns of irritability across early childhood in relation to probabilistic risk of mental health or disorder. Its fundamental premise is that children’s achievement of self-regulatory competence versus enduring dysregulation is a key set-point for resilience or vulnerability to mental health problems across the lifespan. This is because dynamic biologic, social and ecological influences profoundly shape these patterns well beyond early childhood. Thus, Earlier is not a deterministic framework. Rather, as Shonkoff and colleagues have posited in their landmark work, self/dys-regulation in early childhood sets a “sturdy” or “fragile” foundation for ensuing learning, experience, and relationships (Phillips & Shonkoff, 2000). While not all irritable young children develop mental health problems, enduring patterns of early irritability exponentially increase the risk that they will. Nor does Earlier imply that the improved developmental precision of a science of when to worry will identify all individuals who will have mental health problems by the time they are five years old. Rather, it leverages a strong science base to identify the substantial group of children who have or at are high risk for impairing mental health problems due to difficulty managing emotions and behavior and concomitant impact on health and environmental experience to alter mental health trajectories at the population level.
Figure 1.
Earlier Pillar: Harnessing development variation in irritability patterns to forecast lifespan clinical risk1
1 Trajectories shaped by dynamic biologic, social, and ecological influences
Embracing Development Within and Across Individuals and Time
The Mental Health, Earlier roadmap proposes that future research must rest on a developmental specification approach, pinpointing features that enhance normal/abnormal distinctions (Wakschlag, Perlman, et al., 2018). Developmental specification encompasses rigorous accounting for aspects of individual differences and developmental patterning that differentiate normative variation from atypical manifestations. For example, many features of the brain reach essentially adult-like levels very early in childhood, e.g., folding patterns (gyrification), and cortical thickness which reaches 97% of adult levels by around age 2 (Grayson & Fair, 2017; Lyall et al., 2015). While challenging to account for this rapid pace, the crux of this science of when to worry is built by empirically defining atypicality as deviation from age-graded normative patterns at both brain and behavioral levels (Wakschlag, Perlman, et al., 2018). To date, developmental specification has largely been applied to young children’s disruptive behavior and is more mature for behavioral rather than brain-based markers, but this framework is equally relevant for other common emotional and behavioral syndromes (Dougherty et al., 2015).
Distinguishing Normative (Mis)Behavior from Clinical Markers
Actualizing developmentally-generated clinical thresholds requires a reorientation from traditional symptom-based models (present/absent) to more fine-grained distinction of features of behavior that mark atypicality across a spectrum within the developmental context of early childhood (Kaat et al., 2018; Wakschlag, Leventhal, et al., 2007). These key features of behavior that are most informative for the normal:abnormal distinction in early childhood are: (a) quality; (b) developmentally expectable and matched to context; (c) dimensional and narrow-band; and (d) regularity of occurrence.
Quality.
Quality of behavior reflects the extent to which it is modulated and responsive to environmental intervention, and distinguishes typical from atypical manifestations (Wakschlag et al., 2008). For example, high and persistent irritability (i.e., tendency to respond to frustration with intense, easily elicited, and dysregulated anger and/or chronic negative mood), is perhaps the best developmental and transdiagnostic indicator of a host of psychopathologies (Biedzio & Wakschlag, in press; Vidal-Ribas, Brotman, Valdivieso, Leibenluft, & Stringaris, 2016). Yet one of the foremost expressions of irritability in children is temper tantrums, which occur regularly in the vast majority of young children, are a core feature of several DSM disorders (Wakschlag et al., 2012). Dysregulated tantrum features include destructiveness, resistance to efforts to help calm, and duration lasting more than five minutes (Belden, Thompson, & Luby, 2008; Wakschlag et al., 2012). Although tantrums are endemic to early childhood, dysregulated tantrums occur in fewer than 3% of preschoolers making them excellent, easily detectable, risk markers. This pattern has been demonstrated and replicated in two independent samples of more than 3,000 sociodemographically diverse preschoolers (Wakschlag, Perlman, et al., 2018).
Developmentally expectable and matched to context.
Although traditional nosologic systems are typically context-agnostic (Dirks et al., 2012), the extent to which behavior is developmentally expectable and matched to context is clinically differentiating for young children (Buss, 2011; Wakschlag, Briggs-Gowan, et al., 2007). For example, dysregulated fear exhibited in non-threatening versus threatening contexts predicts which preschoolers will develop anxiety symptoms (Buss, 2011). Further, preschoolers who do not display contextually-matched regulation of behavior are at heightened risk of clinical problems (Petitclerc et al., 2015).
Dimensional, narrow-band approaches.
Dimensional, narrow-band approaches are a cornerstone of characterizing developmental variation. These assume that clinical patterns cannot be defined by a single extreme threshold, but rather are expressed along a continuum ranging from normative variation to increasing probabilistic risk. Continuous approaches have long been a mainstay within the developmental psychopathology approach (Achenbach, 1997). However, these have typically relied on counts of problem behaviors rather than characterization along an ordered spectrum from normative to problematic in a developmentally-informed manner. We developed the Multidimensional Assessment Profile for Disruptive Behavior (MAP-DB) dimensional approach to assess the normal-to-abnormal spectrum of preschool behavior via parent report, with atypical behaviors defined as deviation from normative variation within a developmental period (Nichols et al., 2014; Wakschlag et al., 2014). Severity of each item is determined psychometrically using item response theory. This scale has psychometrically characterized dimensional spectra for irritability (“Temper Loss”), noncompliance, aggression, callous behavior (“Low Concern for Others”), and punishment insensitivity. These spectra differentiate those behaviors that occur in the majority of young children and are severe by virtue of frequent occurrence from those that rarely occur and mark dysregulation at lower frequencies.
A burgeoning body of work demonstrates the methodologic, clinical, and mechanistic utility of this dimensional approach (Briggs-Gowan et al., 2014; Grabell et al., 2017; Kaat et al., 2018). For example, we have shown that the fear processing deficits widely established as a mechanism of callousness and psychopathy are evident and specific as early as preschool-age (White et al., 2016). Using the MAP-DB Temper Loss scale, we have also demonstrated that probabilistic risk for clinical disorder occurs at levels of irritability falling within the normal range on traditional checklist ratings (Wakschlag et al., 2015). This same irritability spectrum differentiates variations in recruitment of prefrontal resources during frustration for impaired versus non-impaired irritable children (Grabell et al., 2017). Dimensional approaches are particularly well suited for capturing the rapid developmental change of early childhood because they capture a fuller spectrum of behavioral variation, not merely behaviors at the extremes.
Regularity of occurrence.
Regularity of occurrence has also proven clinically informative in young children. Traditional symptom thresholds have been defined via subjective frequency (e.g., “often loses temper”). These have also been “one size fits all,” treating all behaviors identically without regard to developmental stage. As a result, clinicians assessing young children have long been vexed by the question, “How often is too often?” Such reliance on subjective judgement about commonly occurring behaviors creates a high risk of over-identification. The MAP-DB was designed to address this issue via an objective frequency scale (from “never in past month” to “many times per day”) in order to psychometrically derive frequency thresholds demarcating abnormality in a manner sensitive to the extensive developmental variation of this period. Data from two large community samples of diverse preschoolers indicate that misbehavior is common (i.e., most young children do it), but not predominant (Wakschlag, Perlman, et al., 2018). Clinical risk varies based on the type of behavior although both high frequency normative misbehaviors and pathognomonic indicators of dysregulation are key to sensitive and specific early identification(Wiggins, Briggs-Gowan, et al., 2018). For example, normative misbehaviors only become abnormal if they occur very frequently (e.g., daily tantrums). In contrast, dysregulated tantrums (e.g., destructive) are pathognomonic, occur much less commonly and are abnormal at lower frequencies. Rigorous psychometric analysis is necessary to specify thresholds of atypicality across early childhood (Biedzio & Wakschlag, in press). The absence of developmentally-tuned thresholds impedes identification at the earliest phase of the clinical sequence.
Development matters.
A fundamental principle of Mental Health, Earlier is that earlier identification requires a move away from static assessments that treat development as random noise (e.g., the DSM exclusion of children under six for its key irritability related syndrome, Disruptive Mood Dysregulation Disorder). We recognize the challenge of doing so as methods for detecting within-person change often require a very large change to be greater than measurement error, or require multiple assessment occasions (de Vet et al., 2006; Lin et al., 2016). Natural developmental processes compound this problem, as change is inherent. Further, any developmentally varying threshold for diagnostic status or intervention effect requires a difference in the change over time distinguishable from expectable variation. Nonetheless, the state-of–the-art science enables such differentiation once development is deliberately taken into account.
We posit that accounting for developmental change over time ( both within and across individuals) will substantially improve reliability of risk estimation. A repeated measures approach is critical to ensuring sufficiently stable patterns for probablistic risk estimates. When optimized, this type of longitudinal approach will provide empirical parameters (e.g., how many time points; at what ages; probability estimates that a child will develop impairing problems). Multiple repeated assessments allow for evaluation of two types of stability and change over time; (a) average level of behavior (i.e., an intercept), and/or (b) individual trajectory (i.e., slope), which may have differential predictive utility (Wakschlag et al., 2015).
While inter-individual differences are fairly stable even in very young children, as they reflect relative tendencies, intra-individual instability is developmentally normative (Bornstein, 2014). This is particularly true for problem behaviors; changes in expression and frequency of occurrence manifest over relatively short time frames. For example, the majority of 17-month-olds who frequently exhibit problem behavior, no longer do so a year later (change in the intercept), and vice versa (Baillargeon et al., 2007). Using MAP-DB dimensional characterization, we have also shown that about 33% of preschool children vary substantially in their irritability profiles over time, moving across typical and atypical levels (Wakschlag et al., 2015). Accounting for such intrapersonal longitudinal variation improves clinical prediction (Wakschlag et al., 2015; Whalen et al., 2016). For example, less than 30% of behaviorally inhibited toddlers remain so across early childhood, with stability over time increasing probabilistic risk of anxiety disorder (Henderson, Pine, & Fox, 2015). Requisite frequency of repeated assessment should be somewhat regular (e.g., during well-child visits), with interim assessments conditioned on occurrence of life events (e.g., birth of a sibling) and milestone acquisition (e.g., capacity to say “no”) that may cause transient development perturbations (Biedzio & Wakschlag, in press).
Transdiagnostic Risk Identification
The Mental Health, Earlier roadmap posits the need for transdiagnostic approaches at the earliest identifiable vulnerability phase of the pediatric clinical sequence. Of note, such vulnerability begins well before birth; mental health prevention that begins during or prior to the prenatal period is a key future direction (Tremblay & Japel, 2003; Wakschlag, Krogh-Jespersen, et al., 2018). Broadly writ, this transdiagnostic approach aims to prevent early markers of dysregulation (i.e., vulnerabilities) from worsening to the point of persistent and pervasive patterns that impede adaptive functioning (i.e., syndromes). This is consistent with National Academy of Medicine (NAM) recommendations for prevention: emphasizing risk reduction and delay of onset, which is associated with increased future severity and public health burden (Brown & Beardslee, 2016). NAM’s approach has reduced population attributable risk for physical disease, but uptake has been slower for mental disorders (Brown & Beardslee, 2016).
Traditional diagnostically oriented approaches (e.g., the DSM) have been widely validated for common mood and disruptive syndromes in preschoolers. Indeed, of the approximately 20% of children with mental health problems, a majority of these have roots in early childhood (Dougherty et al., 2015; Pennap et al., 2018). Most children exhibiting mental health problems by preschool age have had enduring problems with dysregulation, suggesting that negative cascades impacting child and family functioning are already entrenched (Tremblay & Japel, 2003). However, there has been reluctance to apply a clinical lens at younger ages, impeding validation of preschool psychopathology syndromes for decades and now constraining an earlier emphasis on mental health risk detection in infancy (Task Force on Research Diagnostic Criteria, 2003; Wakschlag, Leventhal, et al., 2007). This is most unfortunate, given evidence that dysregulation as early as the first year of life (e.g., excessive irritability) is linked to increased risk of disruptive behavior and biomarkers for psychopathology risk (Hemmi, Wolke, & Schneider, 2011; Hyde, O’Callaghan, Bor, Williams, & Najman, 2012). It is also at odds with neurodevelopmental understandings of the dynamic unfolding of psychopathological patterns, which are more fluid and less discrete than the bounds of current nosologies and emerge far earlier than traditional symptom-based approaches can detect (McGorry & Nelson, 2016).
There are cogent arguments for adopting the Mental Health, Earlier risk-oriented, transdiagnostic approach. First, in very young children, mental health risk is best captured by well-defined, cross-cutting problems in self-regulation rather than narrowly defined syndromes. Second, it is not feasible to do large-scale screening targeting risk factors for multiple distinct syndromes. Early dysregulation, in contrast, is a salient target that is a prominent parental concern (Brown, Raglin Bignall, & Ammerman, 2018). Targeting shared elements implicated in common pediatric problems—rather than discrete mechanisms specific to a particular syndrome—will have highest population-level impact (Smith, Montaño, Dishion, Shaw, & Wilson, 2015; Walkup, Mathews, & Green, 2017).
Irritability as candidate marker.
Mental Health, Earlier posits that irritability (e.g., dysregulated tantrums) is the optimal early life transdiagnostic marker for large-scale screening of risk for common emotional and behavioral syndromes. Deficits in self-regulation forecast mental health risk. Self-regulation is a multi-faceted construct including executive function, effortful and self-control, and emotional and behavioral regulation, which is not feasibly reducible to a brief pediatric screening assessment (Murray, Rosanbalm, Christopoulous, & Hamouidi, 2015; Raver, 2013). In contract, irritability is one of the most prominent and easily observable aspects of self-regulatory problems that comprise mental health risk (Heckman, Pinto, & Savelyev, 2013; Moffitt, Poulton, & Caspi, 2013). It has robust transdiagnostic predictive utility for common disruptive and mood problems (e.g., Oppositional Defiant Disorder, Attention Deficit/Hyperactivity Disorder, depression, and anxiety) across the lifespan (Vidal-Ribas et al., 2016). Finally, its salient features (e.g., outbursts, moodiness) encompass normative misbehaviors of early childhood that are easily identified with well-developed measurement methods, particularly its discrete behavioral expression (i.e., tantrums) (Wakschlag et al., 2012).
As highlighted above, applying modern measurement science methods with the MAP-DB, we have demonstrated that irritability can be reliably measured along a normal-to-abnormal spectrum as early as 12 months of age, using survey methods in a manner that is informative for clinical and mechanistic identification (Wakschlag, Perlman, et al., 2018). Thus, we posit irritability as a reliable risk marker of vulnerability to mental health problems, beginning by 12 months of age. This is younger than the age targeted by standard early childhood prevention and intervention programs (typically at preschool age, with age 2 years as the lower bound) (Comer, Chow, Chan, Cooper, & Wilson, 2013; Perrin, Sheldrick, McMenamy, Henson, & Carter, 2014).
Of note, we expect that even this lower developmental bound (age 12 months) also reflects a methodologic artifact, which is already dissipating as clinically-informed neurodevelopmental frameworks are increasingly applied to the first year of life (Bosl, Tager-Flusberg, & Nelson, 2018; Hay, 2017; Miller, Iosif, Young, Hill, & Ozonoff, 2016). One informative line of research along these lines has been a focus on excessive crying and “fussy” behaviors in early infancy (Rao, Brenner, Schisterman, Vik, & Mills, 2004). For example, excessive and persistent infant crying predicts dysregulatory problems including externalizing problems through age 28 (Bilgin et al., 2018; DeSantis, Coster, Bigsby, & Lester, 2004; Wolke, Rizzo, & Woods, 2002). This work suggests that extending the rigor of the developmental specification framework to infancy (a period when normative variation in crying is at its peak) may be fruitful for identification of clinically salient irritability markers to the first year of life.
Our focus on irritability as the key transdiagnostic risk marker for mental health problems dovetails with broader trends in developmental surveillance in pediatrics (Marks, Page Glascoe, & Macias, 2011). Many pediatric screening instruments for young children include irritability items as part of broader socio-emotional assessment (Briggs-Gowan, Carter, Irwin, Wachtel, & Cicchetti, 2004; Chen, Filgueiras, Squires, & Landeira-Fernandez, 2016). Moreover, the adverse effects of irritability on mental health are compounded by downstream effects on other aspects of health and functioning, including associations to obesity, poorer dental health, decrements in school readiness, and exacerbation of chronic pediatric conditions, making irritability a high impact target (Marino, Cassedy, Drotar, & Wray, 2016; Sheldrick et al., 2013). It is also aligned with neuroscientific evidence. Dispositional versus clinically impairing irritability in young children can be distinguished as an aberration of frustrative non-reward processes, expressed via differential recruitment of prefrontal resources during frustration (Grabell et al., 2017). These findings bolster irritability as an optimal candidate risk marker as corollary neurobiologic vulnerability markers can be identified, and potentially altered, earlier than previously thought.
Healthier. Clinical Application of Developmentally-Specified Earlier Identification
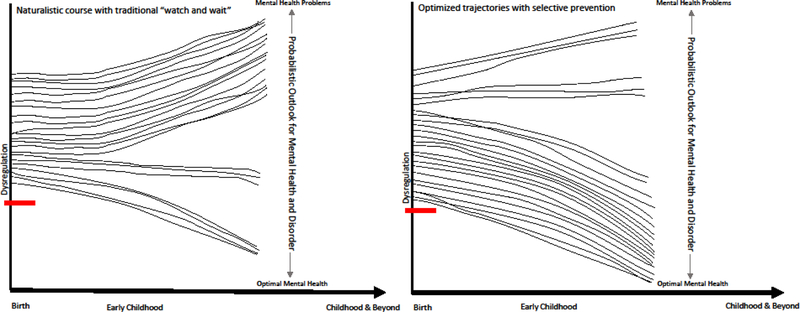
Despite this burgeoning science base for developmentally-based sensitive and specific early identification, implementation of these discoveries into health care delivery systems remains a major gap impeding public health impact (Shonkoff, 2016). Translation of this evidence base to selective prevention targeting transdiagnostic dysregulation to alter lifespan probabilistic clinical risk at the population level is the core of the Healthier Pillar (Figure 2). This has transformative power for significantly reducing the substantial population attributable risk for mental disorder that emanates from early life self-regulatory deficits (Murray et al., 2015; Schaefer et al., 2017). Again we note that this Healthier approach does not suggest that such broad-based selective prevention will effectively eliminate life course risk of mental health problems. Rather, as Figure 2 shows, Healthier aims to alter the set point of risk as early as possible in development for those children already exhibiting clinically-worrisome dysregulation profiles. Improved self-regulation is theorized to have both direct and indirect effects on children’s functioning via their own strengthened capacities and its impact on environmental transactions. For some children and families this may suffice. However, there is some evidence that the use of ongoing “check-up” booster sessions are important for sustained impact (Dishion et al., 2014). Further, those children whose dysregulation is embedded in a high risk ecological context and/or co-occurring with other clinical conditions (e.g., Autism Spectrum Disorder/Inflammatory Bowel Disease) may well require a tiered approach.
Figure 2.
Healthier Pillar: Targeted prevention of early dysregulated irritability moves the dial for young children’s mental health
Reorienting Prevention to the Earliest Phase of the Clinical Sequence
Key components of the Healthier pillar are: (a) leveraging pediatric primary care as a platform for early life prevention with greatest reach; (b) generating dynamic developmentally-based risk models that utilize health information technology and are optimized for integration into clinical care; and (c) providing a scalable roadmap from risk to population-level prevention.
By definition, identifying and intervening with mental health risk at the vulnerability stage must take this process beyond the mental health clinic, since optimally children are identified prior to frank clinical problems. Pediatric primary care medical homes (PCMH) provide a transformative opportunity to advance scalable early identification and prevention of mental health problems at the population-level (Asarnow, Kolko, Miranda, & Kazak, 2017; Shonkoff, 2016). Importantly, embeddedness in routine primary care provides broad population-level access and is the earliest and most frequent point of contact with health professionals given that the vast majority of young children are seen regularly through the first five years of life (Bloom, Jones, & Freeman, 2013). In addition, as PCMH emphasize preventive and continuity care, they provide a non-stigmatized medical home that parents trust (Leslie et al., 2016). Behavioral health issues are also among the most common presenting issue to pediatricians (Boat, 2015).
Developmentally-optimized risk assessment.
We posit that providing clear decision-making parameters for “when to worry” about young children’s mental health requires a dynamic risk assessment process. This must harness advances in health information technology to enable efficient and accurate probabilistic risk estimation within the developmental context. A computer adaptive test (CAT) is one health information technology that could substantially improve screening efficiency and lay the groundwork for optimization of early identification at the population-level (Veldkamp & van der Linden, 2002). CATs maximize precision by only administering those items necessary for clinical determination based on response patterns. CATs provide more efficient testing by maximizing the precision of a score—in this case, probabilistic risk—while minimizing the number of items to reach that precision. There are many methods to accomplish this, but in the context of population health screening, the necessary precision is at the threshold of risk status. That is, it does not matter if a child is well-below or well-above the cutoff since this classification will be accurate regardless. However, for children near the cutoff (traditional area of uncertainty), a CAT can continue asking highly discriminative items to ensure appropriate precision above or below the threshold (McGlohen & Chang, 2008). CATs are of increasing interest in mental health assessment of youth (Gibbons et al., in press). Although not yet applied to assessment of irritability as a transdiagnostic risk marker in early childhood, recent psychometric work shows promise for this efficient screening approach. We recently demonstrated that parental report on only two MAP-DB irritability items (i.e., “easily frustrated” and “has destructive tantrums”) have good predictive utility for irritability-related DSM disorders (Wiggins, Briggs-Gowan, et al., 2018).
Recognizing the importance of brevity in screening, we also underscore that these CATs must be optimized to account for contextual factors external to the child that profoundly shape risk trajectories. For example, it is widely recognized that family stress and ecological adversity exacerbate neurodevelopmental problems and impede intervention uptake, but have only recently been considered in pediatric care (Sheldrick & Perrin, 2009). Co-occurring developmental delays or conditions (e.g., language delays, autism) may also affect risk thresholds (Kaat & Lecavalier, 2013). Optimally, CAT algorithms will evolve to incorporate these developmental and contextual factors as the basis for rapid decision-making about the need for, and level of, mental health prevention services during young children’s pediatric check-ups. CATs could also point to the need for more in-depth assessment prior to clinical determination for those children in the “gray area” (e.g., near the risk threshold for their age or due to the presence of other developmental and contextual factors that substantially increase risk) (McGlohen & Chang, 2008). In the field of early childhood mental health, the developmental complexities of clinical assessment too often make the perfect enemy of the good. There is urgent need to take the existing science base and tools, and partner with health services researchers and implementation scientists to generate robust methods for screening and prevention that can be implemented in real-world settings.
Joint consideration of behavioral indicators and biomarkers in risk determination is a key aspect of multi-level optimization of risk assessment. While its importance is widely recognized, actualization has been impeded by inadequate operationalization for clinical application (Casey et al., 2014). There are hints from recent research with very young children that this avenue of investigation will be clinically fruitful. For example, inattentive/impulsive behaviors as early as 18 months, with atypical pattern of sustained visual attention from 3 to 24 months predict ADHD (Miller et al., 2016). Similarly, atypical brain growth trajectories across the first years of life differentiate infants at high familial risk who do or do not develop autism (Piven, Elison, & Zylka, 2017). Further, neural markers of clinical risk have now been identified as early as 3–6 months of age, including brain structural abnormalities (e.g., enlarged brain volume), functional and information processing atypicalities (e.g., face processing), and non-linear electroencephalogram (EEG) features (e.g., dynamical features of time series such as entropy and fluctuations) (Bosl et al., 2018; Johnson, Gliga, Jones, & Charman, 2015).
Other observational studies highlight the interplay of biobehavioral patterns in shaping individual differences in outcomes for young children at risk (Buss, Davis, Ram, & Coccia, 2018). Joint consideration of irritability and executive control also elucidates heterogeneity in to psychopathology in young children (Healey, Brodzinsky, Bernstein, Rabinovitz, & Halperin, 2009). Towards this future direction, human connectomics that focus on the maturation of segmentation and integration of brain networks across development also show promise (Grayson & Fair, 2017). Neural biomarkers are of particular salience to the Mental Health, Earlier framework because they can be examined from birth, well before internally-directed self-regulation is present and earlier than clinically salient behavioral indicators have been identified (Uddin & Karlsgodt, 2018). Some of these (e.g., EEG) are measured easily and inexpensively, but the resource intensive nature of others (e.g., Magnetic resonance imaging(MRIs)) makes them ill-suited for screening. However, these neural biomarkers may prove to be very informative in second tier direct assessments, particularly for further differentiating children in the “gray area” who fall at the mid-point of the dimensional spectrum of risk. Identifying candidate biomarkers to enhance irritability-based risk assessment in early childhood is a critical step for advancing this line of inquiry. Validating these measures and creating clear, objective processing measures will be crucial steps in deploying them into wider practice.
The use of a transdiagnostic, dimensional irritability spectrum as a risk marker also necessitates a paradigm shift to consideration of multiple identification thresholds identifying probabilistic risk, rather than pass-fail cut-points (Robins et al., 2014). Early evidence also suggests that a repeated assessment over two time-points may yield a sufficiently stable estimate to identify preschoolers with impairing levels of irritability (Wiggins, Estabrook, et al., 2018), but more extensive modeling and population-based work is necessary.
Large-scale selective mental health risk prevention.
We posit that early prevention based on probabilistic risk is the critical next step. This Healthier Pillar points to the need for all families with young children who have a substantial risk of mental health problems to receive low-intensity developmentally oriented parent-training to promote self-regulation in children with early dysregulation. This expansive, selective prevention approach is justifiable based on risk-benefit analysis and the need for transdiagnostic prevention interventions deliverable in non-specialized settings (Walkup et al., 2017). Prevention is far more effective than remediation and has greater impact the earlier it is delivered (Knudsen et al., 2006). Reduction in life course economic and human capital costs are substantial when the adverse, spiraling effects of dysregulation on all aspects of health and development, and on the formative experiences that facilitate positive adaptation and functioning are prevented (Moffitt et al., 2013). Critically, there is virtually no risk of harm, as mental health promotion is beneficial for all children regardless of risk status. Finally, disorder specific evidence-based preventions (EBPs) are often complex and require intensive training, impeding their uptake even within mental health settings, much less primary care, underscoring the need for transdiagnostic, accessible prevention approaches (Walkup et al., 2017).
We propose this broad-brush approach across the early childhood period as a necessary starting point. The specific threshold warranting prevention across the dimensional spectrum remains to be empirically determined. However, clinically we postulate that a >50% risk would warrant receipt of this health promoting intervention. Rigorous empirical testing is needed in narrow-age bands spanning the early childhood period to optimize thresholds by age and to determine whether the intervention has peak and/or attenuated impact at different ages within early childhood. Strengthening children’s capacity to manage emotions and behavior adaptively is the essential shared element of virtually all EBPs for common emotional and behavioral problems of early childhood (Dishion et al., 2014; Mullany et al., 2012).
This first-tier selective intervention for irritable young children would be designed to reduce population attributable risk for early emerging mental disorder as identified by high and persistent irritability. If implemented within extant pediatric networks, this would also rapidly build a registry to generate evidence of large-scale effectiveness and shed light on factors that indicate higher intensity or specialized modality of intervention delivery are needed, following tiered models such as Triple P (Tully & Hunt, 2017). As previously described, the capacity to integrate behavioral and neural markers of risk at very young ages (Bosl et al., 2018) may also sharpen risk determination, beyond what is possible with behavior alone, in the fairly near future and improve the assessment modalities used to quantify that risk. Future research should also extend beyond this broad low intensity application to test a tiered or adaptive approach for those young children and families for whom the first level intervention proves inadequate.
Of course, benefits must be demonstrable in terms of prevention of enduring mental health problems, healthcare savings, and reduction in more intensive and costly service use. Although prevention science, developmental science, and neuroscience have largely proceeded along disparate lines, compelling evidence of the effectiveness of this approach will be derived via alteration of adverse neurodevelopmental trajectories towards adaptive brain:behavior patterns and healthy functioning. This must capitalize on advances in developmentally-sensitive neuroimaging and assessment that provide the capacity to detect neurodevelopmental impacts of intervention and psychopathology biomarkers from very early in life (Graham et al., 2015; Krogh-Jespersen, Kaldy, Valadez, Carter, & Woodward, 2018). Intermediate mechanisms that may explain this link would include reduced rate of escalation of irritability and impairment, and improvements in self-regulation, including executive function supported by optimal functioning of prefrontal regions (e.g., more typical recruitment of neural resources during frustration) (Grabell et al., 2017).
Robust experimental and observational research further supports the Mental Health, Earlier principle that a key to earlier life mental health prevention lays with promotion of early self-regulatory capacities. Improved self-regulation, measured as reductions in children’s externalizing behaviors including irritability, is the key mechanism of lifespan health improvements resulting from early intervention (Heckman et al., 2013). Indeed, the landmark 40-year Dunedin study demonstrated that the level of early childhood self-control is inversely related to the risk of chronic mental health problems, including suicidality, antisocial behavior, and substance abuse, and a broad range of other problems in health and functioning (Moffitt et al., 2013). Self-regulatory capacities are at the core of this early advantage because they impact children’s “fitness” for virtually all other domains of functioning, such as learning, psychological and social functioning, and healthy life styles (Murray et al., 2015; Shonkoff, 2003).
We acknowledge that implementing this broad early mental health promotion approach on a large-scale in primary care will likely require distillation of these self-regulation strategies into a simple accessible brief intervention. These must be available via a menu of modalities with free universal online training for providers. Recently, a number of promising methods along these lines have been developed and/or embedded within primary care, including video-based training, self-administered tablet-based training, and very brief motivational “check-ups” (Breitenstein, Fogg, Ocampo, Acosta, & Gross, 2016; Cates et al., 2016; Dishion et al., 2014). Unfortunately, the majority of the available brief interventions are proprietary and require specialized, often intensive, training that impedes uptake (Walkup et al., 2017). Non-proprietary, simplified, universal self-regulation preventions are requisite to the Mental Health, Earlier imperative to extend beyond the mental health clinic if the hoped for transformative impact is to be realized.
Steps to Population-level Implementation
Although population-level applications are often “backed into” in clinical research with resultant large research to practice gaps (Williams & Beidas, 2018), scalability is a formative Healthier principle. Prevention science has provided the necessary evidence to support scale up of effective programs that align with our model as well as highlighting numerous barriers to wide-scale implementation (Asarnow, Rozenman, Wiblin, & Zeltzer, 2015; Van Ryzin, Roseth, Fosco, Lee, & Chen, 2016). Here we discuss those factors that would facilitate implementation of Mental Health, Earlier in primary care.
Universal CAT-based screening.
Integrating CATs into the electronic medical record and providing multiple methods for caregivers to report (e.g., kiosks or tablets in waiting room, websites for mobile devices/PCs) must be routinized. Instituting screening practices in primary care is a significant challenge but it can be done. Factors that predict success with universal behavioral health screening are feasibility (e.g., CATs are brief and are administered and automatically scored electronically), availability of interventions for those meeting criteria for referral/intervention (e.g., non-proprietary), best practice alerts and other health information technology prompts and reminders are instituted in the electronic medical record regarding screening, and completion rates for screening are monetarily incentivized (via federal insurer mandates and accountable care payment bundles).
Improving population health of young children is incentivized in a value-based payment environment.
Reimbursement and incentive cannot be limited to screening for irritability, as there must be similar incentives for using evidence-based interventions and demonstrating implementation outcomes (e.g., high rates of reach into practice populations, delivery with high fidelity) as well as effectively preventing deleterious outcomes that are both proximal (e.g., reductions in irritability immediately after intervention) and distal (e.g., diagnosis of a mental health syndrome). Existing payment models could be effectively used for our approach, yet other payment models being proposed may be even more germane to early intervention and incentivization of children’s population health (Counts, Smith, & Crowley, 2018).
Behavioral health in primary care prioritizes health promotion and prevention.
Staffing of trained behavioral health providers must be sufficient to provide population level coverage for the large number of children and families in need of low- to moderate-intensity intervention. Providers also serve in their current roles when they are integrated or co-located in pediatric primary care, which is most often providing a mix of brief consultation to providers and caregivers about common behavioral health problems (e.g., crying, toileting, and picky eating), and more traditional mental health services for clinical-level problems (e.g., skills groups, psychotherapy, family counseling). The behavioral health workforce will need to both expand in number in primary care and be restructured. This is consistent with the PCMH model, which restructures the division of labor in primary healthcare settings by having all members of the team perform roles commensurate with their training and education. Nurses and community health workers manage patient education, health promotion, and outside referrals, whereas physicians, psychologists, and social workers focus on clinical issues and those in need of more intensive services in a tiered care model (Yarnall, Pollak, Ostbye, Krause, & Michener, 2003).
Evidence-based preventive interventions are adapted for primary care.
Unfortunately, appreciation for the need to close the practice-research gap by designing interventions with the end-user and system in which they are intended to be implemented in mind, is occurring well after the majority of existing evidence-based programs have been developed and tested (Neta et al., 2015). Thus, effective programs have been designed that cannot be readily translated to primary care given its unique demands, barriers, workflows, and structures. Building on the core components of these effective programs, existing protocols can be systematically adapted for primary care (Smith et al., 2018). The focus of adaptation needs to be on the service context and not on the content of the intervention (e.g., the active ingredients related to effects of the program). The delivery method (e.g., in-person vs. eHealth/mHealth) and in some cases the type of professional delivering the content (e.g., psychologist vs. community health worker/nurse) might not have a notable impact on clinical effectiveness but will markedly contribute to feasibility and sustainability within primary care (Chambers, Glasgow, & Stange, 2013).
Thus, making Mental Health, Earlier a reality will require evidence that this approach achieves the triple aim: better health outcomes, better experience of care for patients, and lower service delivery costs (Berwick, Nolan, & Whittington, 2008). Policy-level changes, payment models and monetary incentives, and a paradigm shift in both primary care providers’ screening and referral practices and perceiving a key role of behavioral health staff in primary care to deliver health promotion interventions will all facilitate implementation of this new approach. The NAM Forum on Children’s Cognitive, Affective, and Behavioral Health has been spearheading an effort on parenting programs in primary care, to advance the science and inform influential policy makers of the need to formally recognize these interventions, which could prompt reimbursement by payers and other facilitators of uptake (Chambers et al., 2013).
Conclusion
Given the robust evidence presented here regarding the antecedents and markers of chronic mental health conditions early in life, it is increasingly important to invest in earlier identification of mental health risk and population-level implementation of screening and evidence-based interventions. These will improve mental health and well-being to prevent illness, beginning in the first months and years of life. We must embrace, rather than distance ourselves from, variations in behavior over time that comprise trajectories of human development. Doing so will make it possible to recognize developmentally abnormal patterns of dysregulation beginning early in life in reliable, reproducible ways that are instrumental to accurate recognition of vulnerability to mental health problems. Such recognition will facilitate early-life interventions at the population-level, which—given the promise of currently available interventions—have the potential to alter the life course pattern of mental health and reduce population attributable risk for early starting and pernicious mental disorder.
Efforts to identify developmentally atypical behavior in early life are challenging and are typically framed in terms of the risk of over- or under-identification. Concerns about the potential for false-positive determinations that may prompt stigmatization and unnecessary utilization of resources have impeded realization of the promise of harnessing developmental vulnerabilities at the earliest possible time. There also remains skepticism about the informativeness and/or stability of developmentally-framed evaluations, including whether developmentally-guided assessments are up to the task. These reinforce the false negatives and trepidation of many pediatricians for taking advantage of early identification metrics for children under six. Such missed opportunities may be the rule rather than the exception in practice today.
We believe that such criticisms are fundamentally biased toward the status quo: children’s healthcare providers are systematically under-assessing children and are operating largely without a developmentally-informed roadmap. Of course, the importance of deliberate, empirically-based approaches cannot be overstated. However, it is also crucial to know when to take action based on the nomological net of evidence propelling us forward. That is, to recognize when the evidence base is “good enough” for clinical, real-world application. Joining a growing and diverse chorus calling for action (Conti & Heckman, 2013; Luby, 2012; Shonkoff, 2016), we must connect the dots across the extensive and rapidly growing developmental, neuroscientific, clinical, and public health science base to transform approaches to mental health promotion and disease prevention. The tolls of waiting on the quality of life and human capital, and the public health burden flowing from mental health problems are too great (Knudsen et al., 2006). Indeed, costs of establishing and changing infrastructure to implement interventions, such as a public health parenting program, are modest and likely to yield savings from reductions in future mental and physical health care-related expenditures (Knudsen et al., 2006; Moffitt et al., 2013; Sanders & Mazzucchelli, 2013).
For too long, the broad range of normal human development has served as a cautionary deterrent to early life mental health identification, prevention, and intervention. With transdisciplinary scientific advances in hand, this can now be recast as an opportunity to identify and prevent malleable risk-conferring behaviors in the first years of life. The Mental Health, Earlier approach rests on probabilistic risk rather than absolute certainty and recognizes that variability in early childhood behavior is the developmental norm. Still this heterogeneity can be meaningfully reduced to specify atypical patterns that are highly likely to result in mental health problems and lifespan reductions in human capital and therefore worthy of our attention and intervention. The public health burden of mental disorders across the lifespan compels us to advance the science of early detection and implement detection and intervention efforts that can achieve and sustain children’s mental health, earlier.
Acknowledgements:
This work was supported in part by funding from the National Institute Mental Health to Wakschlag (2U01MH082830 and 1R01MH107652); the National Institute of Deafness and Communication Disorders to Norton and Wakschlag (1R01DC016273); the American Heart Association to Marino (17SFRN33660752); and the National Institute on Drug Abuse to C. Hendricks Brown, in support of Justin D. Smith (DA027828) that supports the Center for Prevention Implementation Methodology for Drug Abuse and HIV. We thank the many colleagues who have contributed to the evolution of this work through collaboration, support and inspiring conversations including Erica Anderson, James Blair, Margaret BriggsGowan, David Cella, Ryne Estabrook, Ellen Leibenluft, Joan Luby, Vijay Mittal, Susan Perlman, Amelie Petitclerc, Daniel Pine, and Thomas Shanley. The Institute for Innovations in Developmental Sciences (DevSci) has also provided an exceptional transdisciplinary platform for this work.
References
- Achenbach TM (1997). What is normal? What is abnormal? Developmental perspectives on emotional and behavioral problems. In Luthar SS, Burack JA, Cichetti D, & Weisz JR (Eds.), Developmental psychopathology: Perspectives on adjustment, risk and disorder (pp. 93–114). New York, NY, US: Cambridge University Press. [Google Scholar]
- Asarnow JR, Kolko DJ, Miranda J, & Kazak AE (2017). The pediatric patient-centered medical home: Innovative models for improving behavioral health. American Psychologist, 72(1), 13–27. doi: 10.1037/a0040411 [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Rozenman M, Wiblin J, & Zeltzer L (2015). Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: A meta-analysis. JAMA Pediatrics, 169(10), 929–937. doi: 10.1001/jamapediatrics.2015.1141 [DOI] [PubMed] [Google Scholar]
- Baillargeon RH, Normand C, Séguin JR, Zoccolillo M, Japel C, Pérusse D, … Tremblay RE (2007). The evolution of problem and social competence behaviors during toddlerhood: A prospective population-based cohort survey. Infant Mental Health Journal, 28(1), 12–38. doi: 10.1002/imhj.20120 [DOI] [PubMed] [Google Scholar]
- Belden AC, Thompson NR, & Luby JL (2008). Temper tantrums in healthy versus depressed and disruptive preschoolers: Defining tantrum behaviors associated with clinical problems. The Journal of Pediatrics, 152, 117–122. doi: 10.1016/j.jpeds.2007.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM, Nolan TW, & Whittington J (2008). The triple aim: Care, health, and cost. Health Affairs, 27(3), 759–769. doi: 10.1377/hlthaff.27.3.759 [DOI] [PubMed] [Google Scholar]
- Biedzio D, & Wakschlag LS (in press). Developmental emergence of disruptive behaviors beginning in infancy: Delineating normal:abnormal boundaries to enhance early identification. In Zeenah C (Ed.), Handbook of infant mental health (4th ed.). New York: Guilford Press. [Google Scholar]
- Bilgin A, Baumann N, Jaekel J, Breeman LD, Bartmann P, Bauml JG, … Wolke D (2018). Early crying, sleeping, and feeding problems and trajectories of attention problems from childhood to adulthood. Child Development Advance online publication. doi: 10.1111/cdev.13155 [DOI] [PubMed]
- Bloom B, Jones LI, & Freeman G (2013). Summary health statistics for U.S. children: National Health Interview Survey, 2012. Vital and Health Statistics 10(258), 1–81. [PubMed] [Google Scholar]
- Boat TF (2015). Improving lifetime health by promoting behavioral health in children. JAMA, 313(15), 1509–1510. doi: 10.1001/jama.2015.2977 [DOI] [PubMed] [Google Scholar]
- Bornstein MH (2014). Human infancy…and the rest of the lifespan. Annual Review of Psychology, 65, 121–158. doi: 10.1146/annurev-psych-120710-100359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl WJ, Tager-Flusberg H, & Nelson CA (2018). EEG analytics for early detection of autism spectrum disorder: A data-driven approach. Scientific Reports, 8(1), 6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein SM, Fogg L, Ocampo EV, Acosta DI, & Gross D (2016). Parent use and efficacy of a self-administered, tablet-based parent training intervention: A randomized controlled trial. JMIR mHealth and uHealth, 4(2). e36. doi: 10.2196/mhealth.5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, & Cicchetti D (2004). The Brief Infant-Toddler Social and Emotional Assessment (BITSEA): Screening for social-emotional problems and delays in competence. Journal of Pediatric Psychology, 29(2), 143–155. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Nichols S, Voss J, Zobel E, Carter AS, McCarthy K, … Wakschlag LS (2014). Punishment insensitivity and impaired reinforcement learning in preschoolers. Journal of Child Psychology and Psychiatry, 55(2), 154–161. doi: 10.1111/jcpp.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, & Beardslee W (2016). Realizing population-level improvements for all children’s cognitive, affective, and behavioral health: Introduction to the special issue. American Journal of Preventive Medicine, 51(4), S101–S105. doi: 10.1016/j.amepre.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Raglin Bignall W, & Ammerman R (2018). Preventive behavioral health programs in primary care: A systematic review. Pediatrics, 141(5), e20170611. doi: 10.1542/peds.2017-0611 [DOI] [PubMed] [Google Scholar]
- Buss KA (2011). Which fearful toddlers should we worry about: Context, fear regulation and anxiety risk. Developmental Psychology, 47(3), 804–819. doi: 10.1037/a0023227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Ram N, & Coccia M (2018). Dysregulated fear, social inhibition, and respiratory sinus arrhythmia: A replication and extension. Child Development, 89(3), e214–e228. doi: 10.1111/cdev.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, & Insel T (2014). A neurodevelopmental perspective on the Research Domain (RDoC) framework. Biological Psychiatry, 76(5), 350–353. doi: 10.1016/j.biopsych.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Cates CB, Weisleder A, Dreyer BP, Johnson SB, Vlahovicova K, Ledesma J, & Mendelsohn AL (2016). Leveraging healthcare to promote responsive parenting: Impacts of the video interaction project on parenting stress. Journal of Child and Family Studies, 25(3), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DA, Glasgow RE, & Stange KC (2013). The dynamic sustainability framework: Addressing the paradox of sustainment amid ongoing change. Implementation Science, 8(1), 117. doi: 10.1186/1748-5908-8-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Filgueiras A, Squires J, & Landeira-Fernandez J (2016). Examining the factor structure of an early childhood social emotional screening assessment. Journal of Special Education and Rehabilitation, 17(3–4), 89–104. [Google Scholar]
- Comer JS, Chow C, Chan P, Cooper C, & Wilson LA (2013). Psychosocial treatment efficacy for disruptive behavior problems in very young children: A meta-analytic examination. Journal of the American Academy of Child & Adolescent Psychiatry, 52(1), 26–36. doi: 10.1016/j.jaac.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti G, & Heckman JJ (2013). The developmental approach to child and adult health. Pediatrics, 131, S133–S141. doi: 10.1542/peds.2013-0252d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts N, Smith JD, & Crowley DM (2018). (Expected) value-based payment: From total cost of care to net present value of care. Under review [DOI] [PMC free article] [PubMed]
- de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, & Bouter LM (2006). Minimal changes in health status questionnaires: Distinction between minimally detectable change and minimally important change. Health and Quality of Life Outcomes, 4(54). doi: 10.1186/1477-7525-4-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis A, Coster W, Bigsby R, & Lester B (2004). Colic and fussing in infancy, and sensory processing at 3 to 8 years of age. Infant Mental Health Journal, 25(6), 522–539. doi: 10.1002/imhj.20025 [DOI] [Google Scholar]
- Dirks M, De Los Reyes A, Briggs-Gowan MJ, Cella D, & Wakschlag LS (2012). Annual research review: Embracing not erasing contextual variability in children’s behavior--theory and utility in the selection and use of methods and informants in developmental psychopathology. Journal of Child Psychology and Psychiatry, 53(5), 558–574. doi: 10.1111/j.1469-7610.2012.02537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Brennan LM, Shaw DS, McEachern AD, Wilson MN, & Jo B (2014). Prevention of problem behavior through annual family check-ups in early childhood: Intervention effects from home to early elementary school. Journal of Abnormal Child Psychology, 42(3), 343–354. doi: 10.1007/s10802-013-9768-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Leppert KA, Merwin SM, Smith VC, Bufferd SJ, & Kushner MR (2015). Advances and directions in preschool mental health research. Child Development Perspectives, 9(1), 14–19. doi: 10.1111/cdep.12099 [DOI] [Google Scholar]
- Gibbons R, Kupfer D, Frank E, Lahey B, George-Milford B, Biernesser C, … Brent D (in press). A new statistical paradigm for measuring psychopathology dimensions in youth [DOI] [PMC free article] [PubMed]
- Grabell AS, Li Y, Barker JW, Wakschlag LS, Huppert TJ, & Perlman SB (2017). Evidence of non-linear associations between frustration-related prefrontal cortex activation and the normal:abnormal spectrum of irritability in young children. Journal of Abnormal Child Psychology, 46(1), 137–147. doi: 10.1007/s10802-017-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, & Fair DA (2015). The potential of infant fMRI research and the study of early life stress as a promising exemplar. Developmental Cognitive Neuroscience, 12, 12–39. doi: 10.1016/j.dcn.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, & Fair DA (2017). Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage, 160, 15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DF (2017). The early development of human aggression. Child Development Perspectives, 11(2), 102–106. doi: 10.1111/cdep.12220 [DOI] [Google Scholar]
- Healey DM, Brodzinsky L, Bernstein B, Rabinovitz B, & Halperin J (2009). Moderating effects of neurocognitive abilities on the relationship between temperament and global functioning. Child NeuroPsychology, 16(1), 20–31. doi: 10.1080/09297040902984490 [DOI] [PubMed] [Google Scholar]
- Heckman JJ, Pinto R, & Savelyev P (2013). Understanding the mechanisms through which an influential early childhood program boosted adult outcomes. American Economic Review, 103(6), 2052–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi MH, Wolke D, & Schneider S (2011). Associations between problems with crying, sleeping and/or feeding in infancy and long-term behavioural outcomes in childhood: A meta-analysis. Archives of Disease in Childhood, 96(7), 622–629. doi: 10.1136/adc.2010.191312 [DOI] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: A dual-processing perspective. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(1), 207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R, O’Callaghan MJ, Bor W, Williams GM, & Najman JM (2012). Long-term outcomes of infant behavioral dysregulation. Pediatrics, 130(5), e1243–1251. doi: 10.1542/peds.2010-3517 [DOI] [PubMed] [Google Scholar]
- Insel TR (2009). Disruptive insights in psychiatry: Transforming a clinical discipline. Journal of Clinical Investigation, 119(4), 700–705. doi: 10.1172/JCI38832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Gliga T, Jones E, & Charman T (2015). Annual research review: Infant development, autism, and ADHD–early pathways to emerging disorders. Journal of Child Psychology and Psychiatry, 56(3), 228–247. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Blackwell C, Estabrook R, Briggs-Gowan MJ, Petitclerc A, Burns J, … Wakschlag LS (2018). Linking the Child Behavior Checklist (CBCL) with the Multidimensional Assessment Profile of Disruptive Behavior (MAP-DB): Advancing a dimensional spectrum approach to disruptive behavior. Journal of Child and Family Studies, 1–11. 10.1007/s10826-018-1272-4 [DOI] [PMC free article] [PubMed]
- Kaat AJ, & Lecavalier L (2013). Disruptive behavior disorders in children and adolescents with autism spectrum disorders: A review of the prevalence, presentation, and treatment. Research in Autism Spectrum Disorders, 7(12), 1579–1594. doi: 10.1016/j.rasd.2013.08.012 [DOI] [Google Scholar]
- Knudsen E, Heckman JJ, Cameron JL, & Shonkoff JP (2006). Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proceedings of the National Academy of Sciences, 103(27), 10155–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh-Jespersen S, Kaldy Z, Valadez AG, Carter AS, & Woodward AL (2018). Goal prediction in 2-year-old children with and without autism spectrum disorder: An eye-tracking study. Autism Research, 11(6), 870–882. doi: 10.1002/aur.1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie LK, Mehus CJ, Hawkins JD, Boat T, McCabe MA, Barkin S, … Beardslee W (2016). Primary health care: Potential home for family-focused preventive interventions. American Journal of Preventive Medicine, 51(4), S106–118. doi: 10.1016/j.amepre.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SX, Morrison L, Smith PW, Hargood C, Weal M, & Yardley L (2016). Properties of bootstrap tests for N-of-1 studies. The British Journal of Mathematical and Statistical Psychology, 69(3), 276–290. doi: 10.1111/bmsp.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL (2012). Dispelling the “they’ll grow out of it” myth: Implications for intervention. American Journal of Psychiatry, 169(11), 1127–1129. doi: 10.1176/appi.ajp.2012.12081037 [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, … Gilmore JH (2015). Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex, 25(8), 2204–2212. doi: 10.1093/cercor/bhu027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino BS, Cassedy A, Drotar D, & Wray J (2016). The impact of neurodevelopmental and psychosocial outcomes on health-related quality of life in survivors of congenital heart disease. The Journal of Pediatrics, 174, 11–22. [DOI] [PubMed] [Google Scholar]
- Marks KP, Page Glascoe F, & Macias MM (2011). Enhancing the algorithm for developmental–behavioral surveillance and screening in children 0 to 5 years. Clinical Pediatrics, 50(9), 853–868. [DOI] [PubMed] [Google Scholar]
- McGlohen M, & Chang HH (2008). Combining computer adaptive testing technology with cognitively diagnostic assessment. Behavior Research Methods, 40(3), 808–821. [DOI] [PubMed] [Google Scholar]
- McGorry P, & Nelson B (2016). Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry, 73(3), 191–192. doi: 10.1001/jamapsychiatry.2015.2868 [DOI] [PubMed] [Google Scholar]
- Medical Home Initiatives for Children With Special Needs Project Advisory Committee. (2002). The medical home. Pediatrics, 110(1 Pt 1), 184–186.12093969 [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, & Koretz DS (2010). Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics, 125(1), 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, Hill MM, & Ozonoff S (2016). Early detection of ADHD: Insights from infant siblings of children with autism. Journal of Clinical Child & Adolescent Psychology, 47(5), 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, & Wakschlag LS (2017). Research domain criteria (RDoC) grows up: Strengthening neurodevelopmental investigation within the RDoC framework. Journal of Affective Disorders, 216, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Poulton R, & Caspi A (2013). Lifelong impact of early self-control. American Scientist, 101(5), 352. [Google Scholar]
- Mullany B, Barlow A, Neault N, Billy T, Jones T, Tortice I, … Reid R (2012). The Family Spirit trial for American Indian teen mothers and their children: CBPR rationale, design, methods and baseline characteristics. Prevention Science, 13(5), 504–518. [DOI] [PubMed] [Google Scholar]
- Murray D, Rosanbalm K, Christopoulous C, & Hamouidi A (2015). Self-regulation and toxic stress: Foundations for understanding self-regulation from an applied developmental perspective. OPRE Report #2015–21
- Neta G, Glasgow RE, Carpenter CR, Grimshaw JM, Rabin BA, Fernandez ME, & Brownson RC (2015). A framework for enhancing the value of research for dissemination and implementation. American Journal of Public Health, 105(1), 49–57. doi: 10.2105/AJPH.2014.302206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols S, Briggs-Gowan MJ, Estabrook R, Henry DB, Burns JL, Kestler J, & Wakschlag LS (2014). Punishment insensitivity in early childhood: A developmental, dimensional approach. Journal of Abnormal Child Psychology, 43(6), 1011–1023. doi: 10.1007/s10802-014-9950-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennap D, Zito JM, Santosh PJ, Tom SE, Onukwugha E, & Magder LS (2018). Patterns of early mental health diagnosis and medication treatment in a medicaid-insured birth cohort. JAMA Pediatrics, 172(6), 576–584. doi: 10.1001/jamapediatrics.2018.0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin EC, Sheldrick R, McMenamy JM, Henson BS, & Carter AS (2014). Improving parenting skills for families of young children in pediatric settings: A randomized clinical trial. JAMA Pediatrics, 168(1), 16–24. doi: 10.1001/jamapediatrics.2013.2919 [DOI] [PubMed] [Google Scholar]
- Petitclerc A, Briggs-Gowan MJ, Estabrook R, Burns J, Anderson E, McCarthy K, & Wakschlag LS (2015). Contextual variation in young children’s observed disruptive behavior on the DB-DOS: implications for early identification. Journal of Child Psychology and Psychiatry, 56(9), 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DA, & Shonkoff JP (2000). From neurons to neighborhoods: The science of early childhood development Washington DC: National Academies Press. [PubMed] [Google Scholar]
- Pine D, & Fox NA (2015). Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology, 66, 459–485. [DOI] [PubMed] [Google Scholar]
- Piven J, Elison JT, & Zylka MJ (2017). Toward a conceptual framework for early brain and behavior development in autism. Molecular Psychiatry, 22(10), 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MR, Brenner RA, Schisterman EF, Vik T, & Mills JL (2004). Long term cognitive development in children with prolonged crying. Archives of Disease in Childhood, 89(11), 989–992. doi: 10.1136/adc.2003.039198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC (2013). Recommendation for measurement of self-regulation in early childhood. In Memos on measures of social-emotional development in early childhood (pp. 36–42). New York, New York. [Google Scholar]
- Robins DL, Casagrande K, Barton M, Chen CMA, Dumont-Mathieu T, & Fein D (2014). Validation of the Modified Checklist for Autism in Toddlers, Revised With Follow-up (M-CHAT-R/F). Pediatrics, 133(1), 37–45. doi: 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MR, & Mazzucchelli TG (2013). The promotion of self-regulation through parenting interventions. Clinical Child and Family Psychology Review, 16(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Schaefer JD, Caspi A, Belsky DW, Harrington H, Houts R, Horwood LJ, … Moffitt TE (2017). Enduring mental health: Prevalence and prediction. Journal of Abnormal Psychology, 126(2), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick RC, Henson BS, Neger EN, Merchant S, Murphy JM, & Perrin EC (2013). The baby pediatric symptom checklist: Development and initial validation of a new social/emotional screening instrument for very young children. Academic Pediatrics, 13(1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick RC, & Perrin EC (2009). Surveillance of children’s behavior and development: Practical solutions for primary care. Journal of Developmental and Behavioral Pediatrics, 30(2), 151–153. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP (2003). From neurons to neighborhoods: Old and new challenges for developmental and behavioral pediatrics. Journal of Developmental and Behavioral Pediatrics, 24(1), 70–76. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP (2016). Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatrics, 170, 1003–1007. doi: 10.1001/jamapediatrics.2016.1559 [DOI] [PubMed] [Google Scholar]
- Smith JD, Berkel C, Rudo-Stern J, Montaño Z, St., George SM, Prado G, … Dishion TJ (2018). The Family Check-Up 4 Health (FCU4Health): Applying implementation science frameworks to the process of adapting an evidence-based parenting program for prevention of pediatric obesity and excess weight gain in primary care. Frontiers in Public Health, 6(293). doi: 10.3389/fpubh.2018.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Montaño Z, Dishion TJ, Shaw DS, & Wilson MN (2015). Preventing weight gain and obesity: Indirect effects of the family check-up in early childhood. Prevention Science, 16(3), 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force on Research Diagnostic Criteria: Infancy, Preschool. (2003). Research diagnostic criteria for infants and preschool children: The process and empirical support. Journal of the American Academy of Child & Adolescent Psychiatry, 42, 1504–1512. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, & Japel C (2003). Prevention during pregnancy, infancy, and the preschool years. In Farrington DP & Coid JW (Eds.), Early prevention of adult antisocial behaviour (pp. 205–242). Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Tully LA, & Hunt C (2017). A randomized controlled trial of a brief versus standard group parenting program for toddler aggression. Aggressive Behavior, 43(3), 291–303. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, & Karlsgodt KH (2018). Future directions for examination of brain networks in neurodevelopmental disorders. Journal of Clinical Child & Adolescent Psychology, 47(3), 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryzin MJ, Roseth CJ, Fosco GM, Lee YK, & Chen IC (2016). A component-centered meta-analysis of family-based prevention programs for adolescent substance use. Clinical Psychology Review, 45, 72–80. doi: 10.1016/j.cpr.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp B, & van der Linden W (2002). Multidimensional adaptive testing with constraints on test content. Psychometrika, 67, 575–588. [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The status of irritability in psychiatry: A conceptual and quantitative review. Journal of the American Academy of Child & Adolescent Psychiatry, 55(7), 556–570. doi: 10.1016/j.jaac.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Carter AS, Hill C, Danis B, Keenan K, … Leventhal BL (2007). A developmental framework for distinguishing disruptive behavior from normative misbehavior in preschool children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(Special Issue on Preschool Psychopathology), 976–987. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi S, Nichols S, Kestler J, Burns J, … Henry D (2014). Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 82–96. doi: 10.1016/j.jaac.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Hill C, Danis B, Leventhal B, Keenan K, … Carter A (2008). Observational assessment of preschool disruptive behavior, Part II: Validity of the Disruptive Behavior Diagnostic Observation Schedule (DB-DOS). Journal of the American Academy of Child & Adolescent Psychiatry, 47(6), 632–641. doi: 10.1097/CHI.0b013e31816c5c10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Choi SW, Carter AS, Hullsiek H, Burns J, McCarthy K, … Briggs-Gowan MJ (2012). Defining the developmental parameters of temper loss in young children: Implications for developmental psychopathology. Journal of Child Psychiatry and Psychology, 53, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, … Briggs-Gowan MJ (2015). Clinical implications of a dimensional approach: The normal:abnormal spectrum of early irritability. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 626–634. doi: 10.1016/j.jaac.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Krogh-Jespersen S, Reed T, Heard-Garris N, Kessler C, Moskowitz J, … Norton L (2018). Contribution of prenatal stress to mental health risk: A systematic review. In preparation
- Wakschlag LS, Leventhal B, & Thomas B (2007). Disruptive behavior disorders & ADHD in preschool children: Characterizing heterotypic continuities for a developmentally informed nosology for DSM V. In Narrow W, First M, Sirovatka P, & Regier D (Eds.), Age and gender considerations in psychiatric diagnosis: A research agenda for DSM-V (pp. 243–258). Arlington, VA: American Psychiatric Association [Google Scholar]
- Wakschlag LS, Perlman S, Blair R, Leibenluft E, Briggs-Gowan M, & Pine D (2018). The neurodevelopmental basis of early childhood disruptive behavior: Irritable and callous phenotypes as exemplars. American Journal of Psychiatry, 175, 114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Tolan P, & Leventhal B (2010). “Ain’t misbehavin”: Towards a developmentally-specified nosology for preschool disruptive behavior. Journal of Child Psychology and Psychiatry, 51, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Mathews T, & Green C (2017). Transdiagnostic behavioral therapies in pediatric primary care: Looking ahead. JAMA Psychiatry, 74(6), 557–558. doi: 10.1001/jamapsychiatry.2017.0448 [DOI] [PubMed] [Google Scholar]
- Whalen DJ, Luby JL, Tilman R, Mike A, Barch D, & Belden AC (2016). Latent class profiles of depressive symptoms from early to middle childhood: Predictors, outcomes, and gender effects. Journal of Child Psychology and Psychiatry, 57(7), 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Briggs-Gowan MJ, Voss JL, Petitclerc A, McCarthy K, Blair R, R. J, & Wakschlag LS (2016). Can the fear recognition deficits associated with callous-unemotional traits be identified in early childhood? Journal of Clinical and Experimental Neuropsychology, 38(6), 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Briggs-Gowan MJ, Estabrook R, Brotman M, Pine DS, Leibenluft E, & Wakschlag LS (2018). Identifying clinically significant irritability in early childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 57(3), 191–199. doi: 10.1016/j.jaac.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Estabrook R, Leibenluft E, Brotman MP, D.S, Briggs-Gowan MJ, & Wakschlag LS (2018). Generating developmentally-based empirical parameters for DMDD in early childhood. Under review [Google Scholar]
- Williams NJ, & Beidas RS (2018). Annual research review: The state of implementation science in child psychology and psychiatry: A review and suggestions to advance the field. Journal of Child Psychology and Psychiatry Advance online publication. doi: 10.1111/jcpp.12960 [DOI] [PMC free article] [PubMed]
- Wolke D, Rizzo P, & Woods S (2002). Persistent infant crying and hyperactivity problems in middle childhood. Pediatrics, 109(6), 1054–1060. [DOI] [PubMed] [Google Scholar]
- Yarnall KS, Pollak KI, Ostbye T, Krause KM, & Michener JL (2003). Primary care: Is there enough time for prevention? American Journal of Public Health, 93(4), 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]